Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake
Abstract
1. Introduction
2. Results
2.1. Radiochemistry
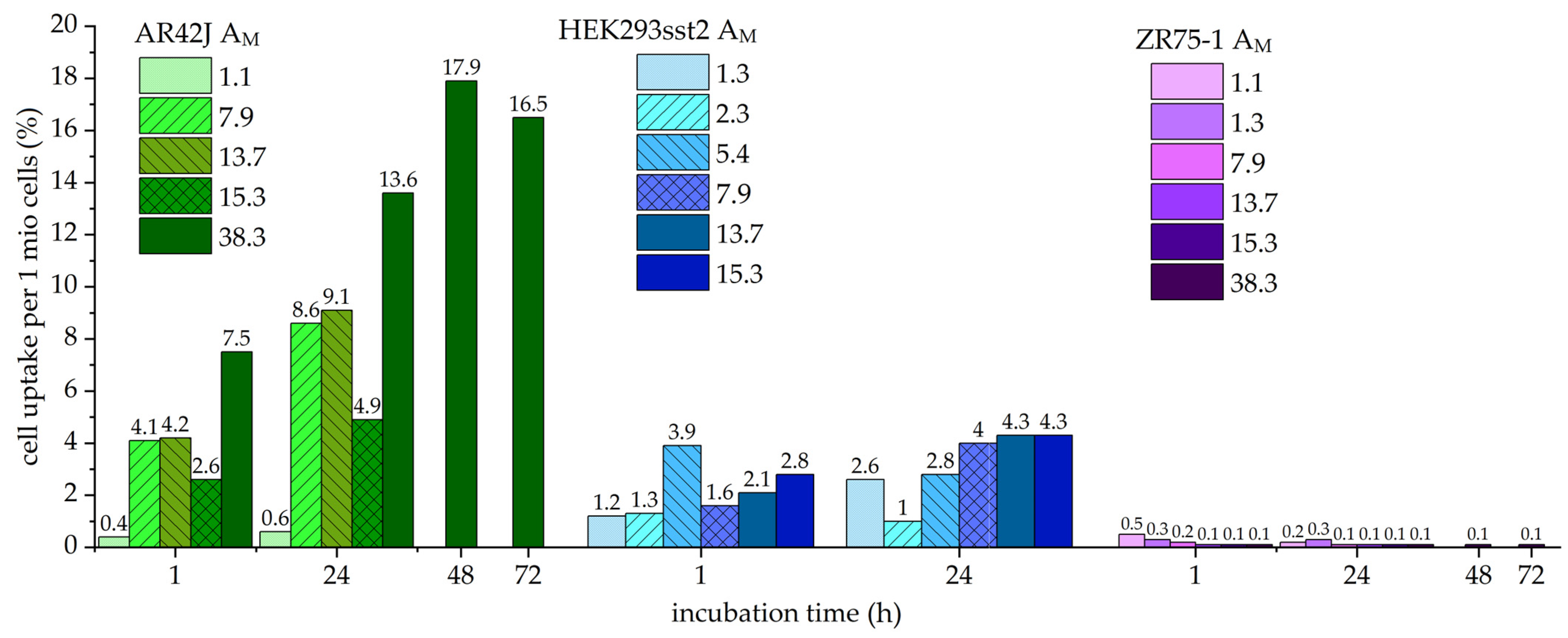
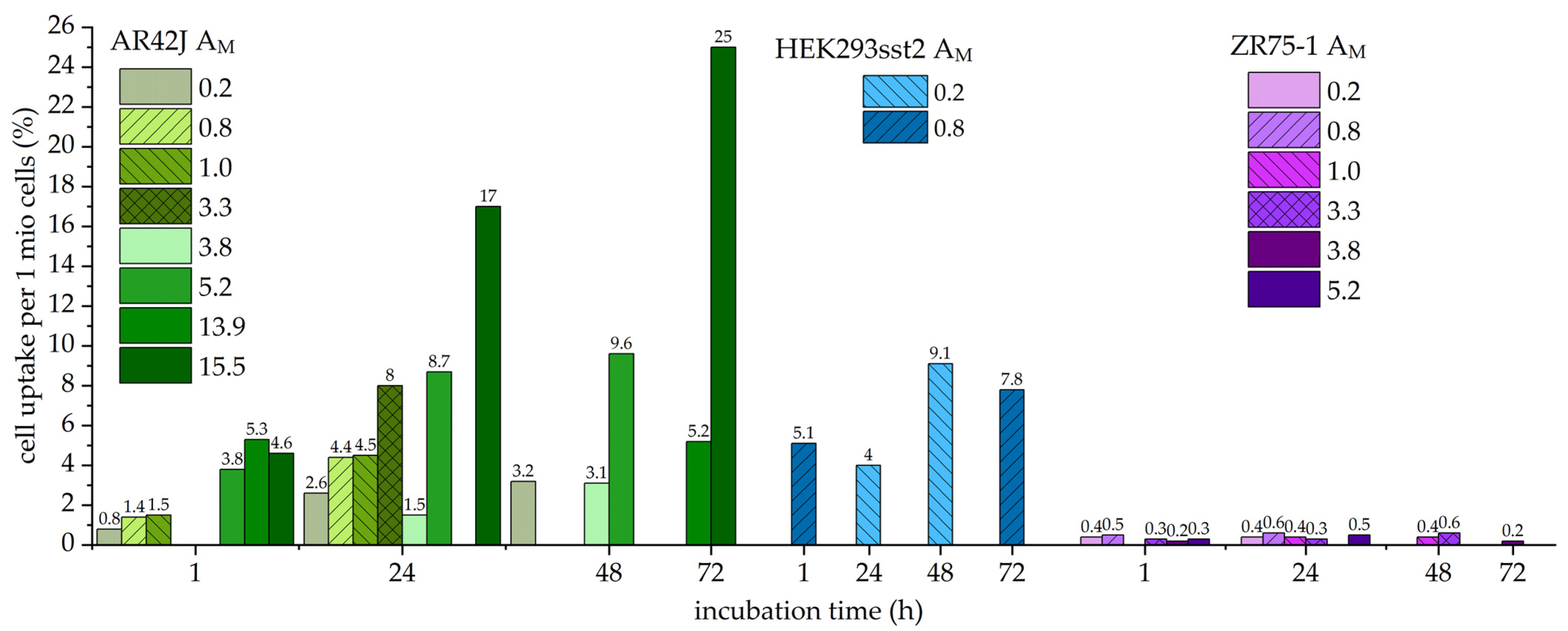
2.2. Cell Uptake Studies with Different Molar Activities
3. Discussion
3.1. Radiochemistry
3.2. Influence of Molar Activity on Cell Uptake
4. Materials and Methods
4.1. Radiochemistry
4.2. Quality Control of Radiotracer
- TLC with eluent 0.1 M Na-citrate pH 5 (Start: 203/212Pb-PSC-TOC and particles (Rf < 0.4), end: 203/212Pb-chloride) (Figure 3).
- TLC with eluent 1MNH4 Ac: MeOH1: 1 (Start: 203/212Pb-particles, end: 203/212Pb-PSC-TOC and 203/212Pb-chloride) (Figure 4).
- HPLC 203/212Pb-PSC-TOC tR = 7.4 min. 203/212Pb-DOTA-TATE tR = 7.1 min.
- pH value: 5.3 ± 0.5.
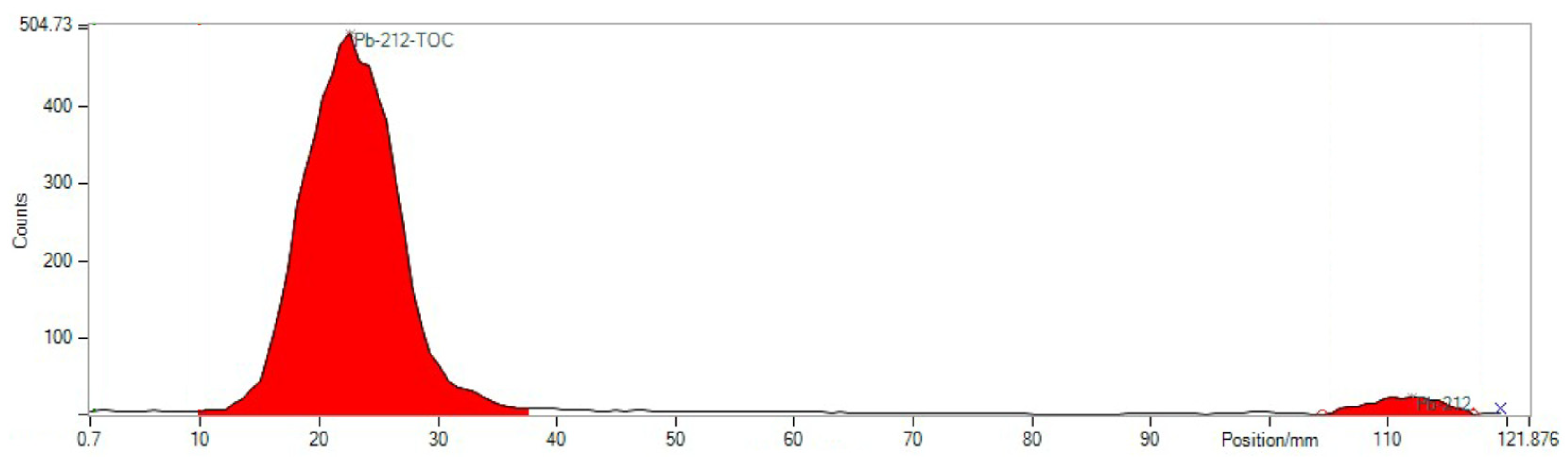
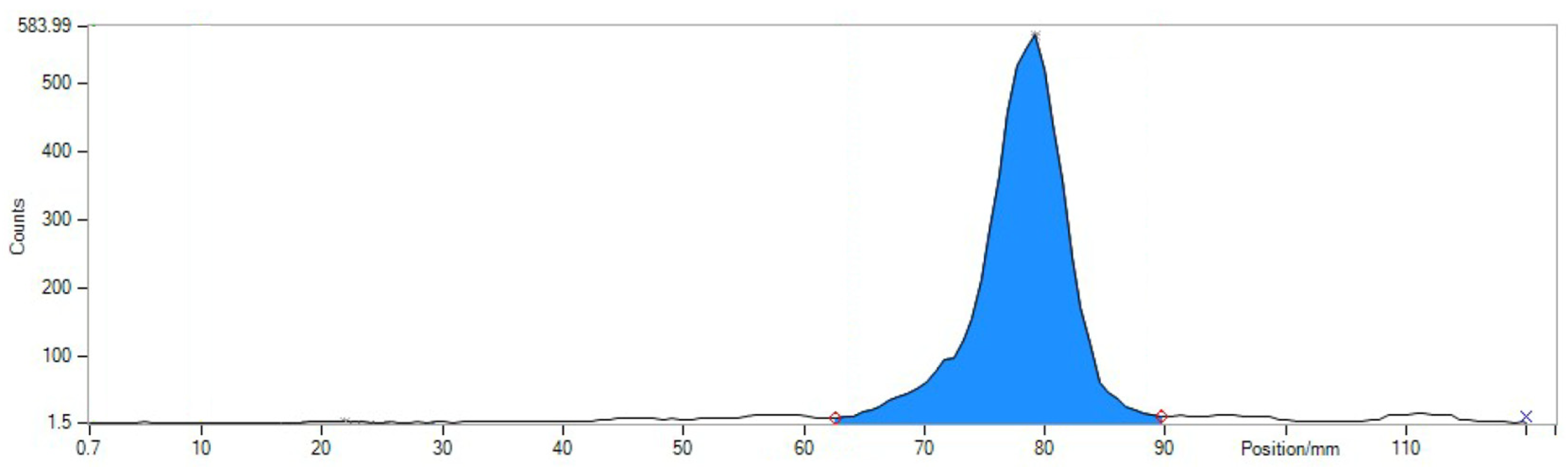
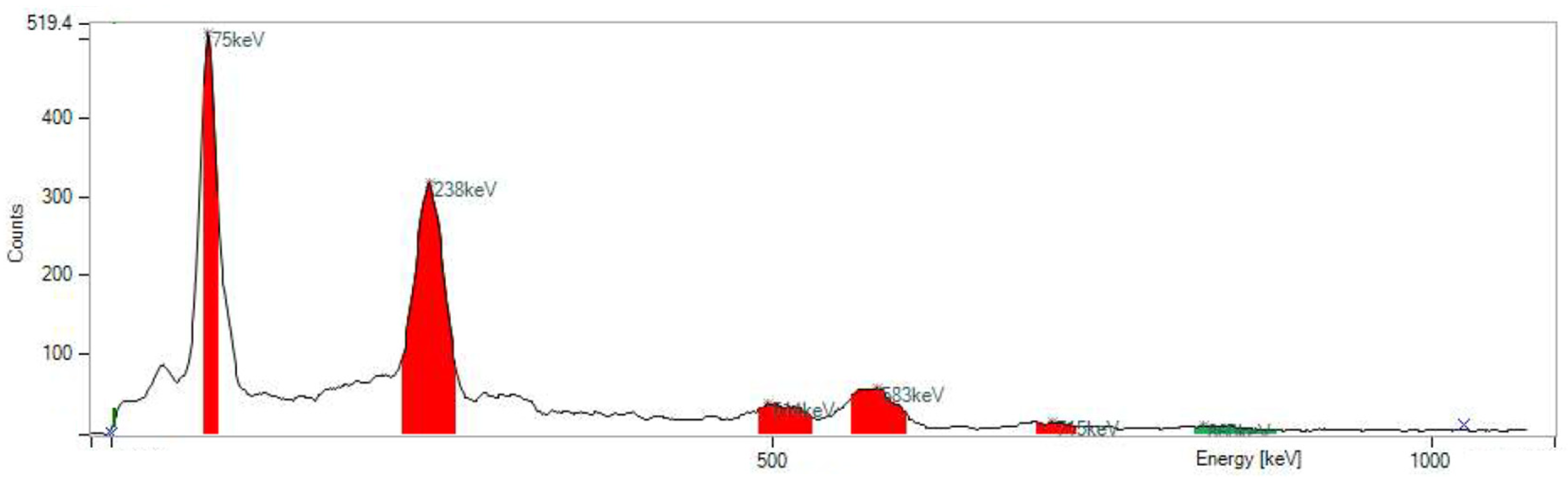
- RNP: 212Pb: 75 and 238 keV; 212Bi: 727 keV (6.7%); and 208Tl: 510 (22.6%), 583 (85.0%), and 860 (12.5%) keV (Figure 5).
4.3. Cell Uptake Experiments
- AR42J: Gibco RPMI 1640 medium (ATCC-Modification) supplemented with 10% fetal calf serum (FCS)
- HEK293 sst2 (stably transfected HEK293 cells): Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (FCS), l-glutamine (2 mM = 1%), G-418 (50 mg/mL)
- ZR75-1: Gibco RPMI 1640 medium (w/o glutamine) supplemented with 10% fetal calf serum (FCS), 1% NEAA, 1 mM sodium pyruvate (1%), and 2 mM N-acetyl-alanyl-l-glutamine (1%)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted alpha therapy: Current clinical applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Brogsitter, C.; Hartmann, H.; Wunderlich, G.; Schottelius, M.; Wester, H.J.; Kotzerke, J. Twins in spirit part IV—[177Lu] high affinity DOTATATE. A promising new tracer for peptide receptor radiotherapy? Nuklearmedizin 2017, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef]
- Li, M.; Sagastume, E.A.; Lee, D.; McAlister, D.; DeGraffenreid, A.J.; Olewine, K.R.; Graves, S.; Copping, R.; Mirzadeh, S.; Zimmerman, B.E.; et al. 203/212Pb theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Curr. Med. Chem. 2020, 27, 7003–7031. [Google Scholar] [CrossRef]
- McNeil, B.L.; Mastroianni, S.A.; McNeil, S.W.; Zeisler, S.; Kumlin, J.; Borjian, S.; McDonagh, A.W.; Cross, M.; Schaffer, P.; Ramogida, C.F. Optimized production, purification, and radiolabeling of the 203Pb/212Pb theranostic pair for nuclear medicine. Sci. Rep. 2023, 13, 10623. [Google Scholar] [CrossRef]
- Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M.; et al. Preclinical evaluation of a lead specific chelator (PSC) conjugated to radiopeptides for 203Pb and 212Pb-Based theranostics. Pharmaceutics 2023, 15, 414. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The chemical fate of 212Bi-DOTA formed by β- decay of 212Pb(DOTA)2-. Radiochim. Acta 1993, 60, 1–10. [Google Scholar] [CrossRef]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núnez, R. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: First-in-humans dose-escalation clinical trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef]
- Müller, D.; Herrmann, H.; Schultz, M.K.; Solbach, C.; Ettrich, T.; Prasad, V. 203Pb-VMT-α-NET scintigraphy of a patient with neuroendocrine tumor. Clin. Nucl. Med. 2023, 48, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Mikalsen, L.T.G.; Kvassheim, M.; Stokke, C. Optimized SPECT Imaging of 224Ra α-particle therapy by 212Pb photon emissions. J. Nucl. Med. 2023, 64, 1131–1137. [Google Scholar] [CrossRef]
- Kotzerke, J.; Runge, R.; Braune, A.; Wunderlich, G. Different Radionuclides in DOTA-EB-TATE Effect Different Uptake in Somatostatin Receptor-Positive HEK293 Cells. J. Nucl. Med. 2019, 60, 436. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, V.Y.; Juzeniene, A.; Chen, Q.; Yang, X.; Bruland, O.S.; Larsen, R.H. Preparation of the alpha-emitting prostate-specific membrane antigen targeted radioligand [212Pb]Pb-NG001 for prostate cancer. J. Label. Comp. Radiopharm. 2020, 63, 129–143. [Google Scholar] [CrossRef]
- Durand-Panteix, S.; Monteil, J.; Sage, M.; Garot, A.; Clavel, M.; Saidi, A.; Torgue, J.; Cogne, M.; Quelven, I. Preclinical study of 212Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma. Br. J. Cancer 2021, 125, 1657–1665. [Google Scholar] [CrossRef]
- Li, J.; Huang, T.; Hua, J.; Wang, Q.; Su, Y.; Chen, P.; Bidlingmaier, S.; Li, A.; Xie, Z.; Bidkar, A.P.; et al. CD46 targeted 212Pb alpha particle radioimmunotherapy for prostate cancer treatment. J. Exp. Clin. Cancer Res. 2023, 42, 61. [Google Scholar] [CrossRef] [PubMed]
- Rathke, H.; Bruchertseifer, F.; Kratochwil, C.; Keller, H.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. First patient exceeding 5-year complete remission after 225Ac-PSMA-TAT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 311–312. [Google Scholar] [CrossRef]
- Wood, V.; Ackerman, N.L. Cherenkov light production from the alpha-emitting decay chains of 223Ra, 212Pb, and 149Tb for Cherenkov luminescence imaging. Appl. Radiat. Isot. 2016, 118, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Luurtsema, G.; Pichler, V.; Bongarzone, S.; Seimbille, Y.; Elsinga, P.; Gee, A.; Vercouillie, J. EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: Impact on safety and imaging quality. EJNMMI Radiopharm. Chem. 2021, 6, 34. [Google Scholar] [CrossRef]
- Orcutt, K.D.; Henry, K.E.; Habjan, C.; Palmer, K.; Heimann, J.; Cupido, J.M.; Gottumukkala, V.; Cissell, D.D.; Lyon, M.C.; Hussein, A.I.; et al. Dosimetry of [212Pb]VMT01, a MC1R-targeted alpha therapeutic compound, and effect of free 208Tl on tissue absorbed doses. Molecules 2022, 27, 5831. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.R.; Menda, Y.; et al. Automated cassette-based production of high specific activity [203/212Pb] peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bobba, K.N.; Bidkar, A.P.; Meher, N.; Fong, C.; Wadhwa, A.; Dhrona, S.; Sorlin, A.; Bidlingmaier, S.; Shuere, B.; He, J.; et al. Evaluation of 134Ce/134La as a PET imaging theranostic pair for 225Ac α-radiotherapeutics. J. Nucl. Med. 2023, 64, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Sabet, A.; Nagarajah, J.; Dogan, A.S.; Biersack, H.-J.; Sabet, A.; Guhlke, S.; Ezziddin, S. Does PRRT with standard activities of 177Lu-octreotate really achieve relevant somatostatin receptor saturation in target tumor lesions?: Insights from intra-therapeutic receptor imaging in patients with metastatic gastroenteropancreatic neuroendocrine tumors. EJNMMI Res. 2013, 3, 82. [Google Scholar] [PubMed]
- Tian, R.; Jacobson, O.; Niu, G.; Kiesewetter, D.O.; Wang, Z.; Zhu, G.; Ma, Y.; Liu, G.; Chen, X. Evans Blue attachment enhances somatostatin receptor subtype-2 imaging and radiotherapy. Theranostics 2018, 8, 735–745. [Google Scholar] [CrossRef]
- Waser, B.; Tamma, M.L.; Cescato, R.; Maecke, H.R.; Reubi, J.C. Highly efficient in vivo agonist-induced internalization of sst2 receptors in somatostatin target tissues. J. Nucl. Med. 2009, 50, 936–941. [Google Scholar] [CrossRef]
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in patients with somatostatin-receptor-positive solid tumors: 5-year follow-up of hematological and renal toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63. [Google Scholar] [CrossRef]
- Nicolas, G.P.; Schreiter, N.; Kaul, F.; Uiters, J.; Bouterfa, H.; Kaufmann, J.; Erlanger, T.E.; Cathomas, R.; Christ, E.; Fani, M.; et al. Sensitivity Comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: A prospective phase II imaging study. J. Nucl. Med. 2018, 59, 915–921. [Google Scholar] [CrossRef]
- Kotzerke, J.; Buesser, D.; Naumann, A.; Runge, R.; Huebinger, L.; Kliewer, A.; Freudenberg, R.; Brogsitter, C. Epigenetic-like stimulation of receptor expression in SSTR2 transfected HEK293 cells as a new therapeutic strategy. Cancers 2022, 14, 2513. [Google Scholar] [CrossRef]
- von Hacht, J.L.; Erdmann, S.; Niederstadt, L.; Prasad, S.; Wagener, A.; Exner, S.; Beindorff, N.; Brenner, W.; Grötzinger, C. Increasing molar activity by HPLC purification improves 68Ga-DOTA-NAPamide tumor accumulation in a B16/F1 melanoma xenograft model. PLoS ONE 2019, 14, e0217883. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef]
- Piron, S.; Verhoeven, J.; De Coster, E.; Descamps, B.; Kersemans, K.; Pieters, L.; Vral, A.; Vanhove, C.; De Vos, F. Impact of the molar activity and PSMA expression level on [18F]AlF-PSMA-11 uptake in prostate cancer. Sci. Rep. 2021, 11, 22623. [Google Scholar] [CrossRef] [PubMed]
| Reactions 1 | Starting Activity (MBq) | Product Activity (MBq) | RCY (%) | AM (MBq/nmol) |
|---|---|---|---|---|
| 1 | 74 | 72 | 97 | 2.3 |
| 2 | 174 | 128 | 74 | 5.4 |
| 3 | 40 | 36 | 90 | 1.3 |
| 4 | 302 | 249 | 82 | 7.9 |
| 5 | 536 | 488 | 91 | 15.3 |
| 6 | 455 | 438 | 96 | 13.7 |
| 7 | 2105 | 1972 | 94 | 61.6 |
| 8 2 | 125 | 109 | 87 | 1.1 |
| 9 | 1301 | 1226 | 94 | 38.3 |
| Reactions 1 | Starting Activity (MBq) | Product Activity (MBq) | RCY (%) | AM (MBq/nmol) |
|---|---|---|---|---|
| 1 | 24 | 10 | 40 | 0.8 |
| 2 | 4 | 2 | 53 | 0.2 |
| 3 | 490 * | 176 * | 36 | 13.9 |
| 4 | 214 * | 48 * | 22 | 3.8 |
| 5 2 | 24 | 11 | 48 | 0.8 |
| Reactions 1 | Starting Activity (MBq) | Product Activity (MBq) | RCY (%) | AM (MBq/nmol) |
|---|---|---|---|---|
| 1 | 522 * | 490 * | 98 | 15.5 |
| 2 | 328 | 315 | 97 | 9.9 |
| 3 | 274 | 260 | 99 | 8.2 |
| 4 | 177 | 165 * | 93 | 5.2 |
| 5 | 146 | 104 * | 71 | 3.3 |
| 6 | 95 | 78 * | 82 | 2.5 |
| 7 | 32 | 31 | 99 | 1.0 |
| Time after Separation (min) | Normalization Factor |
|---|---|
| 0 | 1.86 |
| 5 | 1.80 |
| 30 | 1.58 |
| 60 | 1.37 |
| 90 | 1.26 |
| 120 | 1.18 |
| Cells | Incubation Time (h) | Molar Activity (MBq/nmol) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1.3 | 2.3 | 5.4 | 7.9 | 13.7 | 15.3 | 38.3 | ||
| AR42J uptake (%) | 1 | 0.4 ± 0.1 | 4.1 ± 0.2 | 4.2 ± 0.0 | 2.6 ± 0.2 | 7.5 ± 0.4 | |||
| 24 | 0.6 ± 0.0 | 8.6 ± 0.5 | 9.1 ± 0.4 | 4.9 ± 0.6 | 13.6 ± 0.2 | ||||
| 48 | 17.9 ± 0.7 | ||||||||
| 72 | 16.5 ± 1.3 | ||||||||
| HEK293 sst2 uptake (%) | 1 | 1.2 ± 0.3 | 1.3 ± 0.1 | 3.9 ± 0.2 | 1.6 ± 0.3 | 2.1 ± 0.1 | 2.8 ± 0.1 | 1.2 ± 0.3 | |
| 24 | 2.6 ± 0.6 | 1.0 ± 0.0 | 2.8 ± 0.2 | 4.0 ± 0.3 | 4.3 ± 0.2 | 4.3 ± 0.9 | 2.6 ± 0.6 | ||
| Cells | Incubation Time (h) | Molar Activity (MBq/nmol) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.8 | 1.0 | 3.3 | 3.8 | 5.2 | 13.9 | 15.5 | ||
| AR42J uptake (%) | 1 | 0.8 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | n.d. | 3.8 ± 0.2 | 5.3 ± 0.3 | 4.6 ± 0.3 | |
| 24 | 2.6 ± 0.2 | 4.4 ± 0.4 | 4.5 ± 0.0 | 8.0 ± 1.0 | 1.5 ± 0.1 | 8.7 ± 0.7 | 17.0 ± 0.7 | ||
| 48 | 3.2 ± 0.4 | 3.1 ± 0.1 | 9.6 ± 0.7 | ||||||
| 72 | 5.2 ± 0.4 | 25.0 ± 0.5 | |||||||
| HEK293 sst2 uptake (%) | 1 | 5.1 ± 0.5 | |||||||
| 24 | 4.0 ± 0.5 | ||||||||
| 48 | 9.1 ± 0.5 | ||||||||
| 72 | 7.8 ± 0.0 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretze, M.; Michler, E.; Runge, R.; Wetzig, K.; Tietze, K.; Brandt, F.; Schultz, M.K.; Kotzerke, J. Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake. Pharmaceuticals 2023, 16, 1605. https://doi.org/10.3390/ph16111605
Pretze M, Michler E, Runge R, Wetzig K, Tietze K, Brandt F, Schultz MK, Kotzerke J. Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake. Pharmaceuticals. 2023; 16(11):1605. https://doi.org/10.3390/ph16111605
Chicago/Turabian StylePretze, Marc, Enrico Michler, Roswitha Runge, Kerstin Wetzig, Katja Tietze, Florian Brandt, Michael K. Schultz, and Jörg Kotzerke. 2023. "Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake" Pharmaceuticals 16, no. 11: 1605. https://doi.org/10.3390/ph16111605
APA StylePretze, M., Michler, E., Runge, R., Wetzig, K., Tietze, K., Brandt, F., Schultz, M. K., & Kotzerke, J. (2023). Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake. Pharmaceuticals, 16(11), 1605. https://doi.org/10.3390/ph16111605






