Phytochemicals as Immunomodulatory Molecules in Cancer Therapeutics
Abstract
1. Introduction
2. Phytochemicals: History and Classification
2.1. Phenolics
2.2. Terpenoids
2.3. Alkaloids
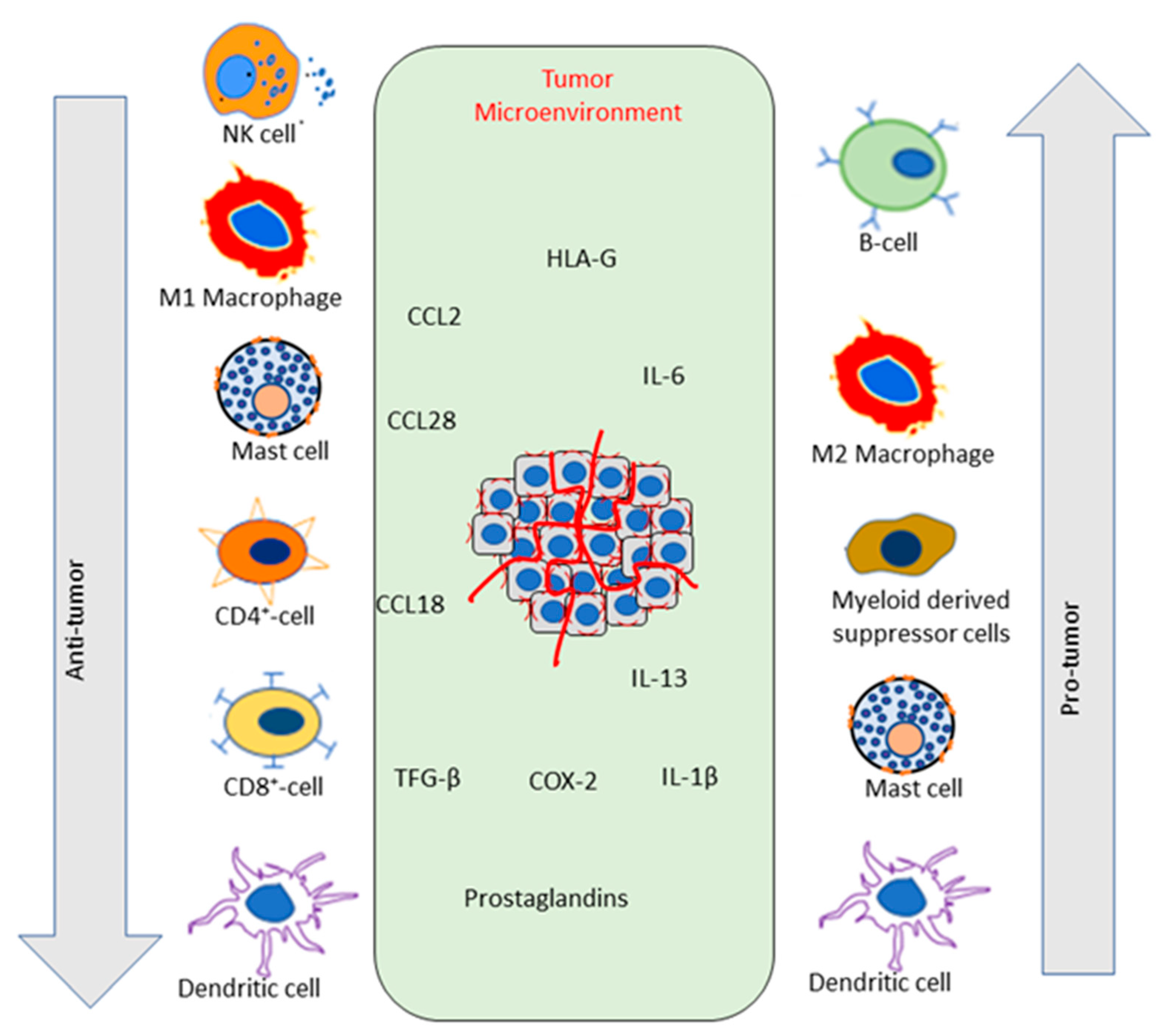
3. Cancer Microenvironment: Immunological Milieu
3.1. Overview of the Immune System in Cancer
3.1.1. Innate Immune Response in Cancer
3.1.2. Adaptive Immune Response in Cancer
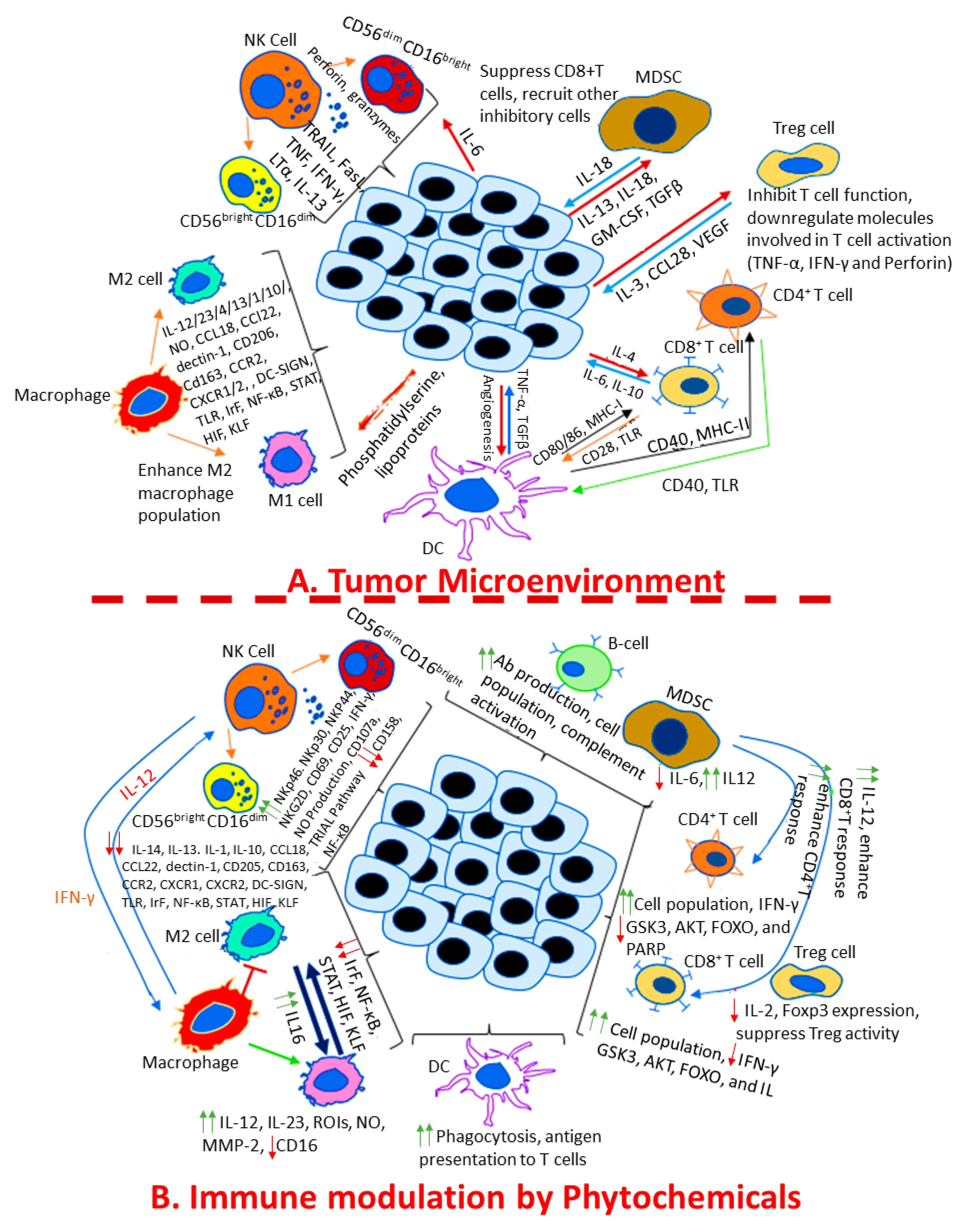
4. Role of Phytochemicals in Modulating Immune Functions in Cancer
4.1. Regulation of the Innate Immune Response in Cancer by Phytochemicals
4.2. Regulation of Adaptive Immunity in Cancer by Phytochemicals
4.3. Phytochemicals in Cancer: Clinical Trials and Other Studies with Human Patients
5. Challenges and Future Prospectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paudel, S.; Mehtani, D.; Puri, N. Mast Cells May Differentially Regulate Growth of Lymphoid Neoplasms by Opposite Modulation of Histamine Receptors. Front. Oncol. 2019, 9, 1280. [Google Scholar] [CrossRef]
- Paudel, S.; Sharma, P.; Puri, N. Immunosenescence, Inflammaging, and Their Implications for Cancer and Anemia. In Models, Molecules and Mechanisms in Biogerontology: Physiological Abnormalities, Diseases and Interventions; Rath, P.C., Ed.; Springer: Singapore, 2019. [Google Scholar]
- Kandhari, K.; Paudel, S.; Raina, K.; Agarwal, C.; Kant, R.; Wempe, M.F.; O’Bryant, C.; Agarwal, R. Comparative Pre-clinical Efficacy of Chinese and Indian Cultivars of Bitter Melon (Momordica charantia) against Pancreatic Cancer. J. Cancer Prev. 2021, 26, 266–276. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 2021, 18, 663–672. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of mTOR Pathway Signaling Activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.; Kerbel, R.S. Immunology of metastasis. Can the immune response cope with disseminated tumor? Cancer Metastasis Rev. 1983, 2, 239–256. [Google Scholar] [CrossRef]
- Baldwin, R.W.; Embleton, M.J.; Price, M.R. Monoclonal antibody-defined antigens on tumor cells. Biomembranes 1983, 11, 285–312. [Google Scholar]
- Baldwin, R.W. Specific and non-specific responses in host resistance to tumors. Tokai J. Exp. Clin. Med. 1983, 8, 419–428. [Google Scholar] [PubMed]
- Baldwin, R.W.; Pimm, M.V. BCG in tumor immunotherapy. Adv. Cancer Res. 1978, 28, 91–147. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.M.; Kim, S.J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Paudel, S.; Joshi, D.; Joshi, K.; Ranjan, R.; Gupta, A.; Bhattacharya, K.; Varshney, A. Anti-Inflammatory and Anti-Arthritic Efficacies of an Indian Traditional Herbo-Mineral Medicine “Divya Amvatari Ras” in Collagen Antibody-Induced Arthritis (CAIA) Mouse Model Through Modulation of IL-6/IL-1β/TNF-α/NFκB Signaling. Front. Pharmacol. 2019, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Paudel, S.; Joshi, D.; Joshi, K.; Ranjan, R.; Gupta, A.; Bhattacharya, K.; Varshney, A. Herbo-mineral formulation ‘Ashwashila’ attenuates rheumatoid arthritis symptoms in collagen-antibody-induced arthritis (CAIA) mice model. Sci. Rep. 2019, 9, 8025. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Yang, N.S.; Lin, T.J. Phytochemicals Approach for Developing Cancer Immunotherapeutics. Front. Pharmacol. 2017, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Deep, G.; Jain, A.K.; Raina, K.; Agarwal, C.; Wempe, M.F.; Agarwal, R. Bitter melon juice activates cellular energy sensor AMP-activated protein kinase causing apoptotic death of human pancreatic carcinoma cells. Carcinogenesis 2013, 34, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.D.; Maurya, A.K.; Ibrahim, H.; Rao, S.; Hove, P.R.; Kumar, D.; Kant, R.; Raina, B.; Agarwal, R.; Kuhn, K.A. Dietary rice bran-modified human gut microbial consortia confers protection against colon carcinogenesis following fecal transfaunation. Biomedicines 2021, 9, 144. [Google Scholar] [CrossRef]
- Hasler, C.M.; Blumberg, J.B. Phytochemicals: Biochemistry and physiology. Introduction. J. Nutr. 1999, 129, 756s–757s. [Google Scholar] [CrossRef]
- Gibson, E.L.; Wardle, J.; Watts, C.J. Fruit and vegetable consumption, nutritional knowledge and beliefs in mothers and children. Appetite 1998, 31, 205–228. [Google Scholar] [CrossRef]
- Rao, B.N. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac. J. Clin. Nutr. 2003, 12, 9–22. [Google Scholar] [PubMed]
- Catic, T.; Oborovic, I.; Redzic, E.; Sukalo, A.; Skrbo, A.; Mašić, I. Traditional Chinese Medicine—An Overview. Int. J. Biomed. Health 2018, 6, 35–50. [Google Scholar] [CrossRef]
- Balachandran, P.; Govindarajan, R. Cancer—An ayurvedic perspective. Pharmacol. Res. 2005, 51, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Baqri, S.S.R.; Alsulimani, A.; Fagoonee, S.; Slama, P.; Kesari, K.K.; Roychoudhury, S.; Haque, S. Phytochemicals from Indian Ethnomedicines: Promising Prospects for the Management of Oxidative Stress and Cancer. Antioxidants 2021, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P. European materia medica in historical texts: Longevity of a tradition and implications for future use. J. Ethnopharmacol. 2010, 132, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Ogunwenmo, K.O.; Idowu, O.A.; Innocent, C.; Esan, E.B.; Oyelana, O.A. Cultivars of Codiaeum variegatum (L.) Blume (Euphorbiaceae) show variability in phytochemical and cytological characteristics. Afr. J. Biotechnol. 2007, 6, 2400–2405. [Google Scholar] [CrossRef]
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Muhsinah, A.B.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704. [Google Scholar] [CrossRef]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Gupta, K.; Taneja, S.; Dhar, K.; Atal, C. Flavonoids of Andrographis paniculata. Phytochemistry 1983, 22, 314–315. [Google Scholar] [CrossRef]
- Muralidhar, A.; Sudhakar, B.; Ravishankar, T.; Reddanna, P.; Reddy, G.; Latha, J. Anti-inflammatory activity of flavonoid fraction isolated from the stem bark of Butea monosperma (LAM): A mechanism based study. Int. J. Phytopharm. 2010, 1, 124–132. [Google Scholar]
- Sankaranarayanan, S.; Bama, P.; Ramachandran, J.; Kalaichelvan, P.; Deccaraman, M.; Vijayalakshimi, M.; Dhamotharan, R.; Dananjeyan, B.; Sathya Bama, S. Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamil Nadu, India. J. Med. Plants Res. 2010, 4, 1089–1101. [Google Scholar]
- Sannomiya, M.; Fonseca, V.B.; Da Silva, M.; Rocha, L.; Dos Santos, L.; Hiruma-Lima, C.; Brito, A.S.; Vilegas, W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J. Ethnopharmacol. 2005, 97, 1–6. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Stephane, F.F.Y.; Jules, B.K.J. Terpenoids as Important Bioactive Constituents of Essential Oils. In Essential Oils; Mozaniel Santana de, O., Wanessa Almeida da, C., Sebastião Gomes, S., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. Ch. 5. [Google Scholar]
- Zakaria, K.N.; Amid, A.; Zakaria, Z.; Jamal, P.; Ismail, A. Anti-Proliferative Activity of Triterpenes Isolated from Clinicanthus nutans on Hep-G2 Liver Cancer Cells. Asian Pac. J. Cancer Prev. 2019, 20, 563–567. [Google Scholar] [CrossRef]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H. Therapeutic and Biomedical Potentialities of Terpenoids-A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Costa, M.A.; Xia, Z.; Davin, L.B.; Lewis, N.G. Toward Engineering the Metabolic Pathways of Cancer-Preventing Lignans in Cereal Grains and Other Crops. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense. Recent Advances in Phytochemistry; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity-An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Lee, S.T.; Gardner, D.R.; Panter, K.E.; James, L.F. Phytochemicals: The good, the bad and the ugly? Phytochemistry 2007, 68, 2973–2985. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Akin-Ajani, O.D.; Ajala, T.O.; Ogunniyi, Q.A.; Fettke, J.; Odeku, O.A. Nutritional and pharmacological potentials of orphan legumes: Subfamily faboideae. Heliyon 2023, 9, e15493. [Google Scholar] [CrossRef]
- Fukuda, T.; Sudoh, Y.; Tsuchiya, Y.; Okuda, T.; Igarashi, Y. Isolation and biosynthesis of preussin B, a pyrrolidine alkaloid from Simplicillium lanosoniveum. J. Nat. Prod. 2014, 77, 813–817. [Google Scholar] [CrossRef]
- Hamad, M. Investigation of alkaloids of Anabasis aphylla (Chenopodiaceae). Ibn AL-Haitham J. Pure Appl. Sci. 2017, 23, 297–304. [Google Scholar]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Zhang, J.Y.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Qaredashi, R.; Hashemzaei, M.; Hosseinzadeh, H. Inhibitory effect of Berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e16145. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Mojarad, T.B.; Roghani, M. The anticonvulsant and antioxidant effects of berberine in kainate-induced temporal lobe epilepsy in rats. Basic Clin. Neurosci. 2014, 5, 124. [Google Scholar]
- Zhu, H.L.; Wan, J.B.; Wang, Y.T.; Li, B.C.; Xiang, C.; He, J.; Li, P. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia 2014, 55, 3–16. [Google Scholar] [CrossRef]
- Dall’Acqua, S. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer’s disease. Bot. Targets Ther. 2013, 3, 19–28. [Google Scholar] [CrossRef]
- Tyroller, S.; Zwickenpflug, W.; Richter, E. New sources of dietary myosmine uptake from cereals, fruits, vegetables, and milk. J. Agric. Food Chem. 2002, 50, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The’seed and soil’hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Becknell, B.; Caligiuri, M.A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005, 86, 209–239. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and relevance to human disease. Blood 2001, 97, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.W.; Vaidya, S.A.; Cheng, G. The art of war: Innate and adaptive immune responses. Cell. Mol. Life Sci. 2003, 60, 2604–2621. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, G. Cancer and innate immune system interactions: Translational potentials for cancer immunotherapy. J. Immunother. 2012, 35, 299–308. [Google Scholar] [CrossRef]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Screpanti, V.; Wallin, R.P.; Ljunggren, H.G.; Grandien, A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J. Immunol. 2001, 167, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Forconi, C.S.; Oduor, C.I.; Oluoch, P.O.; Ong’echa, J.M.; Münz, C.; Bailey, J.A.; Moormann, A.M. A New Hope for CD56(neg)CD16(pos) NK Cells as Unconventional Cytotoxic Mediators: An Adaptation to Chronic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef]
- Sim, G.C.; Radvanyi, L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 377–390. [Google Scholar] [CrossRef]
- Huntington, N.D. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol. Cell Biol. 2014, 92, 210–213. [Google Scholar] [CrossRef]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Chang, W.-A.; Huang, M.-S.; Kuo, P.-L. Tumor microenvironment: A new treatment target for cancer. Int. Sch. Res. Not. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Lu, T.; Ramakrishnan, R.; Altiok, S.; Youn, J.I.; Cheng, P.; Celis, E.; Pisarev, V.; Sherman, S.; Sporn, M.B.; Gabrilovich, D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Investig. 2011, 121, 4015–4029. [Google Scholar] [CrossRef] [PubMed]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.S.; Borregaard, N.; Bundgaard, J.R.; Timshel, S.; Sehested, M.; Kjeldsen, L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut 1996, 38, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef]
- Cines, D.B.; Pollak, E.S.; Buck, C.A.; Loscalzo, J.; Zimmerman, G.A.; McEver, R.P.; Pober, J.S.; Wick, T.M.; Konkle, B.A.; Schwartz, B.S.; et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998, 91, 3527–3561. [Google Scholar]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Classen, A.; Lloberas, J.; Celada, A. Macrophage activation: Classical versus alternative. Methods Mol. Biol. 2009, 531, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Wu, M.; Lou, J.; Zhang, S.; Huang, P.; Sun, R.; Huang, L.; Xie, E.; Wang, F.; Gu, B. Activation of Toll-like receptors signaling in non-small cell lung cancer cell line induced by tumor-associated macrophages. Chin. J. Cancer Res. 2015, 27, 181. [Google Scholar]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Koh, Y.C.; Yang, G.; Lai, C.S.; Weerawatanakorn, M.; Pan, M.H. Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2208. [Google Scholar] [CrossRef]
- Monteiro, L.N.; Rodrigues, M.A.; Gomes, D.A.; Salgado, B.S.; Cassali, G.D. Tumour-associated macrophages: Relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Vet. J. 2018, 234, 119–125. [Google Scholar] [CrossRef]
- Vinogradov, S.; Warren, G.; Wei, X. Macrophages associated with tumors as potential targets and therapeutic intermediates. Nanomedicine 2014, 9, 695–707. [Google Scholar] [CrossRef]
- Dandekar, R.C.; Kingaonkar, A.V.; Dhabekar, G.S. Role of macrophages in malignancy. Ann. Maxillofac. Surg. 2011, 1, 150–154. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.X.; Qiao, S.L.; An, H.W.; Ma, Y.; Qiao, Z.Y.; Rajapaksha, R.P.; Wang, H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials 2017, 112, 153–163. [Google Scholar] [CrossRef]
- Shrivastava, R.; Asif, M.; Singh, V.; Dubey, P.; Ahmad Malik, S.; Lone, M.U.; Tewari, B.N.; Baghel, K.S.; Pal, S.; Nagar, G.K.; et al. M2 polarization of macrophages by Oncostatin M in hypoxic tumor microenvironment is mediated by mTORC2 and promotes tumor growth and metastasis. Cytokine 2019, 118, 130–143. [Google Scholar] [CrossRef]
- Ding, L.; Liang, G.; Yao, Z.; Zhang, J.; Liu, R.; Chen, H.; Zhou, Y.; Wu, H.; Yang, B.; He, Q. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget 2015, 6, 36441. [Google Scholar] [CrossRef]
- Schneider, T.; Hoffmann, H.; Dienemann, H.; Schnabel, P.A.; Enk, A.H.; Ring, S.; Mahnke, K. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J. Thorac. Oncol. 2011, 6, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Karthaus, N.; Torensma, R.; Tel, J. Deciphering the message broadcast by tumor-infiltrating dendritic cells. Am. J. Pathol. 2012, 181, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Krempski, J.; Karyampudi, L.; Behrens, M.D.; Erskine, C.L.; Hartmann, L.; Dong, H.; Goode, E.L.; Kalli, K.R.; Knutson, K.L. Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J. Immunol. 2011, 186, 6905–6913. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Faget, J.; Gobert, M.; Goutagny, N.; Vey, N.; Treilleux, I.; Renaudineau, S.; Poyet, G.; Labidi-Galy, S.I.; Goddard-Leon, S. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012, 72, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- McBlane, J.F.; van Gent, D.C.; Ramsden, D.A.; Romeo, C.; Cuomo, C.A.; Gellert, M.; Oettinger, M.A. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell 1995, 83, 387–395. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S33–S40. [Google Scholar] [CrossRef]
- Wands, J.; Mann, E.; Alpert, E.; Isselbacher, K. The pathogenesis of arthritis associated with acute hepatitis-B surface antigen-positive hepatitis. Complement activation and characterization of circulating immune complexes. J. Clin. Investig. 1975, 55, 930–936. [Google Scholar] [CrossRef]
- Knutson, K.L.; Disis, M.L. Augmenting T helper cell immunity in cancer. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.-C.S.; Rancan, C. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 2020, 181, 1612–1625.e1613. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Frigola, X.; Bonne-Annee, S.; Mercader, M.; Kuntz, S.M.; Krambeck, A.E.; Sengupta, S.; Dong, H.; Cheville, J.C.; Lohse, C.M. Tumor-infiltrating Foxp3− CD4+ CD25+ T cells predict poor survival in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Leffers, N.; Gooden, M.J.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009, 58, 449–459. [Google Scholar] [CrossRef]
- Karpisheh, V.; Mousavi, S.M.; Sheykholeslami, P.N.; Fathi, M.; Saray, M.M.; Aghebati-Maleki, L.; Jafari, R.; Zolbanin, N.M.; Jadidi-Niaragh, F. The role of regulatory T cells in the pathogenesis and treatment of prostate cancer. Life Sci. 2021, 284, 119132. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, J.; Qin, Y.; Wu, Y.; Zhu, L.; Lu, L.; Tang, G.; Shen, Q. Increased expression of immunosuppressive molecules on intratumoral and circulating regulatory T cells in non-small-cell lung cancer patients. Am. J. Cancer Res. 2015, 5, 2190–2201. [Google Scholar]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef]
- Veatch, J.R.; Jesernig, B.L.; Kargl, J.; Fitzgibbon, M.; Lee, S.M.; Baik, C.; Martins, R.; Houghton, A.M.; Riddell, S.R. Endogenous CD4(+) T Cells Recognize Neoantigens in Lung Cancer Patients, Including Recurrent Oncogenic KRAS and ERBB2 (Her2) Driver Mutations. Cancer Immunol. Res. 2019, 7, 910–922. [Google Scholar] [CrossRef]
- Geng, Y.; Shao, Y.; He, W.; Hu, W.; Xu, Y.; Chen, J.; Wu, C.; Jiang, J. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: A Meta-Analysis. Cell. Physiol. Biochem. 2015, 37, 1560–1571. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Wang, Y.; Zhang, W.; Ma, K.; Hu, C.; Zhu, H.; Liang, S.; Liu, M.; Xu, N. IL-6 influences the polarization of macrophages and the formation and growth of colorectal tumor. Oncotarget 2018, 9, 17443–17454. [Google Scholar] [CrossRef] [PubMed]
- Frydrychowicz, M.; Boruczkowski, M.; Kolecka-Bednarczyk, A.; Dworacki, G. The Dual Role of Treg in Cancer. Scand. J. Immunol. 2017, 86, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Korkolopoulou, P.; Kaklamanis, L.; Pezzella, F.; Harris, A.L.; Gatter, K.C. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br. J. Cancer 1996, 73, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Naseri, G.; Rezaee, R.; Mohammadi, M.; Banikazemi, Z.; Mirzaei, H.R.; Salehi, H.; Peyvandi, M.; Pawelek, J.M.; Sahebkar, A. Curcumin: A new candidate for melanoma therapy? Int. J. Cancer 2016, 139, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef]
- Huang, Q.; Xia, J.; Wang, L.; Wang, X.; Ma, X.; Deng, Q.; Lu, Y.; Kumar, M.; Zhou, Z.; Li, L.; et al. miR-153 suppresses IDO1 expression and enhances CAR T cell immunotherapy. J. Hematol. Oncol. 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Attili, I.; Karachaliou, N.; Bonanno, L.; Berenguer, J.; Bracht, J.; Codony-Servat, J.; Codony-Servat, C.; Ito, M.; Rosell, R. STAT3 as a potential immunotherapy biomarker in oncogene-addicted non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918763744. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Forghani, P.; Khorramizadeh, M.R.; Waller, E.K. Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer. Cancer Med. 2014, 3, 215–224. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, J.Y.; Kim, M.J.; Chang, S.H.; Park, Y.S.; Son, C.H.; Park, S.J.; Chung, J.S.; Lee, E.Y.; Kim, S.H.; et al. Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70. J. Immunother. 2010, 33, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Szekeres, K.; Iclozan, C.; Rivera, I.O.; McGill, A.; Johnson, G.; Nwogu, O.; Ghansah, T. Apigenin: Selective CK2 inhibitor increases Ikaros expression and improves T cell homeostasis and function in murine pancreatic cancer. PLoS ONE 2017, 12, e0170197. [Google Scholar] [CrossRef] [PubMed]
- Fraker, L.D.; Halter, S.A.; Forbes, J.T. Effects of orally administered retinol on natural killer cell activity in wild type BALB/c and congenitally athymic BALB/c mice. Cancer Immunol. Immunother. 1986, 21, 114–118. [Google Scholar] [CrossRef]
- Cerwenka, A.; Bakker, A.B.; McClanahan, T.; Wagner, J.; Wu, J.; Phillips, J.H.; Lanier, L.L. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000, 12, 721–727. [Google Scholar] [CrossRef]
- Kim, H.; Jang, M.; Kim, Y.; Choi, J.; Jeon, J.; Kim, J.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016, 68, 406–420. [Google Scholar] [CrossRef]
- Al-Jaderi, Z.; Maghazachi, A.A. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins 2013, 5, 1932–1947. [Google Scholar] [CrossRef]
- Wei, J.; Bhatt, S.; Chang, L.M.; Sampson, H.A.; Masilamani, M. Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS ONE 2012, 7, e47979. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.T.; Cunnick, J.E.; Murphy, P.A.; Hendrich, S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J. Nutr. 1999, 129, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Kim, H.; Liu, C.; Yu, S.; Wang, J.; Grizzle, W.E.; Kimberly, R.P.; Barnes, S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim. Biophys. Acta 2007, 1773, 1116–1123. [Google Scholar] [CrossRef]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M. Curcumin and omega-3 fatty acids enhance NK cell-induced apoptosis of pancreatic cancer cells but curcumin inhibits interferon-γ production: Benefits of omega-3 with curcumin against cancer. Molecules 2015, 20, 3020–3026. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sa, G. Curcumin as an Adjuvant to Cancer Immunotherapy. Front. Oncol. 2021, 11, 675923. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, H.A.; Mahbub, A.A.; Le Maitre, C.L.; Jordan-Mahy, N. Molecular Action of Polyphenols in Leukaemia and Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 3085. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Spivak, A.M.; Soriano-Sarabia, N.; Checkley, M.A.; Barker, E.; Karn, J.; Planelles, V.; Margolis, D.M. HIV Latency-Reversing Agents Have Diverse Effects on Natural Killer Cell Function. Front. Immunol. 2016, 7, 356. [Google Scholar] [CrossRef]
- Quoc Trung, L.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE 2013, 8, e55183. [Google Scholar] [CrossRef]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Partridge, M.A.; Johnson, G.E.; Huang, S.X.; Zhou, H.; Hei, T.K. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp. Cell Res. 2008, 314, 1163–1176. [Google Scholar] [CrossRef]
- Shankar, S.; Siddiqui, I.; Srivastava, R.K. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol. Cell. Biochem. 2007, 304, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Chen, Q.; Siddiqui, I.; Sarva, K.; Srivastava, R.K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4′, 5 tri-hydroxystilbene): Molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.M. Resveratrol-mediated sensitisation to TRAIL-induced apoptosis depends on death receptor and mitochondrial signalling. Eur. J. Cancer 2005, 41, 786–798. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.; Geisler, S.; Lisandrelli, R.; Nguyen Ngoc, H.; Ganzera, M.; Schennach, H.; Fuchs, D.; Fuchs, J.E.; Gostner, J.M.; Kurz, K. Pharmacological Targets of Kaempferol Within Inflammatory Pathways-A Hint Towards the Central Role of Tryptophan Metabolism. Antioxidants 2020, 9, 180. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol-from Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef]
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunol. Immunother. 2014, 63, 889–900. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef]
- Miller, S.C. Echinacea: A miracle herb against aging and cancer? Evidence in vivo in mice. Evid. Based Complement. Altern. Med. 2005, 2, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Kim, K.H.; Lee, S.H.; Yoon, M.S.; Lee, H.J.; Moon, D.O.; Lee, C.M.; Ahn, S.C.; Park, Y.C.; Park, Y.M. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J. Immunol. 2005, 174, 8116–8124. [Google Scholar] [CrossRef]
- Rao, C.V.; Rivenson, A.; Simi, B.; Reddy, B.S. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995, 55, 259–266. [Google Scholar] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Pratap, K.; Verma, P.K.; Singh, B.; Padwad, Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol. 2015, 175, 131–137. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Bukhari, S.N. Immunostimulatory effects of the standardized extract of Tinospora crispa on innate immune responses in Wistar Kyoto rats. Drug Des. Dev. Ther. 2015, 9, 2961–2973. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Wood, S.M.; Beckham, C.; Yosioka, A.; Darban, H.; Watson, R.R. β-Carotene and selenium supplementation enhances immune response in aged humans. Integr. Med. 2000, 2, 85–92. [Google Scholar] [CrossRef]
- Garcia, A.L.; Rühl, R.; Herz, U.; Koebnick, C.; Schweigert, F.J.; Worm, M. Retinoid- and carotenoid-enriched diets influence the ontogenesis of the immune system in mice. Immunology 2003, 110, 180–187. [Google Scholar] [CrossRef]
- Kang, T.H.; Lee, J.H.; Song, C.K.; Han, H.D.; Shin, B.C.; Pai, S.I.; Hung, C.F.; Trimble, C.; Lim, J.S.; Kim, T.W.; et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res. 2007, 67, 802–811. [Google Scholar] [CrossRef]
- Hood, J.L. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med. Hypotheses 2016, 94, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Riganò, R. Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: Potential therapeutic implications in atherosclerosis. Oxid. Med. Cell. Longev. 2014, 2014, 257543. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Belmonte, R.; Erkert, L.; Wirtz, S.; Becker, C. The regulation of intestinal inflammation and cancer development by type 2 immune responses. Int. J. Mol. Sci. 2020, 21, 9772. [Google Scholar] [CrossRef]
- Deng, Y.-M.; Zhao, C.; Wu, L.; Qu, Z.; Wang, X.-Y. Cannabinoid Receptor-1 suppresses M2 macrophage polarization in colorectal cancer by downregulating EGFR. Cell Death Discov. 2022, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Aikun, F.; Wang, Y.; Wu, Y.; Chen, H.; Zheng, S.; Li, Y.; Xu, X.; Li, W. Echinacea purpurea Extract Polarizes M1 Macrophages in Murine Bone Marrow-Derived Macrophages Through the Activation of JNK. J. Cell. Biochem. 2017, 118, 2664–2671. [Google Scholar] [CrossRef]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014, 9, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, X.G. Lycopene enhances antioxidant enzyme activities and immunity function in N-methyl-N′-nitro-N-nitrosoguanidine-enduced gastric cancer rats. Int. J. Mol. Sci. 2011, 12, 3340–3351. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell. Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef]
- Hussain, A.R.; Al-Rasheed, M.; Manogaran, P.S.; Al-Hussein, K.A.; Platanias, L.C.; Al Kuraya, K.; Uddin, S. Curcumin induces apoptosis via inhibition of PI3’-kinase/AKT pathway in acute T cell leukemias. Apoptosis 2006, 11, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhu, W.; Da, J.; Xu, M.; Wang, Y.; Zhou, J.; Wang, Z. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther. 2017, 10, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Focaccetti, C.; Benvenuto, M.; Ciuffa, S.; Fazi, S.; Scimeca, M.; Nardi, A.; Miele, M.T.; Battisti, A.; Bonanno, E.; Modesti, A.; et al. Curcumin Enhances the Antitumoral Effect Induced by the Recombinant Vaccinia Neu Vaccine (rV-neuT) in Mice with Transplanted Salivary Gland Carcinoma Cells. Nutrients 2020, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Lu, Z.Q.; Tang, L.M.; Wu, Z.S.; Wang, D.W.; Zheng, J.Y.; Qiu, Q.M. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. Int. Immunopharmacol. 2012, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Giaisi, M.; Treiber, M.K.; Palfi, K.; Merling, A.; Spring, H.; Krammer, P.H.; Li-Weber, M. Rocaglamide derivatives are immunosuppressive phytochemicals that target NF-AT activity in T cells. J. Immunol. 2005, 174, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.M.; Darwish, Z.E.; El Nouaem, M.I.; Fayed, N.A.; Mourad, G.M.; Ramadan, O.R. The potential preventive effect of dietary phytochemicals In Vivo. BDJ Open 2023, 9, 30. [Google Scholar] [CrossRef]
- Paudel, S.; Mishra, N.; Raina, K.; Agarwal, C.; Agarwal, R. Inhibition of ultraviolet B radiation-induced mast cell recruitment by silibinin in its efficacy against basal cell carcinoma in Ptch+/− mouse model. Cancer Res. 2023, 83 (Suppl. S7), 5271. [Google Scholar] [CrossRef]
- Mishra, N.; Paudel, S.; Agarwal, C.; Agarwal, R. Silibinin modulates migration and survival pathways in bone marrow mast cells via RAC2: Implications in its anti-cancer activity in basal cell carcinoma growth and progression. Cancer Res. 2023, 83 (Suppl. S7), 5270. [Google Scholar] [CrossRef]
- Raina, K.; Paudel, S.; Mishra, N.; Kumar, S.; Orlicky, D.J.; You, Z.; Kant, R.; Agarwal, C.; Agarwal, R. Silibinin: A novel potential therapeutic agent against UVB-induced basal cell carcinoma. Cancer Res. 2022, 82 (Suppl. S12), 716. [Google Scholar] [CrossRef]
- Paudel, S.; Raina, K.; Tiku, V.R.; Maurya, A.; Orlicky, D.J.; You, Z.; Rigby, C.M.; Deep, G.; Kant, R.; Raina, B.; et al. Chemopreventive efficacy of silibinin against basal cell carcinoma growth and progression in UVB-irradiated Ptch+/– mice. Carcinogenesis 2022, 43, 557–570. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Epelbaum, R.; Schaffer, M. Curcumin as an anti-cancer agent: Review of the gap between basic and clinical applications. Curr. Med. Chem. 2010, 17, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef]
- Guerra, N.; Tan, Y.X.; Joncker, N.T.; Choy, A.; Gallardo, F.; Xiong, N.; Knoblaugh, S.; Cado, D.; Greenberg, N.M.; Raulet, D.H. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008, 28, 571–580. [Google Scholar] [CrossRef]
- Bui, J.D.; Schreiber, R.D. Cancer immunosurveillance, immunoediting and inflammation: Independent or interdependent processes? Curr. Opin. Immunol. 2007, 19, 203–208. [Google Scholar] [CrossRef]
- Wu, A.H.; Yu, M.C. Tea, hormone-related cancers and endogenous hormone levels. Mol. Nutr. Food Res. 2006, 50, 160–169. [Google Scholar] [CrossRef]
- Hakim, I.A.; Harris, R.B.; Brown, S.; Chow, H.H.; Wiseman, S.; Agarwal, S.; Talbot, W. Effect of increased tea consumption on oxidative DNA damage among smokers: A randomized controlled study. J. Nutr. 2003, 133, 3303s–3309s. [Google Scholar] [CrossRef]
- Hakim, I.A.; Chow, H.H.; Harris, R.B. Green tea consumption is associated with decreased DNA damage among GSTM1-positive smokers regardless of their hOGG1 genotype. J. Nutr. 2008, 138, 1567s–1571s. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tang, L.; Tang, M.; Billam, M.; Huang, T.; Yu, J.; Wei, Z.; Liang, Y.; Wang, K.; Zhang, Z.Q.; et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: Modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis 2006, 27, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, N.; Tajima, K.; Tominaga, S.; Matsuura, A.; Kuwabara, M.; Okuma, K. Tea polyphenol intake and changes in serum pepsinogen levels. Jpn. J. Cancer Res. 1999, 90, 136–143. [Google Scholar] [CrossRef]
- Santos, M.S.; Gaziano, J.M.; Leka, L.S.; Beharka, A.A.; Hennekens, C.H.; Meydani, S.N. Beta-carotene-induced enhancement of natural killer cell activity in elderly men: An investigation of the role of cytokines. Am. J. Clin. Nutr. 1998, 68, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D.; Heinonen, O.P.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Rautalahti, M.; Hartman, A.M.; Palmgren, J.; Freedman, L.S.; Haapakoski, J.; et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 1996, 88, 1560–1570. [Google Scholar] [CrossRef]
- Blot, W.J.; Li, J.Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.Q.; Yang, C.S.; Zheng, S.F.; Gail, M.; Li, G.Y.; et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993, 85, 1483–1492. [Google Scholar] [CrossRef]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136 (Suppl. S3), 816s–820s. [Google Scholar] [CrossRef]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006, 136 (Suppl. S3), 821s–826s. [Google Scholar] [CrossRef]
| Phenolics | Structural Backbone | Representative Flavonoid | Dietary Sources | Medical Plants | Properties | Refs. |
|---|---|---|---|---|---|---|
| Flavanonol | 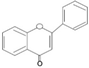 | Taxifolin | Tea | Brysonima crassa, Pongamia pinnata | Antioxidant, anti-inflammatory | [27] |
| Flavone | 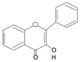 | Apigenin, Rutin, Luteolin, Leteolin Glucosides, Chrysin, Apigenin, | Buckwheat, redpepper, fruits and tomato skin, beets, artichokes, lemongrass, chamomile | Aloe vera, Acalypha indica, Bocopa moneirra, Glyccheriza glabra, Limnophila indica, Mentha longifolia, Momordica charantia, | Antioxidant | [27] |
| Flavanols | 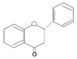 | Kaempferol, Quercetin, Tamarixetin, Myricetin, Galangin | Grapefruit, berries, olive oil, red and yellow onion, brassicatees, walnuts | Azadirachta indica, Betula pendula, Bauhinia monandra, Cannabis sativa, Clitoria ternatea, Mimosa pudica | Antioxidant, cardioprotection, antibacterial, antiviral, anticancer | [33,34,35,36,37] |
| Flavanone | 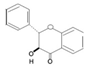 | Naringin, Naringenin, Hesperetin, Silybin | Orange, lemon, grapefruit, milk thistel | Citrus media | Antioxidant, antiinflammatory | [31] |
| Isoflavone | 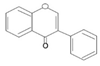 | Daidzin, Genistin, Glycitein | Soybean, chickpeas, peanuts, alfalfa sprouts, red clover, soy | Butea monospermea | Immunomodulatory, antioxidant | [35] |
| Flavan-3-ols |  | Catechin, Epictechin, Gallate, Proanthocyanidins, Theaflavins, Thearubigins, Epigallocatechin | Black tea, green tea, lentils, wine, cocoas, apple juice | Atunu raacemosa, Camellia sinensis | Antioxidant, anti-inflammatory, anticancer, immunemodulatory | [31,32] |
| Hydroxybenzoic acids | 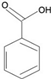 | Salicylic Acid, Salicin | Tea, potato, rosaceous fruit, red wine | Piper marginatum, Pandanus Odorus | Antioxidant | [31] |
| Hydroxycinnamic acid | 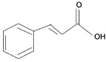 | Caffeic, Ferulic Acid, Coumaric Acid | Coffee, apple, plums, cherries, peaches, eggplant, artichoke, cabbage | Pinuseldarica, Rheumemodi, cyperus rotundus, Euphorbia tirucalli | Antioxidant, anti-tumor, anti-inflammatory, antimicrobial, antidiabetic | [31,32] |
| Trepenoids | Structural Backbone | Trepenoids | Dietary Sources | Medical Plants | Properties | Refs. |
|---|---|---|---|---|---|---|
| Hemiterpenoids | 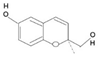 | Isovaleric Acid, Prenol, Isoperene | Grapefruit, hops, orange | Prinsepia utilis, Cananga odorata, Humulus lupulus | Antioxidants | [43,44] |
| Monoterpenoids | 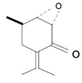 | Geranyl Pyrophosphate, Eucalytol, Limonene, Citral, Camphor, Pinene | Mints, garlic, maize, rosemary, ginger, citrus oils | MenthaLongifolia, Anetheumgraveolens, Magnolia officinalis, Cannabis saativa, Cannabis indica | Antioxidant, anticancer, antidiabetic, immunostimulant | [43,44] |
| Sesquiterpenes | 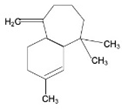 | Artemisinin, Bisabolol, Fernesol, Eudesmol | Ceylon cinnamon, pepper, turmeric, ginger, lettuce, and potatos | Cyperus edulis, Aframomumarundinaceum, Artemisia annua, Thapsia garganica | Antitumor/anticancer, anti-inflammatory, analgesic, antiulcer, antibacterial, antifungal, antiviral, antiparasitic | [43,44] |
| Diterpenes | 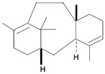 | Cembrene, Kahweol, Taxadiene, Cafestol | Coffee | Coffea arabica, Taxusbrevifolia, | Anti-inflammatory, immunomodulatory | [43,44] |
| Triterpenes | 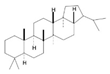 | Lanosterol, Squalene, Saponins, Oleanolic Acid, Ursolic Acid, Betulinic Acid | Soyabeans, legumes, alfalfa, java apple, garlic, lavender, caranberries, winged beans, white birch | Triphyophyllum peltatum, Diospyros leucomelas, Tetracera boiviniana | Anticancer, anti-inflammatory, antioxidant, anti-viral, antibacterial, antifungal | [43,44] |
| Tetraterpenoids |  | Lycopene, Carotene, Phytofluene, Phytoene | Carrots, pumpkins, orange, sweet potato, orange, autumn olive | Mauritia Vinifera, Myrciaria dubia, Spondias lutea | Anti-inflammatory, anti-ulcer, antibacterial, antiviral, hepatoprotective, immunomodulatory, anti-atherosclerotic, wound healing | [43,44] |
| Alkaloids | Structure Backbone | Alkaloids | Dietary Sources | Medical Plants | Properties | Refs. |
|---|---|---|---|---|---|---|
| Pyrrolidine | 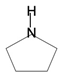 | Piperine, Coniine, Isope-lletierine, Preussin B | Barley, bine, peppers, apple, spinach celery, celeriac | Apium graveolens, Spinacia oleracea, Malus domestica, Capsicum annuum, Humulus lupulus, Hordeum vulgare, Simplicillium lanosoniveum | Antimicrobial, antitumor, anticonvulsant, anti-tubercular, analgesic | [49] |
| Pyridine-piperidine | 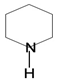 | Anabasine | Tobacco | Anabasis aphyllan | Antitumor, antimicrobial, antiviral, analgesic, anticonvulsant, antiinflammatory, antioxidant, anti-Alzheimer’s, anti-ulcer, anti-diabetic | [50] |
| Quinoline | 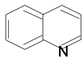 | Quinine, Quinidine, Cinchonine, Cinchonidine, Ellipticine | Cocoa, black tea, scotch whiskey | Cinchona succirubra, Ochrosia Elliptica | Antimalarial, antibacterial, antifungal, anthelmintic, cardiotonic, anticonvulsant, anti-inflammatory, analgesic | [51,52] |
| Isoquinoline | 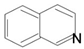 | Berberine, Morphine, Montanine, Salsoline, Galantamine | Goldthread, Oregon grape, phellodendron, turmeric, barberry | Hydrastis Canadensis, Papaver somniferun, Narcissus tazetta, Salsola oppositefolia, Hippeastrum Bittatum | Anti-inflammatory, improves digestion | [53,54,55,56,57] |
| Pyrrolidine-pyridine | 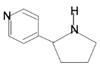 | Myosmine, Nicotine | Kiwi, millet, potato, milk, maize, rice, pineapple | Nicotianatabaccum | Antitumor, antimicrobial, anticonvulsant, anti-tubercular, analgesic | [58] |
| Phytochemicals | Immunomodulatory Effects | Type of Cancer | Study Type | Refs. |
|---|---|---|---|---|
| Kaempferol | ↑↑↑NFκB signaling ↑↑↑IL-1β ↑↑↑TNF ↓↓↓IL6 | Skin, liver, colon, ovary, pancreas, stomach, and bladder cancers | In vitro (PBMC and cell HaCaT, THP1-Blue, THP1-Blue-CD14) | [160] |
| Crude Garlic Extract | ↑↑↑CD4+/CD8+ ratio ↑↑↑IFN-γ ↑↑↑IL-2, IL-4 ↑↑↑Th1/Th2 response ↑↑↑Lymphocyte proliferation | Liver, colon, prostate, and breast cancers | In vivo (Wister rats and chickens) | [161] |
| Cannabinoids | ↑↑↑T-cells and Macrophage ↓↓↓T-helper 2 cells ↓↓↓IL-10 ↓↓↓TNFα and IL-1β expression in macrophages | Breast, lung, colon, prostate, skin, and brain cancers | In vivo (female athymic nude mice) In vitro (cell lines MCF-7, MDA-MB-231, DU-145, CaCo-2, AGS) | [162] |
| Flaxseed Lignans | ↑↑↑NFκB signaling ↓↓↓Proinflammatory cytokines (IL-1ß, IL-6, TNFα, HMGB1, TGFß1, TNFαR1, TGFßR1) ↓↓↓COX-2 level and activity | Breast and prostate cancers | In vivo (female athymic nude mice) In vitro (cell lines MCF-7, MDA-MB-231, DU-145, CaCo-2, AGS) | [163] |
| Anthocyanin | ↑↑↑T-cell proliferation, survival, MDSC differentiation ↓↓↓Cytokine-induced STAT protein phosphorylation | Oral and cervical cancers | PBMCs (healthy adult donors) | [164] |
| Quercetin | ↓↓↓Pro-inflammatory cytokines/chemokines ↓↓↓MHC class II and co-stimulatory molecule ↓↓↓Ag-specific T-cell activation by reducing LPS-stimulated DC activity -Leukocyte biology and Th1/Th2 balance regulation | Oral, cervical, and lung cancers | PBMCs (healthy adult donors) | [6,164] |
| Echinacea | ↑↑↑Macrophages ↑↑↑Phagocytosis ↑↑↑TNF-α, IL-1, IFN-β ↑↑↑Leukocyte mobility ↑↑↑NK cell stimulants and NK cell activation ↑↑↑Murine bone-marrow derived macrophage by increasing CD80, CD86, MHCII expression | Leukemias and lymphomas | In vivo (Leukemic mice) | [165,166] |
| Curcumin | ↑↑↑Apoptosis of malignant cells ↑↑↑T cells ability to kill cancer cells ↑↑↑ CD4+ T-cell and B cell numbers ↑↑↑Lymphocyte-mediated immune functions ↑↑↑Progenitor, effecter, and circulating T-cells ↓↓↓Treg cell activity ↓↓↓TGFβ and IL-10 -Th1/Tc1-type cytokine-producing effector T–cell population normalizes in tumor-bearing hosts ↓↓↓CD80, CD86, MHC class II in DCs. ↓↓↓IL-12 expression in DCs ↓↓↓IL-1β, IL-6, and TNFα in DCs ↓↓↓Metastasis ↓↓↓NFĸB signaling | Breast, colon, colorectal, head and neck, bladder, skin, ovarian pancreatic, and prostate cancers | In vivo (female athymic nude mice) In vitro (cell lines MDA-MB-435, CCL23, CAL27, UM-SCC1, UM-SCCC14A) | [138,167,168] |
| Tinospora cordifolia | ↑↑↑T- and B-lymphocyte proliferation ↑↑↑T-lymphocytes subsets (CD4+ and CD8+) ↑↑↑Th1 and Th2 cytokine secretion | Oral squamous carcinoma, colon, and cervical cancers | In vivo (male Wistar Kyoto rats) In vitro (cell lines KB, CHOK-1, HT-29, SiHa and murine primary cells) | [169] |
| Apigenin | ↑↑↑IFN-γ-induced activation of STAT1 ↑↑↑T-cell immunity ↑↑↑Sensitive to T cell-mediated cell death ↑↑↑CD4+CD8+ T-cells ↓↓↓PD-L1 in DCs ↓↓↓Tregs ↓↓↓Tumor weights and splenomegaly stabilized Ikaros expression in vitro and in vivo by targeting CK2 | Melanoma, colorectal, breast, lung, prostate, leukemia, ovarian cancers | In vivo (C57BL/6 mice) In vitro (cell lines A375, A2058, RPMI-7951, Jurkat cells) | [170,171] |
| Carotenoids | ↑↑↑B- and T-lymphocyte proliferation ↑↑↑Macrophage activity ↑↑↑Cytotoxic T-cells and effector T-cell function ↑↑↑Cytokines | Breast, cervical, ovarian, and Colorectal cancers | In vivo (SJL/J mice) | [172,173] |
| β-carotene | ↑↑↑CD4+ T-cell ↑↑↑NK cells ↑↑↑Cells with markers for IL-2 activation ↑↑NK cell cytotoxicity and total T-cells | Gastric, cervical, prostate, breast, colon cancers, and leukemia | In vivo (SJL/J mice) | [169,174] |
| Lycopene | ↑↑Blood IL-2, IL-4, IL-10, TNF-α levels ↑↑Blood IgA, IgG and IgM levels ↓↓↓IL-6 | Prostate, breast, and lung cancers | In vivo (female Wistar rats) In vitro (cell lines MCF-10a, MCF-7, MDA-MB-231, HBL-100) | [6] |
| β-carotene and Lycopene | ↑↑CD3+, CD4+, CD8+ cells ↑↑β cells and T-helper cells (CD4+ total cell numbers) ↑↑ IgG | Breast adenocarcinoma | In vivo (SJL/J mice) | [175] |
| Flavonoids (chalcones, flavones, isoflavones, flavanones, flavanols, anthocyanins) | ↑↑ T regulatory subset ↓↓↓ mTOR activity | Breast, stomach, and lung cancers | In vivo (SJL/J mice) | [6] |
| Luteolin | ↑↑↑COX-2 ↓↓↓Total cell, neutrophil, eosinophil counts ↓↓↓IL-4 ↓↓↓IFN-γ ↓↓↓ TNF-α ↓↓↓ T-cell proliferation and antigen-specific ↓↓↓ Mast cell histamine secretion | Breast cancer | In vivo (C57BL/6 mice) In vitro (cell lines TC-1, B16, B16E7) | [6] |
| Epigallocatechin-3-Gallate | ↑↑CD8+ and CD4+ T cell-mediated immune responses | Head and neck, breast, prostate, stomach, esophagus, colon, pancreas, skin, lung cancers | [176] |
| Study Type | Phtochemical (s) | Cancer | Refs. |
|---|---|---|---|
| Study with Human Participants | Allium sativum | Colorectal, liver, pancreatic cancer | [212,213] |
| Colon adenoma | |||
| Phase I Clinical Trail | Curcumin alone, curcumin + quercetin | Pancreatic cancer | [188] |
| Oral leukoplakia | [189] | ||
| Cervical intraepithelial neo-plasia | [188] | ||
| Multiple myeloma | [189] | ||
| Advanced colorectal cancer | [189] | ||
| Phase II Clinical Trial | Curcumin | Aberrant crypt foci | [161] |
| Study with Human Participants | Resveratrol |
| [172] |
| Phase II Clinical Trail | Green Tea | Prostate cancer | [198] |
| Study with Human Participants | β-carotene, α-tocopherol, selenium | Gastric cancer | [199] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, S.; Mishra, N.; Agarwal, R. Phytochemicals as Immunomodulatory Molecules in Cancer Therapeutics. Pharmaceuticals 2023, 16, 1652. https://doi.org/10.3390/ph16121652
Paudel S, Mishra N, Agarwal R. Phytochemicals as Immunomodulatory Molecules in Cancer Therapeutics. Pharmaceuticals. 2023; 16(12):1652. https://doi.org/10.3390/ph16121652
Chicago/Turabian StylePaudel, Sandeep, Neha Mishra, and Rajesh Agarwal. 2023. "Phytochemicals as Immunomodulatory Molecules in Cancer Therapeutics" Pharmaceuticals 16, no. 12: 1652. https://doi.org/10.3390/ph16121652
APA StylePaudel, S., Mishra, N., & Agarwal, R. (2023). Phytochemicals as Immunomodulatory Molecules in Cancer Therapeutics. Pharmaceuticals, 16(12), 1652. https://doi.org/10.3390/ph16121652






