Neuroprotective Effects of Davallia mariesii Roots and Its Active Constituents on Scopolamine-Induced Memory Impairment in In Vivo and In Vitro Studies
Abstract
1. Introduction
2. Results
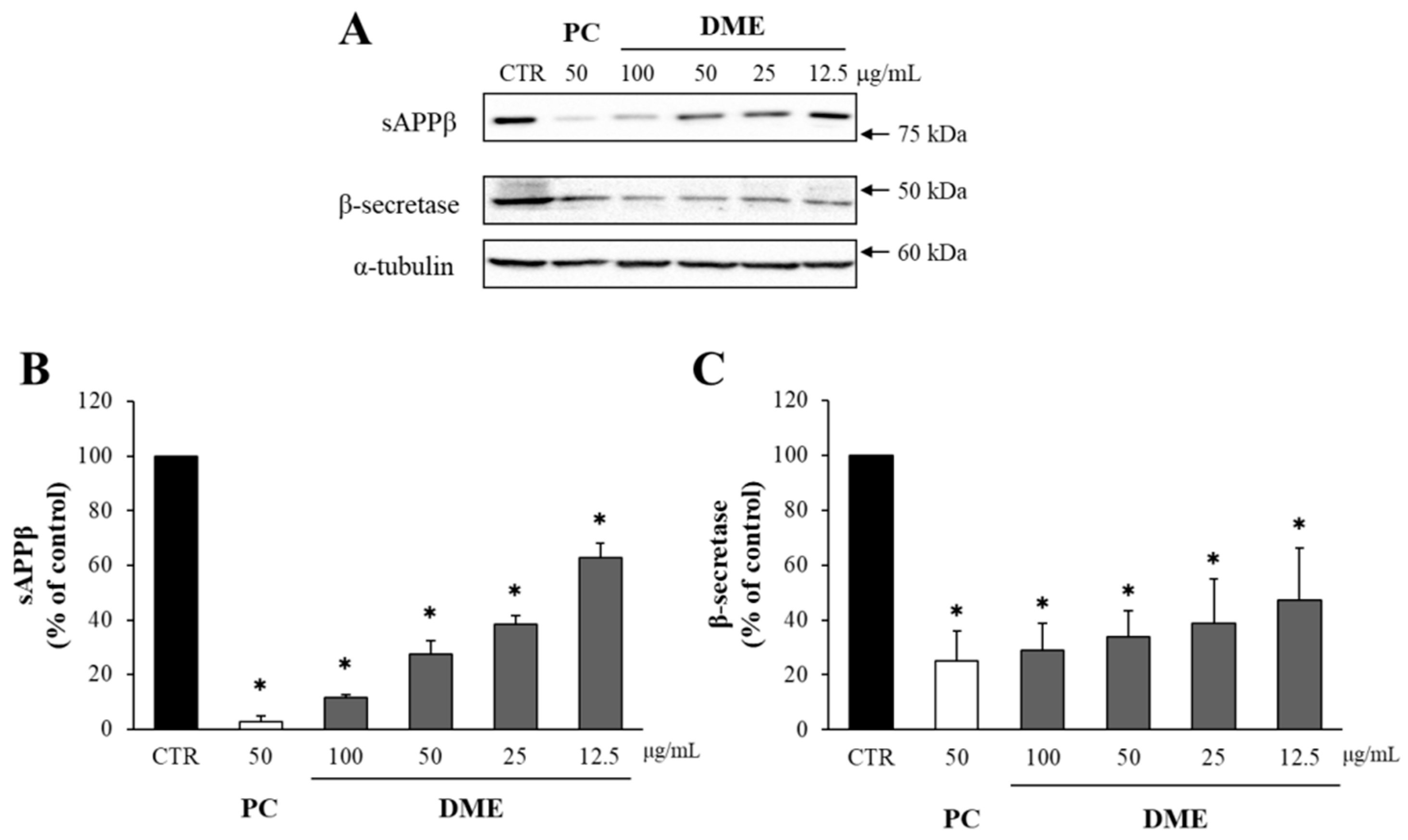
2.1. DME Decreased the Levels of sAPPβ and β-Secretase in APP–CHO Cells
2.2. The Solvent-Partitioned Fractions of DME Decreased the Levels of sAPPβ and β-Secretase
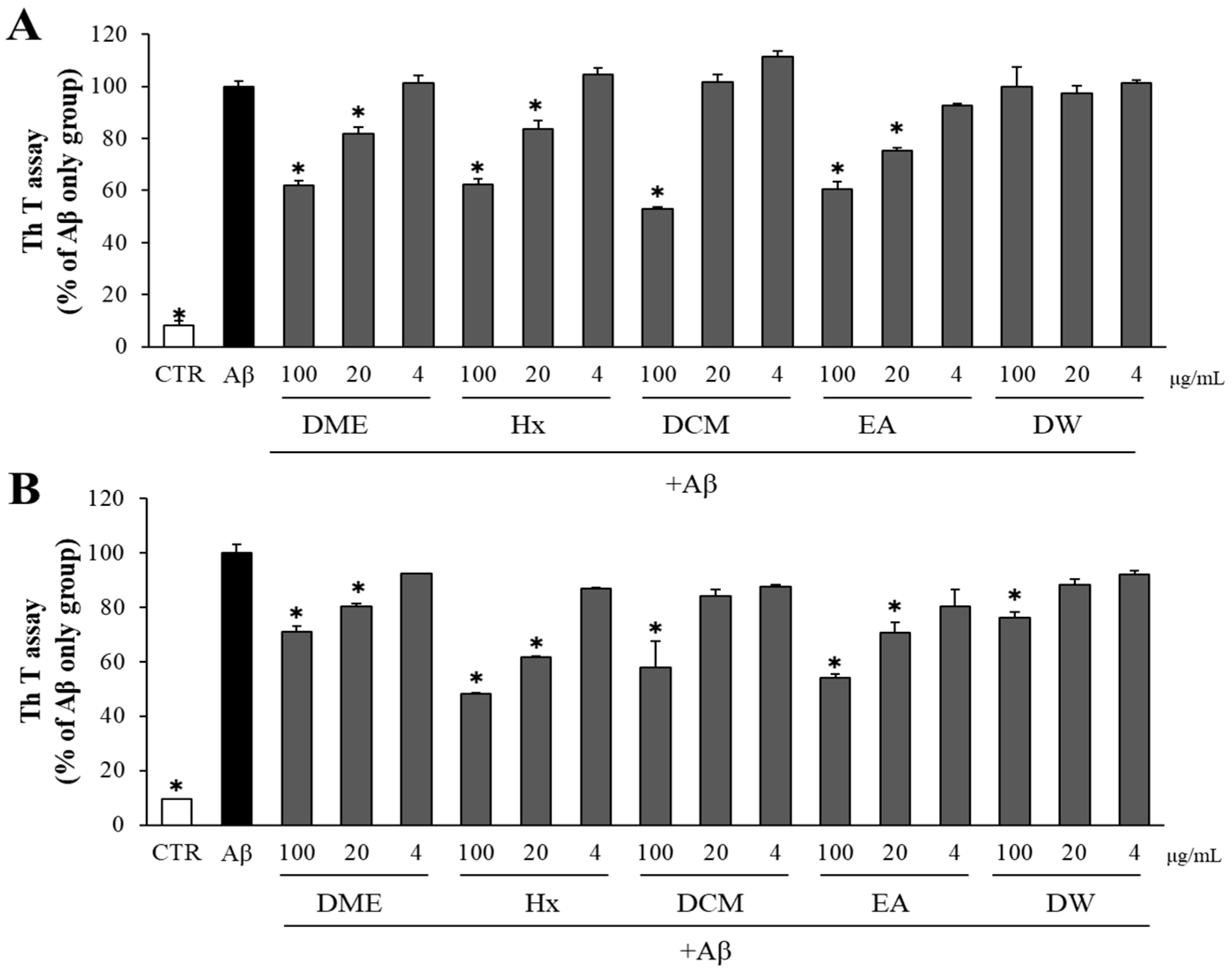
2.3. Both DME and Its Solvent-Partitioned Fractions Decreased Aβ Aggregation and Enhanced the Disaggregation of Pre-Formed Aβ Aggregates
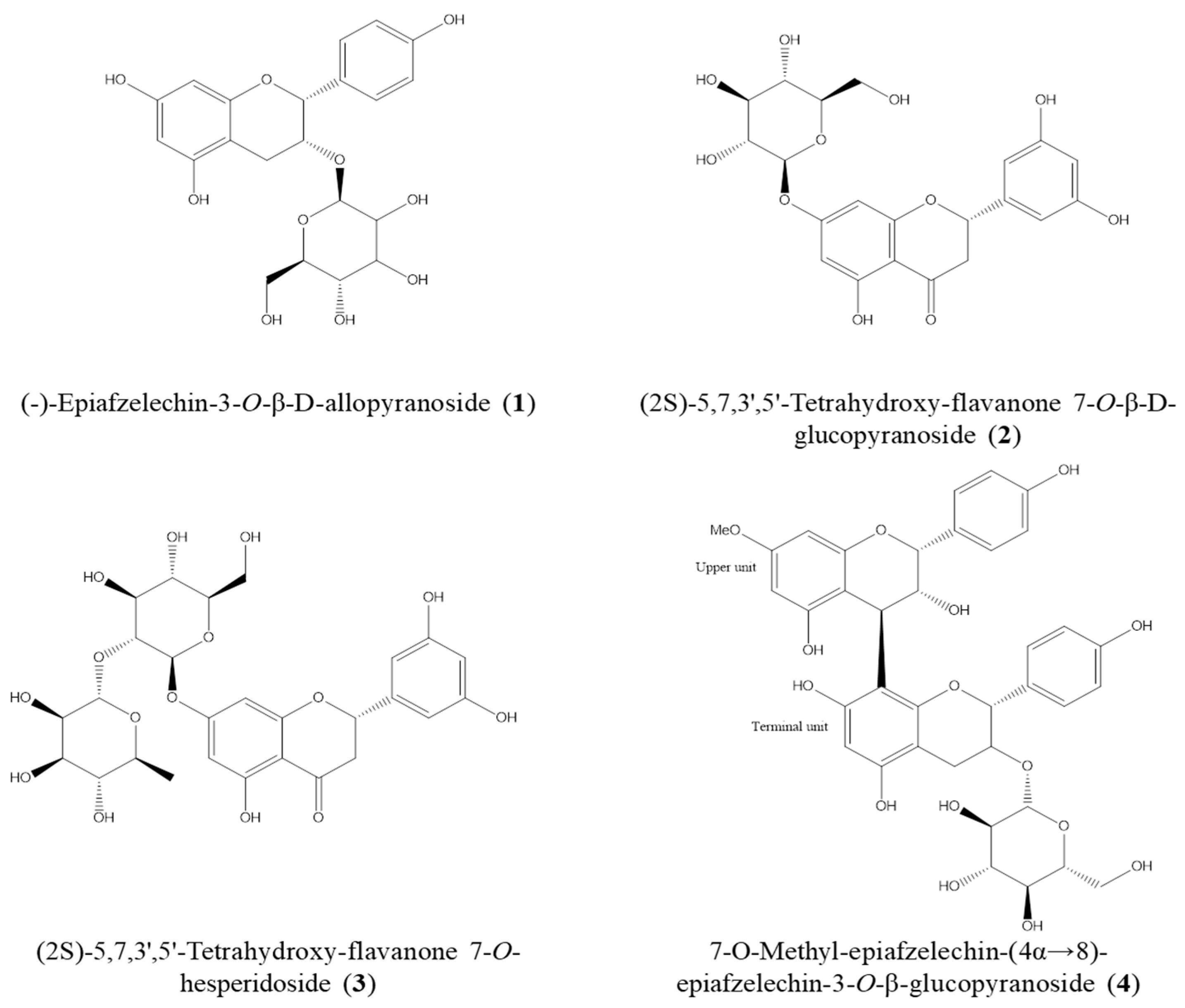
2.4. Isolation of Active Compounds from DME and the Structure Elucidation
2.5. Inhibitory Effects of the Isolated Compounds on sAPPβ and β-Secretase Levels
2.6. Compounds Isolated from DME Decreased Aβ Aggregation and Promoted the Disaggregation of Pre-Formed Aβ Aggregates
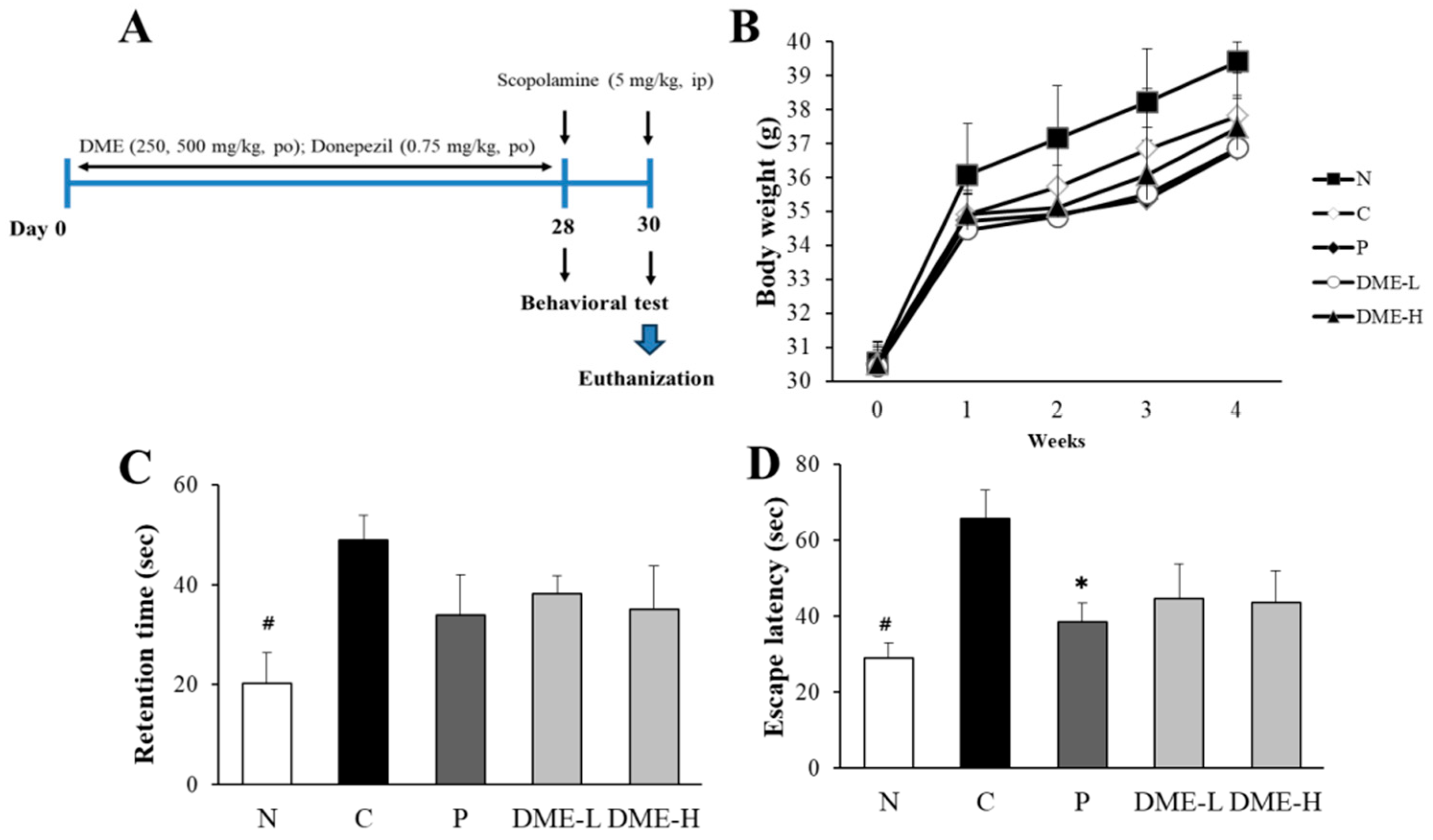
2.7. Evaluation of Changes in Body Weight and Behavioral Tests
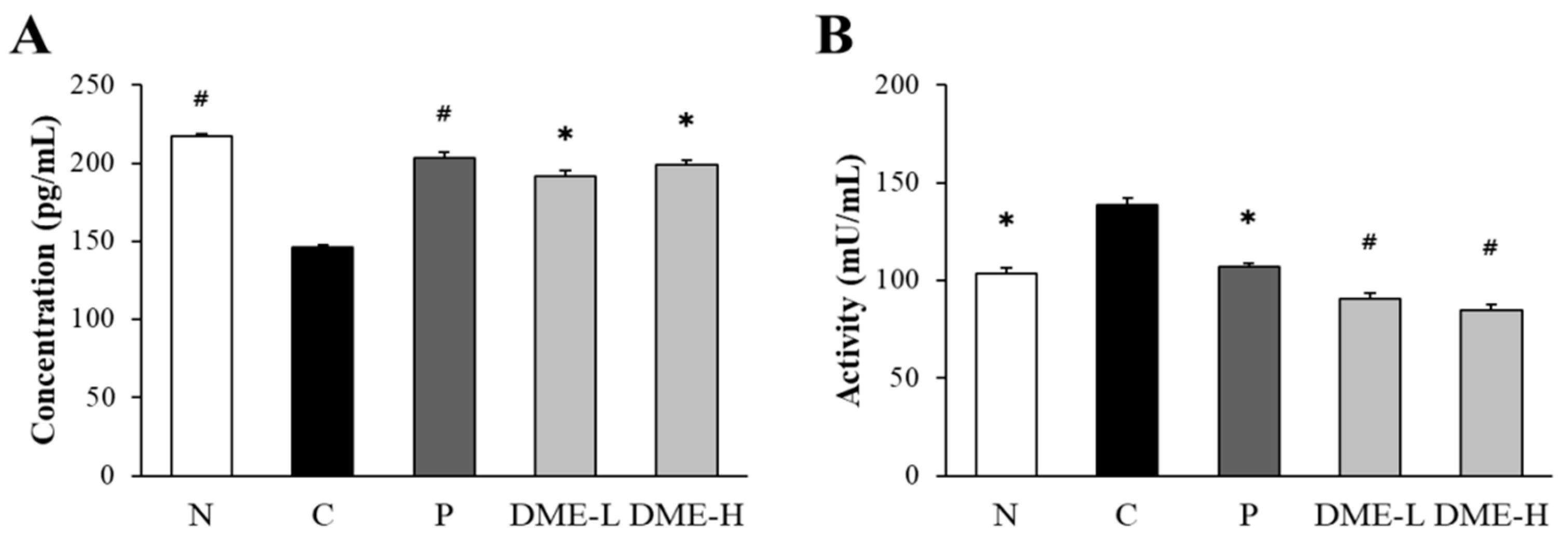
2.8. Inhibitory Effect of DME on the Levels of AChE and ACh in Mice
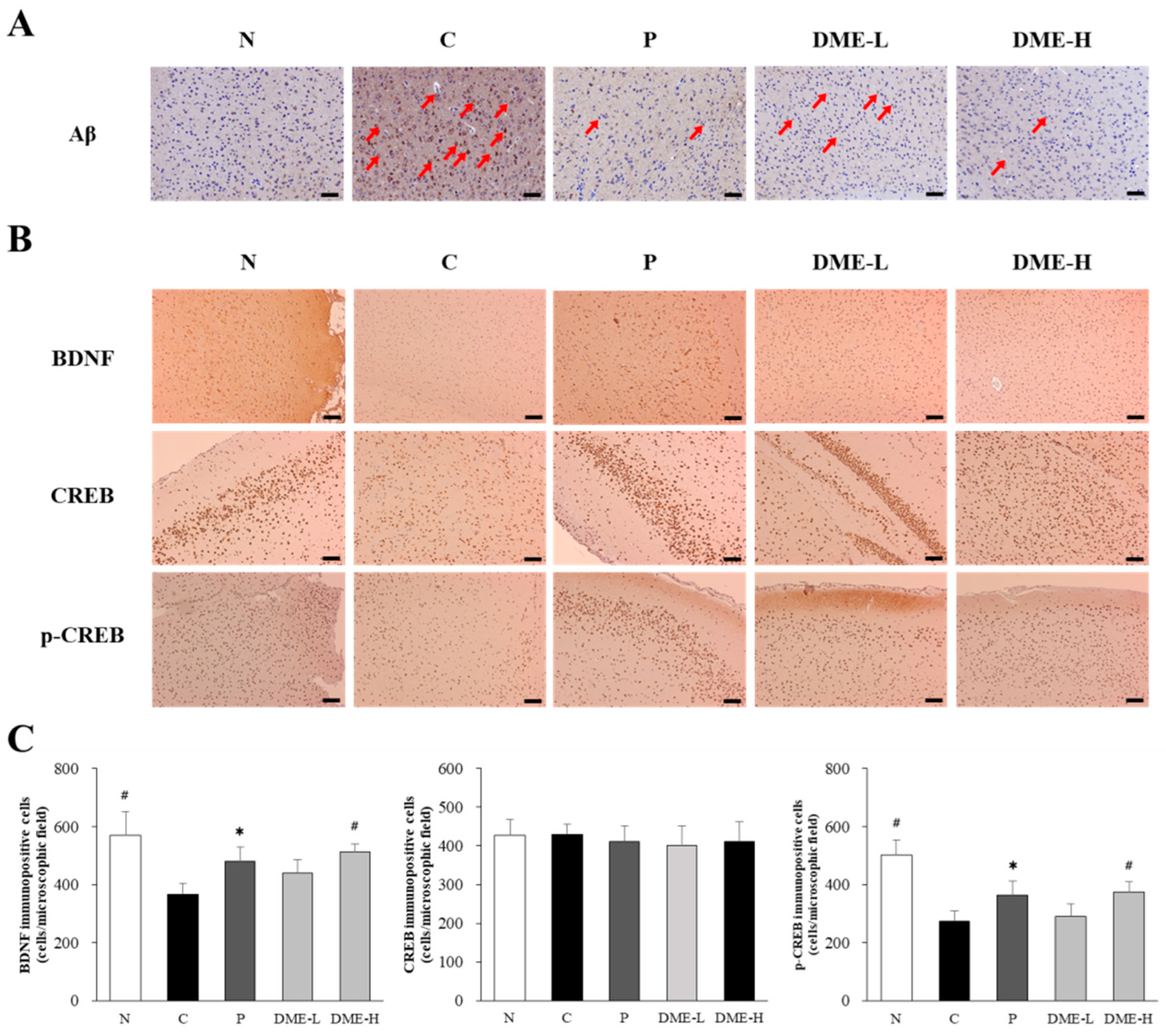
2.9. Effect of DME on Aβ Deposition, BDNF, and CREB Phophorylation
3. Discussion
4. Materials and Methods
4.1. Preparation of D. mariesii Extract (DME)
4.2. Isolation of Active Components
4.3. Cell Culture and MTT Assay
4.4. Western Blot Analysis
4.5. Thioflavin T (Th T) Assay
4.6. Animals and Drug Administration
4.7. Passive Avoidance Test
4.8. Morris Water Maze Test
4.9. Determination of Acetylcholine (ACh) Level and Acetylcholinesterase (AChE) Activity
4.10. Immunohistochemistry (IHC) Staining Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiatr. Psych. Gerichtl. Med. 1907, 64, 146–168. [Google Scholar]
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front. Aging Neurosci. 2022, 14, 937486. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, W.D. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New Approaches for the Treatment of Alzheimer’s Disease. Bioorganic Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef]
- Cummings, J.; Fox, N. Defining Disease Modifying Therapy for Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2017, 4, 109. [Google Scholar] [CrossRef]
- Andrieu, S.; Coley, N.; Lovestone, S.; Aisen, P.S.; Vellas, B. Prevention of Sporadic Alzheimer’s Disease: Lessons Learned from Clinical Trials and Future Directions. Lancet Neurol. 2015, 14, 926–944. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.T.; Hyman, B. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Roher, A.E.; Kokjohn, T.A.; Clarke, S.G.; Sierks, M.R.; Maarouf, C.L.; Serrano, G.E.; Sabbagh, M.S.; Beach, T.G. APP/Aβ structural diversity and Alzheimer’s disease pathogenesis. Neurochem. Int. 2017, 110, 1–13. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Kowalewski, J.M.; Renner, M.; Bousset, L.; Koulakoff, A.; Melki, R.; Giaume, C.; Triller, A. β-amyloid and ATP-induced difusional trapping of astrocyte and neuronal metabotropic glutamate type-5 receptors. Glia 2013, 61, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, S.R.; Lee, H.J. Neurorestorative role of stem cells in Alzheimer’s disease: Astrocyte involvement. Curr. Alzheimer Res. 2016, 13, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. Testing times for the “amyloid cascade hypothesis”. Neurobiol. Aging 2002, 23, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, S.; Tagami, S.; Okoch, M.; Morishima-Kawashima, M. Successive cleavage of β-amyloid precursor protein by γ-secretase. Semin. Cell Dev. Biol. 2020, 105, 64–74. [Google Scholar] [CrossRef]
- Kang, J.; Lemaire, H.G.; Unterbeck, A.; Salbaum, J.M.; Masters, C.L.; Grzeschik, K.H.; Multhaup, G.; Beyreuther, K.; Müller-Hill, B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 1987, 325, 733–736. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Moan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashier, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E.; et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature 1999, 402, 533–537. [Google Scholar] [CrossRef]
- Hussain, I.; Powell, D.; Howlett, D.R.; Tew, D.G.; Meek, T.D.; Chapman, C.; Gloger, I.S.; Murphy, K.E.; Southan, C.D.; Ryan, D.M.; et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell Neurosci. 1999, 14, 419–427. [Google Scholar] [CrossRef]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and regulated α-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Staging of Alzheimer’s Disease-related neurofibrillary changes. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.; Yatin, S.; Akenova, M.; Butterfield, D.A. Alzheimer’s amyloid b-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000, 130, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L. Alzheimer’s disease. Nature 2009, 461, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a Toxin-Induced Experimental Model, Used for Research in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.M.; Arab, H.H.; Rizk, S.M.; El-Maraghy, S.A. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced Alzheimer-like pathological aberrations. Mol. Neurobiol. 2016, 53, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.K.; Ismail, C.A.; Ghareeb, D.A. Differential metformin dose-dependent effects on cognition in rats: Role of Akt. Psychopharmacology 2016, 233, 2513–2524. [Google Scholar] [CrossRef]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef]
- Demirci, K.; Naziroglu, M.; Ovey, I.S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef]
- Balaban, H.; Naziroglu, M.; Demirci, K.; Ovey, I.S. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: The involvement of TRPM2 and TRPV1 channels. Mol. Neurobiol. 2017, 54, 2852–2868. [Google Scholar] [CrossRef]
- Zhang, S.J.; Luo, D.; Li, L.; Tan, R.R.; Xu, Q.Q.; Qin, J.; Zhu, L.; Luo, N.C.; Xu, T.T.; Zhang, R.; et al. Ethyl acetate extract components of Bushen-Yizhi formula provides neuroprotection against scopolamine-induced cognitive impairment. Sci. Rep. 2017, 7, 9824. [Google Scholar] [CrossRef]
- Arce-Varas, N.; Abate, G.; Prandelli, C.; Martínez, C.; Cuetos, F.; Menéndez, M.; Marziano, M.; Cabrera-García, D.; Fernández-Sánchez, M.T.; Novelli, A.; et al. Comparison of Extracellular and Intracellular Blood Compartments Highlights Redox Alterations in Alzheimer’s and Mild Cognitive Impairment Patients. Curr. Alzheimer Res. 2017, 14, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life. Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Hwang, K.W.; Joo, H.-B.; Park, S.-Y. Anti-amyloidogenic properties of Dryopteris crassirhizoma roots in Alzheimer’s Disease cellular model. J. Food Biochem. 2015, 39, 478–484. [Google Scholar] [CrossRef]
- Yang, S.G.; Choi, G.Y.; Song, J.-H. Morphology, Anatomy, Micromorphology, and Palynology of the Squirrel’s Foot Fern, Davallia mariesii (Davalliaceae). Horticulturae 2023, 9, 939. [Google Scholar] [CrossRef]
- Lin, Y.T.; Peng, S.W.; Imtiyaz, Z.; Ho, C.W.; Chiou, W.F.; Lee, M.H. In vivo and in vitro evaluation of the osteogenic potential of Davallia mariesii T. Moore ex Baker. J. Ethnopharmacol. 2021, 264, 113–126. [Google Scholar] [CrossRef]
- Shin, S.L.; Lee, C.H. Antimicrobial Activities of Methanol Extracts Obtained from Several Ferns. Korean J. Plant Resour. 2010, 23, 436–444. [Google Scholar]
- Wu, C.R.; Chang, H.C.; Cheng, Y.D.; Lan, W.C. Aqueous extract of Davallia mariesii attenuates 6-hydroxydopamine-induced oxidative damage and apoptosis in B35 cells through inhibition of caspase cascade and activation of PI3K/AKT/GSK-3β pathway. Nutrients 2018, 10, 1449. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, G.J.; Agrawal, D.C.; Kuo, C.L.; Wu, C.R.; Tsay, H.S. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu”. Biochemistry 2007, 48, 397–406. [Google Scholar]
- Do, H.J.; Oh, T.W.; Yang, J.H.; Park, K.I.; Ma, J.Y. Davallia mariesii moore improves FcεRI-mediated allergic responses in the rat basophilic leukemia mast cell line RBL-2H3 and passive cutaneous anaphylaxis in mice analgesic. Mediat. Inflamm. 2017, 2017, 8701650. [Google Scholar] [CrossRef]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii moore. I. isolation and structures of davallialactone and a new flavanone glucuronide. Chem. Pharm. Bull. 1990, 38, 3218–3225. [Google Scholar] [CrossRef]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii moore. II. Identification and 1H- and 13C-nuclear magnetic resonance spectra of procyanidin B-5, epicatechin-(4β→8)-epicatechin-(4β→6)-epicatechin, and epicatechin-(4β→6)-epicatechin-(4β→8)-epicatechin-(4β→6)-epicatechin. Chem. Pharm. Bull. 1992, 40, 889–898. [Google Scholar]
- Cui, C.B.; Tezuka, Y.; Yamashita, H.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii moore. III. Revised structure and absolute configuration of davallialactone. Chem. Pharm. Bull. 1992, 40, 1711–1717. [Google Scholar] [CrossRef]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii moore. IV. Isolation and structures of a novel norcarotane sesquiterpene glycoside, a chromone glucuronide, and two epicatechin glycosides. Chem. Pharm. Bull. 1992, 40, 2035–2040. [Google Scholar] [CrossRef]
- Cui, C.B.; Tezuka, Y.; Yamashita, H.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a fern, Davallia mariesii moore. V. Isolation and structures of davallin, a new tetrameric proanthocyanidin, and two new phenolic glycosides. Chem. Pharm. Bull. 1993, 41, 1491–1497. [Google Scholar] [CrossRef][Green Version]
- Pfundstein, G.; Nikoeneko, A.G.; Sytnyk, V. Amyloid precursor protein (APP) and amyloid β (Aβ) interact with cell adhesion molecules: Implications in Alzheimer’s disease and normal physiology. Front. Cell Dev. Biol. 2022, 10, 969547. [Google Scholar] [CrossRef]
- Chang, E.J.; Lee, W.J.; Cho, S.H.; Choi, S.W. Proliferative Effects of Flavan-3-ols and Propelargonidins from Rhizomes of Drynaria fortunei on MCF-7 and Osteoblastic Cells. Arch. Pharm. Res. 2003, 26, 620–630. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, N.L.; Zhang, Y.; Gao, H.; Pang, W.Y.; Wong, M.S.; Zhang, G.; Qin, L.; Yao, X.S. Effects of eleven flavonoids from the osteoprotective fraction of Drynaria fortunei (KUNZE) J. SM. on osteoblastic proliferation using an osteoblast-like cell line. Chem. Pharm. Bull. 2008, 56, 46–51. [Google Scholar] [CrossRef]
- Sun, J.M.; Yang, J.S.; Zhang, H. Two New Flavanone Glycosides of Jasminum lanceolarium and Their Anti-oxidant Activities. Chem. Pharm. Bull. 2007, 55, 474–476. [Google Scholar] [CrossRef]
- Liang, Y.H.; Ye, M.; Yang, W.Z.; Qiao, X.; Wang, Q.; Yang, H.J.; Wang, X.I.; Guo, D.A. Flavan-3-ols from the rhizomes of Drynaria fortune. Chem. Pharm. Bull. 2011, 72, 1876–1882. [Google Scholar]
- Yoon, H.; Eom, S.L.; Hyun, J.Y.; Jo, G.h.; Hwang, D.S.; Lee, S.H.; Yong, Y.J.; Park, J.C.; Lee, Y.H.; Lim, Y.H. 1H and 13C NMR data on hydroxy/methoxy flavonoids and the effects of substituents on chemical shifts. Bull. Korean Chem. Soc. 2011, 2011, 2101–2104. [Google Scholar] [CrossRef][Green Version]
- Wilquet, V.; Strooper, B.D. Amyloid-beta precursor protein processing in neurodegeneration. Curr. Opin. Neurobiol. 2004, 14, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chem, W.D.; Wang, Y.D. β-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Rajamani, S.; Kaylor, J.; Han, S.; Zhou, F.; Fink, A.L. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004, 279, 26846–26857. [Google Scholar] [CrossRef] [PubMed]
- Velander, P.; Wu, L.; Henderson, F.; Zhang, S.; Bevan, D.R.; Xu, B. Natural product-based amyloid inhibitors. Biochem. Pharmacol. 2017, 139, 40–55. [Google Scholar] [CrossRef]
- Lee, Y.K.; Yuk, D.Y.; Lee, J.W.; Lee, S.Y.; Ha, T.Y.; Oh, K.W.; Yun, Y.P.; Hong, J.T. (−)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res. 2009, 1250, 164–174. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef]
- Minocha, T.; Birla, H.; Obaid, A.A.; Rai, V.; Sushma, P.; Shivamallu, C.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Tikhonova, M.A.; et al. Flavonoids as Promising Neuroprotectants and Their Therapeutic Potential against Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 6038996. [Google Scholar] [CrossRef]
- Ayza, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 115. [Google Scholar] [CrossRef]
- Tamagno, E.; Bardini, P.; Obbili, A.; Vitali, A.; Borghi, R.; Zaccheo, D.; Pronzato, M.A.; Danni, O.; Smith, M.A.; Perry, G.; et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 2002, 10, 279–288. [Google Scholar] [CrossRef]
- Fukumoto, H.; Cheung, B.S.; Hyman, B.T.; Irizarry, M.C. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002, 59, 1381–1389. [Google Scholar] [CrossRef]
- Katalinić, M.; Rusak, G.; Barović, J.D.; Sinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef]
- Gargari, S.A.; Barzegar, A.; Tarinejad, A. The role of phenolic OH groups of flavonoid compounds with H-bond formation ability to suppress amyloid mature fibrils by destabilizing β-sheet conformation of monomeric Aβ17-42. PLoS ONE 2018, 13, e0199541. [Google Scholar]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef]
- Barnes, C.A.; Danysz, W.; Parsons, C.G. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation spatial memory in awake, freely moving rats. Eur. J. Neurosci. 1996, 8, 565–571. [Google Scholar] [CrossRef]
- Blokland, A. Scopolamine-induced deficits in cognitive performance: A review of animal studies. Scopolamine Rev. 2005, 1, 1–76. [Google Scholar]
- Sakaguchi, M.; Koseki, M.; Wakamatsu, M.; Matsumura, E. Effects of beta-casomorphin-5 on passive avoidance response in mice. Biosci. Biotechnol. Biochem. 2003, 67, 2501–2504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.G.; Boccia, M.M.; Krawczyk, M.C.; Delorenzi, A.; Baratti, C.M. Choline reverses scopolamine-induced memory impairment by improving memory reconsolidation. Neurobiol. Learn. Mem. 2012, 98, 112–121. [Google Scholar] [CrossRef]
- Bejar, C.; Wang, R.H.; Weinstock, M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur. J. Pharmacol. 1999, 383, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Huang, F.I.; Yang, C.R. Moscatilin Ameliorates Tau Phosphorylation and Cognitive Deficits in Alzheimer’s Disease Models. J. Nat. Prod. 2019, 82, 1979–1988. [Google Scholar] [CrossRef]
- Lee, S.B.; Yang, S.Y.; Thao, N.P.; Seo, D.G.; Kim, S.G.; Ma, C.T.; Park, S.-Y.; Kim, Y.H.; Yang, H.O. Protective Effects of Compounds from Cimicifuga dahurica against Amyloid Beta Production in Vitro and Scopolamine-Induced Memory Impairment in Vivo. J. Nat. Prod. 2020, 83, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef] [PubMed]
- García-Ayllón, M.S.; Riba-Llena, I.; Serra-Basante, C.; Alom, J.; Boopathy, R.; Sáez-Valero, J. Altered levels of acetylcholinesterase in Alzheimer plasma. PLoS ONE 2010, 5, e8701. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Mizui, T.; Ishikawa, Y.; Kumanogoh, H.; Lume, M.; Matsumoto, T.; Hara, T.; Yamawaki, S.; Takahashi, M.; Shiosaka, S.; Itami, C.; et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc. Natl. Acad. Sci. USA 2015, 112, 3067–3074. [Google Scholar] [CrossRef]
- Connor, B.; Young, D.; Yan, Q.; Faull, R.L.; Synek, B.; Dragunow, M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Mol. Brain Res. 1997, 49, 71–81. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegenr. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Son, H.J.; Lim, D.W.; Yoon, M.S.; Lee, J.K.; Kim, Y.T.; Han, D.S.; Lee, C.H.; Um, M.Y. Memory-enhancing effects of Ishige foliacea extract: In vitro and in vivo study. J. Food. Biochem. 2020, 44, e13162. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.H.; Ko, M.S.; Kim, Y.S.; Ham, J.E.; Choi, J.Y.; Hwang, K.W.; Park, S.-Y. Neuroprotective Effects of Davallia mariesii Roots and Its Active Constituents on Scopolamine-Induced Memory Impairment in In Vivo and In Vitro Studies. Pharmaceuticals 2023, 16, 1606. https://doi.org/10.3390/ph16111606
Lee CH, Ko MS, Kim YS, Ham JE, Choi JY, Hwang KW, Park S-Y. Neuroprotective Effects of Davallia mariesii Roots and Its Active Constituents on Scopolamine-Induced Memory Impairment in In Vivo and In Vitro Studies. Pharmaceuticals. 2023; 16(11):1606. https://doi.org/10.3390/ph16111606
Chicago/Turabian StyleLee, Chung Hyeon, Min Sung Ko, Ye Seul Kim, Ju Eon Ham, Jee Yeon Choi, Kwang Woo Hwang, and So-Young Park. 2023. "Neuroprotective Effects of Davallia mariesii Roots and Its Active Constituents on Scopolamine-Induced Memory Impairment in In Vivo and In Vitro Studies" Pharmaceuticals 16, no. 11: 1606. https://doi.org/10.3390/ph16111606
APA StyleLee, C. H., Ko, M. S., Kim, Y. S., Ham, J. E., Choi, J. Y., Hwang, K. W., & Park, S.-Y. (2023). Neuroprotective Effects of Davallia mariesii Roots and Its Active Constituents on Scopolamine-Induced Memory Impairment in In Vivo and In Vitro Studies. Pharmaceuticals, 16(11), 1606. https://doi.org/10.3390/ph16111606



