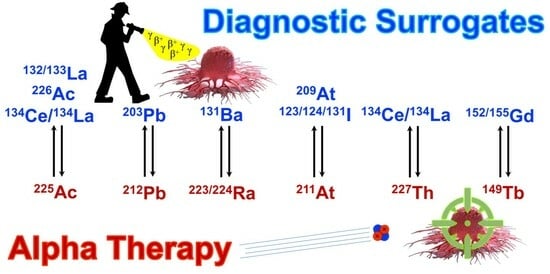
Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications
Abstract
1. Introduction
2. Properties of Ideal Imaging Surrogates for Alpha Emitters
3. Theranostic Imaging Surrogates Proposed for Actinium-225
3.1. Lanthanum-133 (PET)
3.2. Lanthanum-132 (PET)
3.3. Lanthanum-134/Cerium-134 (PET)
3.4. Actinium-226 (SPECT)
4. Theranostic Imaging Surrogates Proposed for Lead-212
Lead-203 (SPECT)
5. Theranostic Imaging Surrogates Proposed for Radium-223/224
Barium-131 (SPECT)
6. Theranostic Imaging Surrogates Proposed for Astatine-211
6.1. Iodine-123 (SPECT)
6.2. Iodine-124 (PET)
6.3. Iodine-131 (SPECT)
6.4. Astatine-209 (SPECT)
7. Theranostic Imaging Surrogates Proposed for Thorium-227
8. Theranostic Imaging Surrogates Proposed for Terbium-149
8.1. Terbium-155 (SPECT)
8.2. Terbium-152 (PET)
9. Summary and Outlook for Alpha-Emitter Imaging Surrogates
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schultz, M.K.; Pouget, J.P.; Wuest, F.; Nelson, B.; Andersson, J.; Cheal, S.; Li, M.; Ianzini, F.; Sangeeta, R.; Graves, S.; et al. Radiobiology of Targeted Alpha Therapy. In Nuclear Medicine and Molecular Imaging; Signore, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 380–403. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted alpha therapy: Progress in radionuclide production, radiochemistry and applications. Pharmaceutics 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. NEJM 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. NEJM 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Mills, K.; Allen, K.J.H.; Causey, P.; Perron, R.W.; Gendron, D.; Sanche, S.; Berman, J.W.; Gorny, M.K.; Dadachova, E. Comparison of various radioactive payloads for a human monoclonal antibody to glycoprotein 41 for elimination of HIV-infected cells. Nucl. Med. Biol. 2020, 82–83, 80–88. [Google Scholar] [CrossRef]
- Elgqvist, J.; Frost, S.; Pouget, J.P.; Albertsson, P. The Potential and Hurdles of Targeted Alpha Therapy–Clinical Trials and Beyond. Front. Oncol. 2014, 3, 324. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2013.00324 (accessed on 29 September 2023). [CrossRef] [PubMed]
- Sollini, M.; Marzo, K.; Chiti, A.; Kirienko, M. The five “W”s and “How” of Targeted Alpha Therapy: Why? Who? What? Where? When? and How? Rend. Lincei 2020, 31, 231–247. [Google Scholar] [CrossRef]
- Lindegren, S.; Albertsson, P.; Bäck, T.; Jensen, H.; Palm, S.; Aneheim, E. Realizing Clinical Trials with Astatine-211: The Chemistry Infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436. [Google Scholar] [CrossRef]
- Sonzogni, A.; Shu, B. Nudat 2.8 (Nuclear Structure and Decay Data). 2020. Available online: https://www.nndc.bnl.gov/nudat2/reCenter.jsp?z=56&n=77> (accessed on 20 September 2023).
- Bannik, K.; Madas, B.; Jarzombek, M.; Sutter, A.; Siemeister, G.; Mumberg, D.; Zitzmann-Kolbe, S. Radiobiological effects of the alpha emitter Ra-223 on tumor cells. Sci. Rep. 2019, 9, 18489. [Google Scholar] [CrossRef]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Kokov, K.V.; Egorova, B.V.; German, M.N.; Klabukov, I.D.; Krasheninnikov, M.E.; Larkin-Kondrov, A.A.; Makoveeva, K.A.; Ovchinnikov, M.V.; Sidorova, M.V.; Chuvilin, D.Y. 212Pb: Production Approaches and Targeted Therapy Applications. Pharmaceutics 2022, 14, 189. [Google Scholar] [CrossRef]
- Abou, D.S.; Fears, A.; Summer, L.; Longtine, M.; Benabdallah, N.; Riddle, R.C.; Ulmert, D.; Michalski, J.; Wahl, R.L.; Chesner, D.; et al. Improved Radium-223 Therapy with Combination Epithelial Sodium Channel Blockade. J. Nucl. Med. 2021, 62, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Hosono, M.; Ikebuchi, H.; Nakamura, Y.; Yanagida, S.; Kinuya, S. Introduction of the targeted alpha therapy (with Radium-223) into clinical practice in Japan: Learnings and implementation. Ann. Nucl. Med. 2019, 33, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Mikalsen, L.T.G.; Kvassheim, M.; Stokke, C. Optimized SPECT Imaging of 224Ra α-Particle Therapy by 212Pb Photon Emissions. J. Nucl. Med. 2023, 64, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Kadonaga, Y.; Ooe, K.; Wang, Y.; Haba, H.; Toyoshima, A.; Cardinale, J.; Giesel, F.L.; et al. Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: A preclinical evaluation as a novel compound. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.J.; Miederer, M.; Vranjes-Durić, S.; Comor, J.J.; Künzi, G.; Hartley, O.; Senekowitsch-Schmidtke, R.; Soloviev, D.; Buchegger, F. Targeted alpha therapy in vivo: Direct evidence for single cancer cell kill using 149Tb-rituximab. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 547–554. [Google Scholar] [CrossRef]
- Umbricht, C.A.; Köster, U.; Bernhardt, P.; Gracheva, N.; Johnston, K.; Schibli, R.; van der Meulen, N.P.; Müller, C. Alpha-PET for Prostate Cancer: Preclinical investigation using 149Tb-PSMA-617. Sci. Rep. 2019, 9, 17800. [Google Scholar] [CrossRef]
- Aliev, R.A.; Zagryadskiy, V.A.; Latushkin, S.T.; Moiseeva, A.N.; Novikov, A.N.; Unezhev, V.N.; Kazakov, A.G. Production of a Short-Lived Therapeutic α-Emitter 149Tb by Irradiation of Europium by 63 MeV α-Particles. At. Energy 2021, 129, 337–340. [Google Scholar] [CrossRef]
- Saini, S.; Bartels, J.L.; Appiah, J.K.; Rider, J.H.; Baumhover, N.; Schultz, M.K.; Lapi, S.E. Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets. J. Nucl. Med. 2023, 64, 1791–1797. [Google Scholar] [CrossRef]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Maecke, H.R. PET of Somatostatin Receptor–Positive Tumors Using 64Cu- and 68Ga-Somatostatin Antagonists: The Chelate Makes the Difference. J. Nucl. Med. 2011, 52, 1110. [Google Scholar] [CrossRef]
- Fani, M.; Braun, F.; Waser, B.; Beetschen, K.; Cescato, R.; Erchegyi, J.; Rivier, J.E.; Weber, W.A.; Maecke, H.R.; Reubi, J.C. Unexpected sensitivity of sst2 antagonists to N-Terminal radiometal modifications. J. Nucl. Med. 2012, 53, 1481. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Müller, C. [225Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [225Ac]Ac-PSMA-617. Cancers 2022, 14, 5651. [Google Scholar] [CrossRef] [PubMed]
- King, A.P.; Gutsche, N.T.; Raju, N.; Fayn, S.; Baidoo, K.E.; Bell, M.M.; Olkowski, C.S.; Swenson, R.E.; Lin, F.I.; Sadowski, S.M. 225Ac-Macropatate: A Novel α-Particle Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 549. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted a-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Rathke, H.; Bruchertseifer, F.; Kratochwil, C.; Keller, H.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. First patient exceeding 5-year complete remission after 225Ac-PSMA-TAT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 311–312. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PSMA-617 radioligand therapy. J. Nucl. Med. 2020, 61, 62–69. [Google Scholar] [CrossRef]
- Zimmermann, R. Is Actinium Really Happening? J. Nucl. Med. 2023, 64, 1516–1518. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of 225Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef]
- Engle, J. The Production of Ac-225. Curr. Radiopharm. 2018, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Grimm, T.; Grimm, A.; Peters, W.; Zamiara, M. High-Purity Actinium-225 Production from Radium-226 using a Superconducting Electron Linac. J. Med. Imaging Radiat. Sci. 2019, 50, S12–S13. [Google Scholar] [CrossRef]
- Augusto, R.S.; Smith, J.; Varah, S.; Paley, W.; Egoriti, L.; McEwen, S.; Day Goodacre, T.; Mildenberger, J.; Gottberg, A.; Trudel, A.; et al. Design and radiological study of the 225Ac medical target at the TRIUMF-ARIEL proton-target station. Radiat. Phys. Chem. 2022, 201, 110491. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Lobbezoo, A.; Moskven, L.; Schaffer, P.; Hoehr, C. Design of a thorium metal target for 225Ac production at TRIUMF. Instruments 2019, 3, 18. [Google Scholar] [CrossRef]
- Deblonde, G.J.P.; Zavarin, M.; Kersting, A.B. The coordination properties and ionic radius of actinium: A 120-year-old enigma. Coord. Chem. Rev. 2021, 446, 214130. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Radiolanthanum: Promising theranostic radionuclides for PET, alpha, and Auger-Meitner therapy. Nucl. Med. Biol. 2022, 110–111, 59–66. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. High yield cyclotron production of a novel 133/135La theranostic pair for nuclear medicine. Sci. Rep. 2020, 10, 22203. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Ferguson, S.; Wuest, M.; Wilson, J.; Duke, M.J.M.; Richter, S.; Soenke-Jans, H.; Andersson, J.D.; Juengling, F.; Wuest, F. First in vivo and phantom imaging of cyclotron produced 133La as a theranostic radionuclide for 225Ac and 135La. J. Nucl. Med. 2022, 63, 584–590. [Google Scholar] [CrossRef]
- Brühlmann, S.A.; Kreller, M.; Pietzsch, H.J.; Kopka, K.; Mamat, C.; Walther, M.; Reissig, F. Efficient Production of the PET Radionuclide 133La for Theranostic Purposes in Targeted Alpha Therapy Using the 134Ba(p,2n)133La Reaction. Pharmaceuticals 2022, 15, 1167. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Yuan, Z.; Rodriguez-Rodriguez, C.; Robertson, A.; Radchenko, V.; Perron, R.; Gendron, D.; Causey, P.; Gao, F.; et al. Synthesis and Evaluation of a Macrocyclic Actinium-225 Chelator, Quality Control and In Vivo Evaluation of 225Ac-crown-αMSH Peptide. Chem. Eur. J. 2020, 26, 11435–11440. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 129, 14904–14909. [Google Scholar] [CrossRef]
- Rochman, D.; Koning, A.J.; Sublet, J.C.; Fleming, M.; Bauge, E.; Hilaire, S.; Romain, P.; Morillon, B.; Duarte, H.; Goriely, S.; et al. The TENDL Library: Hope, Reality, and Future. In Proceedings of the International Conference on Nuclear Data for Science and Technology, Bruges, Belgium, 11–16 September 2016; EDP Sciences: Les Ulis, France, 2017. [Google Scholar]
- Ferguson, S.; Jans, H.S.; Wuest, M.; Riauka, T.; Wuest, F. Comparison of scandium-44 g with other PET radionuclides in preclinical PET phantom imaging. EJNMMI Phys. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Levin, C.S.; Hoffman, E.J. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys. Med. Biol. 1999, 44, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Aluicio-Sarduy, E.; Barnhart, T.E.; Weichert, J.; Hernandez, R.; Engle, J.W. Cyclotron-Produced 132La as a PET Imaging Surrogate for Therapeutic 225Ac. J. Nucl. Med. 2021, 62, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Aluicio-Sarduy, E.; Hernandez, R.; Olson, A.P.; Barnhart, T.E.; Cai, W.; Ellison, P.A.; Engle, J.W. Production and in vivo PET/CT imaging of the theranostic pair 132/135La. Sci. Rep. 2019, 9, 10658. [Google Scholar] [CrossRef] [PubMed]
- Aluicio-Sarduy, E.; Thiele, N.A.; Martin, K.E.; Vaughn, B.A.; Devaraj, J.; Olson, A.P.; Barnhart, T.E.; Wilson, J.J.; Boros, E.; Engle, J.W. Establishing Radiolanthanum Chemistry for Targeted Nuclear Medicine Applications. Chem. Eur. J. 2020, 26, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.P.; Clause, H.K.; Fonslet, J.; Nickles, R.J.; Severin, G.W. Half-lives of 132La and 135La. Phys. Rev. C 2018, 97, 034312. [Google Scholar] [CrossRef]
- Lubberink, M.; Lundqvist, H.; Tolmachev, V. Production, PET performance and dosimetric considerations of 134Ce/134La, an Auger electron and positron-emitting generator for radionuclide therapy. Phys. Med. Biol. 2002, 47, 615–629. [Google Scholar] [CrossRef]
- Bailey, T.A.; Mocko, V.; Shield, K.M.; An, D.D.; Akin, A.C.; Birnbaum, E.R.; Brugh, M.; Cooley, J.C.; Engle, J.W.; Fassbender, M.E.; et al. Developing the 134Ce and 134La pair as companion positron emission tomography diagnostic isotopes for 225Ac and 227Th radiotherapeutics. Nat. Chem. 2021, 13, 284–289. [Google Scholar] [CrossRef]
- Bailey, T.A.; Lakes, A.; An, D.; Gauny, S.; Abergel, R.J. Biodistribution Studies of Chelated Ce-134/La-134 as Positron-Emitting Analogues of Alpha-Emitting Therapy Radionuclides. J. Nucl. Med. 2019, 60 (Suppl. S1), 130. Available online: https://jnm.snmjournals.org/content/60/supplement_1/130 (accessed on 29 September 2023).
- Koniar, H.; Rodríguez-Rodríguez, C.; Radchenko, V.; Yang, H.; Kunz, P.; Rahmim, A.; Uribe, C.; Schaffer, P. SPECT imaging of Ac-226 for radiopharmaceutical development: Performance evaluation as a theranostic isotope pair for Ac-225. J. Nucl. Med. 2022, 63 (Suppl. S2), 2341. Available online: http://jnm.snmjournals.org/content/63/supplement_2/2341.abstract (accessed on 29 September 2023).
- Nagatsu, K.; Suzuki, H.; Fukada, M.; Ito, T.; Ichinose, J.; Honda, Y.; Minegishi, K.; Higashi, T.; Zhang, M.R. Cyclotron production of 225Ac from an electroplated 226Ra target. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Möllenbeck, J.; Morgenstern, A. Cyclotron production of Ac-225 for targeted alpha therapy. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63, 1326. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.; Menda, Y.; et al. Automated cassette-based production of high specific activity [203/212Pb]peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical evaluation of 203/212Pb-labeled low-molecular-weight compounds for targeted radiopharmaceutical therapy of prostate cancer. J. Nucl. Med. 2020, 61, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sagastume, E.A.; Lee, D.; McAlister, D.; DeGraffenreid, A.J.; Olewine, K.R.; Graves, S.; Copping, R.; Mirzadeh, S.; Zimmerman, B.E.; et al. 203/212Pb Theranostic Radiopharmaceuticals for Image-guided Radionuclide Therapy for Cancer. Curr. Med. Chem. 2020, 27, 7003–7031. [Google Scholar] [CrossRef]
- Yong, K.; Brechbiel, M. Application of 212Pb for Targeted α-particle Therapy (TAT): Preclinical and Mechanistic Understanding through to Clinical Translation. AIMS Med. Sci. 2015, 2, 228–245. [Google Scholar] [CrossRef]
- McNeil, B.L.; Robertson, A.K.H.; Fu, W.; Yang, H.; Hoehr, C.; Ramogida, C.F.; Schaffer, P. Production, purification, and radiolabeling of the 203Pb/212Pb theranostic pair. EJNMMI Radiopharm. Chem. 2021, 6, 6. [Google Scholar] [CrossRef]
- McNeil, B.L.; Mastroianni, S.A.; McNeil, S.W.; Zeisler, S.; Kumlin, J.; Borjian, S.; McDonagh, A.W.; Cross, M.; Schaffer, P.; Ramogida, C.F. Optimized production, purification, and radiolabeling of the 203Pb/212Pb theranostic pair for nuclear medicine. Sci. Rep. 2023, 13, 10623. [Google Scholar] [CrossRef]
- Li, R.G.; Stenberg, V.Y.; Larsen, R.H. An Experimental Generator for Production of High-Purity 212Pb for Use in Radiopharmaceuticals. J. Nucl. Med. 2023, 64, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Artun, O. The investigation of the production of Ac-227, Ra-228, Th-228, and U-232 in thorium by particle accelerators for use in radioisotope power systems and nuclear batteries. Nucl. Instrum. Methods Phys. Res. B 2022, 512, 12–20. [Google Scholar] [CrossRef]
- Radchenko, V.; Morgenstern, A.; Jalilian, A.R.; Ramogida, C.F.; Cutler, C.; Duchemin, C.; Hoehr, C.; Haddad, F.; Bruchertseifer, F.; Gausemel, H.; et al. Production and supply of α-particle-emitting radionuclides for targeted α-therapy. J. Nucl. Med. 2021, 62, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Horlock, P.L.; Thakur, M.L.; Watson, I.A. Cyclotron produced lead-203. Postgrad. Med. J. 1975, 51, 751–754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nelson, B.J.B.; Wilson, J.; Schultz, M.K.; Andersson, J.D.; Wuest, F. High-yield cyclotron production of 203Pb using a sealed 205Tl solid target. Nucl. Med. Biol. 2023, 116–117, 108314. [Google Scholar] [CrossRef]
- Nelson, B.; Wilson, J.; Andersson, J.; Wuest, F. O-18-High yield lead-203 cyclotron production using a thallium-205 sealed solid target for diagnostic SPECT imaging. Nucl. Med. Biol. 2022, 114–115, S12. [Google Scholar] [CrossRef]
- Máthé, D.; Szigeti, K.; Hegedűs, N.; Horváth, I.; Veres, D.S.; Kovács, B.; Szűcs, Z. Production and in vivo imaging of 203Pb as a surrogate isotope for in vivo 212Pb internal absorbed dose studies. Appl. Radiat. Isot. 2016, 114, 1–6. [Google Scholar] [CrossRef]
- Miao, Y.; Figueroa, S.D.; Fisher, D.R.; Moore, H.A.; Testa, R.F.; Hoffman, T.J.; Quinn, T.P. 203Pb-labeled α-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J. Nucl. Med. 2008, 49, 823–829. [Google Scholar] [CrossRef]
- Jiao, R.; Allen, K.J.H.; Malo, M.E.; Yilmaz, O.; Wilson, J.; Nelson, B.J.B.; Wuest, F.; Dadachova, E. A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin. Cancers 2023, 15, 3856. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef]
- Okoye, N.; Rold, T.; Berendzen, A.; Zhang, X.; White, R.; Schultz, M.; Li, M.; Dresser, T.; Jurisson, S.; Quinn, T.; et al. Targeting the BB2 receptor in prostate cancer using a Pb-203 labeled peptide. J. Nucl. Med. 2017, 58 (Suppl. S1), 321. Available online: http://jnm.snmjournals.org/content/58/supplement_1/321.abstract (accessed on 29 September 2023).
- Miao, Y.; Hylarides, M.; Fisher, D.R.; Shelton, T.; Moore, H.; Wester, D.W.; Fritzberg, A.R.; Winkelmann, C.T.; Hoffman, T.; Quinn, T.P. Melanoma therapy via peptide-targeted {alpha}-radiation. Clin. Cancer Res. 2005, 11, 5616–5621. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Cheuy, L.; Gonzalez, R.; Fisher, D.R.; Miao, Y. Evaluation of a Novel Pb-203-Labeled Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide for Melanoma Targeting. Mol. Pharm. 2019, 16, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, D.; Lee, D.; Kapoor, S.; Gibson-Corley, K.N.; Quinn, T.P.; Sagastume, E.A.; Mott, S.L.; Walsh, S.A.; Acevedo, M.R.; et al. Enhancing the Efficacy of Melanocortin 1 Receptor-Targeted Radiotherapy by Pharmacologically Upregulating the Receptor in Metastatic Melanoma. Mol. Pharm. 2019, 16, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.H.; Malo, M.E.; Jiao, R.; Dadachova, E. Targeting Melanin in Melanoma with Radionuclide Therapy. Int. J. Mol. Sci. 2022, 23, 9520. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M.; et al. Preclinical Evaluation of a Lead Specific Chelator (PSC) Conjugated to Radiopeptides for 203Pb and 212Pb-Based Theranostics. Pharmaceutics 2023, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. NEJM 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Thiele, N.A.; Gutsche, N.T.; Villmer, A.; Zhang, H.; Woods, J.J.; Baidoo, K.E.; Escorcia, F.E.; Wilson, J.J.; Thorek, D.L.J. Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand. Chem. Sci. 2021, 12, 3733–3742. [Google Scholar] [CrossRef]
- Tornes, A.J.K.; Stenberg, V.Y.; Larsen, R.H.; Bruland, Ø.S.; Revheim, M.E.; Juzeniene, A. Targeted alpha therapy with the 224Ra/212Pb-TCMC-TP-3 dual alpha solution in a multicellular tumor spheroid model of osteosarcoma. Front. Med. 2022, 9, 1058863. [Google Scholar] [CrossRef]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Revheim, M.E.; Larsen, R.H. Dual targeting with 224Ra/212Pb-conjugates for targeted alpha therapy of disseminated cancers: A conceptual approach. Front. Med 2023, 9, 1051825. [Google Scholar] [CrossRef]
- Braun, J.; Lemmel, E.M.; Manger, B.; Rau, R.; Sörensen, H.; Sieper, J. Therapie der ankylosierenden Spondylitis (AS) mit Radiumchlorid (224SpondylAT®). Z. Rheumatol. 2001, 60, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Alpha Tau. AlphaDaRT Revolutionary Alpha-Emitters Radiotherapy. Alpha DaRT Technology Brochure. 2022. Available online: https://www.alphatau.com/_files/ugd/74925d_d8c28da928ba46bdab3f0272d356a8d9.pdf (accessed on 20 September 2023).
- Yang, G.Q.; Harrison, L.B. A Hard Target Needs a Sharper DaRT. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Tal, M.; Raab, S.; Efrati, M.; Reitkopf, S.; Lazarov, E.; Etzyoni, R.; Schmidt, M.; Arazi, L.; Kelson, I.; et al. Intratumoral 224Ra-Loaded Wires Spread Alpha-Emitters Inside Solid Human Tumors in Athymic Mice Achieving Tumor Control. Anticancer Res. 2012, 32, 5315–5321. [Google Scholar] [PubMed]
- Reitkopf-Brodutch, S.; Confino, H.; Schmidt, M.; Cooks, T.; Efrati, M.; Arazi, L.; Rath-Wolfson, L.; Marshak, G.; Kelson, I.; Keisari, Y.; et al. Ablation of experimental colon cancer by intratumoral 224Radium-loaded wires is mediated by alpha particles released from atoms which spread in the tumor and can be augmented by chemotherapy. Int. J. Radiat. Biol. 2015, 91, 179–186. [Google Scholar] [CrossRef]
- Keisari, Y.; Popovtzer, A.; Kelson, I. Effective treatment of metastatic cancer by an innovative intratumoral alpha particle-mediated radiotherapy in combination with immunotherapy: A short review. J. Phys. Conf. Ser. 2020, 1662, 012016. [Google Scholar] [CrossRef]
- Confino, H.; Schmidt, M.; Efrati, M.; Hochman, I.; Umansky, V.; Kelson, I.; Keisari, Y. Inhibition of mouse breast adenocarcinoma growth by ablation with intratumoral alpha-irradiation combined with inhibitors of immunosuppression and CpG. Cancer Immunol. Immunother. 2016, 65, 1149–1158. [Google Scholar] [CrossRef]
- Confino, H.; Hochman, I.; Efrati, M.; Schmidt, M.; Umansky, V.; Kelson, I.; Keisari, Y. Tumor ablation by intratumoral Ra-224-loaded wires induces anti-tumor immunity against experimental metastatic tumors. Cancer Immunol. Immunother. 2015, 64, 191–199. [Google Scholar] [CrossRef]
- Feliciani, G.; Bellia, S.R.; Del Duca, M.; Mazzotti, G.; Monti, M.; Stanganelli, I.; Keisari, Y.; Kelson, I.; Popovtzer, A.; Romeo, A.; et al. A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study. Cancers 2022, 14, 240. [Google Scholar] [CrossRef]
- Domankevich, V.; Efrati, M.; Schmidt, M.; Glikson, E.; Mansour, F.; Shai, A.; Cohen, A.; Zilberstein, Y.; Flaisher, E.; Galalae, R.; et al. RIG-1-Like Receptor Activation Synergizes with Intratumoral Alpha Radiation to Induce Pancreatic Tumor Rejection, Triple-Negative Breast Metastases Clearance, and Antitumor Immune Memory in Mice. Front. Oncol. 2020, 10, 990. [Google Scholar] [CrossRef]
- Nishri, Y.; Vatarescu, M.; Luz, I.; Epstein, L.; Dumančić, M.; Del Mare, S.; Shai, A.; Schmidt, M.; Deutsch, L.; Den, R.B.; et al. Diffusing alpha-emitters radiation therapy in combination with temozolomide or bevacizumab in human glioblastoma multiforme xenografts. Front. Oncol. 2022, 12, 888100. [Google Scholar] [CrossRef]
- Bagheri, R.; Afarideh, H.; Ghannadi-Maragheh, M.; Bahrami-Samani, A.; Shirvani-Arani, S. Production of 223Ra from 226Ra in Tehran Research Reactor for treatment of bone metastases. J. Radioanal. Nucl. Chem. 2015, 304, 1185–1191. [Google Scholar] [CrossRef]
- Pruszyński, M.; Walczak, R.; Rodak, M.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Radiochemical separation of 224Ra from 232U and 228Th sources for 224Ra/212Pb/212Bi generator. Appl. Radiat. Isot. 2021, 172, 109655. [Google Scholar] [CrossRef]
- Abou, D.; Thiele, N.; Villmer, A.; Gustche, N.; Escorcia, F.; Wilson, J.; Thorek, D. MACROPA highly stable chelator of Radium-223 and functionalization attempts for targeted treatment of cancer. J. Nucl. Med. 2020, 61 (Suppl. S1), 587. Available online: http://jnm.snmjournals.org/content/61/supplement_1/587.abstract (accessed on 29 September 2023).
- Bauer, D.; Blumberg, M.; Köckerling, M.; Mamat, C. A comparative evaluation of calix[4]arene-1,3-crown-6 as a ligand for selected divalent cations of radiopharmaceutical interest. RSC Adv. 2019, 9, 32357–32366. [Google Scholar] [CrossRef]
- Steinberg, J.; Bauer, D.; Reissig, F.; Köckerling, M.; Pietzsch, H.J.; Mamat, C. Modified Calix[4]crowns as Molecular Receptors for Barium. ChemistryOpen 2018, 7, 432–438. [Google Scholar] [CrossRef]
- Reissig, F.; Bauer, D.; Ullrich, M.; Kreller, M.; Pietzsch, J.; Mamat, C.; Kopka, K.; Pietzsch, H.J.; Walther, M. Recent insights in barium-131 as a diagnostic match for radium-223: Cyclotron production, separation, radiolabeling, and imaging. Pharmaceuticals 2020, 13, 272. [Google Scholar] [CrossRef]
- Kulage, Z.; Cantrell, T.; Griswold, J.; Denton, D.; Garland, M.; Copping, R.; Mirzadeh, S. Nuclear data for reactor production of 131Ba and 133Ba. Appl. Radiat. Isot. 2021, 172, 109645. [Google Scholar] [CrossRef]
- Feng, Y.; Zalutsky, M.R. Production, purification and availability of 211At: Near term steps towards global access. Nucl. Med. Biol. 2021, 100–101, 12–23. [Google Scholar] [CrossRef]
- Nolen, J.; Mustapha, B.; Gott, M.; Washiyama, K.; Sampathkumaran, U.; Winter, R. Development of 211At Production via Continuous Extraction of 211Rn. J. Med. Imaging Radiat. Sci. 2019, 50, S35–S36. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Zhao, X.G.; Alston, K.L.; Bigner, D. High-level production of alpha-particle-emitting (211)At and preparation of (211)At-labeled antibodies for clinical use. J. Nucl. Med. 2001, 42, 1508–1515. [Google Scholar]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211 based radionuclide therapy: Current clinical trial landscape. Front. Med. 2023, 9, 1076210. [Google Scholar] [CrossRef] [PubMed]
- Urhan, M.; Dadparvar, S.; Mavi, A.; Houseni, M.; Chamroonrat, W.; Alavi, A.; Mandel, S.J. Iodine-123 as a diagnostic imaging agent in differentiated thyroid carcinoma: A comparison with iodine-131 post-treatment scanning and serum thyroglobulin measurement. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Ardisson, V.; Lepareur, N. Chapter 76-Labeling Techniques with 123I: Application to Clinical Settings. In Comprehensive Handbook of Iodine; Preedy, V.R., Burrow, G.N., Watson, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 741–756. [Google Scholar] [CrossRef]
- Costa, O.D.; Barcellos, H.; Matsuda, H.; Sumiya, L.D.; Junqueira, F.C.; Matsuda, M.M.N.; Lapolli, A.L. A new 124Xe irradiation system for 123I production. Appl. Radiat. Isot. 2023, 200, 110926. [Google Scholar] [CrossRef]
- Lamparter, D.; Hallmann, B.; Hänscheid, H.; Boschi, F.; Malinconico, M.; Samnick, S. Improved small scale production of iodine-124 for radiolabeling and clinical applications. Appl. Radiat. Isot. 2018, 140, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Cascini, G.L.; Niccoli Asabella, A.; Notaristefano, A.; Restuccia, A.; Ferrari, C.; Rubini, D.; Altini, C.; Rubini, G. 124 iodine: A longer-life positron emitter isotope-new opportunities in molecular imaging. Biomed. Res. Int. 2014, 2014, 672094. [Google Scholar] [CrossRef]
- Khalafi, H.; Nazari, K.; Ghannadi-Maragheh, M. Investigation of efficient 131I production from natural uranium at Tehran research reactor. Ann. Nucl. Energy 2005, 32, 729–740. [Google Scholar] [CrossRef]
- Crawford, J.R.; Kunz, P.; Yang, H.; Schaffer, P.; Ruth, T.J. 211Rn/211At and 209At production with intense mass separated Fr ion beams for preclinical 211At-based α-therapy research. Appl. Radiat. Isot. 2017, 122, 222–228. [Google Scholar] [CrossRef]
- Crawford, J.R.; Robertson, A.K.H.; Yang, H.; Rodríguez-Rodríguez, C.; Esquinas, P.L.; Kunz, P.; Blinder, S.; Sossi, V.; Schaffer, P.; Ruth, T.J. Evaluation of 209At as a theranostic isotope for 209At-radiopharmaceutical development using high-energy SPECT. Phys. Med. Biol. 2018, 63, 045025. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Wickstroem, K.; Hammer, S.; Bjerke, R.M.; Zitzmann-Kolbe, S.; Ryan, O.B.; Karlsson, J.; Scholz, A.; Hennekes, H.; Mumberg, D.; et al. Advances in Precision Oncology: Targeted Thorium-227 Conjugates as a New Modality in Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2020, 35, 497–510. [Google Scholar] [CrossRef]
- Karlsson, J.; Schatz, C.A.; Wengner, A.M.; Hammer, S.; Scholz, A.; Cuthbertson, A.; Wagner, V.; Hennekes, H.; Jardine, V.; Hagemann, U.B. Targeted thorium-227 conjugates as treatment options in oncology. Front. Med. 2023, 9, 1071086. [Google Scholar] [CrossRef]
- Moiseeva, A.N.; Aliev, R.A.; Unezhev, V.N.; Zagryadskiy, V.A.; Latushkin, S.T.; Aksenov, N.V.; Gustova, N.S.; Voronuk, M.G.; Starodub, G.Y.; Ogloblin, A.A. Cross section measurements of 151Eu(3He,5n) reaction: New opportunities for medical alpha emitter 149Tb production. Sci. Rep. 2020, 10, 508. [Google Scholar] [CrossRef]
- Müller, C.; Vermeulen, C.; Köster, U.; Johnston, K.; Türler, A.; Schibli, R.; van der Meulen, N.P. Alpha-PET with terbium-149: Evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm. Chem. 2016, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Fischer, E.; Behe, M.; Köster, U.; Dorrer, H.; Reber, J.; Haller, S.; Cohrs, S.; Blanc, A.; Grünberg, J.; et al. Future prospects for SPECT imaging using the radiolanthanide terbium-155—Production and preclinical evaluation in tumor-bearing mice. Nucl. Med. Biol. 2014, 41, e58–e65. [Google Scholar] [CrossRef]
- Favaretto, C.; Talip, Z.; Borgna, F.; Grundler, P.V.; Dellepiane, G.; Sommerhalder, A.; Zhang, H.; Schibli, R.; Braccini, S.; Müller, C.; et al. Cyclotron production and radiochemical purification of terbium-155 for SPECT imaging. EJNMMI Radiopharm. Chem. 2021, 6, 37. [Google Scholar] [CrossRef]
- Baum, R.P.; Singh, A.; Benešová, M.; Vermeulen, C.; Gnesin, S.; Köster, U.; Johnston, K.; Müller, D.; Senftleben, S.; Kulkarni, H.R.; et al. Clinical evaluation of the radiolanthanide terbium-152: First-in-human PET/CT with 152Tb-DOTATOC. Dalton Trans. 2017, 46, 14638–14646. [Google Scholar] [CrossRef] [PubMed]
- Naskar, N.; Lahiri, S. Theranostic Terbium Radioisotopes: Challenges in Production for Clinical Application. Front. Med. 2021, 8, 675014. [Google Scholar] [CrossRef]
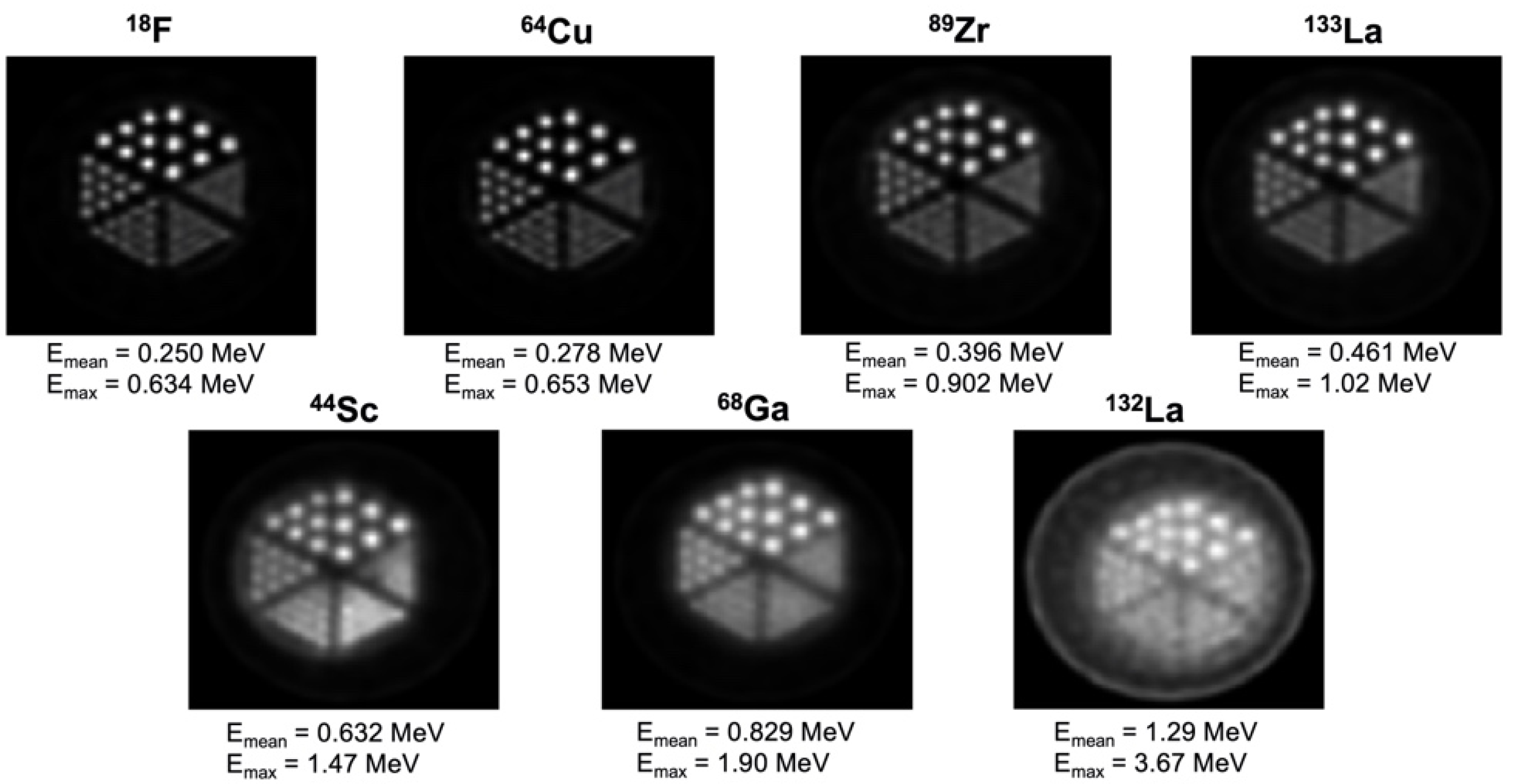
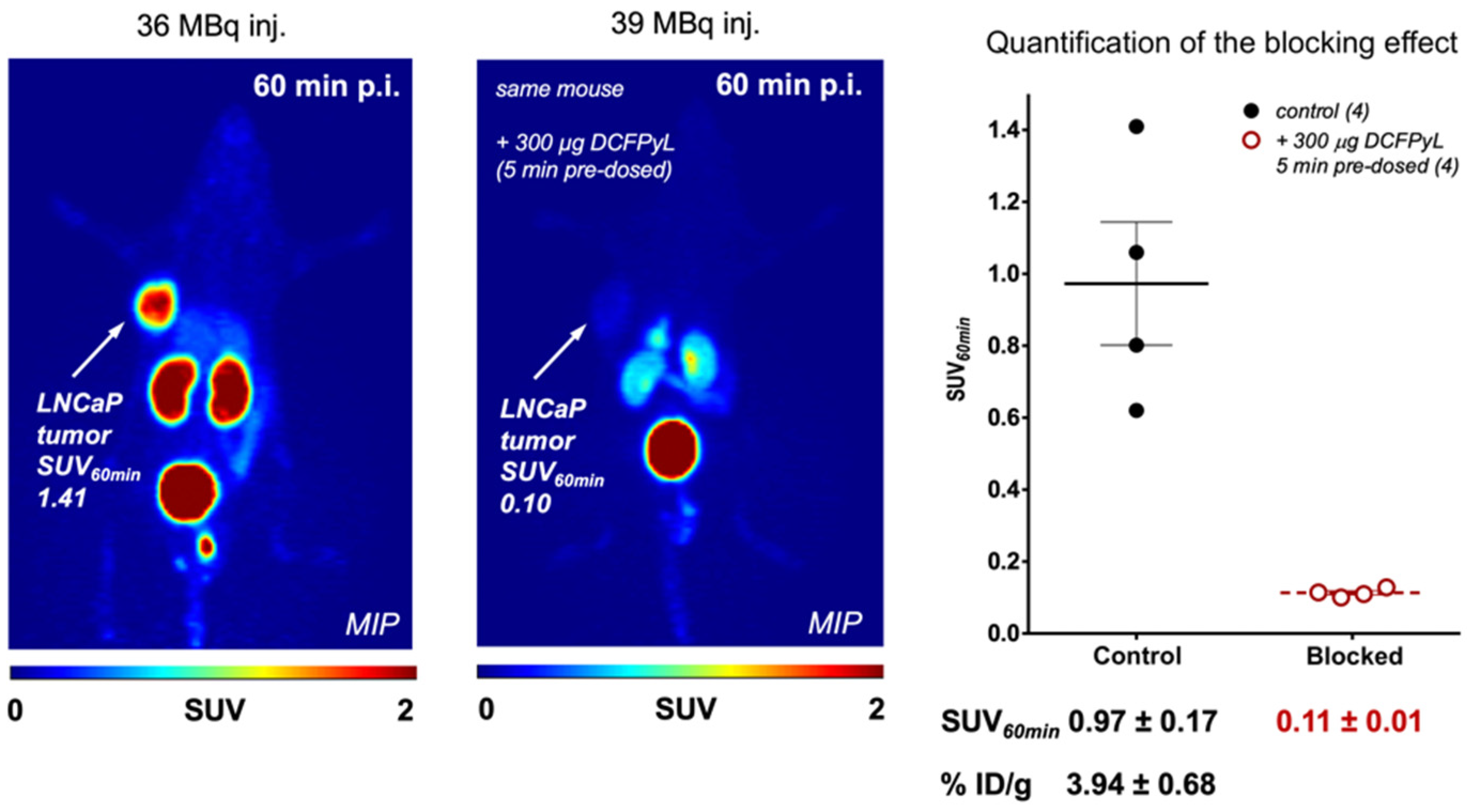

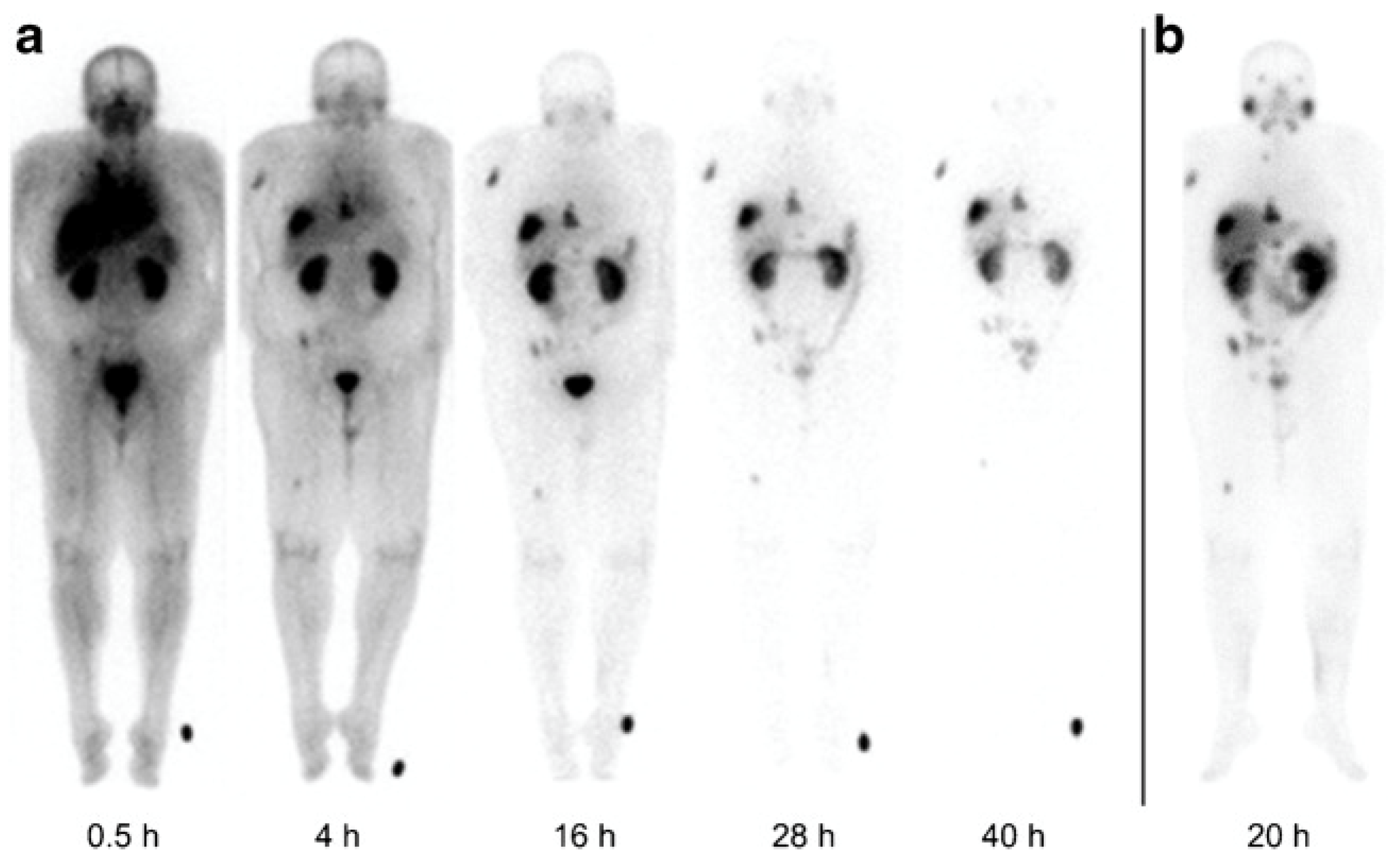

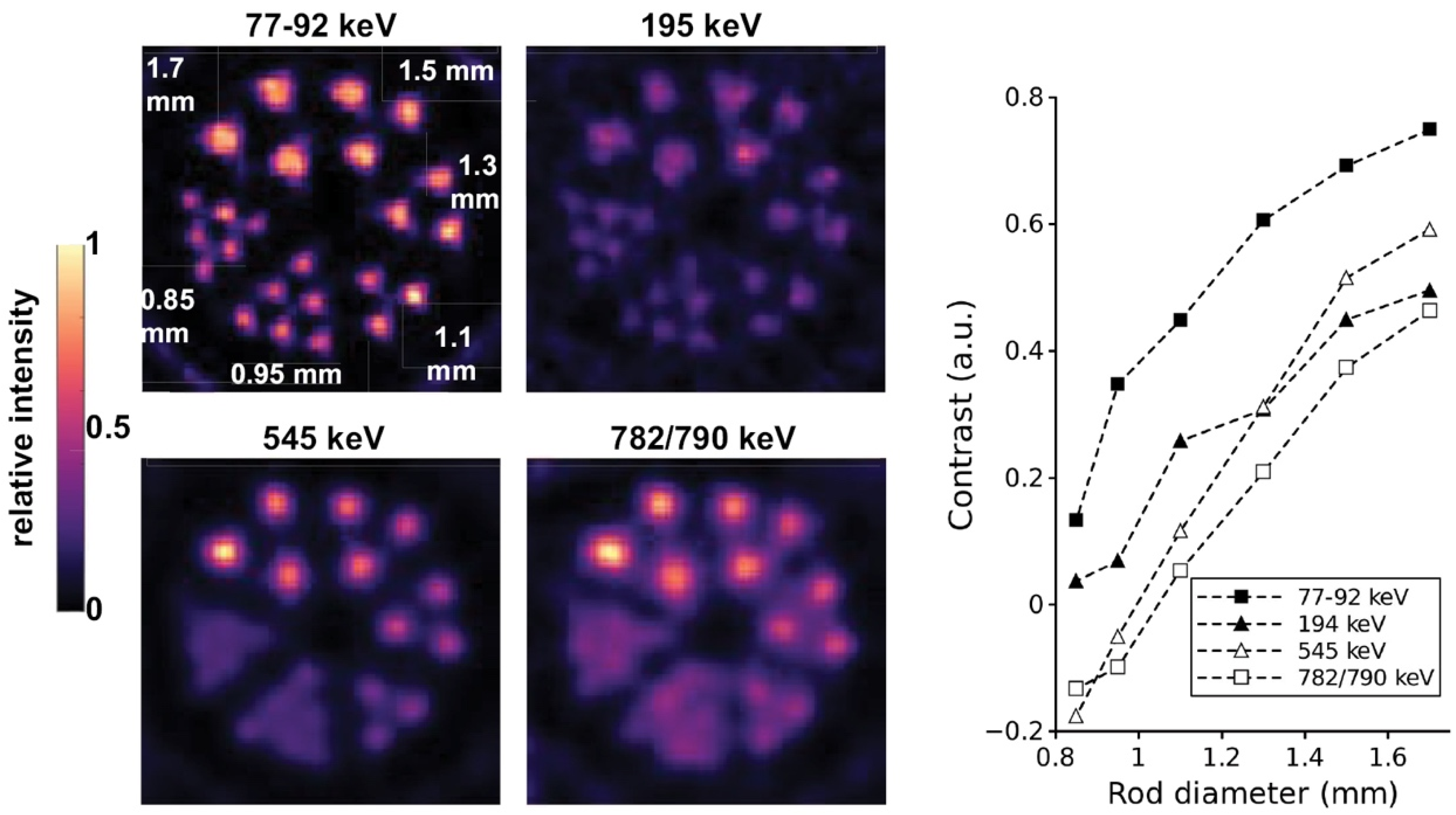
| Alpha Emitter | Proposed Imaging Surrogate | Half-Life | Key Decay Progeny | Key Imaging Emissions | Primary Production Routes | Production Status and References |
|---|---|---|---|---|---|---|
| 225Ac | 9.9 d | 211Fr, 217At, 213Bi, 213Po, 209Tl, 209Pb, 209Bi (stable) | γ: 100 keV (1%), 218 keV (11.4%) | 229Th generator, 226Ra proton/photonuclear reactions, 232Th spallation | Routine production [31,32,33,34] | |
| 133La | 3.9 h | 133Ba | β+: 460 keV (mean), 7.2% | 135Ba or 134Ba proton irradiation | Research [40,41,42] | |
| 132La | 4.8 h | 132Ba (stable) | β+: 1290 keV (mean), 42.1% | 132Ba proton irradiation | Research [48,49,50] | |
| 134Ce/134La | 3.2 d/6.5 min | 134Ba (stable) | β+: 1217 keV (mean), 63.6% | High-energy 139La proton irradiation | Research [52,53,54] | |
| 226Ac | 29.4 h | 226Ra, 226Th, 222Ra, 218Rn, 214Po, 210Pb, 210Bi, 210Po, 206Pb (stable) | γ: 230 keV (26.9%), 158 keV (17.5%) | 226Ra proton irradiation | Research [55] | |
| 212Pb | 10.6 h | 212Bi, 212Po, 208Tl, 208Pb (stable) | γ: 239 keV (44%) | 228Th generator | Routine production [12,63,64,65,66,67] | |
| 203Pb | 51.9 h | 203Tl (stable) | γ: 279 keV (81%) X-ray: 73 keV (37%), 71 keV (22%) | 205Tl proton irradiation, 203Tl proton or deutron irradiation | Routine production [21,63,64,68,69,70,71] | |
| 223Ra | 11.4 d | 219Rn, 215Po, 215At, 211Pb, 211Bi, 211Po, 207Tl, 207Pb (stable) | γ: 269 keV (13%), 154 keV (6%) | 226Ra nuclear reactor irradiation | Routine production [67,96,97] | |
| 224Ra | 3.6 d | 220Rn, 216Po, 212Pb, 212Bi, 212Po, 208Tl, 208Pb (stable) | γ: 241 keV (4%) | 228Th generator | Routine production [67,96,97] | |
| 131Ba | 11.5 d | 131Cs | γ: 496 keV (48%), 124 keV (30%), 216 keV (20%), 371 keV (14%) | 133Cs proton irradiation | Research [101,102] | |
| 211At | 7.2 h | 207Bi, 211Po, 207Pb (stable) | X-ray: 79 keV (21%) | 209Bi alpha particle irradiation | Routine production [8,103,104,105] | |
| 123I | 13.2 h | 123Te (near stable) | γ: 159 keV (83.6%) | 124Xe proton irradiation | Routine production [108,109] | |
| 124I | 4.2 d | 123Te (stable) | β+: 820 keV (mean), 22.7% | 124Te proton or deutron irradiation | Routine production [110,111] | |
| 131I | 8.0 d | 131Xe (stable) | γ: 364 keV (89.6%) | 130Te or uranium nuclear reactor irradiation | Routine production [112] | |
| 209At | 5.4 h | 209Po, 209Bi, 205Bi, 205Pb, 205Tl | γ: 545 keV (91%), 239 keV (12.4%), 195 keV (22.6%) | Proton spallation of uranium carbide | Research [113,114] | |
| 227Th | 18.7 d | 223Ra, 219Rn, 215Po, 215At, 211Pb, 211Bi, 211Po, 207Tl, 207Pb (stable) | γ: 235 keV (12.9%) | 226Ra nuclear reactor irradiation | Routine production [115] | |
| 134Ce/134La | 3.2 d/6.5 min | 134Ba (stable) | β+: 1217 keV (mean), 63.6% | High-energy 139La proton irradiation | Research [52,53,54] | |
| 149Tb | 4.1 h | 149Gd, 149Eu, 149Sm (stable), 145Eu, 145Sm, 145Pm, 145Nd (stable) | β+: 720 keV (mean), 7.1% γ: 165 keV (26.4%) | 151Eu helium-3 bombardment, proton spallation of Ta | Research [19,20,117,118] | |
| 155Tb | 5.3 d | 155Gd (stable) | γ: 87 keV (32%), 105 keV (25%), 180 keV (7.5%), and 262 keV (5%). | 155Gd proton irradiation | Research [119] | |
| 152Tb | 17.5 h | 152Gd (near stable) | β+: 1140 keV (mean), 20.3% | Proton spallation of Ta | Research [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622. https://doi.org/10.3390/ph16111622
Nelson BJB, Wilson J, Andersson JD, Wuest F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals. 2023; 16(11):1622. https://doi.org/10.3390/ph16111622
Chicago/Turabian StyleNelson, Bryce J. B., John Wilson, Jan D. Andersson, and Frank Wuest. 2023. "Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications" Pharmaceuticals 16, no. 11: 1622. https://doi.org/10.3390/ph16111622
APA StyleNelson, B. J. B., Wilson, J., Andersson, J. D., & Wuest, F. (2023). Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals, 16(11), 1622. https://doi.org/10.3390/ph16111622