Identification of Indazole-Based Thiadiazole-Bearing Thiazolidinone Hybrid Derivatives: Theoretical and Computational Approaches to Develop Promising Anti-Alzheimer’s Candidates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro Acetylcholinesterase (AChE) Inhibitory Potential
2.3. In Vitro Butyrylcholinesterase (BuChE) Inhibitory Potential
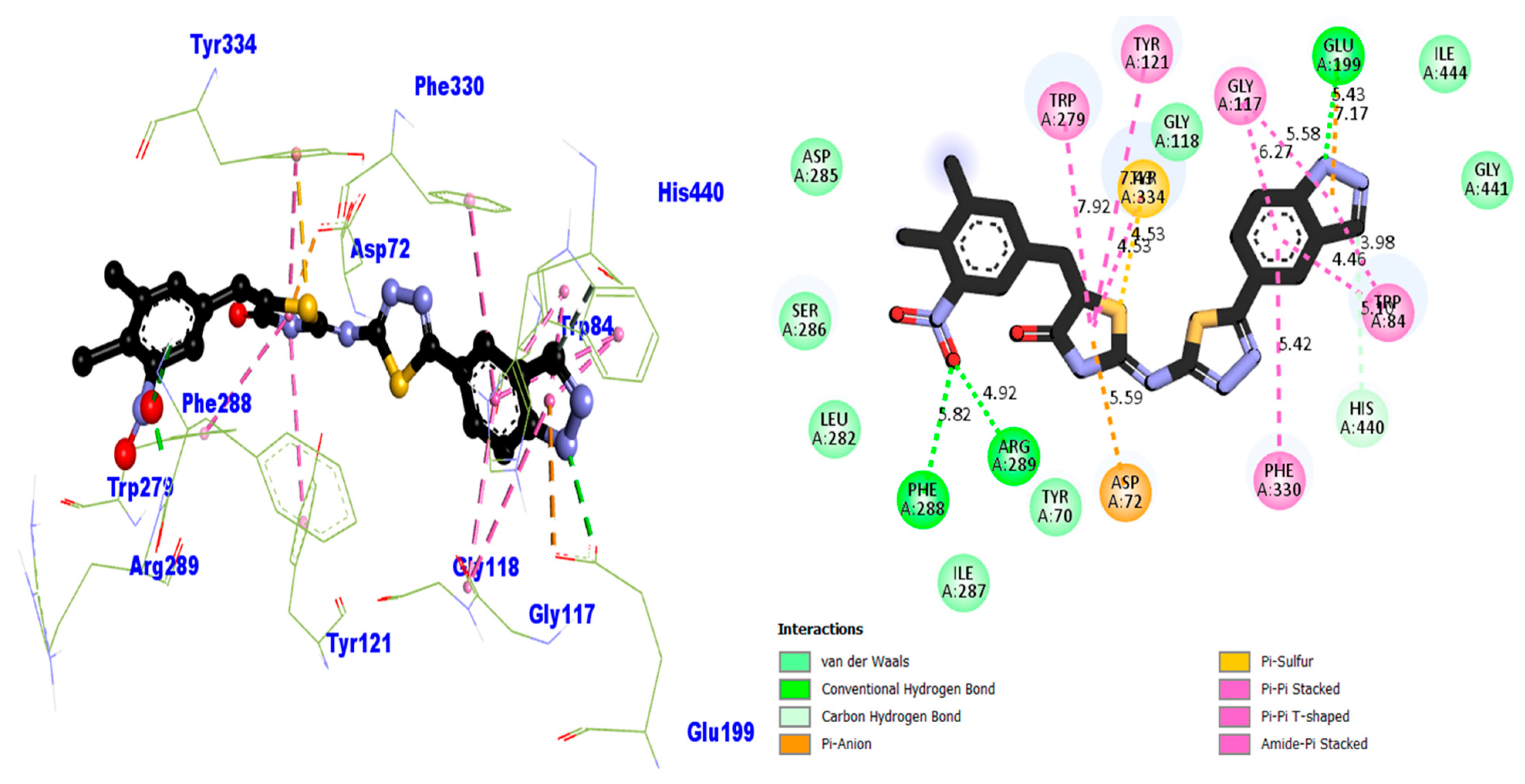
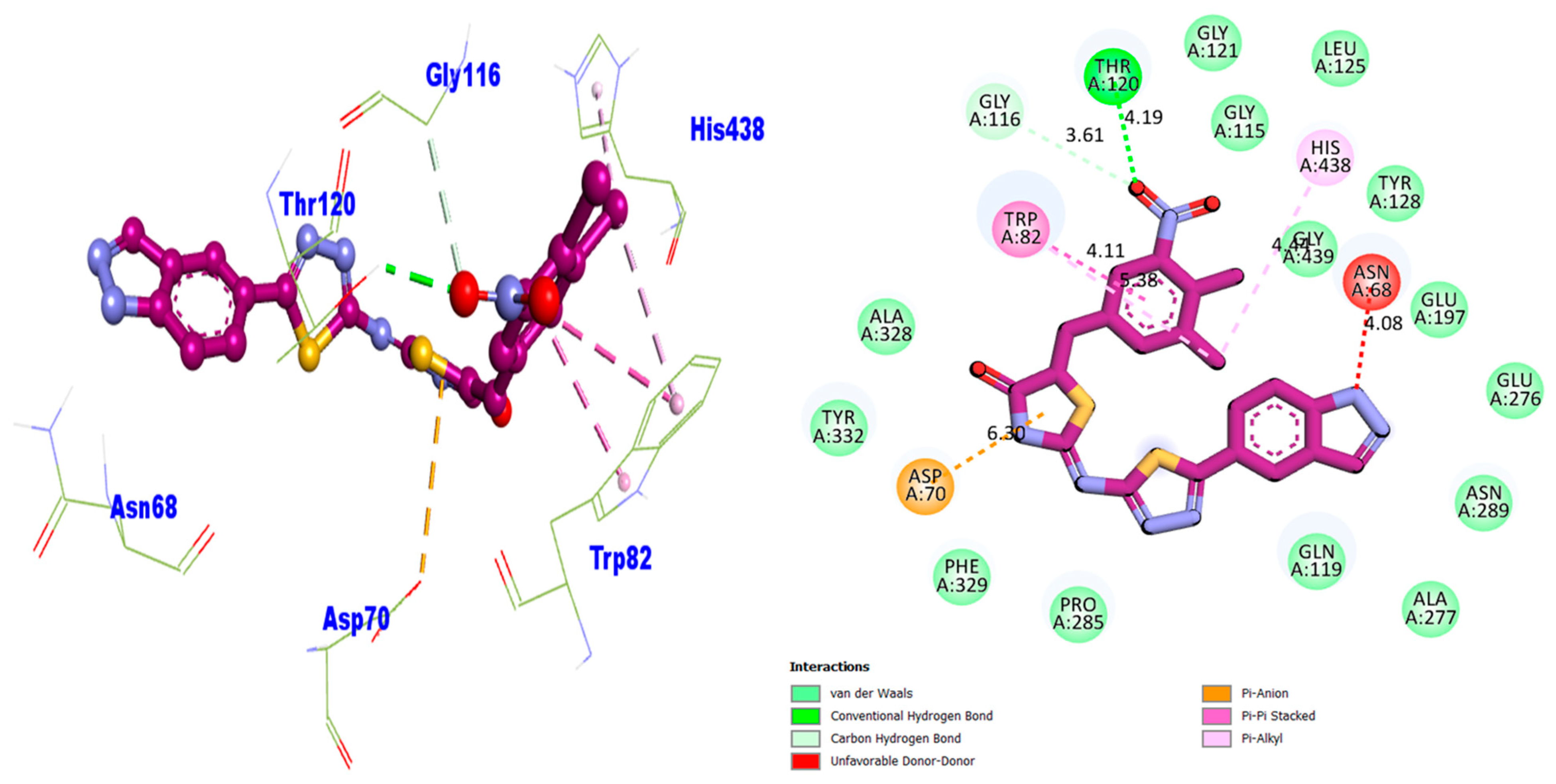
2.4. Docking Study of the Synthesized Compounds (1–17)
3. Materials and Method
3.1. General Information
3.2. General Procedure for the Synthesis of Indazole-Based Thiadiazole-Bearing Thiazolidinone Scaffold
3.3. Spectral Analysis
3.3.1. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3,4-Dichloro-5-Nitrobenzylidene)Thiazolidin-4-One (1)
3.3.2. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-2-Chlorobenzylidene)Thiazolidin-4-One (2)
3.3.3. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-2,4-Dimethylbenzylidene)Thiazolidin-4-One (3)
3.3.4. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3,5-Dimethylbenzylidene)Thiazolidin-4-One (4)
3.3.5. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-4-Methoxybenzylidene)Thiazolidin-4-One (5)
3.3.6. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3-Methoxybenzylidene)Thiazolidin-4-One (6)
3.3.7. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-4-Nitrobenzylidene)Thiazolidin-4-One (7)
3.3.8. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3,4-Dichlorobenzylidene)Thiazolidin-4-one (8)
3.3.9. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-4-(Trifluoromethyl)Benzylidene)Thiazolidin-4-One (9)
3.3.10. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-4-Bromobenzylidene)Thiazolidin-4-One (10)
3.3.11. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3-Bromobenzylidene)Thiazolidin-4-One (11)
3.3.12. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3-Bromo-5-Methylbenzylidene)Thiazolidin-4-One (12)
3.3.13. (2Z,5E)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-(Naphthalen-1-Ylmethylene)Thiazolidin-4-One (13)
3.3.14. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-4-Fluoro-2-Hydroxybenzylidene)Thiazolidin-4-One (14)
3.3.15. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-2-Fluorobenzylidene)Thiazolidin-4-One (15)
3.3.16. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-3-Fluorobenzylidene)Thiazolidin-4-One (16)
3.3.17. (Z)-2-((5-(1H-Indazol-5-yl)-1,3,4-Thiadiazol-2-yl)Imino)-5-((E)-4-Bromo-3-Nitrobenzylidene)Thiazolidin-4-One (17)
3.4. Inhibition Assay of Anticholinesterase/Butyrylcholinesterase Assay
3.5. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chochinov, H.M.; Wilson, K.G.; Enns, M.; Lander, S. Are you depressed? Screening for depression in the terminally ill. Am. J. Psychiatry 1997, 154, 674–676. [Google Scholar] [PubMed]
- Berchtold, N.C.; Cotman, C.W. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol. Aging 1998, 19, 173–189. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke 2002, 33, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer’s disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Holzgrabe, U.; Kapková, P.; Alptüzün, V.; Scheiber, J.; Kugelmann, E. Targeting acetylcholinesterase to treat neurodegeneration. Expert Opin. Ther. Targets 2007, 11, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, V.P. How the cholinesterases got their modern names. Chem.-Biol. Interact. 2010, 187, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Alcantara, V.M.; Chautard-Freire-Maia, E.A.; Scartezini, M.; Cerci, M.S.J.; Braun-Prado, K.; Picheth, G. Butyrylcholinesterase activity and risk factors for coronary artery disease. Scand. J. Clin. Lab. Investig. 2002, 62, 399–404. [Google Scholar] [CrossRef]
- Magarian, E.O.; Dietz, A.J. Correlation of cholinesterase with serum lipids and lipoproteins. J. Clin. Pharmacol. 1987, 27, 819–820. [Google Scholar] [CrossRef]
- Berry, W.T.C.; Cowin, P.J.; Davies, D.R. A relationship between body fat and plasma pseudo-cholinesterase. Br. J. Nutr. 1954, 8, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Pardío, V.T.; Ibarra, N.; Rodríguez, M.A.; Waliszewski, K.N. Use of cholinesterase activity in monitoring organophosphate pesticide exposure of cattle produced in tropical areas. J. Agric. Food Chem. 2001, 49, 6057–6062. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Timchalk, C.; Lin, Y. Carbon nanotube-based electrochemical sensor for assay of salivary cholinesterase enzyme activity: An exposure biomarker of organophosphate pesticides and nerve agents. Environ. Sci. Technol. 2008, 42, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer β-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Woo, Y.J.; Lee, B.H.; Yeun, G.H.; Kim, H.J.; Won, M.H.; Kim, S.H.; Lee, B.H.; Park, J.H. Selective butyrylcholinesterase inhibitors using polyphenol-polyphenol hybrid molecules. Bull. Korean Chem. Soc. 2011, 32, 2593–2598. [Google Scholar] [CrossRef]
- Mills, A.D.; Nazer, M.Z.; Makhluf, J.H.; Kurth, M.J. N,N-Bond-Forming Heterocyclization: Synthesis of 3-Alkoxy-2H-indazoles. J. Org. Chem. 2006, 71, 2687–2689. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, W. Synthesis of 1 H-indazoles and 1 H-pyrazoles via FeBr3/O2 mediated intramolecular C–H amination. J. Org. Chem. 2013, 78, 1317–1322. [Google Scholar] [CrossRef]
- Li, P.; Wu, C.; Zhao, J.; Rogness, D.C.; Shi, F. Synthesis of substituted 1 H-indazoles from arynes and hydrazones. J. Org. Chem. 2012, 77, 3149–3158. [Google Scholar] [CrossRef]
- Spiteri, C.; Keeling, S.; Moses, J.E. New synthesis of 1-substituted-1 H-indazoles via 1, 3-dipolar cycloaddition of in situ generated nitrile imines and benzyne. Org. Lett. 2010, 12, 3368–3371. [Google Scholar] [CrossRef]
- Kumar, M.R.; Park, A.; Park, N.; Lee, S. Consecutive condensation, C–N and N–N bond formations: A copper-catalyzed one-pot three-component synthesis of 2 H-indazole. Org. Lett. 2011, 13, 3542–3545. [Google Scholar] [CrossRef]
- Alomari, M.; Taha, M.; Rahim, F.; SelvaraJ, M.; Iqbal, N.; Chigurupati, S.; Hussain, S.; Uddin, N.; Almandil, N.B.; Nawaz, M.; et al. Synthesis of indole-based-thiadiazole derivatives as a potent inhibitor of α-glucosidase enzyme along with in silico study. Bioorganic Chem. 2021, 108, 104638. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Rahim, F.; Ullah, H.; Wadood, A.; Farooq, R.K.; Shah, S.A.A.; Nawaz, M.; Zakaria, Z.A. Synthesis, in vitro urease inhibitory potential and molecular docking study of benzofuran-based-thiazoldinone analogues. Sci. Rep. 2020, 10, 10673. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Luo, X.Q.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1, 3, 4-thiadiazole and 1, 3, 4-oxadiazole moiety. Bioorganic Med. Chem. 2008, 16, 3632–3640. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, R.A.; Ahmed, B. Syntheses and anti-depressant activity of 5-amino-1, 3, 4-thiadiazole-2-thiol imines and thiobenzyl derivatives. Bioorganic Med. Chem. 2008, 16, 8029–8034. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Uddin, I.; Gollapalli, M.; Almandil, N.B.; Rahim, F.; Farooq, R.K.; Nawaz, M.; Ibrahim, M.; Alqahtani, M.A.; Bamarouf, Y.A.; et al. Synthesis, anti-leishmanial and molecular docking study of bis-indole derivatives. BMC Chem. 2019, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Uddin, N.; Ali, M.; Rahim, F.; Khan, G.; Farooq, R.K.; Gollapalli, M.; Iqbal, N.; Farooq, M.; Khan, K.M. Inhibition potential of phenyl linked benzimidazole-triazolothiadiazole modular hybrids against β-glucuronidase and their interactions thereof. Int. J. Biol. Macromol. 2020, 161, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Almandil, N.B.; Rashid, U.; Ali, M.; Ibrahim, M.; Gollapalli, M.; Mosaddik, A.; Khan, K.M. 2, 5-Disubstituted thiadiazoles as potent β-glucuronidase inhibitors; Synthesis, in vitro and in silico studies. Bioorganic Chem. 2019, 91, 103126. [Google Scholar] [CrossRef]
- Alomari, M.; Taha, M.; Imran, S.; Jamil, W.; Selvaraj, M.; Uddin, N.; Rahim, F. Design, synthesis, in vitro evaluation, molecular docking and ADME properties studies of hybrid bis-coumarin with thiadiazole as a new inhibitor of Urease. Bioorganic Chem. 2019, 92, 103235. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorganic Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef]
- Khan, Y.; Rehman, W.; Hussain, R.; Khan, S.; Malik, A.; Khan, M.; Liaqat, A.; Rasheed, L.; Begum, F.; Fazil, S.; et al. New biologically potent benzimidazole-based-triazole derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors along with molecular docking study. J. Heterocycl. Chem. 2022, 59, 2225–2239. [Google Scholar] [CrossRef]
- Hussain, R.; Rehman, W.; Rahim, F.; Khan, S.; Alanazi, A.S.; Alanazi, M.M.; Rasheed, L.; Khan, Y.; Shah, S.A.A.; Taha, M. Synthesis, In Vitro Thymidine Phosphorylase Inhibitory Activity and Molecular Docking Study of Novel Pyridine-derived Bis-Oxadiazole Bearing Bis-Schiff Base Derivatives. Arab. J. Chem. 2023, 16, 104773. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, H.; Hussain, R.; Khan, Y.; Khan, M.U.; Khan, M.; Sattar, A.; Khan, M.S. Synthesis, in Vitro Bio-evaluation, and Molecular Docking Study of Thiosemicarbazone-based Isatin/bis-Schiff base Hybrid Analogues as Effective Cholinesterase Inhibitors. J. Mol. Struct. 2023, 1284, 135351. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, W.; Rahim, F.; Hussain, R.; Obaidullah, A.J.; Alotaibi, H.F.; Alanazi, M.M.; Khan, M.U.; Khan, Y. Bis-indole based triazine derivatives: Synthesis, characterization, in vitro β-glucuronidase anti-cancer and anti-bacterial evaluation along with in silico molecular docking and ADME analysis. Arab. J. Chem. 2023, 16, 104970. [Google Scholar] [CrossRef]
- Anwar, S.; Rehman, W.; Hussain, R.; Khan, S.; Alanazi, M.M.; Alsaif, N.A.; Khan, Y.; Iqbal, S.; Naz, A.; Hashmi, M.A. Investigation of Novel Benzoxazole-Oxadiazole Derivatives as Effective Anti-Alzheimer’s Agents: In Vitro and In Silico Approaches. Pharmaceuticals 2023, 16, 909. [Google Scholar] [CrossRef]

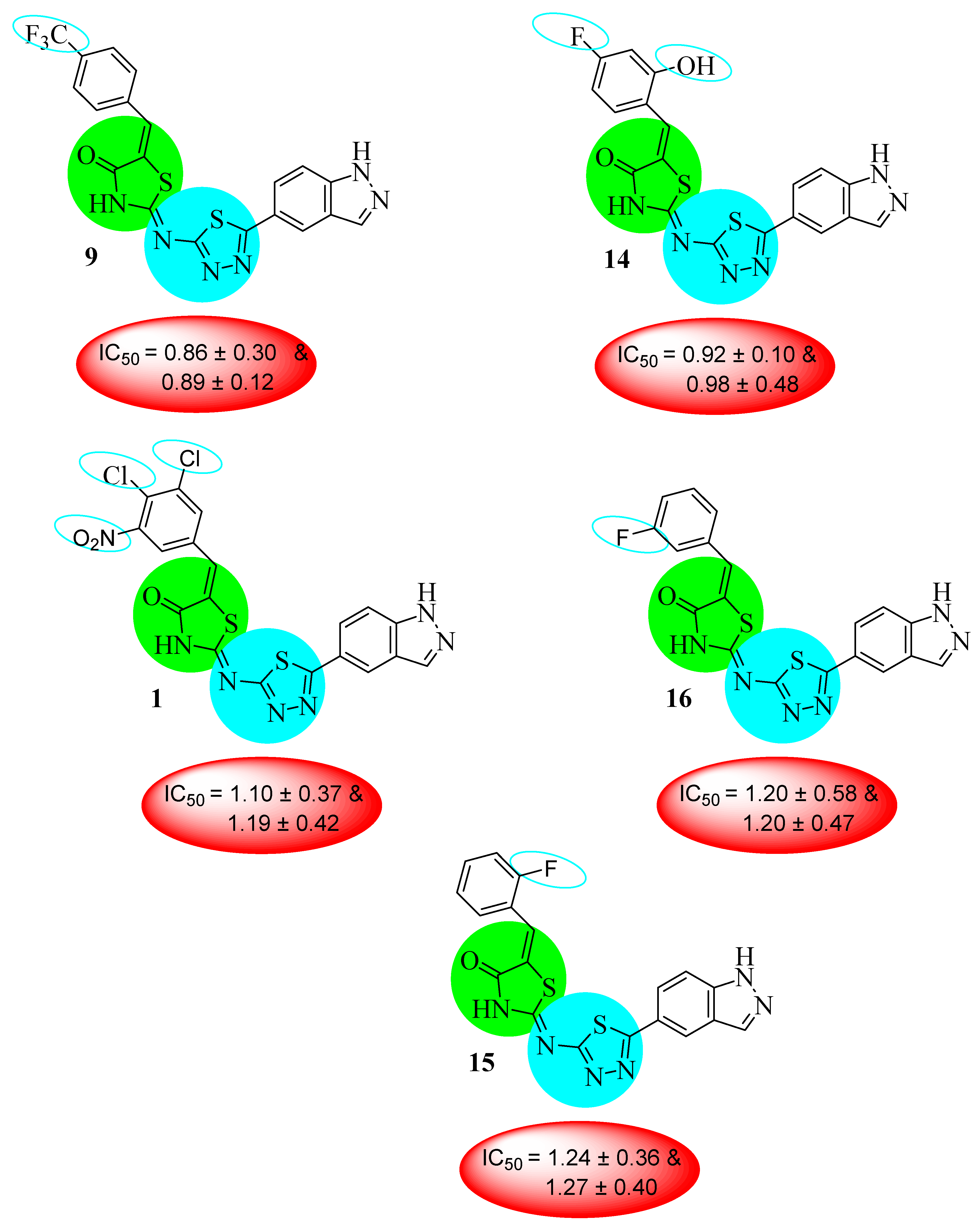

| S. No. | R | AChE IC50 ± SEM (µM) | BuChE IC50 ± SEM (µM) |
|---|---|---|---|
| 1 | 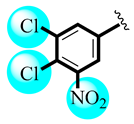 | 1.10 ± 0.37 | 1.19 ± 0.42 |
| 2 | 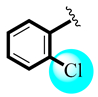 | 3.11 ± 0.59 | 3.36 ± 0.28 |
| 3 | 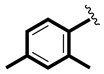 | 8.26 ± 0.40 | 8.80 ± 0.13 |
| 4 |  | 8.60 ± 1.05 | 8.84 ± 0.98 |
| 5 |  | 10.43 ± 0.98 | 10.62 ± 0.74 |
| 6 | 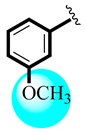 | 12.73 ± 0.33 | 12.98 ± 0.15 |
| 7 | 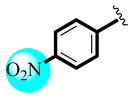 | 1.40 ± 0.31 | 1.57 ± 0.28 |
| 8 | 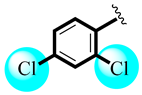 | 1.22 ± 0.64 | 1.29 ± 0.41 |
| 9 |  | 0.86 ± 0.30 | 0.89 ± 0.12 |
| 10 |  | 24.68 ± 0.12 | 25.03 ± 0.44 |
| 11 | 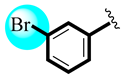 | 26.73 ± 0.84 | 27.08 ± 0.19 |
| 12 | 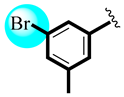 | 25.12 ± 0.18 | 26.20 ± 0.67 |
| 13 |  | N.A. | N.A. |
| 14 | 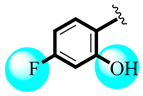 | 0.92 ± 0.10 | 0.98 ± 0.48 |
| 15 |  | 1.24 ± 0.36 | 1.27 ± 0.40 |
| 16 | 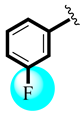 | 1.20 ± 0.58 | 1.20 ± 0.47 |
| 17 | 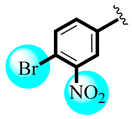 | 22.30 ± 1.00 | 20.46 ± 0.86 |
| Standard drug Donepezil | 1.26 ± 0.18 µM | 1.35 ± 0.37 µM | |
| Active Analog | Distance (A°) | Type of Interaction | Receptor | Docking Score |
|---|---|---|---|---|
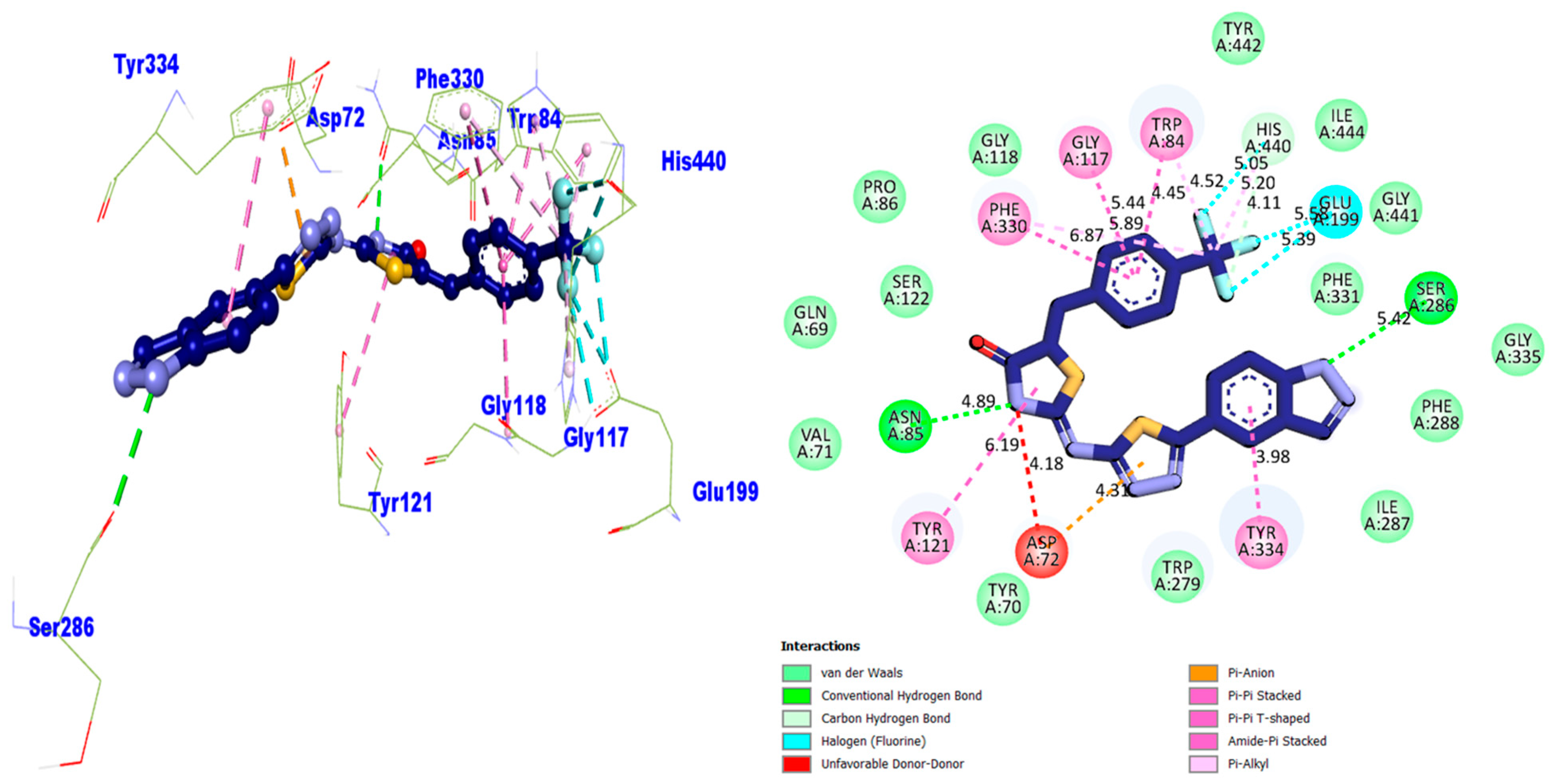
| Compound 1 AChE | 5.82 | Conventional H–B | PHE-A-288 | −11.86 |
| 4.92 | Conventional H–B | ARG-A-289 | ||
| 5.59 | Pi–Anion | ASP-A-72 | ||
| 5.42 | Pi–Pi Stacked | PHE-A-330 | ||
| 5.10 | Carbon H–B | HIS-A-440 | ||
| 4.46 | Pi–Pi Stacked | TRP-A-84 | ||
| 3.98 | Pi–Pi T-shaped | TRP-A-84 | ||
| 7.17 | Pi–Anion | GLU-A-199 | ||
| 5.43 | Conventional H–B | GLU-A-199 | ||
| 5.58 | Pi–Pi T-shaped | GLY-A-117 | ||
| 6.27 | Pi–Pi Stacked | GLY-A-117 | ||
| 4.53 | Pi–Sulfur | TYR-A-334 | ||
| 4.53 | Pi–Pi T-shaped | TYR-A-334 | ||
| 7.49 | Pi–Pi T-shaped | TYR-A-121 | ||
| 7.92 | Pi–Pi T-shaped | TRP-A-279 | ||
| Compound 1 BuChE | 6.30 | Pi–Anion | ASP-A-70 | −7.56 |
| 4.08 | Unfavorable D–D | ASN-A-68 | ||
| 4.44 | Pi–Alkyl | HIS-A-438 | ||
| 4.19 | Conventional H–B | THR-A-120 | ||
| 3.61 | Carbon H–B | GLY-A-116 | ||
| 4.11 | Pi–Pi Stacked | TRP-A-82 | ||
| 5.38 | Pi–Alkyl | TRP-A-82 | ||
| Compound 9 AChE | 4.89 | Conventional H–B | ASN-A-85 | −10.34 |
| 6.19 | Amide–Pi Stacked | TYR-A-121 | ||
| 4.18 | Unfavorable D–D | ASP-A-72 | ||
| 4.31 | Pi–Anion | ASP-A-72 | ||
| 3.98 | Pi–Pi Stacked | TYR-A-334 | ||
| 5.42 | Conventional H–B | SER-A-286 | ||
| 5.39 | H–F | GLU-A-199 | ||
| 5.58 | H–F | GLU-A-199 | ||
| 4.11 | Carbon H–B | HIS-A-440 | ||
| 5.20 | Pi–Alkyl | HIS-A-440 | ||
| 5.05 | H–F | HIS-A-440 | ||
| 4.52 | Pi–Alkyl | TRP-A-84 | ||
| 4.45 | Pi–Pi Stacked | TRP-A-84 | ||
| 5.44 | Pi–Pi Stacked | GLY-A-117 | ||
| 5.89 | Pi–Alkyl | PHE-A-330 | ||
| 6.87 | Pi–Pi Stacked | PHE-A-330 | ||
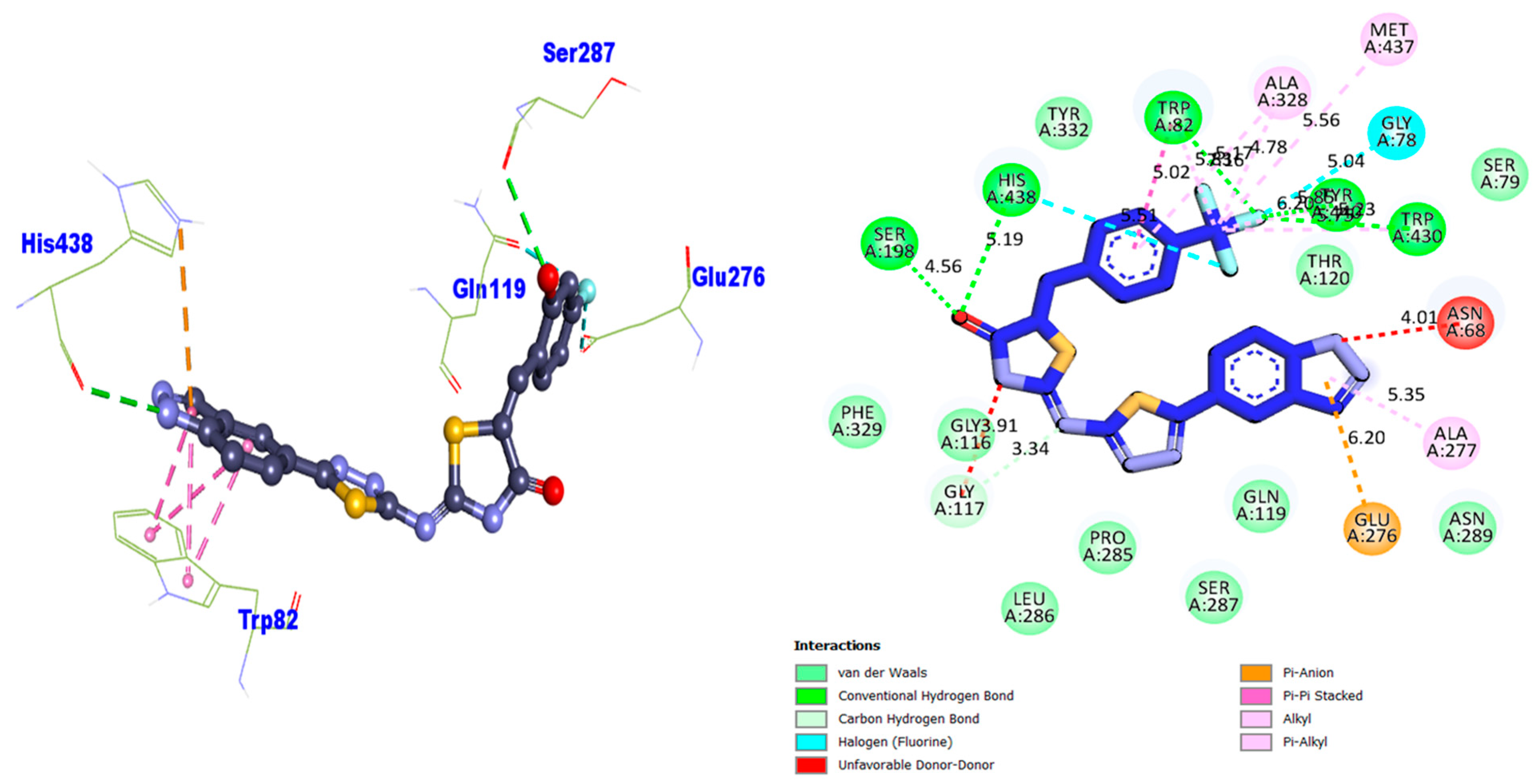
| Compound 9 BuChE | 3.91 | Unfavorable D–D | GLY-A-117 | −12.88 |
| 3.34 | Carbon H–B | GLY-A-117 | ||
| 6.20 | Pi–Anion | GLU-A-276 | ||
| 5.35 | Pi–Alkyl | ALA-A-277 | ||
| 4.01 | Unfavorable D–D | ASN-A-68 | ||
| 5.75 | Alkyl | TRP-A-430 | ||
| 5.23 | Conventional H–B | TRP-A-430 | ||
| 6.20 5.86 | Alkyl Conventional H–B | TYR-A-440 TYR-A-440 | ||
| 5.04 | H–F | GLY-A-78 | ||
| 5.56 | Alkyl | MET-A-437 | ||
| 4.78 | Alkyl | ALA-A-328 | ||
| 5.17 | Pi–Alkyl | ALA-A-328 | ||
| 5.36 | Conventional H–B | TRP-A-82 | ||
| 5.73 | Alkyl | TRP-A-82 | ||
| 5.02 | Pi–Pi Stacked | TRP-A-82 | ||
| 5.51 | H–F | HIS-A-438 | ||
| 5.19 | Conventional H–B | HIS-A-438 | ||
| 4.56 | Conventional H–B | SER-A-198 | ||
| Compound 14 AChE | 7.70 | Pi–Pi Stacked | TRP-A-279 | −6.99 |
| 4.39 | Pi–Pi Stacked | TYR-A-334 | ||
| 4.29 | Pi–Sulfur | TYR-A-334 | ||
| 6.26 | Pi–Pi Stacked | GLY-A-117 | ||
| 5.44 | Pi–Pi Stacked | GLY-A-117 | ||
| 5.23 | Pi–Pi Stacked | PHE-A-330 | ||
| 4.83 | Pi–Pi Stacked | TRP-A-84 | ||
| 4.06 | Pi–Pi Stacked | TRP-A-84 | ||
| 7.14 | Pi–Anion | GLU-A-199 | ||
| 4.17 | H–F | SER-A-286 | ||
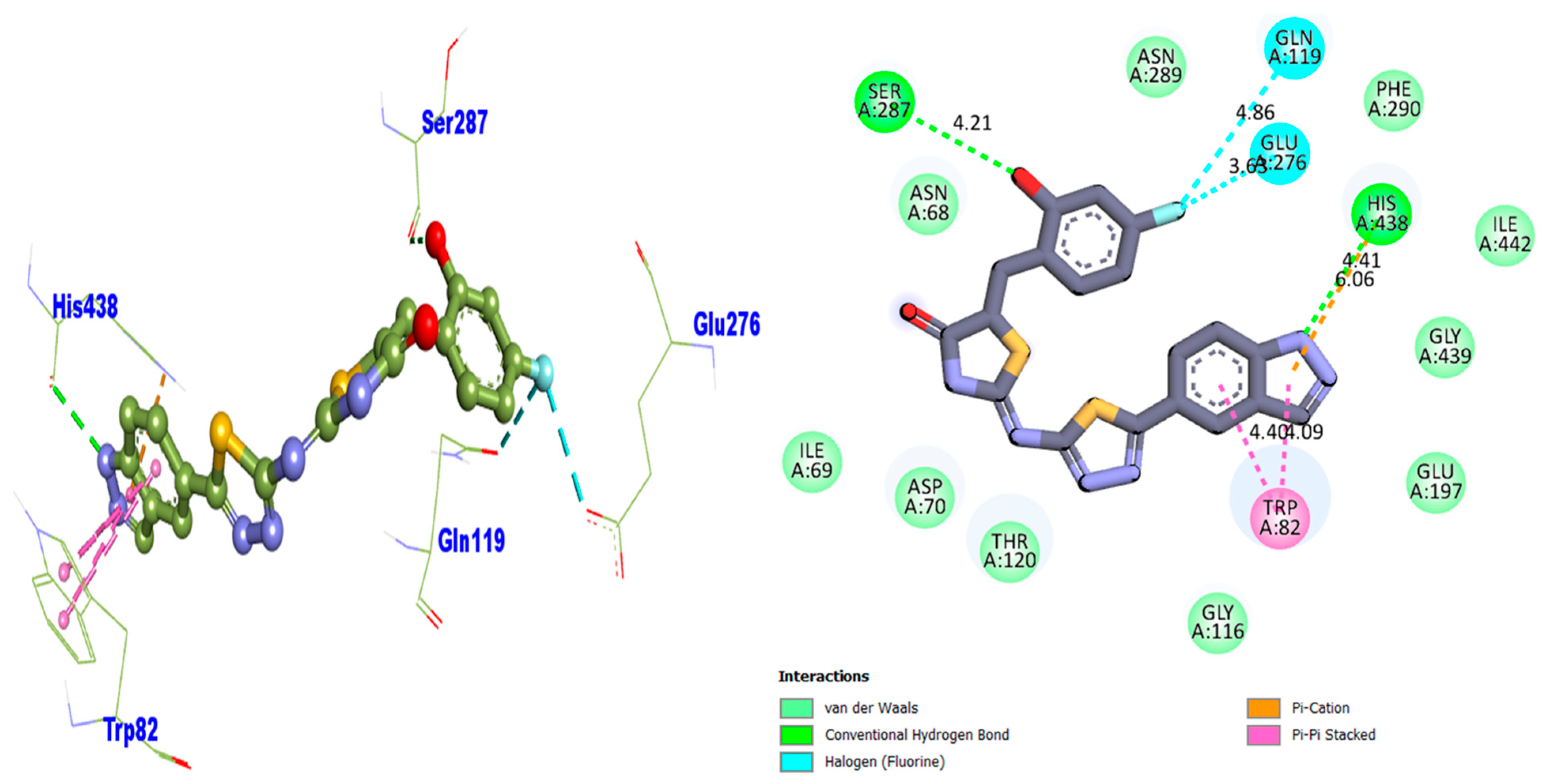
| Compound 14 BuChE | 4.40 | Pi–Pi Stacked | TRP-A-82 | −6.12 |
| 4.09 | Pi–Pi Stacked | TRP-A-82 | ||
| 6.06 | Pi–Cation | HIS-A-438 | ||
| 4.41 | Conventional H–B | HIS-A-438 | ||
| 3.63 | H–F | GLU-A-276 | ||
| 4.86 | H–F | GLN-A-119 | ||
| 4.21 | Conventional H–B | SER-A-287 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, Y.; Khan, S.; Hussain, R.; Rehman, W.; Maalik, A.; Gulshan, U.; Attwa, M.W.; Darwish, H.W.; Ghabbour, H.A.; Ali, N. Identification of Indazole-Based Thiadiazole-Bearing Thiazolidinone Hybrid Derivatives: Theoretical and Computational Approaches to Develop Promising Anti-Alzheimer’s Candidates. Pharmaceuticals 2023, 16, 1667. https://doi.org/10.3390/ph16121667
Khan Y, Khan S, Hussain R, Rehman W, Maalik A, Gulshan U, Attwa MW, Darwish HW, Ghabbour HA, Ali N. Identification of Indazole-Based Thiadiazole-Bearing Thiazolidinone Hybrid Derivatives: Theoretical and Computational Approaches to Develop Promising Anti-Alzheimer’s Candidates. Pharmaceuticals. 2023; 16(12):1667. https://doi.org/10.3390/ph16121667
Chicago/Turabian StyleKhan, Yousaf, Shoaib Khan, Rafaqat Hussain, Wajid Rehman, Aneela Maalik, Urooba Gulshan, Mohamed W. Attwa, Hany W. Darwish, Hazem A. Ghabbour, and Nawab Ali. 2023. "Identification of Indazole-Based Thiadiazole-Bearing Thiazolidinone Hybrid Derivatives: Theoretical and Computational Approaches to Develop Promising Anti-Alzheimer’s Candidates" Pharmaceuticals 16, no. 12: 1667. https://doi.org/10.3390/ph16121667
APA StyleKhan, Y., Khan, S., Hussain, R., Rehman, W., Maalik, A., Gulshan, U., Attwa, M. W., Darwish, H. W., Ghabbour, H. A., & Ali, N. (2023). Identification of Indazole-Based Thiadiazole-Bearing Thiazolidinone Hybrid Derivatives: Theoretical and Computational Approaches to Develop Promising Anti-Alzheimer’s Candidates. Pharmaceuticals, 16(12), 1667. https://doi.org/10.3390/ph16121667









