In Vitro Antibiofilm Effect of N-Acetyl-L-cysteine/Dry Propolis Extract Combination on Bacterial Pathogens Isolated from Upper Respiratory Tract Infections
Abstract
:1. Introduction
2. Results
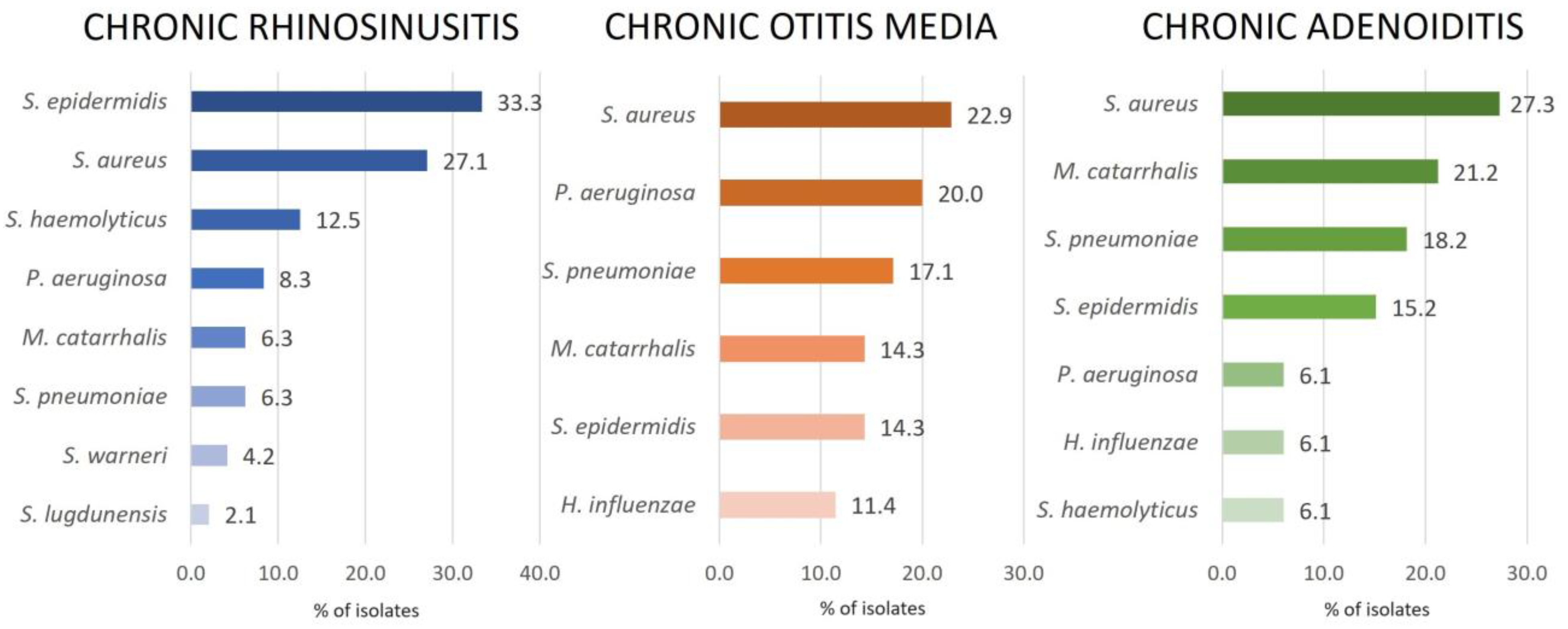
2.1. Bacterial Isolates
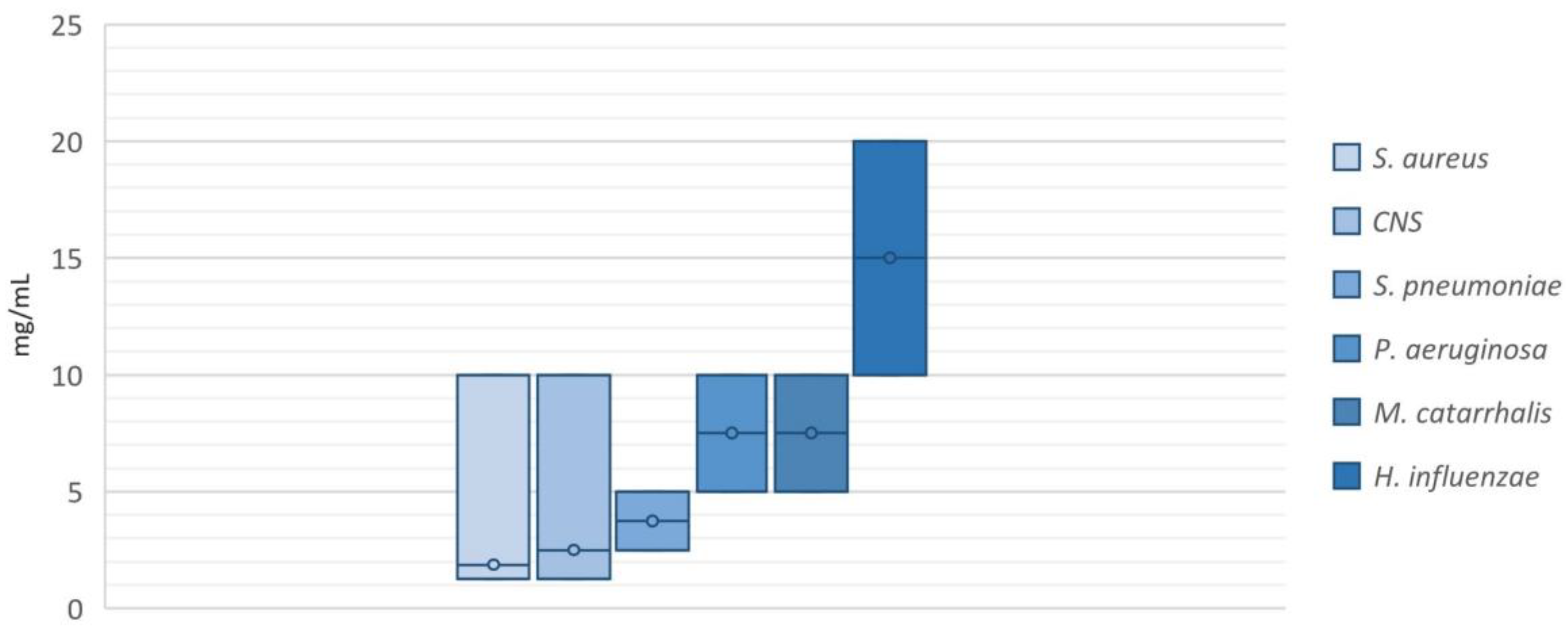
2.2. Antimicrobial Activity of NAC/Dry Propolis Extract Fixed Combination
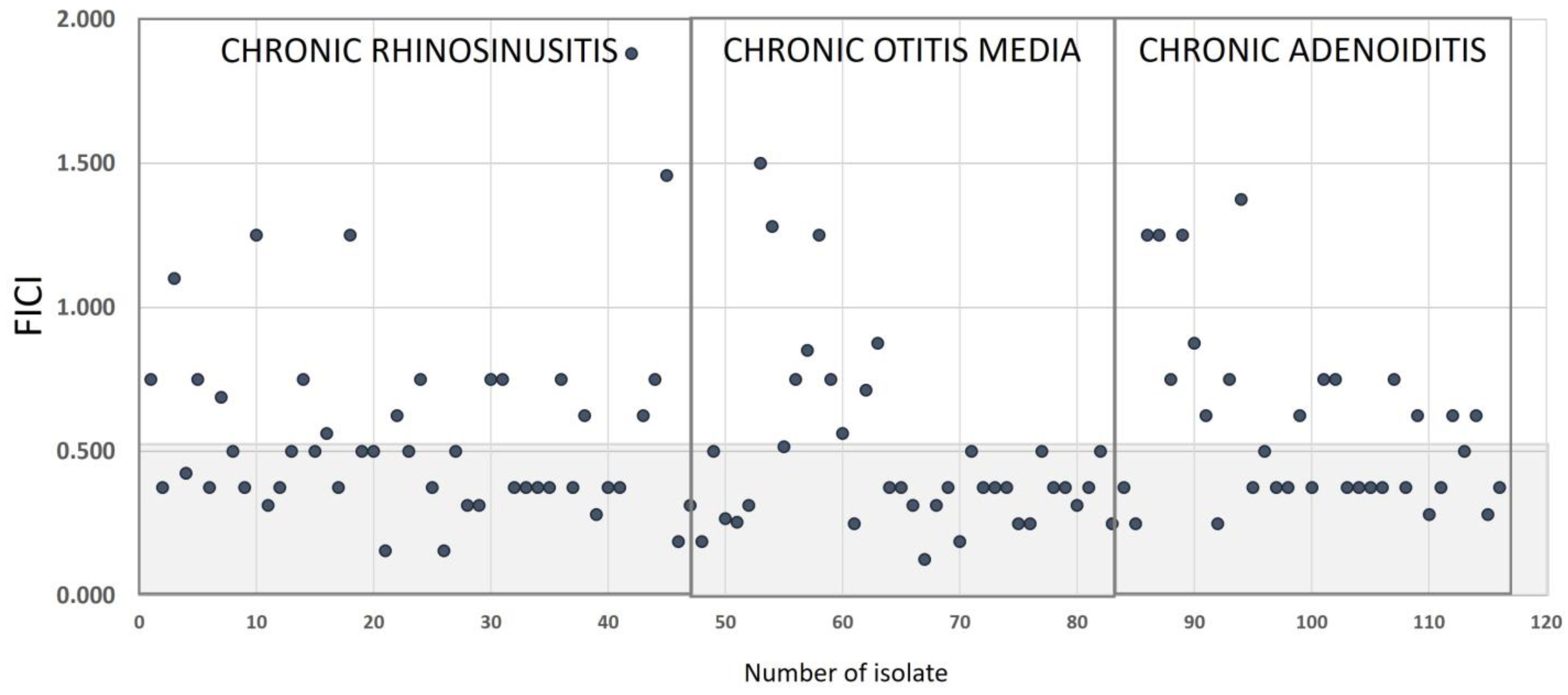
2.3. Synergistic Effect of NAC/Dry Propolis Extract Fixed Combination
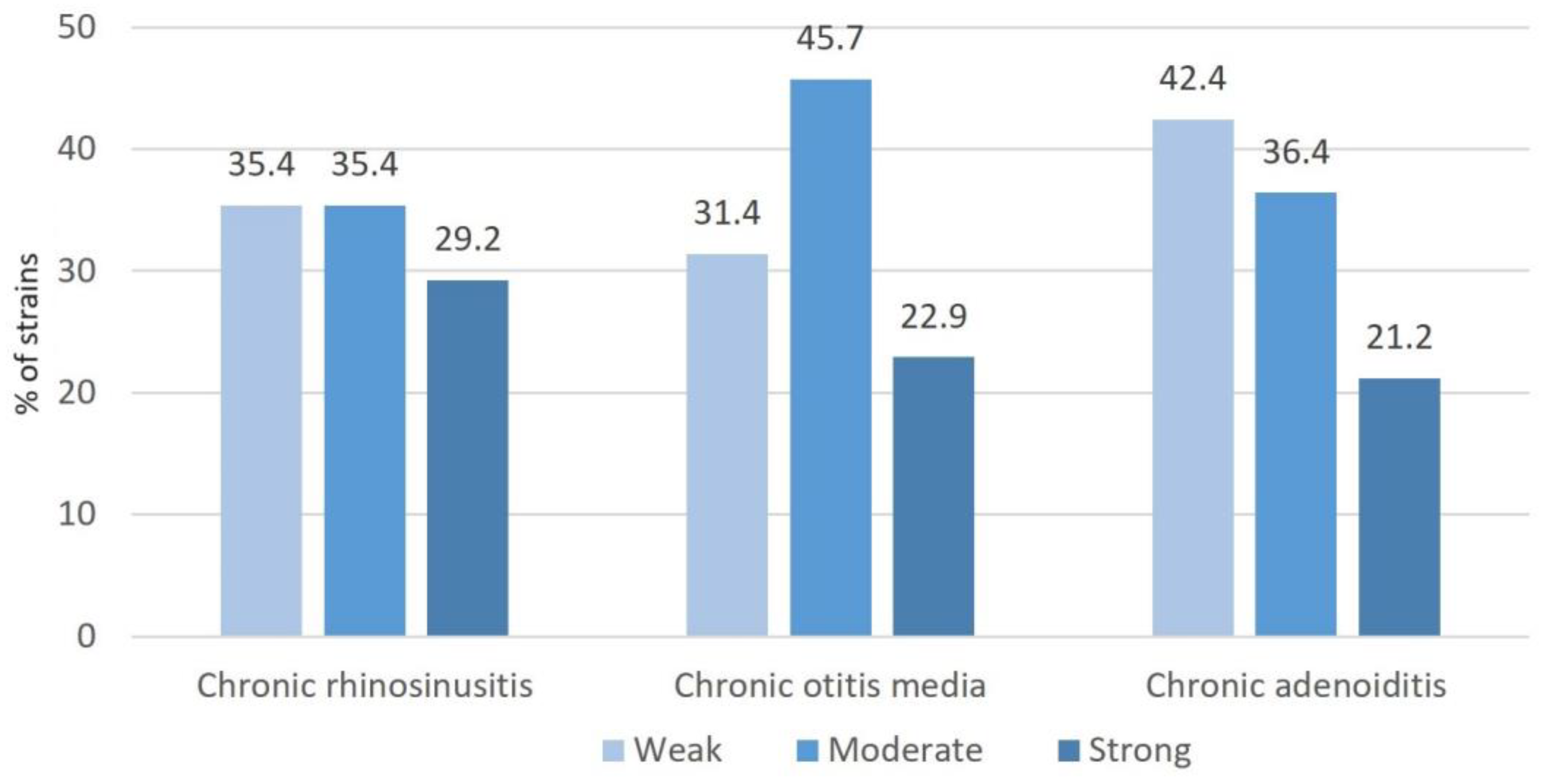
2.4. Biofilm Production
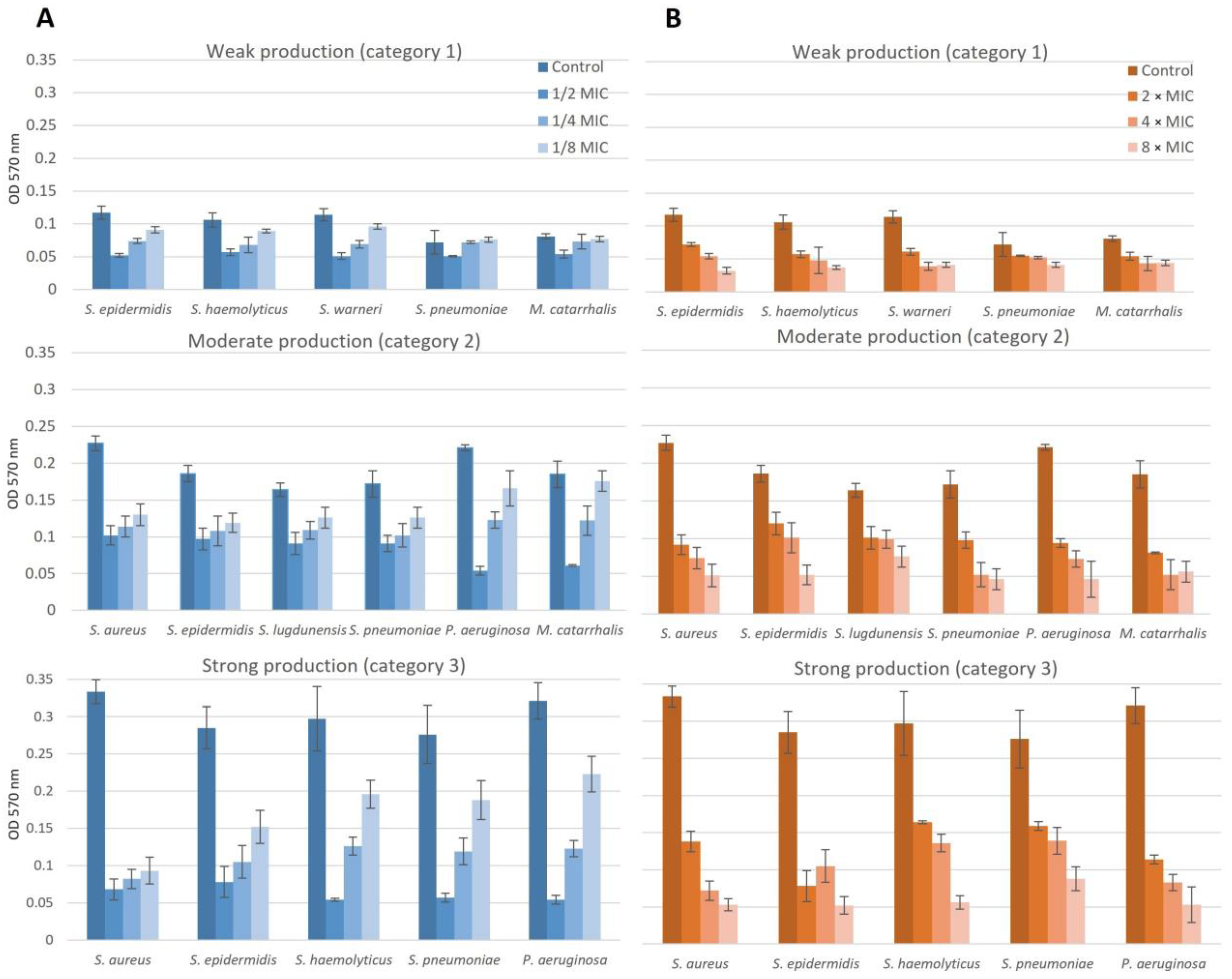
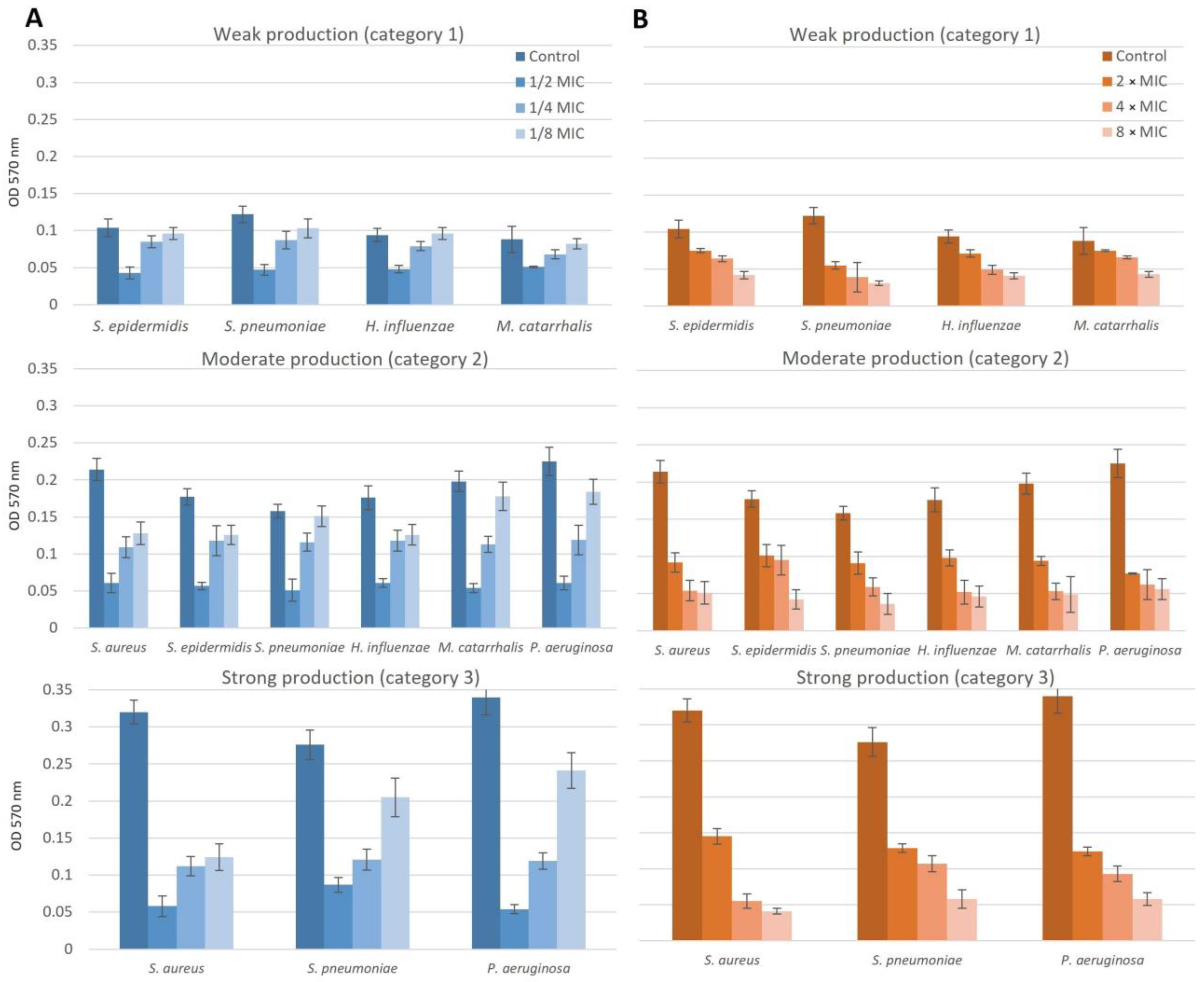
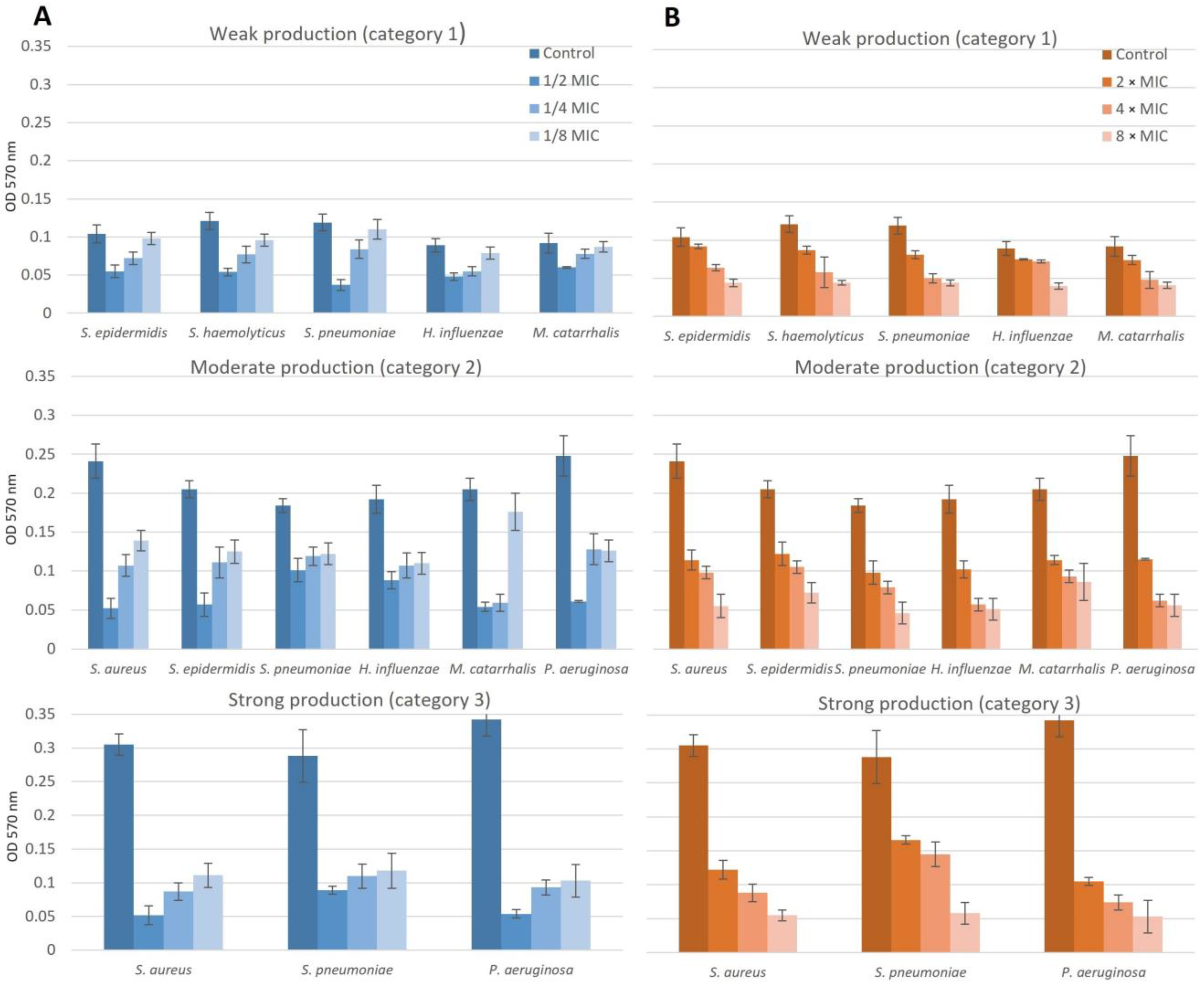
2.5. Effect of NAC/Dry Propolis Extract Combinations on Biofilm Formation and Biofilm Eradication
2.5.1. Chronic Rhinosinusitis
2.5.2. Chronic Otitis Media
2.5.3. Chronic Adenoiditis
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Specimens’ Collection and Bacterial Isolation and Identification
4.3. Determination of Minimum Inhibitory Concentration of NAC/Dry Propolis Extract Fixed Combination
4.4. Synergy Testing
4.5. In Vitro Determination of Bacterial Biofilm-Forming Capacity
4.6. Effect of NAC/Dry Propolis Extract Combinations on Biofilm Formation and Biofilm Eradication
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calò, L.; Passàli, G.C.; Galli, J.; Fadda, G.; Paludetti, G. Role of biofilms in chronic inflammatory diseases of the upper airways. Adv. Otorhinolaryngol. 2011, 72, 93–96. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Gloag, E.S.; Fabbri, S.; Wozniak, D.J.; Stoodley, P. Biofilm mechanics: Implications in infection and survival. Biofilm 2019, 2, 100017. [Google Scholar] [CrossRef]
- Torretta, S.; Drago, L.; Marchisio, P.; Ibba, T.; Pignataro, L. Role of Biofilms in Children with Chronic Adenoiditis and Middle Ear Disease. J. Clin. Med. 2019, 8, 671. [Google Scholar] [CrossRef]
- Singh, G.B.; Malhotra, S.; Yadav, S.C.; Kaur, R.; Kwatra, D.; Kumar, S. The Role of Biofilms in Chronic Otitis Media-Active Squamosal Disease: An Evaluative Study. Otol. Neurotol. 2021, 42, e1279–e1285. [Google Scholar] [CrossRef]
- Al-Mutairi, D.; Kilty, S.J. Bacterial biofilms and the pathophysiology of chronic rhinosinusitis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 18–23. [Google Scholar] [CrossRef]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Sadowska, A.M. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2012, 6, 127–135. [Google Scholar] [CrossRef]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef]
- Zulhendri, F.; Perera, C.O.; Tandean, S.; Abdulah, R.; Herman, H.; Christoper, A.; Chandrasekaran, K.; Putra, A.; Lesmana, R. The Potential Use of Propolis as a Primary or an Adjunctive Therapy in Respiratory Tract-Related Diseases and Disorders: A Systematic Scoping Review. Biomed. Pharmacother. 2022, 146, 112595. [Google Scholar] [CrossRef]
- Inui, S.; Hatano, A.; Yoshino, M.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ahn, M.R.; Tazawa, S.; Araki, Y.; Kumazawa, S. Identification of the phenolic compounds contributing to antibacterial activity in ethanol extracts of Brazilian red propolis. Nat. Prod. Res. 2014, 28, 1293–1296. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Meto, A.; Boaretto, G.; Pinetti, D.; Marchetti, L.; Benvenuti, S.; Pellati, F.; Blasi, E. Propolis Affects Pseudomonas aeruginosa Growth, Biofilm Formation, eDNA Release and Phenazine Production: Potential Involvement of Polyphenols. Microorganisms 2020, 8, 243. [Google Scholar] [CrossRef]
- de Azevedo, M.N.; Marques, N.T.; Fonseca, M.F.L.; Schuch, L.F.; de Arruda, J.A.A.; Santos, V.R.; Mesquita, R.A.; Moreno, A. Disinfectant effects of Brazilian green propolis alcohol solutions on the Staphylococcus aureus biofilm of maxillofacial prosthesis polymers. J. Prosthet. Dent. 2022, 128, 1405–1411. [Google Scholar] [CrossRef]
- Grecka, K.; Xiong, Z.R.; Chen, H.; Pełka, K.; Worobo, R.W.; Szweda, P. Effect of Ethanol Extracts of Propolis (EEPs) against Staphylococcal Biofilm-Microscopic Studies. Pathogens 2020, 9, 646. [Google Scholar] [CrossRef]
- Ripari, N.; Pereira, A.F.M.; Júnior, A.F.; Rall, V.L.M.; Aldana-Mejía, J.A.; Bastos, J.K.; Sforcin, J.M. Brazilian red propolis in combination with β-lactams exerts an efficient antibacterial action over methicillin-resistant Staphylococcus aureus (MRSA) strains. J. Appl. Microbiol. 2023, 134, lxac080. [Google Scholar] [CrossRef]
- Llamosí, M.; Sempere, J.; Coronel, P.; Gimeno, M.; Yuste, J.; Domenech, M. Combination of Cefditoren and N-acetyl-l-Cysteine Shows a Synergistic Effect against Multidrug-Resistant Streptococcus pneumoniae Biofilms. Microbiol. Spectr. 2022, 10, e0341522. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef]
- Žuža, O.; Minić, R.; Kotur-Stevuljević, J.; Žujović, D.; Đorđević, B.; Ilić, A. A combination of N-acetyl cysteine and propolis attenuates oxidative-inflammatory parameters during COPD exacerbation. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2467–2477. [Google Scholar] [CrossRef]
- Vekić, J.; Ivanišević, J.; Zeljković, A.; Spasojević-Kalimanovska, V.; Bogavac-Stanojević, N.; Mihajlović, M.; Janać, J.; Vujčić, S.; Miljković, M.; Zujović, D.; et al. Effect of propolis and N-acetylcysteine supplementation on lipoprotein subclasses distribution and paraoxonase 1 activity in subjects with acute respiratory infection. J. Med. Biochem. 2020, 39, 467–473. [Google Scholar] [CrossRef]
- Lea, J.; Conlin, A.E.; Sekirov, I.; Restelli, V.; Ayakar, K.G.; Turnbull, L.A.; Doyle, P.; Noble, M.; Rennie, R.; Schreiber, W.E.; et al. In vitro efficacy of N-acetylcysteine on bacteria associated with chronic suppurative otitis media. J. Otolaryngol. Head Neck Surg. 2014, 43, 20. [Google Scholar] [CrossRef]
- Domenech, M.; Garcia, E. N-Acetyl-L-Cysteine and Cysteamine as New Strategies against Mixed Biofilms of Nonencapsulated Streptococcus pneumoniae and Nontypeable Haemophilus influenzae. Antimicrob. Agents Chemother. 2017, 61, e01992-16. [Google Scholar] [CrossRef]
- Sempere, J.; Llamosí, M.; Román, F.; Lago, D.; González-Camacho, F.; Pérez-García, C.; Yuste, J.; Domenech, M. Clearance of mixed biofilm of Streptococcus pneumoniae and methicillin-susceptible/resistant Staphylococcus aureus by antioxidants N-acetyl-L-cysteine and cysteamine. Sci. Rep. 2022, 12, 6668. [Google Scholar] [CrossRef]
- Jun, Y.; Youn, C.K.; Jo, E.R.; Cho, S.I. In vitro inhibitory activity of N-acetylcysteine on tympanostomy tube biofilms from methicillin-resistant Staphylococcus aureus and quinolone-resistant Pseudomonas aeruginosa. Int. J. Pediatr. Otorhinolaryngol. 2019, 126, 109622. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibits biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef]
- Chlumsky, O.; Smith, H.J.; Parker, A.E.; Brileya, K.; Wilking, J.N.; Purkrtova, S.; Michova, H.; Ulbrich, P.; Viktorova, J.; Demnerova, K. Evaluation of the Antimicrobial Efficacy of N-Acetyl-l-Cysteine, Rhamnolipids, and Usnic Acid-Novel Approaches to Fight Food-Borne Pathogens. Int. J. Mol. Sci. 2021, 22, 11307. [Google Scholar] [CrossRef]
- Drago, L.; Mombelli, B.; De Vecchi, E.; Fassina, M.C.; Tocalli, L.; Gismondo, M.R. In vitro antimicrobial activity of propolis dry extract. J. Chemother. 2000, 12, 390–395, Erratum in: J. Chemother. 2001, 13, 102. [Google Scholar] [CrossRef]
- EI-Fadaly, H.; EI-Badrawy, E.E.Y. Flavonoids of propolis and their antibacterial activities. Pak. J. Biol. Sci. 2001, 21, 204–207. [Google Scholar] [CrossRef]
- Stepanović, S.; Antić, N.; Dakić, I.; Švabić-Vlahović, M. In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef]
- Almuhayawi, M.S. Propolis as a novel antibacterial agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef] [PubMed]
- Perez-Giraldo, C.; Rodríguez-Benito, A.; Morán, F.J.; Hurtado, C.; Blanco, M.T.; Gómez-García, A.C. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997, 39, 643–646. [Google Scholar] [CrossRef]
- Manoharan, A.; Das, T.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; Manos, J. The effect of N-acetylcysteine in a combined antibiofilm treatment against antibiotic-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2020, 75, 1787–1798. [Google Scholar] [CrossRef]
- Kolaylı, S.; Esertas, U.U.Z.; Kara, Y. The Antimicrobial, Anti-Quorum Sensing, and Anti-Biofilm Activities of Ethanolic Propolis Extracts Used as Food Supplements. Biol. Bull. Russ. Acad. Sci. 2022, 49 (Suppl. S3), S21–S30. [Google Scholar] [CrossRef]
- Queiroga, M.C.; Laranjo, M.; Andrade, N.; Marques, M.; Costa, A.R.; Antunes, C.M. Antimicrobial, Antibiofilm and Toxicological Assessment of Propolis. Antibiotics 2023, 12, 347. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Wąsik, T.J. In vitro antimicrobial activity of ethanolic extract of polish propolis against biofilm forming Staphylococcus epidermidis strains. Evid. Based Complement. Alternat. Med. 2013, 2013, 590703. [Google Scholar] [CrossRef]
- Scazzocchio, F.; D’Auria, F.D.; Alessandrini, D.; Pantanella, F. Multifactorial aspects of antimicrobial activity of propolis. Microbiol. Res. 2006, 161, 327–333. [Google Scholar] [CrossRef]
- Doganli, G.A. Phenolic content and antibiofilm activity of propolis against clinical MSSA strains. Rec. Nat. Prod. 2016, 10, 617–627. [Google Scholar]
- De Marco, S.; Piccioni, M.; Pagiotti, R.; Pietrella, D. Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versus Pseudomonas aeruginosa. Evid. Based Complement Altern. Med. 2017, 2017, 5163575. [Google Scholar] [CrossRef] [PubMed]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Riise, G.C.; Qvarfordt, I.; Larsson, S.; Eliasson, V.; Andersson, B.A. Inhibitory effect of N-acetylcysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human oropharyngeal epithelial cells in vitro. Respiration 2000, 67, 552–558. [Google Scholar] [CrossRef]
- Zheng, C.H.; Ahmed, K.; Rikitomi, N.; Martinez, G.; Nagatake, T. The effects of S-carboxymethylcysteine and N-acetylcysteine on the adherence of Moraxella catarrhalis to human pharyngeal epithelial cells. Microbiol. Immunol. 1999, 43, 107–113. [Google Scholar] [CrossRef]
- Riga, M.G.; Chelis, L.; Kakolyris, S.; Papadopoulos, S.; Stathakidou, S.; Chamalidou, E.; Xenidis, N.; Amarantidis, K.; Dimopoulos, P.; Danielides, V. Transtympanic injections of N-acetylcysteine for the prevention of cisplatin-induced ototoxicity: A feasible method with promissing efficacy. Am. J. Clin. Oncol. 2013, 36, 1–6. [Google Scholar] [CrossRef]
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The use of propolis in dentistry, oral health, and medicine: A review. J. Oral. Biosci. 2021, 63, 23–34. [Google Scholar] [CrossRef]
- Garcia, L. Synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In Clinical Microbiology Procedures Handbook, 3rd ed.; Leber, A.L., Ed.; American Society of Microbiology: Wiley, CO, USA, 2014; Chapter 5.16; pp. 140–162. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božić, D.D.; Ćirković, I.; Milovanović, J.; Bufan, B.; Folić, M.; Savić Vujović, K.; Pavlović, B.; Jotić, A. In Vitro Antibiofilm Effect of N-Acetyl-L-cysteine/Dry Propolis Extract Combination on Bacterial Pathogens Isolated from Upper Respiratory Tract Infections. Pharmaceuticals 2023, 16, 1604. https://doi.org/10.3390/ph16111604
Božić DD, Ćirković I, Milovanović J, Bufan B, Folić M, Savić Vujović K, Pavlović B, Jotić A. In Vitro Antibiofilm Effect of N-Acetyl-L-cysteine/Dry Propolis Extract Combination on Bacterial Pathogens Isolated from Upper Respiratory Tract Infections. Pharmaceuticals. 2023; 16(11):1604. https://doi.org/10.3390/ph16111604
Chicago/Turabian StyleBožić, Dragana D., Ivana Ćirković, Jovica Milovanović, Biljana Bufan, Miljan Folić, Katarina Savić Vujović, Bojan Pavlović, and Ana Jotić. 2023. "In Vitro Antibiofilm Effect of N-Acetyl-L-cysteine/Dry Propolis Extract Combination on Bacterial Pathogens Isolated from Upper Respiratory Tract Infections" Pharmaceuticals 16, no. 11: 1604. https://doi.org/10.3390/ph16111604
APA StyleBožić, D. D., Ćirković, I., Milovanović, J., Bufan, B., Folić, M., Savić Vujović, K., Pavlović, B., & Jotić, A. (2023). In Vitro Antibiofilm Effect of N-Acetyl-L-cysteine/Dry Propolis Extract Combination on Bacterial Pathogens Isolated from Upper Respiratory Tract Infections. Pharmaceuticals, 16(11), 1604. https://doi.org/10.3390/ph16111604






