Cranberry Proanthocyanidins Mitigate Reflux-Induced Transporter Dysregulation in an Esophageal Adenocarcinoma Model
Abstract
1. Introduction
2. Results
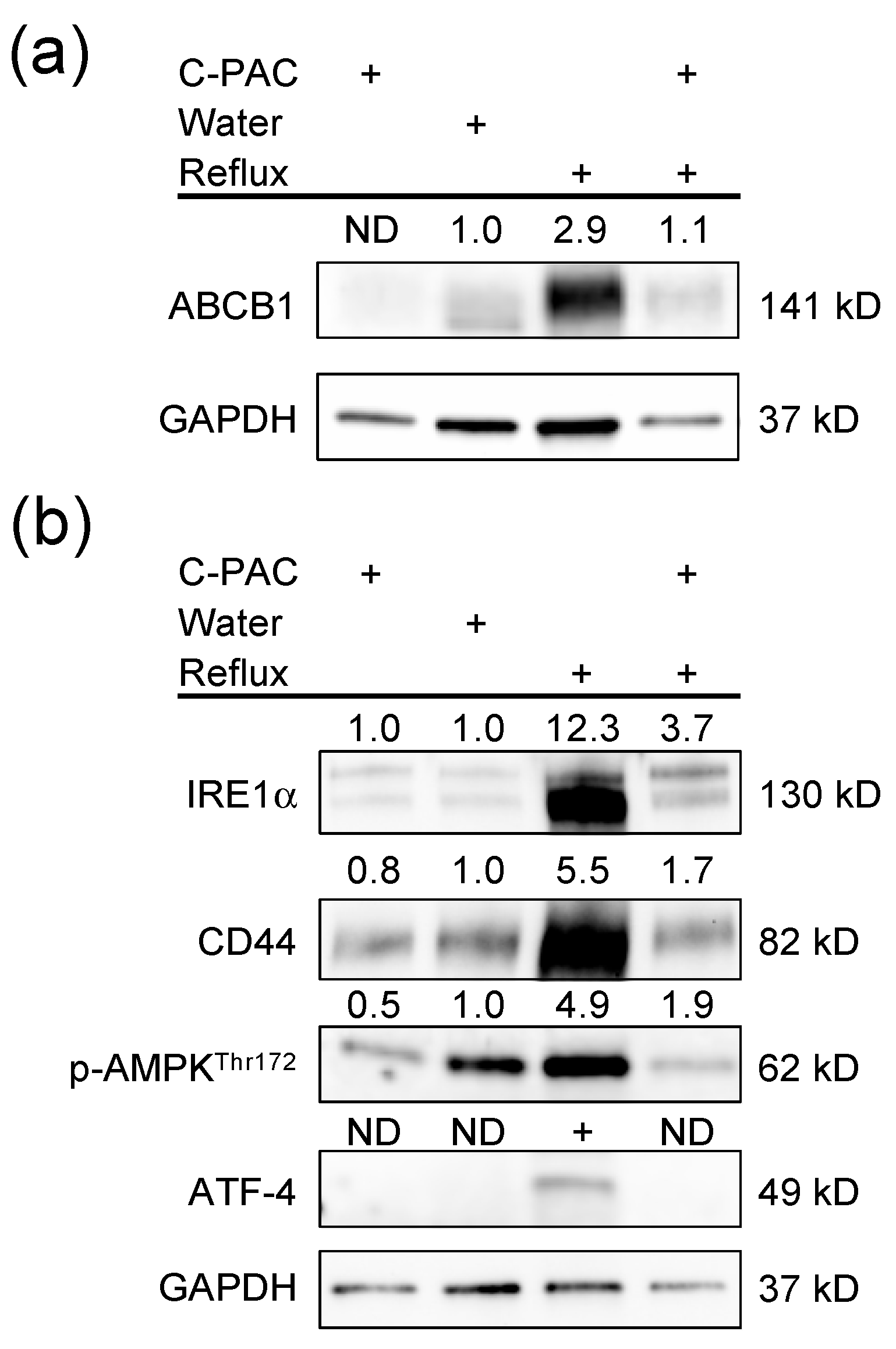
2.1. C-PACs Mitigate Reflux-Induced Alterations in ABC Transporter Expression in the Rat Esophagus
2.2. C-PACs Mitigate Reflux-Induced Alterations in SLC Transporter Expression in the Rat Esophagus
2.3. Aquaporin and Additional Transporters Dysregulated in Reflux-Induced EAC and Restored by C-PACs
2.4. Transporter Expression Altered by C-PACs in the Normal Rat Esophagus
2.5. Transporter Dysregulation Observed in Human Esophageal Cancer and Corresponding Pathway Enrichment
3. Discussion
4. Materials and Methods
4.1. Esophagogastroduodenal Anastomosis (EGDA) Surgical Model of Reflux-Induced EAC and C-PAC Delivery
4.2. Rat Esophageal RNA Isolation, RNA Sequencing and Transporter Expression Analyses
4.3. GEO Dataset Renormalization and Analysis
4.4. Tissue Lysate Isolation and Western Blot Analysis
4.5. Pathway Analysis and Protein Interaction Prediction
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.M.; Devesa, S.S.; Chow, W.H. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J. Natl. Cancer Inst. 2008, 100, 1184–1187. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Hang, T.P.; Spiritos, Z.; Gamboa, A.M.; Chen, Z.; Force, S.; Patel, V.; Chawla, S.; Keilin, S.; Saba, N.F.; El-Rayes, B.; et al. Epidemiology of Early Esophageal Adenocarcinoma. Clin. Endosc. 2022, 55, 372. [Google Scholar] [CrossRef]
- Runge, T.M.; Abrams, J.A.; Shaheen, N.J. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol. Clin. N. Am. 2015, 44, 203–231. [Google Scholar] [CrossRef]
- Coleman, H.G.; Xie, S.H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef]
- Lagergren, J.; Bergstrom, R.; Lindgren, A.; Nyren, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999, 340, 825–831. [Google Scholar] [CrossRef]
- El-Serag, H. The association between obesity and GERD: A review of the epidemiological evidence. Digest Dis. Sci. 2008, 53, 2307–2312. [Google Scholar] [CrossRef]
- Friedenberg, F.K.; Xanthopoulos, M.; Foster, G.D.; Richter, J.E. The association between gastroesophageal reflux disease and obesity. Am. J. Gastroenterol. 2008, 103, 2111–2122. [Google Scholar] [CrossRef]
- Hampel, H.; Abraham, N.S.; El-Serag, H.B. Meta-analysis: Obesity and the risk for gastroesophageal reflux disease and its complications. Ann. Intern. Med. 2005, 143, 199–211. [Google Scholar] [CrossRef]
- Wang, Q.L.; Xie, S.H.; Li, W.T.; Lagergren, J. Smoking Cessation and Risk of Esophageal Cancer by Histological Type: Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2017, 109, djx115. [Google Scholar] [CrossRef]
- Chandar, A.K.; Iyer, P.G. Role of Obesity in the Pathogenesis and Progression of Barrett’s Esophagus. Gastroenterol. Clin. N. Am. 2015, 44, 249–264. [Google Scholar] [CrossRef]
- Drahos, J.; Xiao, Q.; Risch, H.A.; Freedman, N.D.; Abnet, C.C.; Anderson, L.A.; Bernstein, L.; Brown, L.; Chow, W.H.; Gammon, M.D.; et al. Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int. J. Cancer 2015, 138, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Nimptsch, K.; Steffen, A.; Pischon, T. Obesity and Oesophageal Cancer. Recent Results Cancer Res. 2016, 208, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Emerenziani, S.; Rescio, M.P.; Guarino, M.P.; Cicala, M. Gastro-esophageal reflux disease and obesity, where is the link? World J. Gastroenterol. 2013, 19, 6536–6539. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Boeckxstaens, G.; Smout, A.J. Management of the patient with incomplete response to PPI therapy. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 401–414. [Google Scholar] [CrossRef]
- Rubenstein, J.H.; Jiang, L.; Kurlander, J.E.; Chen, J.; Metko, V.; Khodadost, M.; Nofz, K.; Raghunathan, T. Incomplete Response of Gastroesophageal Reflux Symptoms Poorly Predicts Erosive Esophagitis or Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2021, 19, 2284–2292. [Google Scholar] [CrossRef]
- Sohda, M.; Kuwano, H. Current Status and Future Prospects for Esophageal Cancer Treatment. Ann. Thorac. Cardiovas. Surg. 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Haisley, K.R.; Hart, K.D.; Nabavizadeh, N.; Bensch, K.G.; Vaccaro, G.M.; Thomas, C.R.; Schipper, P.H.; Hunter, J.G.; Dolan, J.P. Neoadjuvant chemoradiotherapy with concurrent cisplatin/5-fluorouracil is associated with increased pathologic complete response and improved survival compared to carboplatin/paclitaxel in patients with locally advanced esophageal cancer. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Blum Murphy, M.; Xiao, L.; Patel, V.R.; Maru, D.M.; Correa, A.M.; Amlashi, F.G.; Liao, Z.; Komaki, R.; Lin, S.H.; Skinner, H.D.; et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer Am. Cancer Soc. 2017, 123, 4106–4113. [Google Scholar] [CrossRef]
- Weh, K.M.; Howard, C.L.; Zhang, Y.; Tripp, B.A.; Clarke, J.L.; Howell, A.B.; Rubenstein, J.H.; Abrams, J.A.; Westerhoff, M.; Kresty, L.A. Prebiotic proanthocyanidins inhibit bile reflux-induced esophageal adenocarcinoma through reshaping the gut microbiome and esophageal metabolome. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Ding, W.Y.; Bondoc, F.; Curtis, S.K.; Yang, C.S. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis 1999, 20, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef]
- Okada, Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem. Biophys. 2004, 41, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Pote, M.S.; Gacche, R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lapucci, A.; Landini, I.; Coronnello, M.; Roviello, G.; Mini, E. Role of ATP-binding cassette transporters in cancer initiation and progression. Semin. Cancer Biol. 2020, 60, 72–95. [Google Scholar] [CrossRef]
- Moon, C.S.; Moon, D.; Kang, S.K. Aquaporins in Cancer Biology. Front. Oncol. 2022, 12, 782829. [Google Scholar] [CrossRef]
- Assounga, A.G.; Warner, C.M. Transcription of major histocompatibility complex class I (Kb) and transporter associated with antigen processing 1 and 2 genes is up-regulated with age. Immunology 2004, 113, 378–383. [Google Scholar] [CrossRef]
- Colas, C.; Laine, E. Targeting Solute Carrier Transporters through Functional Mapping. Trends Pharmacol. Sci. 2021, 42, 3–6. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef]
- Anderson, C.M.; Stahl, A. SLC27 fatty acid transport proteins. Mol. Asp. Med. 2013, 34, 516–528. [Google Scholar] [CrossRef]
- Hagenbuch, B.; Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Asp. Med. 2013, 34, 396–412. [Google Scholar] [CrossRef]
- Donowitz, M.; Tse, C.M.; Fuster, D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Asp. Med. 2013, 34, 236–251. [Google Scholar] [CrossRef]
- Quednau, B.D.; Nicoll, D.A.; Philipson, K.D. The sodium/calcium exchanger family—SLC8. Pflug. Arch. Eur. J. Phys. 2004, 447, 543–548. [Google Scholar] [CrossRef]
- Koepsell, H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol. Asp. Med. 2013, 34, 413–435. [Google Scholar] [CrossRef]
- Pelis, R.M.; Wright, S.H. SLC22, SLC44, and SLC47 Transporters-Organic Anion and Cation Transporters: Molecular and Cellular Properties. Curr. Top. Membr. 2014, 73, 233–261. [Google Scholar] [CrossRef]
- Romero, M.F.; Chen, A.P.; Parker, M.D.; Boron, W.F. The SLC4 family of bicarbonate (HCO3−) transporters. Mol. Asp. Med. 2013, 34, 159–182. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. BBA Mol. Cell Res. 2016, 1863, 2531–2539. [Google Scholar] [CrossRef]
- Bodoy, S.; Fotiadis, D.; Stoeger, C.; Kanai, Y.; Palacín, M. The small SLC43 family: Facilitator system L amino acid transporters and the orphan EEG1. Mol. Asp. Med. 2013, 34, 638–645. [Google Scholar] [CrossRef]
- Bröer, S.; Gether, U. The solute carrier 6 family of transporters. Br. J. Pharmacol. 2012, 167, 256–278. [Google Scholar] [CrossRef]
- Kanai, Y.; Hediger, M.A. The glutamate/neutral amino acid transporter family SLC1: Molecular, physiological and pharmacological aspects. Pflug. Arch. Eur. J. Physiol. 2004, 447, 469–479. [Google Scholar] [CrossRef]
- Thwaites, D.T.; Anderson, C.M.H. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011, 164, 1802–1816. [Google Scholar] [CrossRef]
- Blayney, J.K.; Cairns, L.; Li, G.; McCabe, N.; Stevenson, L.; Peters, C.J.; Reid, N.B.; Spence, V.J.; Chisambo, C.; McManus, D.; et al. Glucose transporter 1 expression as a marker of prognosis in oesophageal adenocarcinoma. Oncotarget 2018, 9, 18518–18528. [Google Scholar] [CrossRef]
- Younes, M.; Lechago, J.; Chakraborty, S.; Ostrowski, M.; Bridges, M.; Meriano, F.; Solcher, D.; Barroso, A.; Whitman, D.; Schwartz, J.; et al. Relationship between dysplasia, p53 protein accumulation, DNA ploidy, and Glut1 overexpression in Barrett metaplasia. Scand. J. Gastroenterol. 2000, 35, 131–137. [Google Scholar] [CrossRef]
- Younes, M.; Ertan, A.; Lechago, L.V.; Somoano, J.; Lechago, J. Human erythrocyte glucose transporter (Glut1) is immunohistochemically detected as a late event during malignant progression in Barrett’s metaplasia. Cancer Epidemiol. Biomark. Prev. 1997, 6, 303–305. [Google Scholar]
- Wang, Q.; Ma, C.M.; Kemmner, W. Wdr66 is a novel marker for risk stratification and involved in epithelial-mesenchymal transition of esophageal squamous cell carcinoma. BMC Cancer 2013, 13, 137. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Pritchard, S.A.; McGrath, S.M.; Valentine, H.R.; Price, P.M.; Welch, I.M.; West, C.M. Increasing expression of hypoxia-inducible proteins in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Br. J. Cancer 2007, 96, 1377–1383. [Google Scholar] [CrossRef]
- Dvorak, K.; Watts, G.S.; Ramsey, L.; Holubec, H.; Payne, C.M.; Bernstein, C.; Jenkins, G.J.; Sampliner, R.E.; Prasad, A.; Garewal, H.S.; et al. Expression of bile acid transporting proteins in Barrett’s esophagus and esophageal adenocarcinoma. Am. J. Gastroenterol. 2009, 104, 302–309. [Google Scholar] [CrossRef][Green Version]
- Rumiato, E.; Boldrin, E.; Malacrida, S.; Battaglia, G.; Bocus, P.; Castoro, C.; Cagol, M.; Chiarion-Sileni, V.; Ruol, A.; Amadori, A.; et al. A germline predictive signature of response to platinum chemotherapy in esophageal cancer. Transl. Res. 2016, 171, 29–37.e21. [Google Scholar] [CrossRef] [PubMed]
- Laczko, D.; Rosztoczy, A.; Birkas, K.; Katona, M.; Rakonczay, Z., Jr.; Tiszlavicz, L.; Roka, R.; Wittmann, T.; Hegyi, P.; Venglovecz, V. Role of ion transporters in the bile acid-induced esophageal injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G16–G31. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Schuller, V.; May, A.; Gerges, C.; Anders, M.; Becker, J.; Hess, T.; Kreuser, N.; Thieme, R.; Ludwig, K.U.; et al. Identification of loci of functional relevance to Barrett’s esophagus and esophageal adenocarcinoma: Cross-referencing of expression quantitative trait loci data from disease-relevant tissues with genetic association data. PLoS ONE 2019, 14, e0227072. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Raoof, D.A.; Thomas, D.G.; Greenson, J.K.; Giordano, T.J.; Robinson, G.S.; Bourner, M.J.; Bauer, C.T.; Orringer, M.B.; Beer, D.G. L-type amino acid transporter-1 overexpression and melphalan sensitivity in Barrett’s adenocarcinoma. Neoplasia 2004, 6, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Minegaki, T.; Takara, K.; Hamaguchi, R.; Tsujimoto, M.; Nishiguchi, K. Factors affecting the sensitivity of human-derived esophageal carcinoma cell lines to 5-fluorouracil and cisplatin. Oncol. Lett. 2013, 5, 427–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parcej, D.; Tampé, R. ABC proteins in antigen translocation and viral inhibition. Nat. Chem. Biol. 2010, 6, 572–580. [Google Scholar] [CrossRef]
- Padariya, M.; Kote, S.; Mayordomo, M.; Dapic, I.; Alfaro, J.; Hupp, T.; Fahraeus, R.; Kalathiya, U. Structural determinants of peptide-dependent TAP1–TAP2 transit passage targeted by viral proteins and altered by cancer-associated mutations. Comput. Struct. Biotechnol. J. 2021, 19, 5072–5091. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Narumiya, K.; Bollschweiler, E.; Holscher, A.H.; Yamamoto, M.; Drebber, U.; Alakus, H.; Metzger, R.; Warnecke-Eberz, U. Different response rates to chemotherapy between Japanese and German esophageal squamous cell carcinoma: Patients may be influenced by ERCC1 or ABCB1. Future Oncol. 2020, 16, 2075–2087. [Google Scholar] [CrossRef]
- Jimenez, P.; Chueca, E.; Arruebo, M.; Strunk, M.; Solanas, E.; Serrano, T.; Garcia-Gonzalez, M.A.; Lanas, A. CD24 Expression Is Increased in 5-Fluorouracil-Treated Esophageal Adenocarcinoma Cells. Front. Pharmacol. 2017, 8, 321. [Google Scholar] [CrossRef]
- Frankell, A.M.; Jammula, S.; Li, X.; Contino, G.; Killcoyne, S.; Abbas, S.; Perner, J.; Bower, L.; Devonshire, G.; Ococks, E.; et al. The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat. Genet. 2019, 51, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Pasello, G.; Agata, S.; Bonaldi, L.; Corradin, A.; Montagna, M.; Zamarchi, R.; Parenti, A.; Cagol, M.; Zaninotto, G.; Ruol, A.; et al. DNA copy number alterations correlate with survival of esophageal adenocarcinoma patients. Mod. Pathol. 2009, 22, 58–65. [Google Scholar] [CrossRef] [PubMed]
- van Dekken, H.; Vissers, K.; Tilanus, H.W.; Kuo, W.L.; Tanke, H.J.; Rosenberg, C.; IJszenga, M.; Szuhai, K. Genomic array and expression analysis of frequent high-level amplifications in adenocarcinomas of the gastro-esophageal junction. Cancer Genet. Cytogenet. 2006, 166, 157–162. [Google Scholar] [CrossRef]
- Duggan, S.P.; Garry, C.; Behan, F.M.; Phipps, S.; Kudo, H.; Kirca, M.; Zaheer, A.; McGarrigle, S.; Reynolds, J.V.; Goldin, R.; et al. siRNA Library Screening Identifies a Druggable Immune-Signature Driving Esophageal Adenocarcinoma Cell Growth. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Mari, L.; Hoefnagel, S.J.; Zito, D.; van de Meent, M.; van Endert, P.; Calpe, S.; Serra, M.C.S.; Heemskerk, M.H.M.; van Laarhoven, H.W.M.; Hulshof, M.C.C.M.; et al. microRNA 125a Regulates MHC-I Expression on Esophageal Adenocarcinoma Cells, Associated with Suppression of Antitumor Immune Response and Poor Outcomes of Patients. Gastroenterology 2018, 155, 784–798. [Google Scholar] [CrossRef]
- Hummel, R.; Sie, C.; Watson, D.I.; Wang, T.T.; Ansar, A.; Michael, M.Z.; Van der Hoek, M.; Haier, J.; Hussey, D.J. MicroRNA signatures in chemotherapy resistant esophageal cancer cell lines. World J. Gastroenterol. 2014, 20, 14904–14912. [Google Scholar] [CrossRef]
- Rojek, A.; Praetorius, J.; Frokiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef]
- Kusayama, M.; Wada, K.; Nagata, M.; Ishimoto, S.; Takahashi, H.; Yoneda, M.; Nakajima, A.; Okura, M.; Kogo, M.; Kamisaki, Y. Critical role of aquaporin 3 on growth of human esophageal and oral squamous cell carcinoma. Cancer Sci. 2011, 102, 1128–1136. [Google Scholar] [CrossRef]
- Shimizu, H.; Shiozaki, A.; Ichikawa, D.; Fujiwara, H.; Konishi, H.; Ishii, H.; Komatsu, S.; Kubota, T.; Okamoto, K.; Kishimoto, M.; et al. The expression and role of Aquaporin 5 in esophageal squamous cell carcinoma. J. Gastroenterol. 2014, 49, 655–666. [Google Scholar] [CrossRef]
- Yamazato, Y.; Shiozaki, A.; Ichikawa, D.; Kosuga, T.; Shoda, K.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; et al. Aquaporin 1 suppresses apoptosis and affects prognosis in esophageal squamous cell carcinoma. Oncotarget 2018, 9, 29957–29974. [Google Scholar] [CrossRef][Green Version]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. BBA Biomembr. 2015, 1848, 2576–2583. [Google Scholar] [CrossRef]
- Yu, C.P.; Tsai, P.L.; Li, P.Y.; Hsu, P.W.; Lin, S.P.; Lee Chao, P.D.; Hou, Y.C. Cranberry Ingestion Modulated Drug Transporters and Metabolizing Enzymes: Gefitinib Used as a Probe Substrate in Rats. Molecules 2022, 27, 5772. [Google Scholar] [CrossRef]
- Morita, T.; Akiyoshi, T.; Tsuchitani, T.; Kataoka, H.; Araki, N.; Yajima, K.; Katayama, K.; Imaoka, A.; Ohtani, H. Inhibitory Effects of Cranberry Juice and Its Components on Intestinal OATP1A2 and OATP2B1: Identification of Avicularin as a Novel Inhibitor. J. Agric. Food Chem. 2022, 70, 3310–3320. [Google Scholar] [CrossRef]
- Gomez-Garduno, J.; Leon-Rodriguez, R.; Alemon-Medina, R.; Perez-Guille, B.E.; Soriano-Rosales, R.E.; Gonzalez-Ortiz, A.; Chavez-Pacheco, J.L.; Solorio-Lopez, E.; Fernandez-Perez, P.; Rivera-Espinosa, L. Phytochemicals That Interfere with Drug Metabolism and Transport, Modifying Plasma Concentration in Humans and Animals. Dose Response 2022, 20, 15593258221120485. [Google Scholar] [CrossRef]
- Nabekura, T. Overcoming multidrug resistance in human cancer cells by natural compounds. Toxins 2010, 2, 1207–1224. [Google Scholar] [CrossRef]
- Hussain, S.A.; Sulaiman, A.A.; Alhaddad, H.; Alhadidi, Q. Natural polyphenols: Influence on membrane transporters. J. Intercult. Ethnopharmacol. 2016, 5, 97–104. [Google Scholar] [CrossRef]
- Nabekura, T.; Kawasaki, T.; Furuta, M.; Kaneko, T.; Uwai, Y. Effects of Natural Polyphenols on the Expression of Drug Efflux Transporter P-Glycoprotein in Human Intestinal Cells. ACS Omega 2018, 3, 1621–1626. [Google Scholar] [CrossRef]
- Wang, Y.; Han, A.; Chen, E.; Singh, R.K.; Chichester, C.O.; Moore, R.G.; Singh, A.P.; Vorsa, N. The cranberry flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. Int. J. Oncol. 2015, 46, 1924–1934. [Google Scholar] [CrossRef]
- Zhou, H.P.; Hylemon, P.B. Bile acids are nutrient signaling hormones. Steroids 2014, 86, 62–68. [Google Scholar] [CrossRef]
- Schneider, L.S.; von Schwarzenberg, K.; Lehr, T.; Ulrich, M.; Kubisch-Dohmen, R.; Liebl, J.; Trauner, D.; Menche, D.; Vollmar, A.M. Vacuolar-ATPase Inhibition Blocks Iron Metabolism to Mediate Therapeutic Effects in Breast Cancer. Cancer Res. 2015, 75, 2863–2874. [Google Scholar] [CrossRef]
- Lee, H.M.; Joh, J.W.; Seo, S.R.; Kim, W.T.; Kim, M.K.; Choi, H.S.; Kim, S.Y.; Jang, Y.J.; Sinn, D.H.; Choi, G.S.; et al. Cell-surface major vault protein promotes cancer progression through harboring mesenchymal and intermediate circulating tumor cells in hepatocellular carcinomas. Sci. Rep. 2017, 7, 13201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Y.; Song, Z.; Zhao, R. Pan-Cancer Analysis of Voltage-Dependent Anion Channel (VDAC1) as a Cancer Therapeutic Target or Diagnostic Biomarker. Dis. Markers 2022, 2022, 5946110. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Holmes, R.S.; Vaughan, T.L. Epidemiology and pathogenesis of esophageal cancer. Semin. Radiat. Oncol. 2007, 17, 2–9. [Google Scholar] [CrossRef]
- King, R.J.; Qiu, F.; Yu, F.; Singh, P.K. Metabolic and Immunological Subtypes of Esophageal Cancer Reveal Potential Therapeutic Opportunities. Front. Cell Dev. Biol. 2021, 9, 667852. [Google Scholar] [CrossRef]
- Albrecht, B.; Hausmann, M.; Zitzelsberger, H.; Stein, H.; Siewert, J.R.; Hopt, U.; Langer, R.; Höfler, H.; Werner, M.; Walch, A. Array-based comparative genomic hybridization for the detection of DNA sequence copy number changes in Barrett’s adenocarcinoma. J. Pathol. 2004, 203, 780–788. [Google Scholar] [CrossRef]
- van Eerden, R.A.G.; van Doorn, L.; de Man, F.M.; Heersche, N.; Doukas, M.; van den Bosch, T.P.P.; Oomen-de Hoop, E.; de Bruijn, P.; Bins, S.; Ibrahim, E.; et al. Tissue Type Differences in ABCB1 Expression and Paclitaxel Tissue Pharmacokinetics in Patients with Esophageal Cancer. Front. Pharmacol. 2021, 12, 759146. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Fitzgerald, R.C.; Vaughan, T.L.; Palles, C.; Gockel, I.; Tomlinson, I.; Buas, M.F.; May, A.; Gerges, C.; Anders, M.; et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett’s oesophagus: A large-scale meta-analysis. Lancet Oncol. 2016, 17, 1363–1373. [Google Scholar] [CrossRef]
- Dai, J.Y.; Wang, X.; Buas, M.F.; Zhang, C.; Ma, J.; Wei, B.; Li, Y.; Zhao, B.; Hyun, T.S.; Chen, X.; et al. Whole-genome sequencing of esophageal adenocarcinoma in Chinese patients reveals distinct mutational signatures and genomic alterations. Commun. Biol. 2018, 1, 174. [Google Scholar] [CrossRef]
- Goldman, A.; Chen, H.; Khan, M.R.; Roesly, H.; Hill, K.A.; Shahidullah, M.; Mandal, A.; Delamere, N.A.; Dvorak, K. The Na+/H+ exchanger controls deoxycholic acid-induced apoptosis by a H+-activated, Na+-dependent ionic shift in esophageal cells. PLoS ONE 2011, 6, e23835. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.; Shahidullah, M.; Goldman, D.; Khailova, L.; Watts, G.; Delamere, N.; Dvorak, K. A novel mechanism of acid and bile acid-induced DNA damage involving Na+/H+ exchanger: Implication for Barrett’s oesophagus. Gut 2010, 59, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Norita, K.; Asanuma, K.; Koike, T.; Okata, T.; Fujiya, T.; Abe, Y.; Nakagawa, K.; Hatta, W.; Uno, K.; Nakamura, T.; et al. Impaired Mucosal Integrity in Proximal Esophagus Is Involved in Development of Proton Pump Inhibitor-Refractory Nonerosive Reflux Disease. Digestion 2021, 102, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Dulak, A.M.; Stojanov, P.; Peng, S.; Lawrence, M.S.; Fox, C.; Stewart, C.; Bandla, S.; Imamura, Y.; Schumacher, S.E.; Shefler, E.; et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 2013, 45, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Lagorce-Pages, C.; Paraf, F.; Dubois, S.; Belghiti, J.; Flejou, J.F. Expression of CD44 in premalignant and malignant Barrett’s oesophagus. Histopathology 1998, 32, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, M.M.; O’Riordan, J.; Windle, H.J.; Carton, E.; Ravi, N.; Kelleher, D.; Reynolds, J.V. NF-kappaB activation in esophageal adenocarcinoma: Relationship to Barrett’s metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann. Surg. 2004, 239, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Gomaa, A.; Wang-Bishop, L.; Ballout, F.; Hu, T.; McDonald, O.; Washington, M.K.; Livingstone, A.S.; Wang, T.C.; Peng, D.; et al. Unfolded Protein Response Is Activated by Aurora Kinase A in Esophageal Adenocarcinoma. Cancers 2022, 14, 1401. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.C.; Sarbia, M.; Weber, A.A.; Borchard, F.; Gabbert, H.E.; Schror, K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999, 59, 198–204. [Google Scholar]
- Lagisetty, K.H.; McEwen, D.P.; Nancarrow, D.J.; Schiebel, J.G.; Ferrer-Torres, D.; Ray, D.; Frankel, T.L.; Lin, J.; Chang, A.C.; Kresty, L.A.; et al. Immune determinants of Barrett’s progression to esophageal adenocarcinoma. JCI Insight 2021, 6, e143888. [Google Scholar] [CrossRef]
- Cesar-Razquin, A.; Snijder, B.; Frappier-Brinton, T.; Isserlin, R.; Gyimesi, G.; Bai, X.; Reithmeier, R.A.; Hepworth, D.; Hediger, M.A.; Edwards, A.M.; et al. A Call for Systematic Research on Solute Carriers. Cell 2015, 162, 478–487. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Liu, L.; Ju, Y.C.; Wang, J.; Zhou, R.M. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol. Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Alfahel, R.; Sawicki, T.; Jablonska, M.; Przybylowicz, K.E. Anti-Hyperglycemic Effects of Bioactive Compounds in the Context of the Prevention of Diet-Related Diseases. Foods 2023, 12, 3698. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Abrego, J.; Gunda, V.; Vernucci, E.; Shukla, S.K.; King, R.J.; Dasgupta, A.; Goode, G.; Murthy, D.; Yu, F.; Singh, P.K. GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells. Cancer Lett. 2017, 400, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Huang, Z.; Li, B.; Nice, E.C.; Huang, C.; Wei, L.; Zou, B. Targeting Glucose Metabolism Enzymes in Cancer Treatment: Current and Emerging Strategies. Cancers 2022, 14, 4568. [Google Scholar] [CrossRef]
- de Castro, A.; Fernandes, M.R.; de Carvalho, D.C.; de Souza, T.P.; Rodrigues, J.C.G.; Andrade, R.B.; Modesto, A.A.C.; Santos, S.; Assumpcao, P.P.; Dos Santos, N.P.C. Polymorphisms of xenobiotic-metabolizing and transporter genes, and the risk of gastric and colorectal cancer in an admixed population from the Brazilian Amazon. Am. J. Transl. Res. 2020, 12, 6626–6636. [Google Scholar]
- Czuba, L.C.; Hillgren, K.M.; Swaan, P.W. Post-translational modifications of transporters. Pharmacol. Ther. 2018, 192, 88–99. [Google Scholar] [CrossRef]
- Schlessinger, A.; Yee, S.W.; Sali, A.; Giacomini, K.M. SLC classification: An update. Clin. Pharmacol. Ther. 2013, 94, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.A.; Li, J.; Enkavi, G.; Wen, P.C.; Huang, Z.; Tajkhorshid, E. Visualizing functional motions of membrane transporters with molecular dynamics simulations. Biochemistry 2013, 52, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005, 44, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Walther, D. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput. Biol. 2015, 11, e1004049. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. The age of crosstalk: Phosphorylation, ubiquitination, and beyond. Mol. Cell 2007, 28, 730–738. [Google Scholar] [CrossRef]
- Korkuc, P.; Walther, D. Towards understanding the crosstalk between protein post-translational modifications: Homo- and heterotypic PTM pair distances on protein surfaces are not random. Proteins 2017, 85, 78–92. [Google Scholar] [CrossRef]
- Prabakaran, S.; Lippens, G.; Steen, H.; Gunawardena, J. Post-translational modification: Nature’s escape from genetic imprisonment and the basis for dynamic information encoding. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 565–583. [Google Scholar] [CrossRef]
- Richter, J.E.; Peura, D.; Benjamin, S.B.; Joelsson, B.; Whipple, J. Efficacy of omeprazole for the treatment of symptomatic acid reflux disease without esophagitis. Arch. Intern. Med. 2000, 160, 1810–1816. [Google Scholar] [CrossRef][Green Version]
- Ferrer-Torres, D.; Nancarrow, D.J.; Steinberg, H.; Wang, Z.; Kuick, R.; Weh, K.M.; Mills, R.E.; Ray, D.; Ray, P.; Lin, J.; et al. Constitutively Higher Level of GSTT2 in Esophageal Tissues from African Americans Protects Cells against DNA Damage. Gastroenterology 2019, 156, 1404–1415. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef]
- Son, S.W.; Chau, G.C.; Kim, S.T.; Um, S.H. Vacuolar H+-ATPase Subunit V0C Regulates Aerobic Glycolysis of Esophageal Cancer Cells via PKM2 Signaling. Cells 2019, 8, 1137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Long, G.; Zheng, Y.; Yang, X.; Cai, W.; He, S.; Qin, X.; Liao, H. Correction: Zheng et al. Glycolysis-Related SLC2A1 Is a Potential Pan-Cancer Biomarker for Prognosis and Immunotherapy. Cancers 2022, 14, 5344, Erratum in Cancers 2023, 15, 586. [Google Scholar] [CrossRef]
- Weh, K.M.; Howell, A.B.; Kresty, L.A. Expression, modulation, and clinical correlates of the autophagy protein Beclin-1 in esophageal adenocarcinoma. Mol. Carcinog. 2016, 55, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]


| Gene | Protein/ Alias | Substrate(s); Function(s) in Addition to Transport | Influx or Efflux | Reflux-Induced Changes | C-PAC-Induced Changes Given Reflux | ||
|---|---|---|---|---|---|---|---|
| Log2FC a | p-Value b | Log2FC a | p-Value b | ||||
| Abca1 | ABC1 | Cholesterol, phospholipids, bile salts; Cellular response to cholesterol, LPS, retinoic acid, cytokine and xenobiotic stimulus | Efflux | 1.21 | 0.037 | −0.58 | 0.498 |
| Abca9 | ABCA9 | Lipids, cholesterol, acyl CoA derivatives; Lipid homeostasis, monocyte differentiation | Efflux | −2.20 | 0.002 | 0.73 | 0.018 |
| Abcb1b | MDR1 P-gp | Chemotherapeutics, cholesterol, phospholipids, bile salts, omeprazole, statins, antibiotics, immunosuppressants antivirals; G2/M transition of mitotic cell cycle, xenobiotic detoxification, xenobiotic metabolic process | Efflux | 1.80 | 0.001 | −2.65 | 0.002 |
| Abcb2 | TAP1 | Peptides or antigens; Adaptive immune response, antigen processing and presentation via MHC class I, defense response | Influx | 1.18 | 0.048 | −1.34 | 0.295 |
| Abcb3 | TAP2 | Peptides or antigens; T cell mediated cytotoxicity, antigen processing and presentation via MHC class I, response to molecule of bacterial origin | Influx | 1.96 | 0.019 | −1.71 | 0.188 |
| Abcb4 | MDR3 | Phospholipids, paclitaxel, bile salts; Bile acid secretion, cellular response to bile, lipid homeostasis | Efflux | 0.65 | 0.007 | −1.31 | 0.048 |
| Abcb11 | BSEP | Bile acids, phospholipids, statins; Bile acid metabolic process, bile acid signaling pathway, cholesterol, lipid and phospholipid homeostasis | Efflux | 3.28 | 0.021 | −1.99 | 0.128 |
| Abcc1 | MRP1 | Glutathione, leukotriene C4, estradiol-17-beta-o-glucuronide, methotrexate, chemotherapeutic drugs, xenobiotics; Cell chemotaxis, anti-cancer drug resistance, cellular response to amyloid-beta, oxidative stress, leukotriene metabolic process, heme and xenobiotic catabolic process | Efflux | 1.19 | 0.011 | −1.28 | 0.027 |
| Abcc3 | MRP3 | Bile acids, etoposide, leukotriene C4, glucuronisides, xenobiotics; Xenobiotic metabolic process, metabolism of lipids, steroids and bile, recycling of bile, nuclear receptors meta-pathway, NRF2 | Efflux | 1.54 | 0.001 | −1.47 | 0.042 |
| Abcc4 | MRP4 | cAMP, cGMP, cholate, statins, GSH, bile salts, prostaglandin, urate; Xenobiotic metabolism; cellular detoxification, anti-cancer drug resistance | Efflux | 1.54 | 0.001 | −2.06 | <0.001 |
| Abcc5 | MRP5 | cAMP, cGMP, folate, glutathione, glutamate, heme, bile salts, antiretroviral nucleosides, thiopurine anticancer drugs; Hyaluronan biosynthetic process, xenobiotic metabolic process | Efflux | 1.31 | 0.034 | −1.51 | 0.054 |
| Abcc6 | MRP6 | Glutathione conjugates, ATP, cisplatin, leukotriene C4; Calcium homeostasis, gene expression, inhibition of non-skeletal tissue mineralization, response to xenobiotic stimulus | Efflux | 1.06 | 0.041 | −2.31 | 0.041 |
| Abcc10 | MRP7 | Chemotherapeutics, cholesterol, bile, phospholipids, leukotriene C4, glutathione, peptides; Leukotriene metabolic process; heme synthesis; cellular detoxification | Efflux | 0.15 | 0.040 | −1.77 | 0.003 |
| Abcg2 | BCRP | Xenobiotics, urate, lipids, riboflavin, doxorubicin, estradiol, imatinib, irinotecan, statins, tamoxifen, testosterone; Cellular detoxification, urate metabolic process | Efflux | 1.74 | 0.014 | −1.54 | 0.212 |
| Gene | Protein/ Alias | Substrate(s); Function(s) in Addition to Transport | Influx or Efflux | Reflux-Induced Changes | C-PAC-Induced Changes Given Reflux | ||
|---|---|---|---|---|---|---|---|
| Log2FC | p-Value c | Log2FC | p-Value c | ||||
| Slc2a1 a | GLUT1 | Glucose, galactose, mannose, glucosamine, ranitidine, quercetin, resveratrol; Glycolysis, gluconeogenesis, cellular respiration | Influx | 1.98 | 0.008 | −1.93 | 0.044 |
| Slc3a2 a | CD98 | Large neutral amino acids; Calcium regulation, lymphocyte activation | Both | 1.65 | 0.005 | −1.66 | 0.128 |
| Slc4a9 b | AE4 | Sodium, chloride, bicarbonate; Anion exchange, Intracellular pH | Both | −2.23 | <0.001 | 1.98 | <0.001 |
| Slc4a11 b | BTR1 | Borate, sodium, bicarbonate, protons; Cell proliferation, response to oxidative stress | Both | 2.37 | 0.029 | −2.52 | 0.004 |
| Slc5a1 a | SGLT1 | Glucose, galactose, myo-inositol, sodium; Nuclear receptors meta-pathway, NRF2 | Influx | 1.23 | 0.046 | −1.06 | 0.392 |
| Slc6a14 b | ATB0+ | Neutral and cationic amino acids, glutamine, arginine, glycine, bile salts, metal ions, amines, sodium and chloride neurotransmitters; Response to toxic substance, nuclear receptors meta-pathway, NRF2 | Both | 7.53 | <0.001 | −2.16 | <0.001 |
| Slc6a20 b | SIT1 | Sodium, chloride, amino acids, proline, bile salts, amines, sarcosine, pipecolic acid; Nuclear receptors meta-pathway, NRF2, kidney function | Both | 3.50 | <0.001 | −1.43 | 0.004 |
| Slc7a5 a | LAT1 | Large neutral amino acids, xenobiotics; Aryl hydrocarbon receptor pathway, nuclear receptors meta-pathway, response to LPS, autophagy regulation | Both | 1.40 | 0.004 | −1.59 | 0.017 |
| Slc7a7 a | y + LAT | Cationic amino acids, large neutral amino acids; Regulation of arginine metabolic process | Both | 1.92 | 0.028 | −1.32 | 0.266 |
| Slc7a8 a | LAT2 | Cationic amino acids, large neutral amino acids, glycine, proline, tryptophan, thyroid hormone, toxins; Leukocyte migration, metal ion homeostasis | Both | 1.93 | 0.001 | −2.10 | 0.007 |
| Slc7a11 a | xCT | Cystine, L-glutamate; Oxidative stress response, glutathione metabolic process, nuclear receptors meta-pathway, NRF2, ferroptosis, p53 transcriptional gene network | Both | 2.51 | 0.001 | −2.53 | 0.003 |
| Slc8a3 b | NCX3 | Sodium, calcium; Cell communication, cellular response to hypoxia, memory | Both | −3.19 | <0.001 | 2.13 | 0.008 |
| Slc9a1 b | NHE1 | Protons, sodium, hydrogen; Cellular pH, cell migration, cell volume; Response to hypoxia, response to acidic pH, cell polarity and migration, RhoA, p38, and ErbB1 signaling | Both | 1.00 | <0.001 | −0.84 | <0.001 |
| Slc9a3 b | NHE3 | Protons, sodium, hydrogen; Regulation of intracellular pH | Both | 3.12 | 0.002 | −1.01 | 0.830 |
| Slc9a5 b | NHE5 | Protons, sodium, hydrogen; Regulation of intracellular pH | Both | 1.93 | <0.001 | −1.45 | <0.001 |
| Slc10a2 b | ASBT | Bile salts, sodium, phospholipids; Cholesterol homeostasis, response to bacterium | Influx | 2.37 | 0.003 | −1.42 | 0.047 |
| Slc15a2 a | PEPT2 | Di- and tri- peptides, protons, beta-lactam antibiotics, xenobiotics; Innate immune response, xenobiotic detoxification | Influx | −2.09 | 0.050 | 0.42 | 0.087 |
| Slc16a2 a | MCT8 | Thyroid hormones (T2, rT3, T3, T4), lactate; Amino acid and thyroid hormone metabolic process | Influx | 3.08 | <0.001 | −3.11 | 0.004 |
| Slc22a2 a | OCT2 | Organic cations, oxaliplatin, cisplatin, carboplatin, paclitaxel, 5-fluorouracil, ranitidine, metformin; Kidney function | Influx | −2.64 | 0.026 | 0.92 | 0.038 |
| Slc22a7 a | OAT2 | Organic anions, acyclovir, prostaglandins, xenobiotics; Fluoropyrimidine activity and pathway, xenobiotic metabolism | Influx | 1.57 | 0.023 | −2.97 | 0.018 |
| Slc22a8 a | OAT3 | Organic anions, carboxylate, prostaglandins, xenobiotics; Response to toxic substances | Influx | 2.09 | 0.032 | −2.89 | 0.015 |
| Slc24a3 b | NCKX3 | Calcium, sodium, potassium; Bone mineralization, calcium homeostasis, regulation of gene expression | Influx | −1.67 | <0.001 | 0.85 | 0.021 |
| Slc25a11 b | OGC | Oxoglutarate, malate, glutathione; Gluconeogenesis from lactate; Nitrogen metabolism, apoptosis | Both | −1.17 | <0.001 | 1.00 | <0.001 |
| Slc25a13 a | CTLN2 | Aspartate, glutamate; ATP biosynthetic process, cellular respiration, gluconeogenesis, response to calcium | Both | 1.40 | 0.024 | −1.14 | 0.254 |
| Slc28a3 a | CNT3 | Nucleosides, vitamins, utidine, gemcitabine, fludarabine, ribavirin; Xenobiotic metabolic process | Influx | 0.89 | 0.021 | −1.95 | 0.024 |
| Slc31a1 a | CTR1 | Copper, cisplatin, bile salts, organic acids, metal ions, amines; Copper homeostasis and metabolism, platinum pathway, angiogenesis | Influx | 1.47 | 0.003 | −1.39 | 0.137 |
| Slc35f2 b | HSNOV1 | Amino acids, glucose, nucleotides, lipids, organic anions; Biological process | Influx | 1.50 | <0.001 | −1.05 | 0.001 |
| Slc46a2 b | TSCOT | Cyclic GMP-AMP; T cell homeostasis, innate immune response, regulation of T cell differentiation | Influx | 1.32 | <0.001 | −0.77 | 0.015 |
| Slco2a1 a | OATP2A1 | Prostaglandins, lactate, vitamins, nucleosides; Lipid metabolism in senescent cells | Both | 1.67 | 0.011 | −1.64 | 0.037 |
| Slco4a1 a | OATP4A1 | Thyroid hormones (T3, T4, rT3), esterone-3-sulfate, organic anion, taurocholate; Intracellular pH, regulation of pH | Influx | 1.10 | 0.024 | −2.84 | 0.026 |
| Gene | Protein/ Alias | Substrate(s); Function(s) in Addition to Transport | Influx or Efflux | Reflux-Induced Changes | C-PAC-Induced Changes Given Reflux | ||
|---|---|---|---|---|---|---|---|
| Log2FC | p-Value c | Log2FC | p-Value c | ||||
| Atp6v0a4 b | Subunit of ATPase | Protons for ATP hydrolysis; Intracellular pH reduction, regulation of pH | Influx | 2.73 | <0.001 | −2.34 | <0.001 |
| Atp6v0c b | Subunit of ATPase | Protons for ATP hydrolysis; Acidification, intracellular pH reduction, regulation of macroautophagy and Wnt signaling pathway | Influx | 1.73 | 0.005 | −1.91 | 0.006 |
| Aqp1 a | AQP1 | Water, ammonium, carbon dioxide, glycerol, nitric oxide; cGMP-mediated signaling, cell volume homeostasis, cellular response to UV, cAMP, copper ion, hypoxia, nitric oxide, retinoic acid, Gram-negative bacterium | Both | 0.11 | 0.018 | −1.01 | 0.089 |
| Aqp3 b | AQP3 | Water, glycerol; Cellular response to hypoxia, retinoic acid and vitamin D, regulation of keratinocyte differentiation and immune system process | Both | 2.40 | 0.008 | −1.62 | 0.289 |
| Aqp4 b | AQP4 | Water; Cellular response to type II interferon, intracellular water homeostasis, | Both | −2.72 | <0.001 | 2.10 | 0.200 |
| Mvp b | MVP | Nucleo-cytoplasmic transport, mRNA; ERBB signaling pathway, regulation of: EGFR signaling pathway, protein tyrosine kinase activity, protein autophosphorylation | Efflux | 1.00 | 0.003 | −0.77 | 0.012 |
| Vdac2 a | VDAC2 | Closed: cation-selective; Open: weak anion selectivity; Negative regulation of intrinsic apoptosis signaling pathway | Both | 1.27 | 0.016 | −0.83 | 0.483 |
| Gene | C-PACs versus Water | |
|---|---|---|
| Log2FC | p-Value a | |
| Abcb1b | −1.75 | 0.037 |
| Abcb3 | −1.48 | 0.015 |
| Slc22a8 | −1.99 | 0.016 |
| Atp6v0c | −1.78 | 0.018 |
| Gene Symbol | Log2FC | p-Value | FDR |
|---|---|---|---|
| ABCB2 | 1.71 | 7.14 × 10−6 | 5.92 × 10−5 |
| ABCB3 | 0.50 | 3.90 × 10−2 | 1.04 × 10−1 |
| ABCB4 | 0.96 | 9.77 × 10−3 | 3.37 × 10−2 |
| ABCC1 | 0.46 | 4.38 × 10−2 | 1.14 × 10−1 |
| ABCC3 | 3.92 | 1.77 × 10−15 | 1.80 × 10−13 |
| ABCC4 | 1.29 | 3.56 × 10−3 | 1.43 × 10−2 |
| ABCC6 | 2.18 | 8.78 × 10−5 | 5.55 × 10−4 |
| ABCC10 | 1.49 | 1.52 × 10−8 | 2.40 × 10−7 |
| SLC6A14 | 3.53 | 4.80 × 10−4 | 2.50 × 10−3 |
| SLC6A20 | 4.72 | 1.35 × 10−7 | 1.69 × 10−6 |
| SLC7A7 | 3.64 | 4.95 × 10−13 | 2.50 × 10−11 |
| SLC7A11 | 1.52 | 1.22 × 10−3 | 5.64 × 10−3 |
| SLC9A1 | 1.83 | 2.31 × 10−11 | 7.32 × 10−10 |
| SLC15A2 | −2.04 | 5.43 × 10−6 | 4.63 × 10−5 |
| SLC24A3 | −3.87 | 1.43 × 10−11 | 4.79 × 10−10 |
| SLC25A11 | −1.39 | 1.12 × 10−5 | 8.89 × 10−5 |
| SLC25A13 | 1.92 | 1.49 × 10−5 | 1.15 × 10−4 |
| AQP1 | 1.77 | 2.18 × 10−3 | 9.39 × 10−3 |
| MVP | 1.47 | 1.45 × 10−7 | 1.80 ×10−6 |
| Transporter | Target Tissues or Cell Lines | References | Summary Findings |
| ABCB1 | EAC, GEJ | [55,59,61,62,63,87,88] | Novel driver of EAC; Inc levels (39% in EAC) based on DNA copy number changes; gain reported in EAC; amplification in GEJ tumors compared to gastric samples; elevated expression in EAC compared with normal tissues and higher in EAC compared with SCC; negatively correlated with IC50 for 5-FU in human cell lines; chemotherapeutic resistance |
| ABCB2 | EAC | [65] | Increased expression level in EAC, linked to reduced survival, and immune response; high mRNA expression level significantly linked to poor survival among EAC patients |
| ABCB3 | EAC | [65] | High mRNA expression level significantly linked to poor survival among EAC patients |
| ABCB4 | EAC, EC | [62,63] | Amplification in GEJ tumors compared to gastric samples; gain associated with poor survival |
| ABCC3 | EAC, BE | [50,51,66] | Increased mRNA expression level from esophageal squamous epithelium to BE and with progression to EAC; increased mRNA expression in EAC cell lines linked to 5-FU resistance; SNP associated with response to platinum-based neoadjuvant therapy in EC patients (EAC and ESCC cases combined) |
| ABCC4 | EAC | [62] | Gain in EAC and linked to poor survival |
| ABCC5 | EAC | [89] | Associated with BE progression to EAC |
| ABCC10 | EAC, GEJ | [64] | Amplification in 18% of EAC and GEJ tumors |
| ABCG2 | EAC | [60,61] | Enhancer element in untranslated region identified as a noncoding driver element in EAC; increased mRNA expression in the OE19 EAC cell line following 5-FU treatment |
| SLC2A1 | EAC | [45,46,47] | Associated with EAC and poor prognosis; increased expression in EAC compared with dysplasia’s; increased expression in EAC and high-grade dysplasias compared with non-dysplasias |
| SLC7A5 | EAC | [54] | Increased mRNA expression and decreased LAT1 at the protein level in EAC compared with BE |
| SLC7A8 | EC | [51] | Identified as part of a five-gene signature identifying SNPs impacting the response of esophageal cancer patients (combined ESCC and EAC) to platinum-based neoadjuvant therapy |
| SLC8A3 | EAC | [90] | Mutated in a Chinese cohort of EAC patients and enriched in the protein digestion and absorption pathway (directionality not reported) |
| SLC9A1 | EAC | [52,91,92] | DCA treatment increased levels in BE and EAC human derived cell lines; increased by bile exposure in the BE cell line; increased in BE patient samples and a dysplastic BE cell line |
| SLC9A3 | EAC, BE | [53] | Identified as a potential novel risk loci among associated variants through the integration of expression quantitative trait loci and genetic association data in BE/EAC tissues |
| SLC10A2 | EAC | [50] | Increased mRNA expression in BE and EAC compared to normal squamous esophagus |
| SLC22A2 | EAC | [55] | Expression level impacted sensitivity to 5-FU treatment based on EAC human cell line treatment |
| SLCO2A1 | Reflux-exposed | [93] | Increased expression in patients with reflux extending to the proximal esophagus, concomitant infiltration of CD3-positive lymphocytes and reduction in proximal esophageal TEER, increased in IL-8 and IL-1β and decreased occludin mRNA levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Weh, K.M.; Tripp, B.A.; Clarke, J.L.; Howard, C.L.; Sunilkumar, S.; Howell, A.B.; Kresty, L.A. Cranberry Proanthocyanidins Mitigate Reflux-Induced Transporter Dysregulation in an Esophageal Adenocarcinoma Model. Pharmaceuticals 2023, 16, 1697. https://doi.org/10.3390/ph16121697
Zhang Y, Weh KM, Tripp BA, Clarke JL, Howard CL, Sunilkumar S, Howell AB, Kresty LA. Cranberry Proanthocyanidins Mitigate Reflux-Induced Transporter Dysregulation in an Esophageal Adenocarcinoma Model. Pharmaceuticals. 2023; 16(12):1697. https://doi.org/10.3390/ph16121697
Chicago/Turabian StyleZhang, Yun, Katherine M. Weh, Bridget A. Tripp, Jennifer L. Clarke, Connor L. Howard, Shruthi Sunilkumar, Amy B. Howell, and Laura A. Kresty. 2023. "Cranberry Proanthocyanidins Mitigate Reflux-Induced Transporter Dysregulation in an Esophageal Adenocarcinoma Model" Pharmaceuticals 16, no. 12: 1697. https://doi.org/10.3390/ph16121697
APA StyleZhang, Y., Weh, K. M., Tripp, B. A., Clarke, J. L., Howard, C. L., Sunilkumar, S., Howell, A. B., & Kresty, L. A. (2023). Cranberry Proanthocyanidins Mitigate Reflux-Induced Transporter Dysregulation in an Esophageal Adenocarcinoma Model. Pharmaceuticals, 16(12), 1697. https://doi.org/10.3390/ph16121697









