Intratracheally Administered Peptide-Modified Lipid Admixture Containing Fasudil and/or DETA NONOate Ameliorates Various Pathologies of Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Results
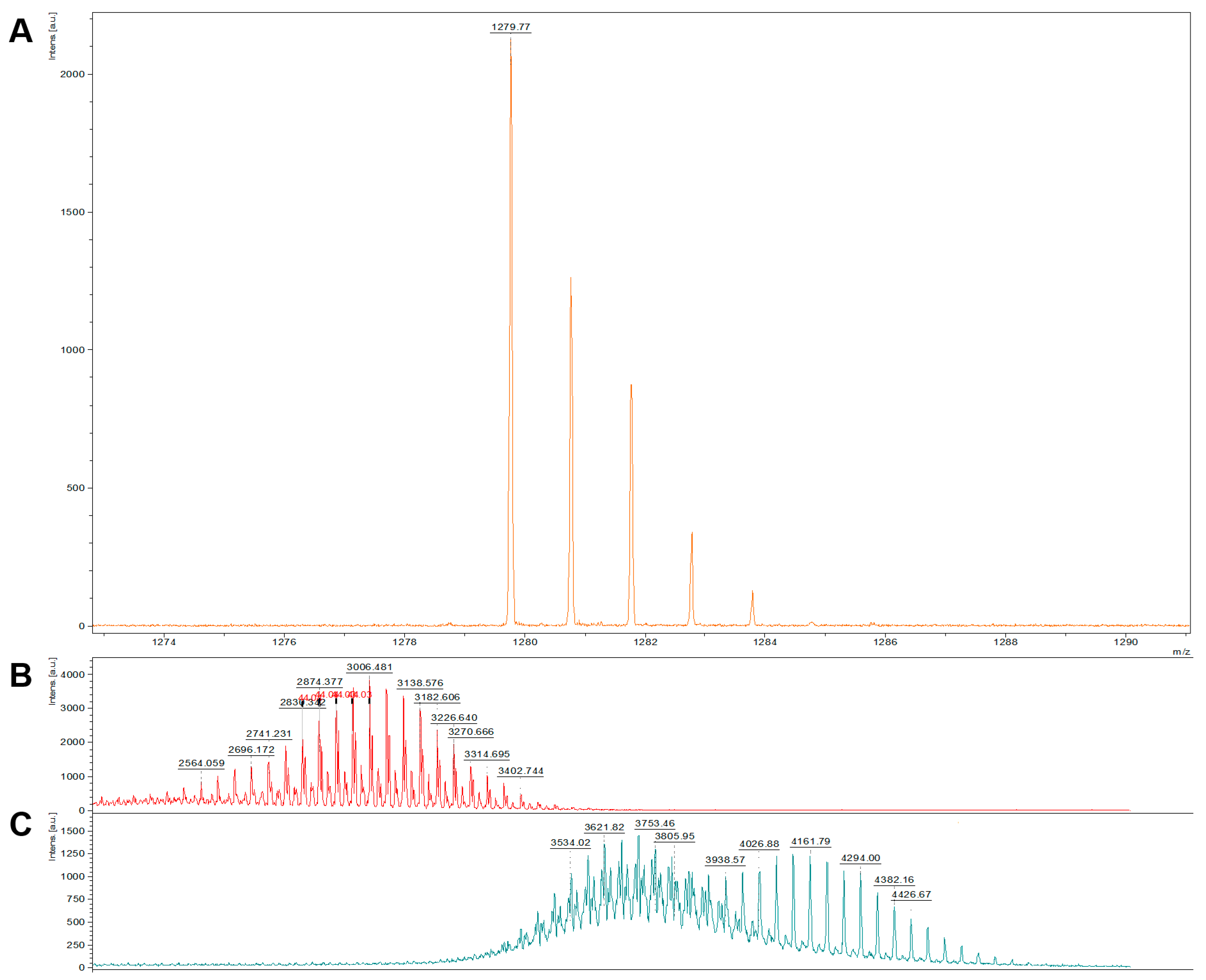
2.1. Characterization of CAR-Lipid Conjugates and CAR-Modified Lipid Mixtures
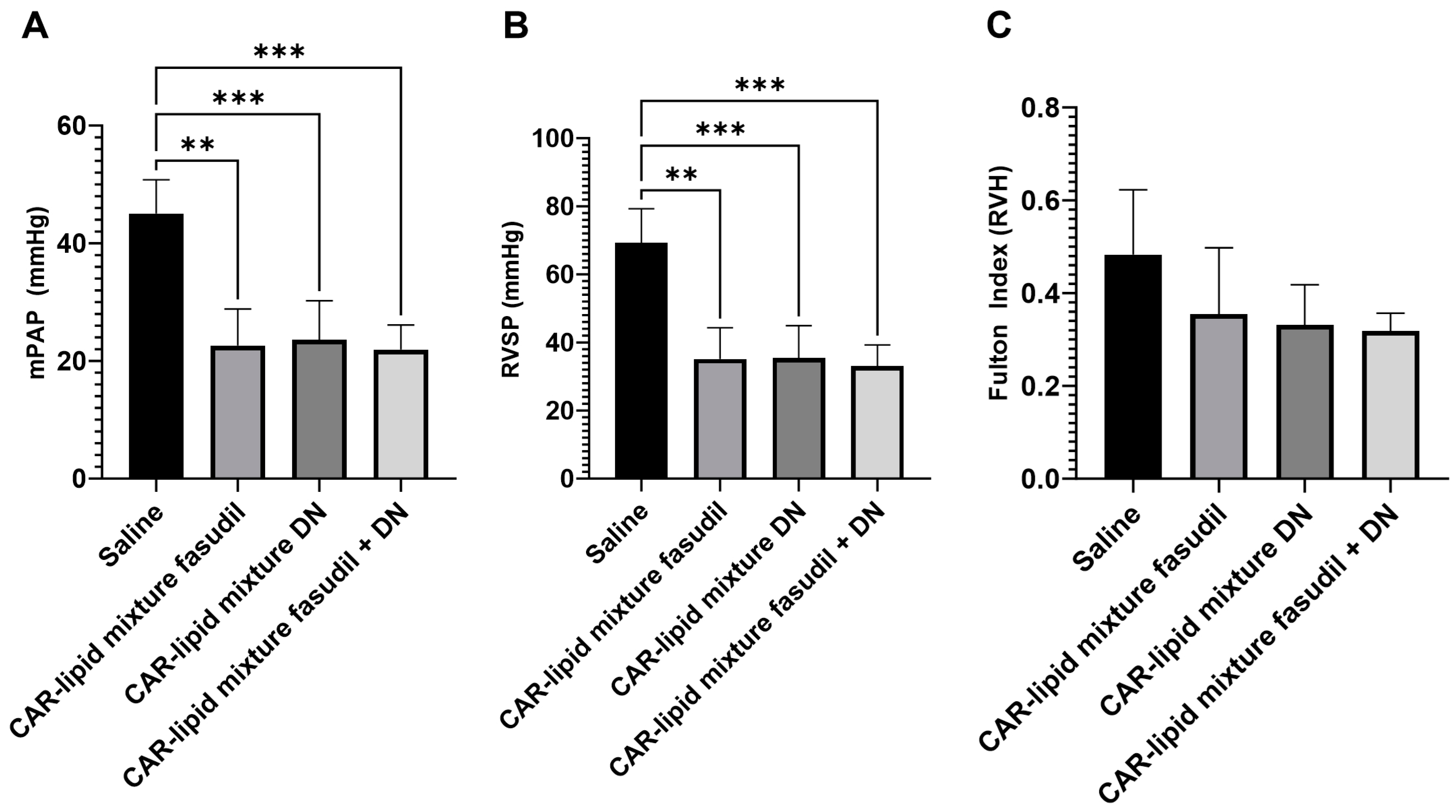
2.2. Evaluation of Pulmonary Hemodynamics and Right Ventricular Hypertrophy
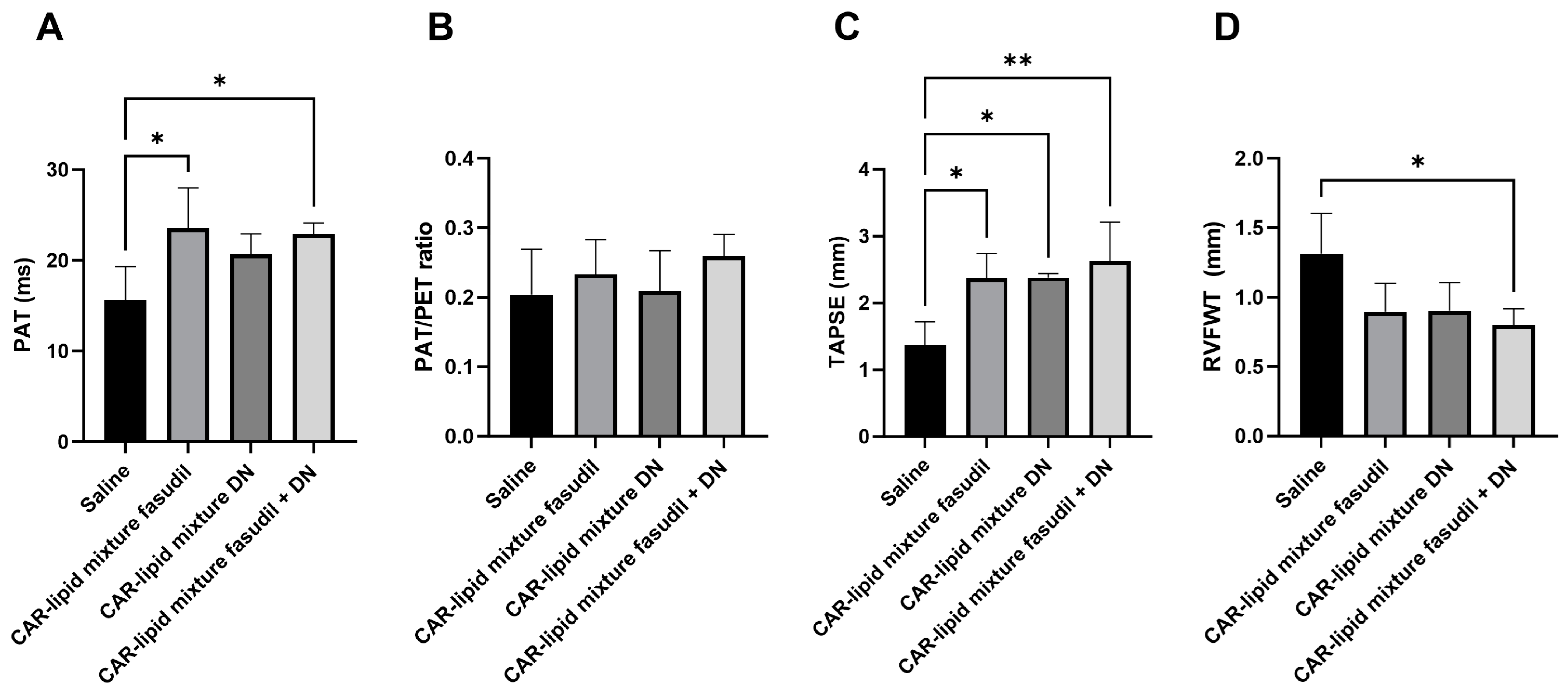
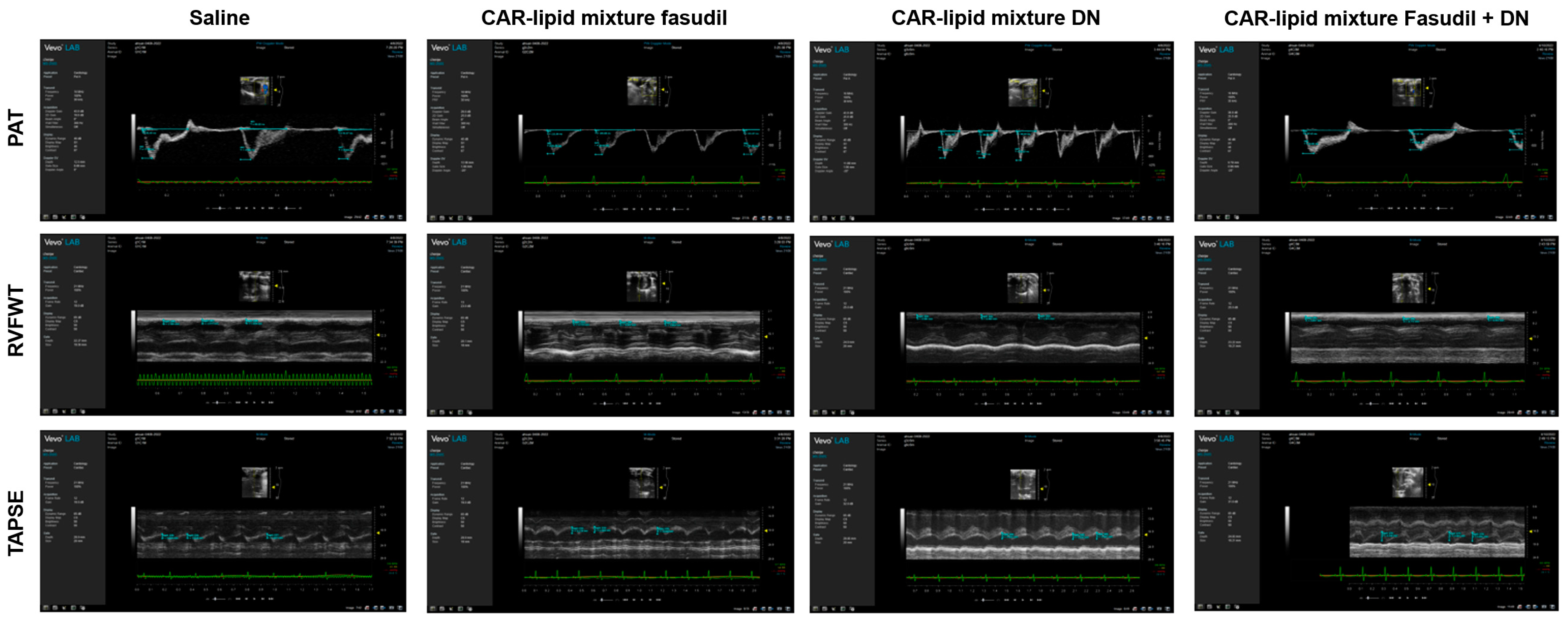
2.3. Echocardiographic Evaluation of RV Functions
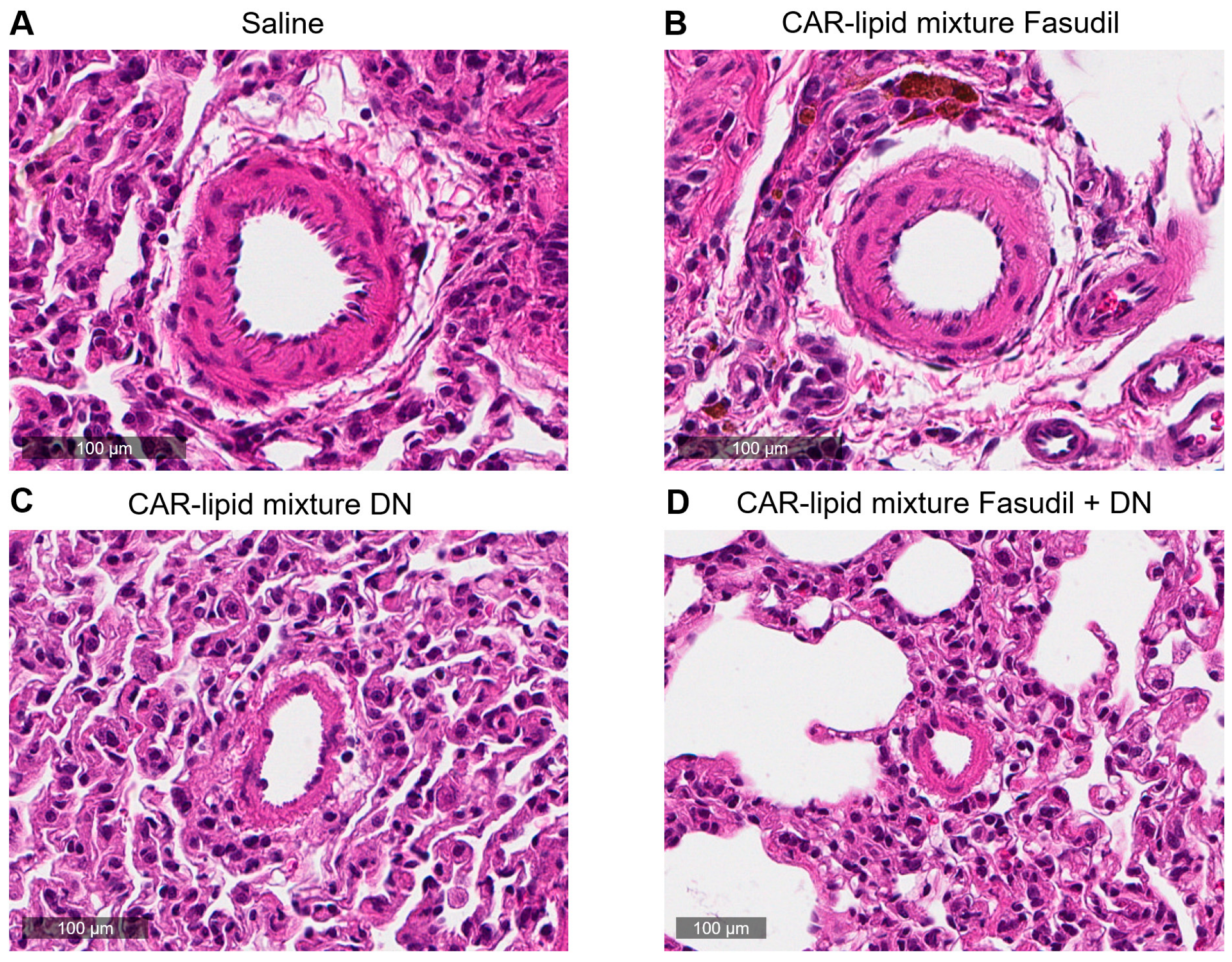
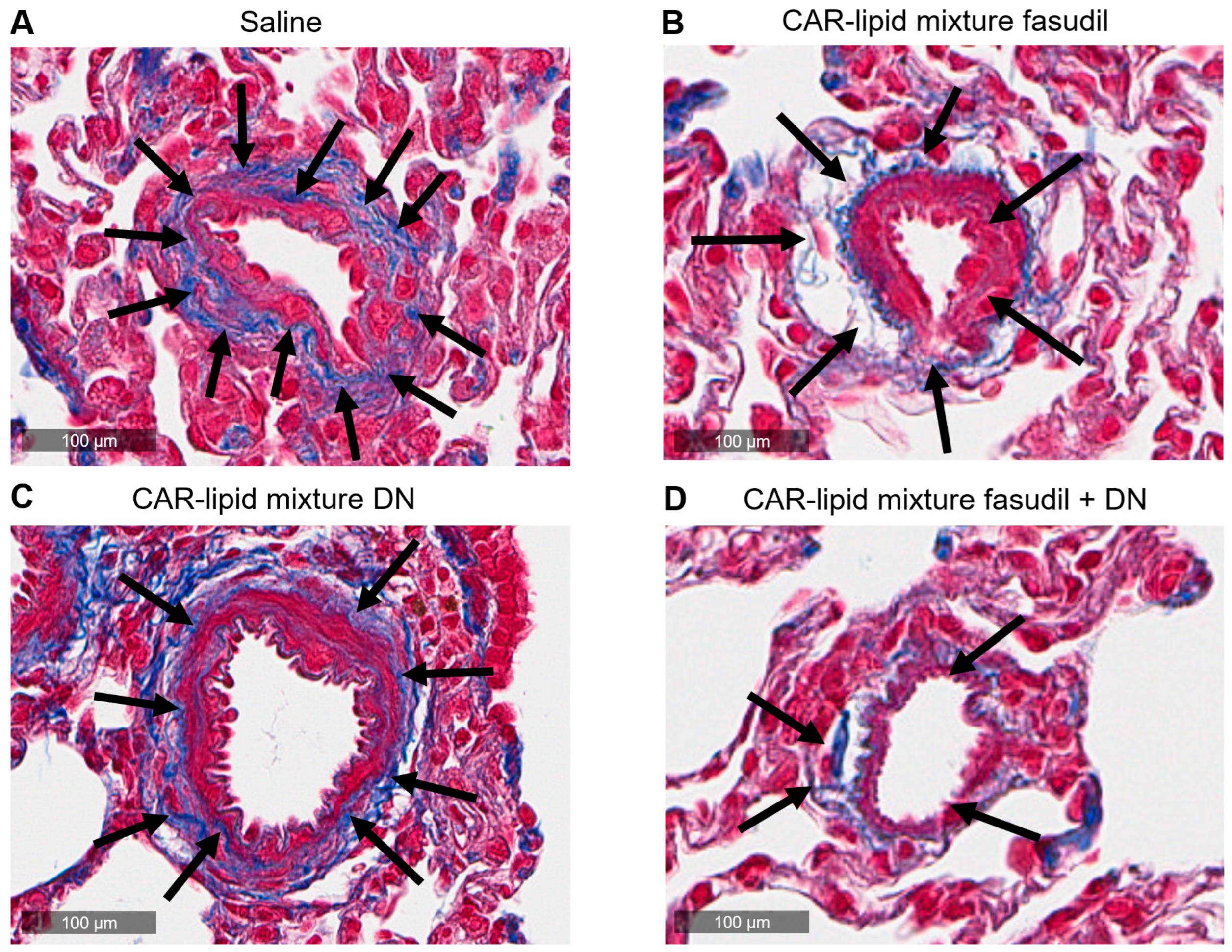
2.4. Histopathological Evaluation of Lung Vasculatures
3. Discussion
4. Material and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of CAR-Modified Lipid Mixture of Fasudil and DN
4.2.2. Conjugation of CAR and DSPE-PEG 2000 Maleimide
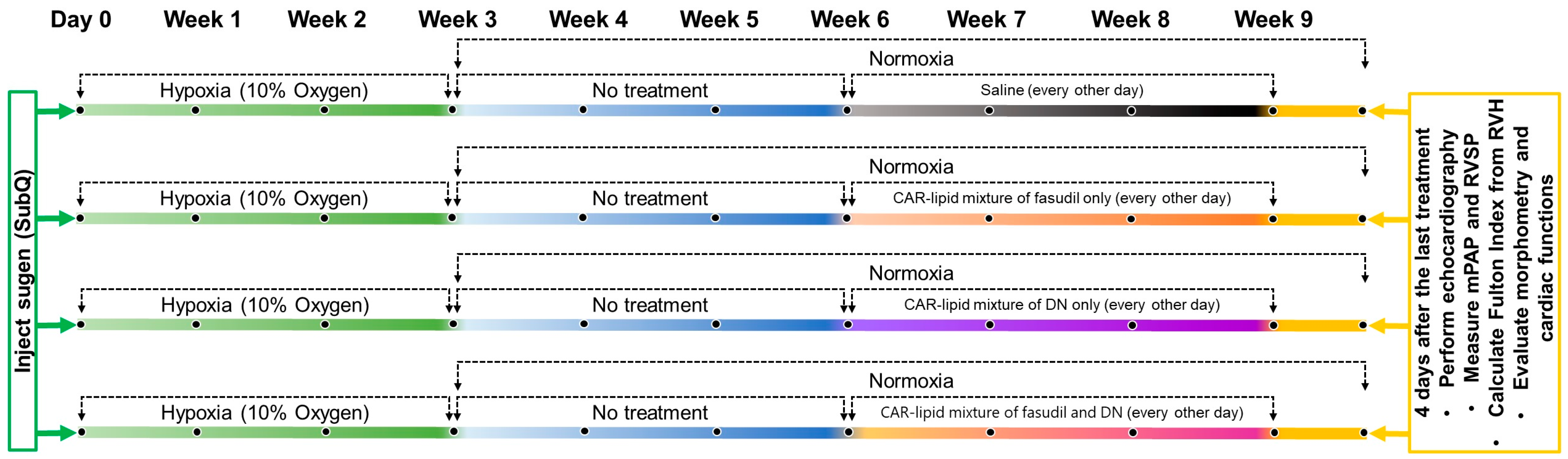
4.2.3. Development of the Sugen/Hypoxia (SuHx) Rat Model of PAH
4.2.4. Evaluation of Right Ventricular Functions
4.2.5. Measurement of Right Ventricular Systemic Pressure (RVSP) and Mean Pulmonary Arterial Pressure (mPAP)
4.2.6. Measurement of Right Ventricular Hypertrophy
4.2.7. Vessel Remodeling and Collagen Deposition in the Pulmonary Vasculature of Rats
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Food and Drug Administration. Orphan Products: Hope for People with Rare Diseases; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019. [Google Scholar]
- Rich, S. The effects of vasodilators in pulmonary hypertension: Pulmonary vascular or peripheral vascular? Circ. Heart Fail. 2009, 2, 145–150. [Google Scholar] [CrossRef]
- Page, R.L., 2nd; O’Bryant, C.L.; Cheng, D.; Dow, T.J.; Ky, B.; Stein, C.M.; Spencer, A.P.; Trupp, R.J.; Lindenfeld, J.; American Heart Association Clinical Pharmacology; et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e32–e69. [Google Scholar] [CrossRef]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [CrossRef]
- Gupta, N.; Al-Saikhan, F.I.; Patel, B.; Rashid, J.; Ahsan, F. Fasudil and SOD packaged in peptide-studded-liposomes: Properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs. Int. J. Pharm. 2015, 488, 33–43. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Judge, E.P.; Gaine, S.P. Management of pulmonary arterial hypertension. Curr. Opin. Crit Care 2013, 19, 44–50. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; McMurtry, I.F.; Oka, M.; Nozik-Grayck, E.; Komatsu, M.; Ahsan, F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J. Control Release 2013, 167, 189–199. [Google Scholar] [CrossRef]
- Li, B.; He, W.; Ye, L.; Zhu, Y.; Tian, Y.; Chen, L.; Yang, J.; Miao, M.; Shi, Y.; Azevedo, H.S.; et al. Targeted Delivery of Sildenafil for Inhibiting Pulmonary Vascular Remodeling. Hypertension 2019, 73, 703–711. [Google Scholar] [CrossRef]
- Burks, M.; Stickel, S.; Galie, N. Pulmonary Arterial Hypertension: Combination Therapy in Practice. Am. J. Cardiovasc. Drugs 2018, 18, 249–257. [Google Scholar] [CrossRef]
- Mandras, S.; Kovacs, G.; Olschewski, H.; Broderick, M.; Nelsen, A.; Shen, E.; Champion, H. Combination Therapy in Pulmonary Arterial Hypertension-Targeting the Nitric Oxide and Prostacyclin Pathways. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 453–462. [Google Scholar] [CrossRef]
- Galie, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.-C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef]
- Shah, A.J.; Beckmann, T.; Vorla, M.; Kalra, D.K. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023, 24, 5850. [Google Scholar] [CrossRef]
- Rossi, S.; Pietrangelo, C.; Pierdomenico, S.D.; Giuliani, L. Upfront triple oral combination therapy including selexipag in a high-risk patient with idiopathic pulmonary arterial hypertension: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Nahar, K.; Rashid, J.; Absar, S.; Al-Saikhan, F.I.; Ahsan, F. Liposomal Aerosols of Nitric Oxide (NO) Donor as a Long-Acting Substitute for the Ultra-Short-Acting Inhaled NO in the Treatment of PAH. Pharm. Res. 2016, 33, 1696–1710. [Google Scholar] [CrossRef]
- Rashid, J.; Nahar, K.; Raut, S.; Keshavarz, A.; Ahsan, F. Fasudil and DETA NONOate, Loaded in a Peptide-Modified Liposomal Carrier, Slow PAH Progression upon Pulmonary Delivery. Mol. Pharm. 2018, 15, 1755–1765. [Google Scholar] [CrossRef]
- He, S.; Zhu, T.; Fang, Z. The Role and Regulation of Pulmonary Artery Smooth Muscle Cells in Pulmonary Hypertension. Int. J. Hypertens. 2020, 2020, 1478291. [Google Scholar] [CrossRef]
- Montagnoli, T.L.; da Silva, J.S.; Sudo, S.Z.; Santos, A.D.; Gomide, G.F.; de Sá, M.P.L.; Zapata-Sudo, G. ROCK Inhibition as Potential Target for Treatment of Pulmonary Hypertension. Cells 2021, 10, 1648. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, J.; Zheng, X.; Hu, X.; He, Y. Prostaglandin and prostaglandin receptors: Present and future promising therapeutic targets for pulmonary arterial hypertension. Respir. Res. 2023, 24, 263. [Google Scholar] [CrossRef]
- Kurakula, K.; Smolders, V.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef]
- Archer, S.L.; Weir, E.K.; Wilkins, M.R. Basic science of pulmonary arterial hypertension for clinicians: New concepts and experimental therapies. Circulation 2010, 121, 2045–2066. [Google Scholar] [CrossRef]
- Hautbergue, T.; Antigny, F.; Boet, A.; Haddad, F.; Masson, B.; Lambert, M.; Delaporte, A.; Menager, J.-B.; Savale, L.; Le Pavec, J.; et al. Right Ventricle Remodeling Metabolic Signature in Experimental Pulmonary Hypertension Models of Chronic Hypoxia and Monocrotaline Exposure. Cells 2021, 10, 1559. [Google Scholar] [CrossRef]
- Gupta, N.; Rashid, J.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Cocktail of Superoxide Dismutase and Fasudil Encapsulated in Targeted Liposomes Slows PAH Progression at a Reduced Dosing Frequency. Mol. Pharm. 2017, 14, 830–841. [Google Scholar] [CrossRef]
- Keshavarz, A.; Alobaida, A.; McMurtry, I.F.; Nozik-Grayck, E.; Stenmark, K.R.; Ahsan, F. CAR, a Homing Peptide, Prolongs Pulmonary Preferential Vasodilation by Increasing Pulmonary Retention and Reducing Systemic Absorption of Liposomal Fasudil. Mol. Pharm. 2019, 16, 3414–3429. [Google Scholar] [CrossRef]
- Zhu, Z.; Godana, D.; Li, A.; Rodriguez, B.; Gu, C.; Tang, H.; Minshall, R.D.; Huang, W.; Chen, J. Echocardiographic assessment of right ventricular function in experimental pulmonary hypertension. Pulm. Circ. 2019, 9, 2045894019841987. [Google Scholar] [CrossRef]
- Zhang, X.; An, H.; Li, J.; Zhang, Y.; Liu, Y.; Jia, Z.; Zhang, W.; Chu, L.; Zhang, H. Selective activation of vascular Kv7.4/Kv7.5 K+ channels by fasudil contributes to its vasorelaxant effect. Br. J. Pharmacol. 2016, 173, 3480–3491. [Google Scholar] [CrossRef]
- Kumar, A.A.; Yeo, N.; Whittaker, M.; Attra, P.; Barrick, T.R.; Bridges, L.R.; Dickson, D.W.; Esiri, M.M.; Farris, C.W.; Graham, D.; et al. Vascular Collagen Type-IV in Hypertension and Cerebral Small Vessel Disease. Stroke 2022, 53, 3696–3705. [Google Scholar] [CrossRef]
- Asano, T.; Ikegaki, I.; Satoh, S.-I.; Seto, M.; Sasaki, Y. A Protein Kinase Inhibitor, Fasudil (AT-877): A Novel Approach to Signal Transduction Therapy. Cardiovasc. Drug Rev. 1998, 16, 76–87. [Google Scholar] [CrossRef]
- Sarkar, T.; Isbatan, A.; Moinuddin, S.M.; Chen, J.; Ahsan, F. Catheterization of Pulmonary and Carotid Arteries for Concurrent Measurement of Mean Pulmonary and Systemic Arterial Pressure in Rat Models of Pulmonary Arterial Hypertension. Bio Protoc. 2023, 13, e4737. [Google Scholar] [CrossRef]
- Ma, W.; Han, W.; Greer, P.A.; Tuder, R.M.; Toque, H.A.; Wang, K.K.; Caldwell, R.W.; Su, Y. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J. Clin. Investig. 2011, 121, 4548–4566. [Google Scholar] [CrossRef]
- Chen, J.; Sysol, J.R.; Singla, S.; Zhao, S.; Yamamura, A.; Valdez-Jasso, D.; Abbasi, T.; Shioura, K.M.; Sahni, S.; Reddy, V.; et al. Nicotinamide Phosphoribosyltransferase Promotes Pulmonary Vascular Remodeling and Is a Therapeutic Target in Pulmonary Arterial Hypertension. Circulation 2017, 135, 1532–1546. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, T.; Moinuddin, S.M.; Isbatan, A.; Chen, J.; Mann, D.; Ahsan, F. Intratracheally Administered Peptide-Modified Lipid Admixture Containing Fasudil and/or DETA NONOate Ameliorates Various Pathologies of Pulmonary Arterial Hypertension. Pharmaceuticals 2023, 16, 1656. https://doi.org/10.3390/ph16121656
Sarkar T, Moinuddin SM, Isbatan A, Chen J, Mann D, Ahsan F. Intratracheally Administered Peptide-Modified Lipid Admixture Containing Fasudil and/or DETA NONOate Ameliorates Various Pathologies of Pulmonary Arterial Hypertension. Pharmaceuticals. 2023; 16(12):1656. https://doi.org/10.3390/ph16121656
Chicago/Turabian StyleSarkar, Tanoy, Sakib M. Moinuddin, Ayman Isbatan, Jiwang Chen, David Mann, and Fakhrul Ahsan. 2023. "Intratracheally Administered Peptide-Modified Lipid Admixture Containing Fasudil and/or DETA NONOate Ameliorates Various Pathologies of Pulmonary Arterial Hypertension" Pharmaceuticals 16, no. 12: 1656. https://doi.org/10.3390/ph16121656
APA StyleSarkar, T., Moinuddin, S. M., Isbatan, A., Chen, J., Mann, D., & Ahsan, F. (2023). Intratracheally Administered Peptide-Modified Lipid Admixture Containing Fasudil and/or DETA NONOate Ameliorates Various Pathologies of Pulmonary Arterial Hypertension. Pharmaceuticals, 16(12), 1656. https://doi.org/10.3390/ph16121656






