RNA Combined with Nanoformulation to Advance Therapeutic Technologies
Abstract
:1. Introduction
2. Nanotechnology’s Impact on Healthcare: Advancements, Applications, and RNA Nanotechnology
2.1. Nanoformulation
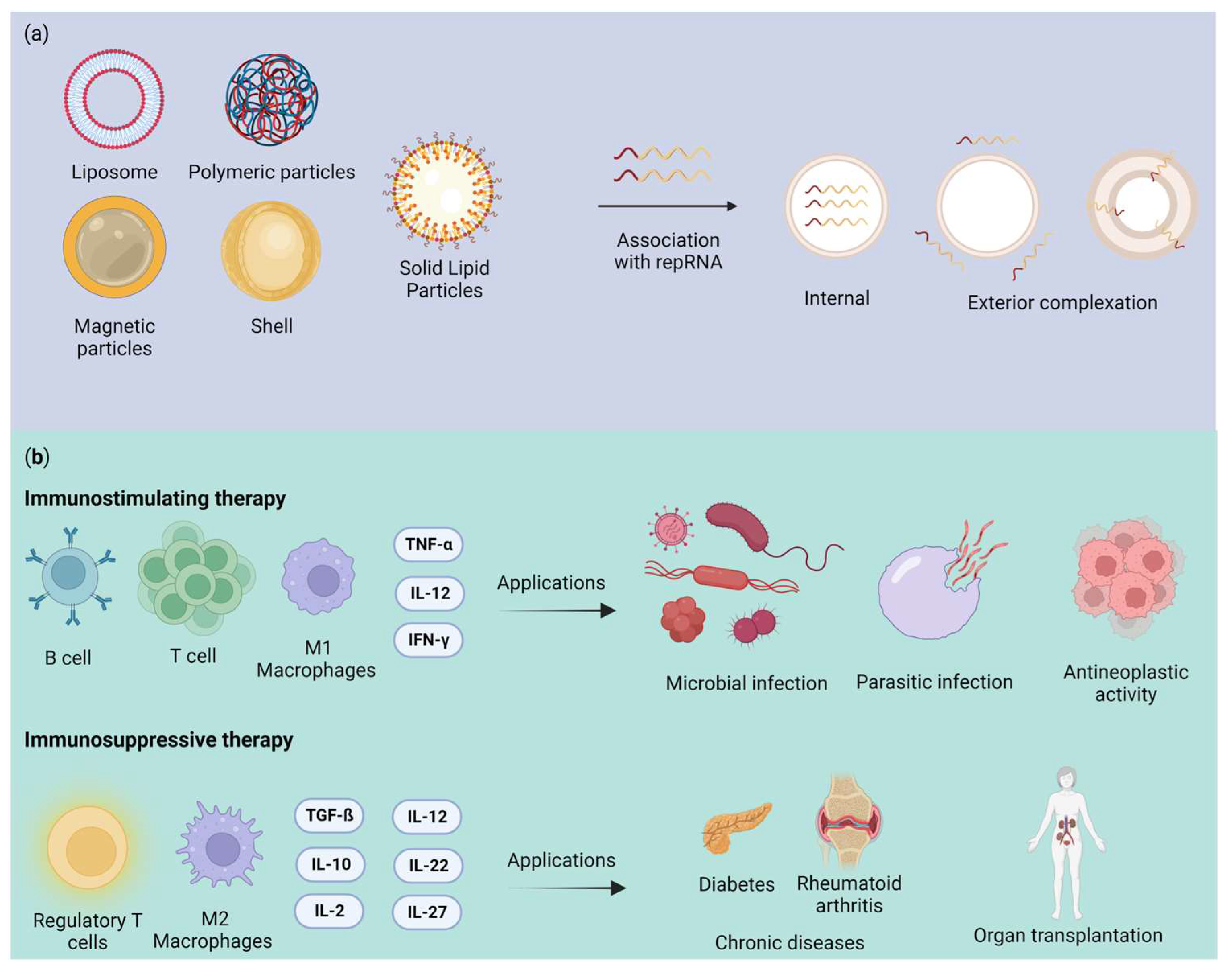
2.1.1. Liposomes
2.1.2. Polymeric Nanoparticle
2.1.3. Magnetic Nanoparticle
2.1.4. Inorganic Nanoparticles
2.1.5. Solid Lipid Nanoparticles (SLNs)
2.2. Nanotoxicology Challenges and Opportunities
3. Virus-Based Delivery System
4. Advantages and Challenges of Replicon RNA Therapy
5. Advances and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundstrom, K. Nanoparticle-Based Delivery of Self-Amplifying RNA. Gene Ther. 2020, 27, 183–185. [Google Scholar] [CrossRef]
- Sergeeva, O.V.; Koteliansky, V.E.; Zatsepin, T.S. MRNA-Based Therapeutics–Advances and Perspectives. Biochemistry 2016, 81, 709–722. [Google Scholar] [CrossRef]
- Guo, P.; Haque, F.; Hallahan, B.; Reif, R.; Li, H. Uniqueness, Advantages, Challenges, Solutions, and Perspectives in Therapeutics Applying RNA Nanotechnology. Nucleic Acid. Ther. 2012, 22, 226–245. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. MRNA Therapeutics in Cancer Immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. MRNA-Based Therapeutics-Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Démoulins, T.; Englezou, P.C.; Milona, P.; Ruggli, N.; Tirelli, N.; Pichon, C.; Sapet, C.; Ebensen, T.; Guzmán, C.A.; McCullough, K.C. Self-Replicating RNA Vaccine Delivery to Dendritic Cells. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1499, pp. 37–75. [Google Scholar]
- Lin, G.; Yan, H.; Sun, J.; Zhao, J.; Zhang, Y. Self-Replicating RNA Nanoparticle Vaccine Elicits Protective Immune Responses against SARS-CoV-2. Mol. Ther. Nucleic Acids 2023, 32, 650–666. [Google Scholar] [CrossRef]
- Lundstrom, K. Self-Replicating RNA Viruses for RNA Therapeutics. Molecules 2018, 23, 3310. [Google Scholar] [CrossRef]
- Nakamura, A.; Kotaki, T.; Nagai, Y.; Takazawa, S.; Tokunaga, K.; Kameoka, M. Construction and Evaluation of a Self-Replicative RNA Vaccine against SARS-CoV-2 Using Yellow Fever Virus Replicon. PLoS ONE 2022, 17, e0274829. [Google Scholar] [CrossRef]
- Dana, H.; Mahmoodi Chalbatani, G.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of SiRNA. Int. J. Biomed. Sci. IJBS 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.A.; Awidi, A. SiRNA: Mechanism of Action, Challenges, and Therapeutic Approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-Targeted Nanocarriers for Therapeutic Delivery to Cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Aigner, A. Therapeutic SiRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Pi, F.; Sharma, A.; Rajabi, M.; Haque, F.; Shu, D.; Leggas, M.; Evers, B.M.; Guo, P. Stable RNA Nanoparticles as Potential New Generation Drugs for Cancer Therapy. Adv. Drug Deliv. Rev. 2014, 66, 74–89. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Li, S.; Jia, L.; Wang, H.; Li, M.; Deng, J.; Zhu, A.; Ma, L.; Li, W.; et al. The Nano Delivery Systems and Applications of MRNA. Eur. J. Med. Chem. 2022, 227, 113910. [Google Scholar] [CrossRef]
- Antunes Filho, S.; Backx, B.P. Nanotecnologia e Seus Impactos Na Sociedade. Rev. Tecnol. Soc. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Fialkoski, D.; Malfatti, C.R.M. Nanotecnologia: Uma Prospecção Tecnológica No Âmbito Nacional e Internacional. Cad. Prospecção 2019, 12, 590. [Google Scholar] [CrossRef]
- Nunes, A.R.; Costa, E.C.; Alves, G.; Silva, L.R. Nanoformulations for the Delivery of Dietary Anthocyanins for the Prevention and Treatment of Diabetes Mellitus and Its Complications. Pharmaceuticals 2023, 16, 736. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-Based Antiviral Therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef]
- Cui, F.; Liu, J.; Pang, S.; Li, B. Recent Advance in Tumor Microenvironment-Based Stimuli-Responsive Nanoscale Drug Delivery and Imaging Platform. Front. Pharmacol. 2022, 13, 929854. [Google Scholar] [CrossRef] [PubMed]
- Guo, P. The Emerging Field of RNA Nanotechnology. Nat. Nanotechnol. 2010, 5, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, D.; Haque, F.; Binzel, D.W.; Guo, P. Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano 2017, 11, 1142–1164. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, C.; Chen, C.; Garver, K.; Trottier, M. Inter-RNA Interaction of Phage Φ29 PRNA to form a Hexameric Complex for Viral DNA Transportation. Mol. Cell 1998, 2, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alharbi, K.S.; Afzal, M.; Alotaibi, N.H.; Yasir, M.; Elmowafy, M.; Alshehri, S. Novel Nanotechnology Approaches for Diagnosis and Therapy of Breast, Ovarian and Cervical Cancer in Female: A Review. J. Drug Deliv. Sci. Technol. 2021, 61, 102198. [Google Scholar] [CrossRef]
- Maji, R.; Dey, N.S.; Satapathy, B.S.; Mukherjee, B.; Mondal, S. Preparation and Characterization of Tamoxifen Citrate Loaded Nanoparticles for Breast Cancer Therapy. Int. J. Nanomed. 2015, 9, 3107–3118. [Google Scholar] [CrossRef]
- Carvalho Lopes, J.; Pereira Torres, M.L. Utilização de Nanopartículas No Tratamento Do Câncer: Aspectos Gerais, Mecanismos de Ação Antineoplásicos e Aplicabilidades Tumorais. Rev. Bras. Cancerol. 2020, 65, e-13400. [Google Scholar] [CrossRef]
- Assadpour, E.; Rezaei, A.; Das, S.S.; Krishna Rao, B.V.; Singh, S.K.; Kharazmi, M.S.; Jha, N.K.; Jha, S.K.; Prieto, M.A.; Jafari, S.M. Cannabidiol-Loaded Nanocarriers and Their Therapeutic Applications. Pharmaceuticals 2023, 16, 487. [Google Scholar] [CrossRef]
- Lashkari, A.; Ranjbar, R. Nanoparticles and Nanoformulated Drugs as Promising Delivery System in Treatment of Microbial-Induced CNS Infection: A Systematic Review of Literature. J. Neurovirol. 2021, 27, 542–549. [Google Scholar] [CrossRef]
- Zanoni, E.T.; Cardoso, W.A.; Baesso, A.S.; Savi, G.D.; Folgueras, M.V.; Mendes, E.; Angioletto, E. Evaluation of Antimicrobial Activity and Adsorption of Silica Nanoparticles Doped with Cuo. Rev. Mater. 2019, 24, 1–11. [Google Scholar] [CrossRef]
- Frézard, F. Liposomes: From Biophysics to the Design of Peptide Vaccines. Braz. J. Med. Biol. Res. 1999, 32, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.A. Nanopartículas na Entrega Eficaz e Segura de Fármacos Ao Cérebro Por via Nasal—Experiência Profissionalizante Na Vertente de Farmácia Comunitária, Hospitalar e Investigação. Master’s Thesis, University of Beira Interior, Covilhã, Portugal, 2015. [Google Scholar]
- Chaves, M.A.; Ferreira, L.S.; Baldino, L.; Pinho, S.C.; Reverchon, E. Current Applications of Liposomes for the Delivery of Vitamins: A Systematic Review. Nanomaterials 2023, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of Liposomes as Drug Delivery System for Therapeutic Applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the Treatment of Brain Cancer—A Review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef]
- Andrade, G.M.; Júnior, W.T.d.S.; De Souza, E.L.; Porto, P.G.; Araújo, C.d.S.; Martins, C.S.; Dos Santos, S.S.; Peixoto, C.C.C. de M.; Hayakawa, T.M.; Rosario, J.F.A. Compilação de Dados Referentes a Síndrome de Grisel: Exposição de Evidências/Compilation of Data Relating to Grisel Syndrome: Exhibition of Evidence. Braz. J. Health Rev. 2022, 5, 5783–5789. [Google Scholar] [CrossRef]
- Rajasegaran, T.; How, C.W.; Saud, A.; Ali, A.; Lim, J.C.W. Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing. Pharmaceuticals 2023, 16, 451. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for Delivery of Antioxidants in Cosmeceuticals: Challenges and Development Strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and Innovation in the Manufacturing Process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Sayour, E.J.; Mendez-Gomez, H.R.; Mitchell, D.A. Cancer Vaccine Immunotherapy with RNA-Loaded Liposomes. Int. J. Mol. Sci. 2018, 19, 2890. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, C.; Gaskill, K.; Pasley, S.; Matosevic, S. Engineering Liposomal Nanoparticles for Targeted Gene Therapy. Gene Ther. 2017, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Saffari, M.; Moghimi, H.R.; Dass, C.R. Barriers to Liposomal Gene Delivery: From Application Site to the Target. Iran. J. Pharm. Res. IJPR 2016, 15, 3. [Google Scholar]
- Ropert, C. Liposomes as a Gene Delivery System. Braz. J. Med. Biol. Res. 1999, 32, 163–169. [Google Scholar] [CrossRef]
- Guillot, A.J.; Martínez-Navarrete, M.; Garrigues, T.M.; Melero, A. Skin Drug Delivery Using Lipid Vesicles: A Starting Guideline for Their Development. J. Control. Release 2023, 355, 624–654. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local Drug Delivery Strategies for Cancer Treatment: Gels, Nanoparticles, Polymeric Films, Rods, and Wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Xu, L.; Anchordoquy, T. Drug Delivery Trends in Clinical Trials and Translational Medicine: Challenges and Opportunities in the Delivery of Nucleic Acid-Based Therapeutics. J. Pharm. Sci. 2011, 100, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W.T. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef]
- Thapa, B.; Narain, R. Mechanism, Current Challenges and New Approaches for Non Viral Gene Delivery. In Polymers and Nanomaterials for Gene Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–27. ISBN 9780081005217. [Google Scholar]
- Uddin, S.N. Cationic Lipids Used in Non-Viral Gene Delivery Systems. Biotechnol. Mol. Biol. Rev. 2007, 2, 58–067. [Google Scholar]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and Lipid Nanoparticles for Delivery of Self-Amplifying RNA Vaccines. J. Control. Release 2021, 338, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Piñón-Segundo, E.; Mendoza-Muñoz, N.; Zambrano-Zaragoza, M.L.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Approaches in Polymeric Nanoparticles for Vaginal Drug Delivery: A Review of the State of the Art. Int. J. Mol. Sci. 2018, 19, 1549. [Google Scholar] [CrossRef]
- MacHtakova, M.; Thérien-Aubin, H.; Landfester, K. Polymer Nano-Systems for the Encapsulation and Delivery of Active Biomacromolecular Therapeutic Agents. Chem. Soc. Rev. 2022, 51, 128–152. [Google Scholar] [CrossRef]
- Lächelt, U.; Wagner, E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef]
- Ita, K. Polyplexes for Gene and Nucleic Acid Delivery: Progress and Bottlenecks. Eur. J. Pharm. Sci. 2020, 150, 105358. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–66. ISBN 9780128139325. [Google Scholar]
- Peltonen, L.; Singhal, M.; Hirvonen, J. Principles of Nanosized Drug Delivery Systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–25. ISBN 9780081029855. [Google Scholar]
- Singh, N.; Joshi, A.; Pal Toor, A.; Verma, G. Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. [Google Scholar]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef]
- Martins, M.G. Encapsulamento de Nanopartículas Magnéticas Em Polímeros Acrílicos e Avaliação de Hipertermia Para Potencial Tratamento de Câncer. In Master’s Thesis in Pharmaceutical Sciences; Federal University of Rio de Janeiro: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic Nanoparticles: Preparation, Physical Properties, and Applications in Biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic Nanoparticles in Biomedical Applications: A Review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.I.; Motelica, L.; Ficai, D.; Semenescu, A.; Oprea, O.C.; Ficai, A. Smart Magnetic Drug Delivery Systems for the Treatment of Cancer. Nanomaterials 2023, 13, 876. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Malmir, M.; Lashgari, N.; Badiei, A. The Role of Hollow Magnetic Nanoparticles in Drug Delivery. RSC Adv. 2019, 9, 25094–25106. [Google Scholar] [CrossRef]
- Shasha, C.; Krishnan, K.M. Nonequilibrium Dynamics of Magnetic Nanoparticles with Applications in Biomedicine. Adv. Mater. 2021, 33, e1904131. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, H.; Song, W.; Ru, X.; Zhou, W.; Yu, X. A Simple Magnetic Nanoparticles-Based Viral RNA Extraction Method for Efficient Detection of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gao, F. An Overview of Surface-Functionalized Magnetic Nanoparticles: Preparation and Application for Wastewater Treatment. ChemistrySelect 2019, 4, 6805–6811. [Google Scholar] [CrossRef]
- Schneider-Futschik, E.K.; Reyes-Ortega, F. Advantages and Disadvantages of Using Magnetic Nanoparticles for the Treatment of Complicated Ocular Disorders. Pharmaceutics 2021, 13, 1157. [Google Scholar] [CrossRef]
- Hobson, D.W. Nanotechnology. In Comprehensive Biotechnology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 3, pp. 683–697. ISBN 9780080885049. [Google Scholar]
- Baghaban-Eslaminejad, M.; Oryan, A.; Kamali, A.; Moshiri, A. The Role of Nanomedicine, Nanotechnology, and Nanostructures on Oral Bone Healing, Modeling, and Remodeling. In Nanostructures for Oral Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 777–832. [Google Scholar]
- Dahman, Y. Nanoshells. In Nanotechnology and Functional Materials for Engineers; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 175–190. [Google Scholar]
- Wang, Y.C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Synthetic Methodologies to Gold Nanoshells: An Overview. Molecules 2018, 23, 2851. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Arami, S. Potential Applications of Nanoshells in Biomedical Sciences. J. Drug Target. 2014, 22, 175–190. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.-H.; Halas, N.; West, J.; Drezek, R. Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, A.; Rishitha, N.; Mehdi, S. Role of Nanoparticles in Bioimaging, Diagnosis and Treatment of Cancer Disorder. In Design of Nanostructures for Theranostics Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 529–562. ISBN 9780128136690. [Google Scholar]
- Lee, S.Y.; Shieh, M.J. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4254–4264. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Arai, S.; Hou, Y.; Degl’Innocenti, A.; Cappello, V.; Mazzolai, B.; Chang, Y.T.; Mattoli, V.; Suzuki, M.; Ciofani, G. Gold Nanoshell-Mediated Remote Myotube Activation. ACS Nano 2017, 11, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Nouri, S.; Mohammadi, E.; Mehravi, B.; Majidi, F.; Ashtari, K.; Neshasteh-Riz, A.; Einali, S. NIR Triggered Glycosylated Gold Nanoshell as a Photothermal Agent on Melanoma Cancer Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2316–2324. [Google Scholar] [CrossRef]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Yaghmur, A.; Mu, H. Recent Advances in Drug Delivery Applications of Cubosomes, Hexosomes, and Solid Lipid Nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid Lipid Nanoparticles: A Review on Recent Perspectives and Patents. Expert. Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- De Jesus, M.B.; Zuhorn, I.S. Solid Lipid Nanoparticles as Nucleic Acid Delivery System: Properties and Molecular Mechanisms. J. Control. Release 2015, 201, 1–13. [Google Scholar] [CrossRef]
- Subhan, M.A.; Filipczak, N.; Torchilin, V.P. Advances with Lipid-Based Nanosystems for SiRNA Delivery to Breast Cancers. Pharmaceuticals 2023, 16, 970. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, V.J.; Chen, Y. Nanoparticles-A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef]
- Newton, A.M.J. Sukhjinder Kaur Solid Lipid Nanoparticles for Skin and Drug Delivery. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–334. ISBN 9780128162002. [Google Scholar]
- Mandal, A.; Bisht, R.; Pal, D.; Mitra, A.K. Diagnosis and Drug Delivery to the Brain: Novel Strategies. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 59–83. ISBN 9780323429979. [Google Scholar]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid Lipid Nanoparticles for Hydrophilic Drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Wang, Z.G.; Liu, S.L. Lipid Nanoparticles for MRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 27, 5607. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.N.; Lee, S.Y.; Lee, S.; Youn, H.; Im, H.J. Lipid Nanoparticles for Delivery of RNA Therapeutics: Current Status and the Role of in Vivo Imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-Loaded Solid Lipid Nanoparticles: Preparation, Characterisation, Stability and Transdermal Behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef]
- Badilli, U.; Gumustas, M.; Uslu, B.; Ozkan, S.A. Lipid-Based Nanoparticles for Dermal Drug Delivery. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 369–413. ISBN 9780128136638. [Google Scholar]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Sguizzato, M.; Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Prepared by Microwave and Ultrasound-Assisted Synthesis: Promising Green Strategies for the Nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (Slns): An Advanced Drug Delivery System Targeting Brain through Bbb. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, S. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian. J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent Advances in Lipid Nanoparticle Formulations with Solid Matrix for Oral Drug Delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Lopalco, A.; Denora, N. Nanoformulations for Drug Delivery: Safety, Toxicity, and Efficacy. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; Volume 1800, pp. 347–365. [Google Scholar]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Lee, Y.H.; Hsu, Y.H.; Liao, C.T.; Lin, Y.F.; Chiu, H.W. Current Strategies in Assessment of Nanotoxicity: Alternatives to in Vivo Animal Testing. Int. J. Mol. Sci. 2021, 22, 4216. [Google Scholar] [CrossRef]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1894, pp. 1–29. [Google Scholar]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Elsaesser, A.; Howard, C.V. Toxicology of Nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in the Development of Gene Delivery Systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Eshka, S.F.A.; Bahador, M.; Gordan, M.M.; Karbasi, S.; Tabar, Z.M.; Basiri, M. A Systematic Review of Gene Editing Clinical Trials. medRxiv 2022. [Google Scholar] [CrossRef]
- Kontogiannis, O.; Karalis, V. On the in Vivo Kinetics of Gene Delivery Vectors. medRxiv 2022. [Google Scholar] [CrossRef]
- Nayerossadat, N.; Ali, P.; Maedeh, T. Viral and Nonviral Delivery Systems for Gene Delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Abinaya, R.V.; Viswanathan, P. Biotechnology-Based Therapeutics. In Translational Biotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–52. [Google Scholar]
- Trobridge, G.D. Foamy Virus Vectors for Gene Transfer. Expert. Opin. Biol. Ther. 2009, 9, 1427–1436. [Google Scholar] [CrossRef]
- Deregowski, V.; Canalis, E. Gene Delivery by Retroviruses. In Osteoporosis. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2008; Volume 455. [Google Scholar]
- Cavalieri, V.; Baiamonte, E.; Lo Iacono, M. Non-Primate Lentiviral Vectors and Their Applications in Gene Therapy for Ocular Disorders. Viruses 2018, 10, 316. [Google Scholar] [CrossRef]
- Worgall, S.; Crystal, R.G. Gene Therapy. In Principles of Tissue Engineerin, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 657–686. ISBN 9780123983589. [Google Scholar]
- Wong, L.-F.; Goodhead, L.; Kyriacos, C.P.; Mitrophanous, K.A.; Kingsman, S.M.; Mazarakis, N.D. Lentivirus-Mediated Gene Transfer to the Central Nervous: Therapeutic and Research Applications. Hum. Gene Ther. 2006, 17, 1–9. [Google Scholar] [CrossRef]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes. Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Kasala, D.; Yoon, A.R.; Hong, J.; Kim, S.W.; Yun, C.O. Evolving Lessons on Nanomaterial-Coated Viral Vectors for Local and Systemic Gene Therapy. Nanomedicine 2016, 11, 1689–1713. [Google Scholar] [CrossRef]
- Ahmadi, S.E.; Soleymani, M.; Shahriyary, F.; Amirzargar, M.R.; Ofoghi, M.; Fattahi, M.D.; Safa, M. Viral Vectors and Extracellular Vesicles: Innate Delivery Systems Utilized in CRISPR/Cas-Mediated Cancer Therapy. Cancer Gene Ther. 2023, 30, 936–954. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; An, Y.; Chen, Z. Construction and Application of Adenoviral Vectors. Mol. Ther. Nucleic Acids 2023, 34, 102027. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.E.; Chicaybam, L.; Stein, R.T.; Tanuri, A.; Delgado-Cañedo, A.; Bonamino, M.H. Retroviral Vectors and Transposons for Stable Gene Therapy: Advances, Current Challenges and Perspectives. J. Transl. Med. 2016, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Garofalo, M.; Hirvinen, M.; Cerullo, V. The Evolution of Adenoviral Vectors through Genetic and Chemical Surface Modifications. Viruses 2014, 6, 832–855. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, T.; Naldini, L.; Collen, D.; Chuah, M.K.L. Oncoretroviral and Lentiviral Vector-Mediated Gene Therapy. Methods Enzym. 2002, 346, 573–589. [Google Scholar]
- Cevher, E.; Demir, A.; Sefik, E. Gene Delivery Systems: Recent Progress in Viral and Non-Viral Therapy. In Recent Advances in Novel Drug Carrier Systems; InTech: London, UK, 2012. [Google Scholar]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Minnaert, A.K.; Vanluchene, H.; Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Raemdonck, K.; Sanders, N.N.; Remaut, K. Strategies for Controlling the Innate Immune Activity of Conventional and Self-Amplifying MRNA Therapeutics: Getting the Message Across. Adv. Drug Deliv. Rev. 2021, 176, 113900. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. MRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef]
- Ballesteros-Briones, M.C.; Silva-Pilipich, N.; Herrador-Cañete, G.; Vanrell, L.; Smerdou, C. A New Generation of Vaccines Based on Alphavirus Self-Amplifying RNA. Curr. Opin. Virol. 2020, 44, 145–153. [Google Scholar] [CrossRef]
- Blom, D.J.; Marais, A.D.; Moodley, R.; van der Merwe, N.; van Tonder, A.; Raal, F.J. RNA-Based Therapy in the Management of Lipid Disorders: A Review. Lipids Health Dis. 2022, 21, 41. [Google Scholar] [CrossRef]
- Magadum, A.; Kaur, K.; Zangi, L. MRNA-Based Protein Replacement Therapy for the Heart. Mol. Ther. 2019, 27, 785–793. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; O’Connor, M.A.; Walls, A.C.; Hemann, E.A.; Murapa, P.; Archer, J.; Leventhal, S.; Fuller, J.T.; Lewis, T.B.; et al. An Alphavirus-Derived Replicon RNA Vaccine Induces SARS-CoV-2 Neutralizing Antibody and T Cell Responses in Mice and Nonhuman Primates. Sci. Transl. Med. 2020, 12, eabc9396. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.C.; Bassi, I.; Milona, P.; Suter, R.; Thomann-Harwood, L.; Englezou, P.; Démoulins, T.; Ruggli, N. Self-Replicating Replicon-Rna Delivery to Dendritic Cells by Chitosan-Nanoparticles for Translation in Vitro and in Vivo. Mol. Ther. Nucleic Acids 2014, 3, e173. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Yus, B.I.; Aldon, Y.; Shattock, R.J. Inside out: Optimization of Lipid Nanoparticle Formulations for Exterior Complexation and in Vivo Delivery of SaRNA. Gene Ther. 2019, 26, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, W.; Li, Z.; Song, W.; Ding, J.; Chen, X. Immunomodulatory Nanosystems. Adv. Sci. 2019, 6, 1900101. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Hodel, K.V.S.; Fonseca, L.M.D.S.; Mascarenhas, L.A.B.; Andrade, L.P.C.d.S.; Rocha, V.P.C.; Soares, M.B.P.; Berglund, P.; Duthie, M.S.; Reed, S.G.; et al. The Importance of Rna-Based Vaccines in the Fight against COVID-19: An Overview. Vaccines 2021, 9, 1345. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of Mrna-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic MRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Garcia, A.B.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.J.; Ulmer, J.B.; et al. Neutralization of the Plasmodium-Encoded MIF Ortholog Confers Protective Immunity against Malaria Infection. Nat. Commun. 2018, 9, 2714. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Archer, J.; Fuerte-Stone, J.; Khandhar, A.P.; Voigt, E.; Granger, B.; Bombardi, R.G.; Govero, J.; Tan, Q.; Durnell, L.A.; et al. Intramuscular Delivery of Replicon RNA Encoding ZIKV-117 Human Monoclonal Antibody Protects against Zika Virus Infection. Mol. Ther. Methods Clin. Dev. 2020, 18, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, S.S.; Meade-White, K.; Rao, D.; Haddock, E.; Leung, J.; Scott, D.; Archer, J.; Randall, S.; Erasmus, J.H.; Feldmann, H.; et al. Replicating RNA Vaccination Elicits an Unexpected Immune Response That Efficiently Protects Mice against Lethal Crimean-Congo Hemorrhagic Fever Virus Challenge. Lancet 2022, 82, 104188. [Google Scholar] [CrossRef] [PubMed]
- Fotoran, W.L.; da Silva, J.R.; Glitz, C.; Ferreira, L.C.d.S.; Wunderlich, G. Establishment of an Antiplasmodial Vaccine Based on PfRH5-Encoding RNA Replicons Stabilized by Cationic Liposomes. Pharmaceutics 2023, 15, 1223. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; O’hagan, D.T.; Adamo, R.; Baudner, B.C. Conjugation of Mannans to Enhance the Potency of Liposome Nanoparticles for the Delivery of Rna Vaccines. Pharmaceutics 2021, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA Nanoparticles Generate Protective Immunity against Lethal Ebola, H1N1 Influenza, and Toxoplasma Gondii Challenges with a Single Dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.C.; Milona, P.; Thomann-Harwood, L.; Démoulins, T.; Englezou, P.; Suter, R.; Ruggli, N. Self-Amplifying Replicon RNA Vaccine Delivery to Dendritic Cells by Synthetic Nanoparticles. Vaccines 2014, 2, 735–754. [Google Scholar] [CrossRef]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A Cationic Nanoemulsion for the Delivery of Next-Generation RNA Vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; Guderian, J.; Granger, B.; Archer, J.; Archer, M.; Gage, E.; Fuerte-Stone, J.; Larson, E.; Lin, S.; et al. A Nanostructured Lipid Carrier for Delivery of a Replicating Viral RNA Provides Single, Low-Dose Protection against Zika. Mol. Ther. 2018, 26, 2507–2522. [Google Scholar] [CrossRef]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-Targeted Therapeutics. Cell Metab. 2018, 27, 714–739. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid Nanoparticles for Nucleic Acid Delivery: Current Perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Massaad-Massade, L.; Boutary, S.; Caillaud, M.; Gracia, C.; Parola, B.; Gnaouiya, S.B.; Stella, B.; Arpicco, S.; Buchy, E.; Desmaële, D.; et al. New Formulation for the Delivery of Oligonucleotides Using “Clickable” SiRNA-Polyisoprenoid-Conjugated Nanoparticles: Application to Cancers Harboring Fusion Oncogenes. Bioconjug Chem. 2018, 29, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vieweger, M.; Zhang, K.; Yin, H.; Wang, H.; Li, X.; Li, S.; Hu, S.; Sparreboom, A.; Evers, B.M.; et al. Ultra-Thermostable RNA Nanoparticles for Solubilizing and High-Yield Loading of Paclitaxel for Breast Cancer Therapy. Nat. Commun. 2020, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Thiruvengadam, M.; Farooqui, Z.; Rajakumar, G.; Sajid Jamal, Q.M.; Alzohairy, M.A.; Almatroudi, A.; Alomary, M.N.; Chung, I.M.; Al-Suhaimi, E.A. Nanotechnology, in Silico and Endocrine-Based Strategy for Delivering Paclitaxel and MiRNA: Prospects for the Therapeutic Management of Breast Cancer. Semin. Cancer Biol. 2021, 69, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.P. Antitumour Activity of ANG1005, a Conjugate between Paclitaxel and the New Brain Delivery Vector Angiopep-2. Br. J. Pharmacol. 2008, 155, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Mumper, R.J. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J. Nanomed. Nanotechnol. 2013, 4, 6. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic Acid (FA)-Conjugated Mesoporous Silica Nanoparticles Combined with MRP-1 SiRNA Improves the Suppressive Effects of Myricetin on Non-Small Cell Lung Cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Attarwala, H.; Sweetser, M.T.; Clausen, V.A.; Robbie, G.J. Patisiran Pharmacokinetics, Pharmacodynamics, and Exposure-Response Analyses in the Phase 3 APOLLO Trial in Patients With Hereditary Transthyretin-Mediated (HATTR) Amyloidosis. J. Clin. Pharmacol. 2020, 60, 37–49. [Google Scholar] [CrossRef]
- Majeed, C.N.; Ma, C.D.; Xiao, T.; Rudnick, S.; Bonkovsky, H.L. Spotlight on Givosiran as a Treatment Option for Adults with Acute Hepatic Porphyria: Design, Development, and Place in Therapy. Drug Des. Devel Ther. 2022, 16, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Bonkovsky, H.L.; Gouya, L.; Aguilera-Peiró, P.; Montgomery Bissell, D.; Stein, P.E.; Balwani, M.; Anderson, D.K.E.; Parker, C.; Kuter, D.J.; et al. Efficacy and Safety of Givosiran for Acute Hepatic Porphyria: 24-Month Interim Analysis of the Randomized Phase 3 ENVISION Study. Liver Int. 2022, 42, 161–172. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and MRNA Delivery. Adv. Healthc. Mater. 2021, 10, 2001812. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic Nanoparticles for RNA-Based Cancer Treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
| Classification | Name (Trade Tame) | Main Component | Delivery Route | Indication | Approval (Year) |
|---|---|---|---|---|---|
| Liposome | AmBisome | Liposomal amphotericin B | Intravenous | Fungal/protozoal infections | FDA (1997) EMA (2006) ANVISA (1997) |
| Doxil/ Caelyx | Liposomal doxorubicin | Intravenous | Antineoplastic agents (ovarian and breast cancer; multiple myeloma; Karposi’s Sarcoma) | FDA (1995, 2005, 2008) EMA (1996) ANVISA (2011) | |
| Myocet (Myoce liposomal) | Liposomal doxorubicin | Intravenous | Antineoplastic agents (breast neoplasms) | FDA (2000) EMA (2000) | |
| Visudyne | Liposomal verteporfin | Intravenous | Ophthalmic agents (myopia; ocular histoplasmosis; macular degeneration, wet age-related) | FDA (2000) ANVISA (2004) EMA (2007) | |
| Marqibo | Liposomal vincristine | Intravenous | Antineoplastic agents (hematologic malignancies and solid tumors) | FDA (2012) | |
| Onivyde (Onivyde pegylated liposomal) | Liposomal irinotecan | Intravenous | Antineoplastic agents (pancreatic cancer) | FDA (2015) EMA (2016) | |
| Polymer-based nanoparticles | Eligard | Leuprolide acetate and polymer (PLGH (poly (DL-Lactide-co-glycolide))) | Subcutaneous | Antineoplastic agents (prostate cancer) | FDA (2002) ANVISA (2006) |
| Mircera | Methoxy polyethylene glycol-epoetin beta | Subcutaneous/ Intravenous | Anemia associated with chronic kidney disease | FDA (2007) EMA (2007) ANVISA (2008) | |
| Cimzia | PEGylated antibody fragment (Certolizumab) | Subcutaneous | Anti-inflammatory action (Crohn’s disease; rheumatoid arthritis; psoriatic arthritis; ankylosing spondylitis) | FDA (2008, 2009, 2013) EMA (2009) ANVISA (2017) | |
| PegIntron | PEGylated IFN alpha-2b protein | Subcutaneous | Immunomodulator (hepatitis C) | FDA (2001) EMA (2000) ANVISA (2011) | |
| Magnetic nanoparticles | NanoTherm | Iron oxide coated with amino silane | Intratumoral injection | Antineoplastic agents (glioblastoma) | FDA (2010) EMA (2013) |
| Feraheme | Iron oxide and a polyglucose sorbitol carboxymethyether | Intravenous | Treatment of anemia | FDA (2009) EMA (2012) | |
| Lipid nanoparticle | Patisiran (Onpattro) | Phospholipids, cholesterol, ionizable cationic lipid (DLin-MC3-DMA), and polyethylene glycol-modified lipid | Intravenous | Polyneuropathy | FDA (2018) |
| Carrier | RNA Replicon | Results | Reference |
|---|---|---|---|
| Cationic nanocarrier | RepRNA PMIF (macrophage migration inhibitory factor and cytokine). | It improved host cellular and humoral immunity against Plasmodium infection in the liver and blood and conferred complete protection against malaria reinfection in murine mice. | [150] |
| Nanostructured lipid transporters (NLCs) | RepRNA ZIKV-117 mAb. | Rapid protection against Zika virus infection in mice. | [151] |
| Lipid InOrganic Nanoparticles (LION) | LION/repRNA-CoV2S | LION/repRNA-CoV2S vaccine intramuscularly to mice, a significant amount of anti-SARS-CoV-2 S protein IgG antibody isotypes, resembling a Type 1 T helper cell response, were produced. | [142] |
| Cationic nanocarrier | RepRNA CCHFV (Crimean–Congo hemorrhagic fever virus) encoding NP (nucleoprotein), GPC (glycoprotein precursor) or both | It provided robust protection against Crimean–Congo hemorrhagic fever virus in lethal mice. | [152] |
| Cationic liposomes | samPfRH5 replicon (Plasmodium falciparum reticulocyte binding protein homologue 5) | The liposome–replicon complexes showed high transfection efficiencies. They elicited antibodies capable of inhibiting the growth of the parasite in vitro | [153] |
| Mannosylation of lipid nanoparticles (LNPs) | Self-amplifying mRNA encoded an influenza (hemagglutinin) | Compared to LNPs, mannnosylated lipid nanoparticles (MLNPs) showed higher levels of IgG1 and IgG2a. | [154] |
| Polymeric nanoparticle | Nanoparticle (MDNP)-delivered VEEV replicon RNA encoding the hemagglutinin protein (HA) of an H1N1 influenza virus (A/WSN/33) or the Ebola virus (EBOV) glycoprotein (GP) | The vaccine elicits both CD8+ T-cell and antibody responses and can be created with numerous antigen-expressing replicons. | [155] |
| Technology | Application | Type of Nanocarrier | References |
|---|---|---|---|
| siRNA-SQ and siRNA-SOLA | Cancers Harboring Fusion Oncogenes | Polyisoprenoid chains | [161] |
| RNA-paclitaxel | Breast cancer treatment | Lipidic | [162,163,164,165] |
| MRP-1 siRNA | Non-small cell lung cancer | Mesoporous silica | [166] |
| ALN-18328 (Patisiran) | Transthyretin amyloidosis (ATTR) | Lipid nanoparticle | [167,168] |
| GalNAc-siRNA (Givosiran) | Acute hepatic porphyria | Lipid nanoparticle | [169,170] |
| siRNA-LNP | Vaccine for COVID-19 | Lipid nanoparticle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.S.; dos Santos, D.; Souza, A.L.; Macedo, M.E.; Bandeira, M.E.; Junior, S.S.S.; Fiuza, B.S.D.; Rocha, V.P.C.; dos Santos Fonseca, L.M.; Nunes, D.D.G.; et al. RNA Combined with Nanoformulation to Advance Therapeutic Technologies. Pharmaceuticals 2023, 16, 1634. https://doi.org/10.3390/ph16121634
Lima ES, dos Santos D, Souza AL, Macedo ME, Bandeira ME, Junior SSS, Fiuza BSD, Rocha VPC, dos Santos Fonseca LM, Nunes DDG, et al. RNA Combined with Nanoformulation to Advance Therapeutic Technologies. Pharmaceuticals. 2023; 16(12):1634. https://doi.org/10.3390/ph16121634
Chicago/Turabian StyleLima, Eduarda Santos, Déborah dos Santos, Atena Liriel Souza, Maria Eduarda Macedo, Mariana Evangelista Bandeira, Sérgio Santos Silva Junior, Bianca Sampaio Dotto Fiuza, Vinicius Pinto Costa Rocha, Larissa Moraes dos Santos Fonseca, Danielle Devequi Gomes Nunes, and et al. 2023. "RNA Combined with Nanoformulation to Advance Therapeutic Technologies" Pharmaceuticals 16, no. 12: 1634. https://doi.org/10.3390/ph16121634
APA StyleLima, E. S., dos Santos, D., Souza, A. L., Macedo, M. E., Bandeira, M. E., Junior, S. S. S., Fiuza, B. S. D., Rocha, V. P. C., dos Santos Fonseca, L. M., Nunes, D. D. G., Hodel, K. V. S., & Machado, B. A. S. (2023). RNA Combined with Nanoformulation to Advance Therapeutic Technologies. Pharmaceuticals, 16(12), 1634. https://doi.org/10.3390/ph16121634






