Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review
Abstract
:1. Introduction
1.1. Plukenetia volubilis Linneo
1.2. Cardiovascular Diseases
1.3. The Role of Oxidative Stress and Inflammation in CVD
1.4. The Potential of SI for Cardiovascular Health
2. Literature Search
3. Effects of SI on CVD Risk Factors
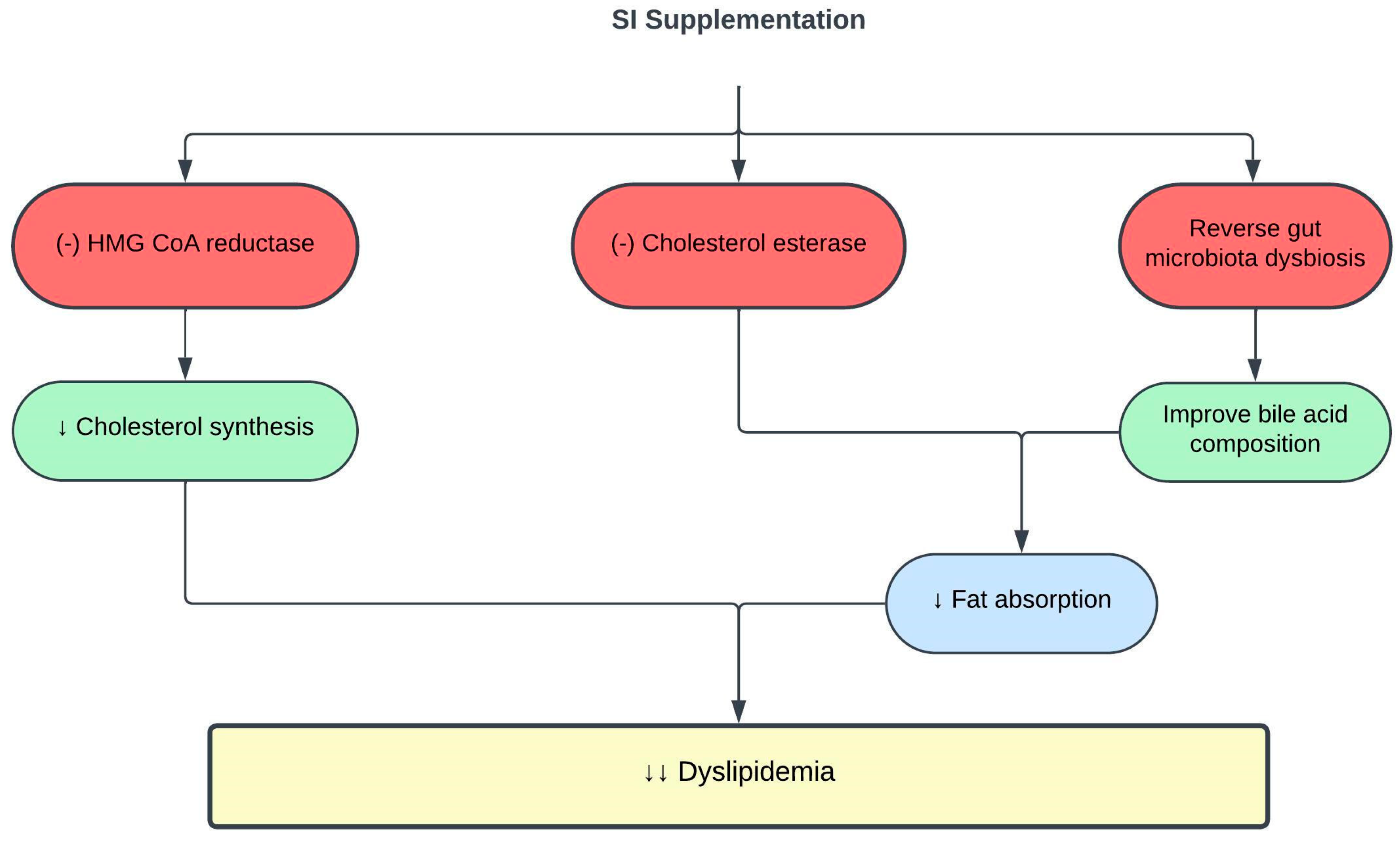
3.1. Effects of SI on Dyslipidemia
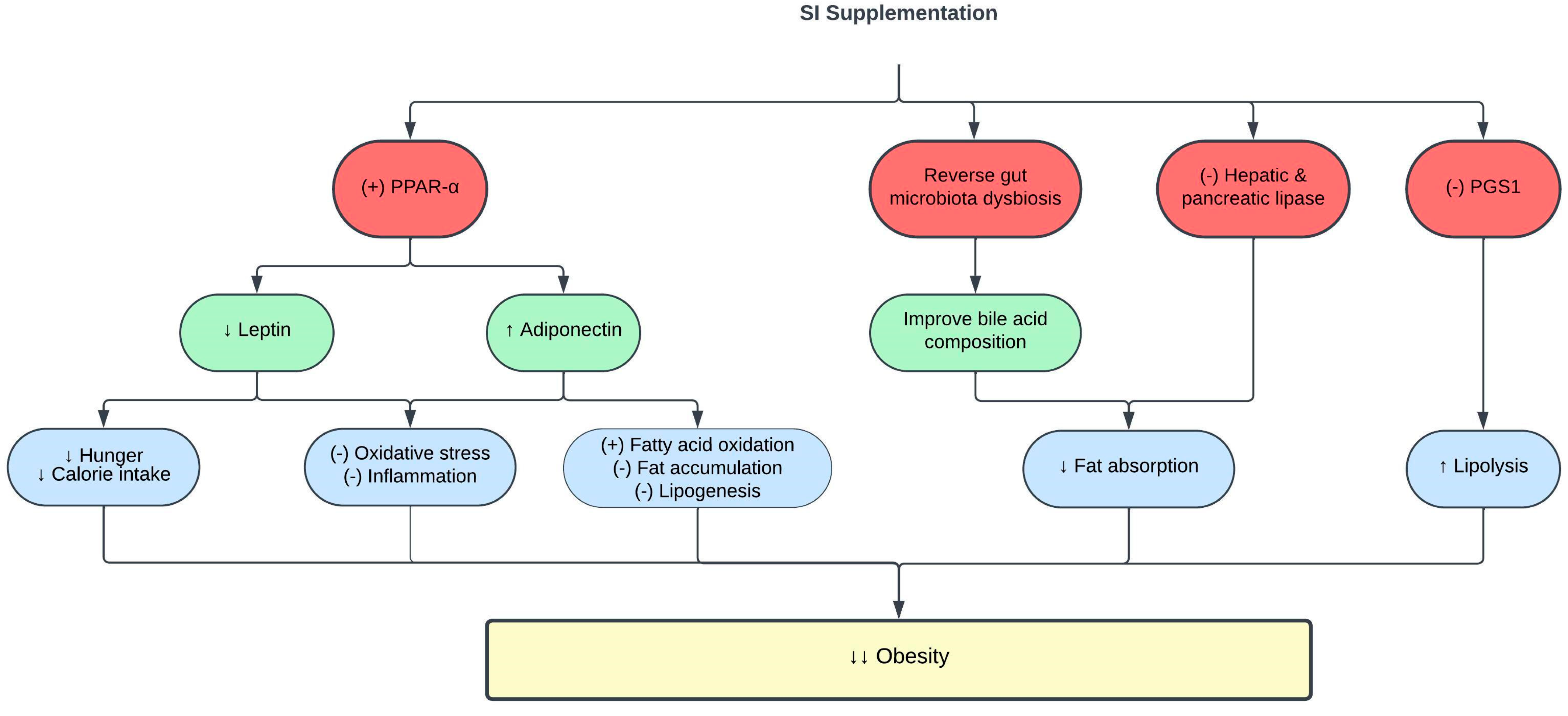
3.2. Effects of SI on Obesity
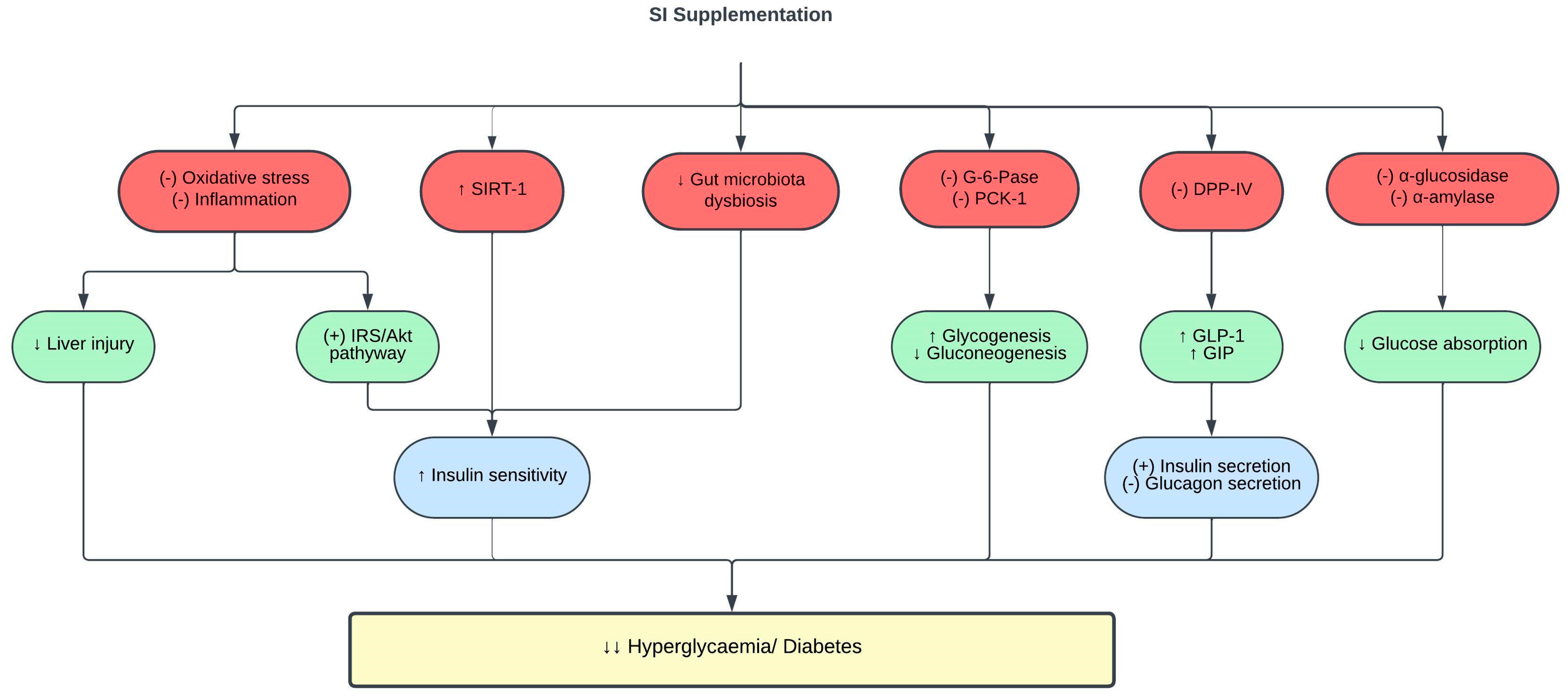
3.3. Effects of SI on Glucose Metabolism and Diabetes
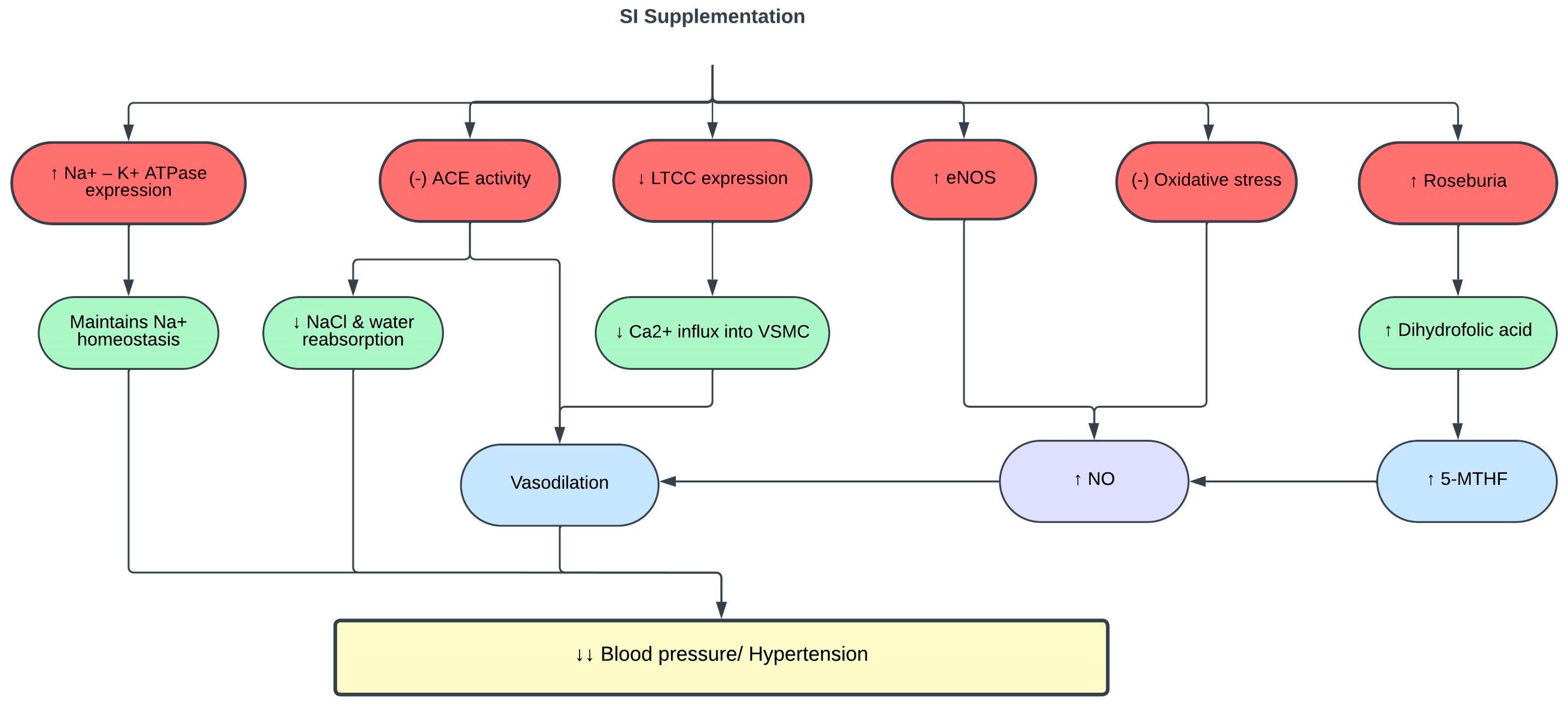
3.4. Effects of SI on Blood Pressure
4. Safety of SI
5. Strength, Limitations, and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- del-Castillo, Á.M.R.; Gonzalez-Aspajo, G.; de Fátima Sánchez-Márquez, M.; Kodahl, N. Ethnobotanical Knowledge in the Peruvian Amazon of the Neglected and Underutilized Crop Sacha Inchi (Plukenetia volubilis L.). Econ. Bot. 2019, 73, 281–287. [Google Scholar] [CrossRef]
- Silalahi, M. Sacha Inchi (Plukenetia volubilis L.): Its Potential as Foodstuff and Traditional Medicine. GSC Biol. Pharm. Sci. 2022, 18, 213–218. [Google Scholar] [CrossRef]
- Flores, S.; Flores, A.; Calderón, C.; Obregón, D. Synthesis and Characterization of Sacha Inchi (Plukenetia volubilis L.) Oil-Based Alkyd Resin. Prog. Org. Coat. 2019, 136, 105289. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Quiñones-Segura, Y.; Sanchez-Reinoso, Z.; Díaz, D.L.; Abril, J.I. Physicochemical Properties of Oils Extracted from γ-Irradiated Sacha Inchi (Plukenetia volubilis L.) Seeds. Food Chem. 2017, 237, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Dugo, L.; Cacciola, F.; Beccaria, M.; Grasso, S.; Dachà, M.; Dugo, P.; Mondello, L. Chemical Characterization of Sacha Inchi (Plukenetia volubilis L.) Oil. J. Agric. Food Chem. 2011, 59, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, H.P.; Schmitz-Hübsch, M. Sacha Inchi (Plukenetia volubilis L.) Nut Oil and Its Therapeutic and Nutritional Uses. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 991–994. ISBN 9780123756886. [Google Scholar]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha Inchi (Plukenetia volubilis L.): Nutritional Composition, Biological Activity, and Uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Tanwar, B.; Kumar Sihag, M.; Sharma, V. Sacha Inchi (Plukenetia volubilis L.): An Emerging Source of Nutrients, Omega-3 Fatty Acid and Phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Ramos Escudero, M.; Viñas-Ospino, A.; Morales, M.T.; Asuero, A.G. Characterization of Commercial Sacha Inchi Oil According to Its Composition: Tocopherols, Fatty Acids, Sterols, Triterpene and Aliphatic Alcohols. J. Food Sci. Technol. 2019, 56, 4503–4515. [Google Scholar] [CrossRef]
- Mai, H.C.; Nguyen, D.C.; Thuong Nhan, N.P.; Bach, L.G. Physico-Chemical Properties of Sacha Inchi (Plukenetia volubilis L.) Seed Oil from Vietnam. Asian J. Chem. 2020, 32, 335–338. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Nascimento, A.K.L.; Melo-Silveira, R.F.; Dantas-Santos, N.; Fernandes, J.M.; Zucolotto, S.M.; Rocha, H.A.O.; Scortecci, K.C. Antioxidant and Antiproliferative Activities of Leaf Extracts from Plukenetia volubilis Linneo (Euphorbiaceae). Evid.-Based Complement. Altern. Med. 2013, 2013, 950272. [Google Scholar] [CrossRef]
- Mishra, V.K.; Kumar Bachheti, R.; Husen, A.; Mishra, V.K.; Bacheti, R.K. Medicinal Uses of Chlorophyll: A Critical Overview. In Chlorophyll: Structure, Function and Medicinal Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 177–196. [Google Scholar]
- Torres Sánchez, E.G.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Sacha Inchi Oil Press-Cake: Physicochemical Characteristics, Food-Related Applications and Biological Activity. Food Rev. Int. 2023, 39, 148–159. [Google Scholar] [CrossRef]
- Cárdenas, D.M.; Rave, L.J.G.; Soto, J.A. Biological Activity of Sacha Inchi (Plukenetia volubilis Linneo) and Potential Uses in Human Health: A Review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Suttisansanee, U.; Sahasakul, Y. Effects of Maturity and Thermal Treatment on Phenolic Profiles and In Vitro Health-Related Properties of Sacha Inchi Leaves. Plants 2022, 11, 1515. [Google Scholar] [CrossRef]
- Pagidipati, N.J.; Gaziano, T.A. Estimating Deaths from Cardiovascular Disease: A Review of Global Methodologies of Mortality Measurement. Circulation 2013, 127, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Ekinci, G. Economic Impacts of Cardiovascular Diseases: An Econometric Evaluation in Turkey. Iran J. Public Health 2023, 52, 118. [Google Scholar] [CrossRef] [PubMed]
- Gulyamova, S.T.; Abdul Aziz, S.F.; Omar, N.H.; Mohd, R.H. Workplace-Related Socioeconomic Issues Associated with Job Performance and Productivity among Employees with Various Impairments: A Systematic Literature Review. Soc. Sci. 2023, 12, 275. [Google Scholar] [CrossRef]
- Vuong, T.D.; Wei, F.; Beverly, C.J. Absenteeism Due to Functional Limitations Caused by Seven Common Chronic Diseases in US Workers. J. Occup. Environ. Med. 2015, 57, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Karam, C.; Beauchet, A.; Czernichow, S.; de Roquefeuil, F.; Bourez, A.; Mansencal, N.; Dubourg, O. Trends in Cardiovascular Disease Risk Factor Prevalence and Estimated 10-Year Cardiovascular Risk Scores in a Large Untreated French Urban Population: The CARVAR 92 Study. PLoS ONE 2015, 10, e0124817. [Google Scholar] [CrossRef]
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular Disease in the Developing World. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Sahril, N.; Rezali, M.S.; Kuang Kuay, L.; Baharudin, A.; Abd Razak, M.A.; Azlan Kassim, M.S.; Mohd Yusoff, M.F.; Omar, M.A.; Ahmad, N.A. Self-Reported Modifiable Risk Factors of Cardiovascular Disease among Older Adults in Malaysia: A Cross-Sectional Study of Prevalence and Clustering. Int. J. Environ. Res. Public Health 2021, 18, 7941. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Almeida Rezende, M.S.; Dantas, S.H.; de Lima Silva, S.; de Oliveira, J.C.P.L.; de Lourdes Assunção Araújo de Azevedo, F.; Alves, R.M.F.R.; de Menezes, G.M.S.; dos Santos, P.F.; Gonçalves, T.A.F.; et al. Unveiling the Role of Inflammation and Oxidative Stress on Age-Related Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1954398. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Schulz, E.; Münzel, T. Crosstalk of Mitochondria with NADPH Oxidase via Reactive Oxygen and Nitrogen Species Signalling and Its Role for Vascular Function. Br. J. Pharmacol. 2017, 174, 1670–1689. [Google Scholar] [CrossRef]
- Li, H.; Horke, S.; Förstermann, U. Vascular Oxidative Stress, Nitric Oxide and Atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Fiordelisi, A.; Iaccarino, G.; Morisco, C.; Coscioni, E.; Sorriento, D. NFkappaB Is a Key Player in the Crosstalk between Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 1599. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. The Noncanonical NF-κB Pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef]
- Groenen, A.G.; Halmos, B.; Tall, A.R.; Westerterp, M. Cholesterol Efflux Pathways, Inflammation, and Atherosclerosis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis—From Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Norhazlindah, M.F.; Jahurul, M.H.A.; Norliza, M.; Shihabul, A.; Islam, S.; Nyam, K.L.; Zaidul, I.S.M. Techniques for Extraction, Characterization, and Application of Oil from Sacha Inchi (Plukenetia volubilis L.) Seed: A Review. J. Food Meas. Charact. 2023, 17, 904–915. [Google Scholar] [CrossRef]
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Naina Mohamed, I. Interdependence of Anti-Inflammatory and Antioxidant Properties of Squalene–Implication for Cardiovascular Health. Life 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and Cardiovascular Disease Risk among the MASHAD Study Population. Lipids Health Dis. 2020, 19, 42. [Google Scholar] [CrossRef]
- Ambulay, J.P.; Rojas, P.A.; Timoteo, O.S.; Barreto, T.V.; Colarossi, A. Effect of the Emulsion of Sacha Inchi (Plukenetia Huayabambana) Oil on Oxidative Stress and Inflammation in Rats Induced to Obesity. J. Funct. Foods 2020, 64, 103631. [Google Scholar] [CrossRef]
- Garmendia, F.; Pando, R.; Ronceros, G. Effect of Sacha Inchi Oil (Plukenetia volúbilis L.) on the Lipid Profile of Patients with Hyperlipoproteinemia. Rev. Peru. Med. Exp. Salud Publica 2011, 28, 628–632. [Google Scholar]
- Gonzales, G.F.; Gonzales, C. A Randomized, Double-Blind Placebo-Controlled Study on Acceptability, Safety and Efficacy of Oral Administration of Sacha Inchi Oil (Plukenetia volubilis L.) in Adult Human Subjects. Food Chem. Toxicol. 2014, 65, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Alayón, A.N.; Ortega Ávila, J.G.; Echeverri Jiménez, I. Metabolic Status Is Related to the Effects of Adding of Sacha Inchi (Plukenetia volubilis L.) Oil on Postprandial Inflammation and Lipid Profile: Randomized, Crossover Clinical Trial. J Food Biochem 2019, 43, e12703. [Google Scholar] [CrossRef]
- Suwanangul, S.; Aluko, R.E.; Sangsawad, P.; Kreungngernd, D.; Ruttarattanamongkol, K. Antioxidant and Enzyme Inhibitory Properties of Sacha Inchi (Plukenetia volubilis) Protein Hydrolysate and Its Peptide Fractions. J. Food Biochem. 2022, 46, e14464. [Google Scholar] [CrossRef]
- Bellosta, S.; Corsini, A. Statin Drug Interactions and Related Adverse Reactions: An Update. Expert Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef]
- Prasongsub, W.; Pimsan, N.; Buranapattarachote, C.; Punturee, K. Anti-HMG-CoA Reductase and Antioxidant Activities of Sacha Inchi (Plukenetia volubilis L.) Nutshell Extract. Thai-J. Cit. Index Cent. (TCI) ASEAN Cit. Index 2021, 54, 18–26. [Google Scholar] [CrossRef]
- da Costa, R.F.; Freire, V.N.; Bezerra, E.M.; Cavada, B.S.; Caetano, E.W.S.; de Lima Filho, J.L.; Albuquerque, E.L. Explaining Statin Inhibition Effectiveness of HMG-CoA Reductase by Quantum Biochemistry Computations. Phys. Chem. Chem. Phys. 2012, 14, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Asmaa, B.H.; Ream, N. In Vitro Screening of the Pancreatic Cholesterol Esterase Inhibitory Activity of Some Medicinal Plants Grown in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1432–1436. Available online: www.ijppr.com (accessed on 18 September 2023).
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The Modulatory Effect of Infusions of Green Tea, Oolong Tea, and Black Tea on Gut Microbiota in High-Fat-Induced Obese Mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Just, S.; Mondot, S.; Ecker, J.; Wegner, K.; Rath, E.; Gau, L.; Streidl, T.; Hery-Arnaud, G.; Schmidt, S.; Lesker, T.R.; et al. The Gut Microbiota Drives the Impact of Bile Acids and Fat Source in Diet on Mouse Metabolism. Microbiome 2018, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Xu, Y.; Luo, T.; Ge, Y.; Jiang, Y.; Shi, Y.; Sun, J.; Le, G. Dietary Methionine Restriction Improves the Gut Microbiota and Reduces Intestinal Permeability and Inflammation in High-Fat-Fed Mice. Food Funct. 2019, 10, 5952–5968. [Google Scholar] [CrossRef]
- Li, P.; Huang, J.; Xiao, N.; Cai, X.; Yang, Y.; Deng, J.; Zhang, L.H.; Du, B. Sacha Inchi Oil Alleviates Gut Microbiota Dysbiosis and Improves Hepatic Lipid Dysmetabolism in High-Fat Diet-Fed Rats. Food Funct. 2020, 11, 5827–5841. [Google Scholar] [CrossRef]
- Nie, Q.; Xing, M.; Chen, H.; Hu, J.; Nie, S. Metabolomics and Lipidomics Profiling Reveals Hypocholesterolemic and Hypolipidemic Effects of Arabinoxylan on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 10614–10623. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.; Patlas, M.; Pinthus, J.H.; Mourtzakis, M. The Clinical Importance of Visceral Adiposity: A Critical Review of Methods for Visceral Adipose Tissue Analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Balla, T.; Sengupta, N.; Kim, Y.J. Lipid Synthesis and Transport Are Coupled to Regulate Membrane Lipid Dynamics in the Endoplasmic Reticulum. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158461. [Google Scholar] [CrossRef]
- Fang, L.; Harkewicz, R.; Hartvigsen, K.; Wiesner, P.; Choi, S.-H.; Almazan, F.; Pattison, J.; Deer, E.; Sayaphupha, T.; Dennis, E.A.; et al. Oxidized Cholesteryl Esters and Phospholipids in Zebrafish Larvae Fed a High Cholesterol Diet. J. Biol. Chem. 2010, 285, 32343–32351. [Google Scholar] [CrossRef]
- Kayser, B.D.; Lhomme, M.; Prifti, E.; Da Cunha, C.; Marquet, F.; Chain, F.; Naas, I.; Pelloux, V.; Dao, M.; Kontush, A.; et al. Phosphatidylglycerols Are Induced by Gut Dysbiosis and Inflammation, and Favorably Modulate Adipose Tissue Remodeling in Obesity. FASEB J. 2019, 33, 4741–4754. [Google Scholar] [CrossRef]
- Mukherjee, M. Human Digestive and Metabolic Lipases—A Brief Review. J. Mol. Catal. B Enzym. 2003, 22, 369–376. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the Preparation of Fish Protein Anti-Obesity Hydrolysates Using Response Surface Methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Lipid-Lowering Peptides from Sacha Inchi Meal. Int. J. Mol. Sci. 2023, 24, 1529. [Google Scholar] [CrossRef]
- Suwanangul, S.; Sangsawad, P.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Chittrakorn, S.; Ruttarattanamongkol, K. Antioxidant Activities of Sacha Inchi (Plukenetia volubilis L.) Protein Isolate and Its Hydrolysates Produced with Different Proteases. Maejo Int. J. Sci. Technol. 2021, 15, 48–60. [Google Scholar]
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits. Horticulturae 2022, 8, 344. [Google Scholar] [CrossRef]
- Ugusman, A.; Shahrin, S.A.S.; Azizan, N.H.; Pillai, S.B.; Krishnan, K.; Salamt, N.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Mokhtar, M.H. Role of Honey in Obesity Management: A Systematic Review. Front. Nutr. 2022, 9, 924097. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E.; Pap, D. The Role of Oxidative Stress in the Development of Obesity and Obesity-Related Metabolic Disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Savini, I.; Catani, M.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-Associated Oxidative Stress: Strategies Finalized to Improve Redox State. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in Cardiovascular Diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Duryee, M.J.; Clemens, D.L.; Opperman, P.J.; Thiele, G.M.; Duryee, L.M.; Garvin, R.P.; Anderson, D.R. Malondialdehyde-Acetaldehyde Modified (MAA) Proteins Differentially Effect the Inflammatory Response in Macrophage, Endothelial Cells and Animal Models of Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 12948. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable Oils Rich in Alpha Linolenic Acid Increment Hepatic N-3 LCPUFA, Modulating the Fatty Acid Metabolism and Antioxidant Response in Rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35. [Google Scholar] [CrossRef]
- Yan, K.; Deng, X.; Zhai, X.; Zhou, M.; Jia, X.; Luo, L.; Niu, M.; Zhu, H.; Qiang, H.; Zhou, Y. P38 Mitogen-Activated Protein Kinase and Liver X Receptor-α Mediate the Leptin Effect on Sterol Regulatory Element Binding Protein-1c Expression in Hepatic Stellate Cells. Mol. Med. 2012, 18, 10–18. [Google Scholar] [CrossRef] [PubMed]
- de Faria, A.P.; Ritter, A.M.V.; Gasparetti, C.S.; Corrêa, N.B.; Brunelli, V.; Almeida, A.; Pires, N.F.; Modolo, R.; Moreno Junior, H. A Proposed Inflammatory Score of Circulating Cytokines/Adipokines Associated with Resistant Hypertension, but Dependent on Obesity Parameters. Arq. Bras. Cardiol. 2019, 112, 383–389. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The Role of Leptin and Ghrelin in the Regulation of Food Intake and Body Weight in Humans: A Review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia Is Required for the Development of Leptin Resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef] [PubMed]
- Almabrouk, T.A.M.; White, A.D.; Ugusman, A.B.; Skiba, D.S.; Katwan, O.J.; Alganga, H.; Guzik, T.J.; Touyz, R.M.; Salt, I.P.; Kennedy, S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front. Physiol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.S.; Aminuddin, A.; Zainal Abidin, S.; Syafruddin, S.E.; Ahmad, M.F.; Mohd Mokhtar, N.; Kumar, J.; Hamid, A.A.; Ugusman, A. Profiling of Differentially Expressed MicroRNAs in Human Umbilical Vein Endothelial Cells Exposed to Hyperglycemia via RNA Sequencing. Life 2023, 13, 1296. [Google Scholar] [CrossRef] [PubMed]
- Attjioui, M.; Ryan, S.; Ristic, A.K.; Higgins, T.; Goñi, O.; Gibney, E.R.; Tierney, J.; O’Connell, S. Comparison of Edible Brown Algae Extracts for the Inhibition of Intestinal Carbohydrate Digestive Enzymes Involved in Glucose Release from the Diet. J. Nutr. Sci. 2021, 10, e5. [Google Scholar] [CrossRef] [PubMed]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Li, Y.; Zhang, K.; Zheng, X.; Wong, V.K.W.; Liu, W. Inhibitory Effect of Plant Essential Oils on α-Glucosidase. Food Sci. Biotechnol. 2022, 31, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between Phenolic Compounds, Amylolytic Enzymes and Starch: An Updated Overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Taslimi, P.; Gulçin, İ. Antidiabetic Potential: In Vitro Inhibition Effects of Some Natural Phenolic Compounds on α-Glycosidase and α-Amylase Enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and Chlorogenic Acids Inhibit Key Enzymes Linked to Type 2 Diabetes (in Vitro): A Comparative Study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- TADERA, K.; MINAMI, Y.; TAKAMATSU, K.; MATSUOKA, T. Inhibition of ALPHA.-Glucosidase and ALPHA.-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, K.; Liu, X.; Zhang, X. Isolation and Identification of Dipeptidyl Peptidase-IV Inhibitory Peptides from Sacha Inchi Meal. J. Sci. Food Agric. 2023, 103, 2926–2938. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the Mechanism of Cell Death Resulting from Streptozotocin Challenge in Experimental Animals, Its Practical Use and Potential Risk to Humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A Practical Guide for Induction of Type-2 Diabetes in Rat: Incorporating a High-Fat Diet and Streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Zhu, B.T. Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells. Cells 2022, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Mathias Akinlade, O.; Victor Owoyele, B.; Olufemi Soladoye, A. Streptozotocin-Induced Type 1 and 2 Diabetes in Rodents: A Model for Studying Diabetic Cardiac Autonomic Neuropathy. Afr. Health Sci. 2021, 21, 719–727. [Google Scholar] [CrossRef]
- Rojanaverawong, W.; Wongmanee, N.; Hanchang, W. Sacha Inchi (Plukenetia volubilis L.) Oil Improves Hepatic Insulin Sensitivity and Glucose Metabolism through Insulin Signaling Pathway in a Rat Model of Type 2 Diabetes. Prev. Nutr. Food Sci. 2023, 28, 30–42. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Jiang, S.; Young, J.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced Alterations in Hepatic Glucose and Lipid Metabolism: The Role of Type 1 and Type 2 Diabetes Mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin Regulation of Gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Yan, J.; Sheng, L.; Li, H. Akkermansia muciniphila: Is It the Holy Grail for Ameliorating Metabolic Diseases? Gut Microbes 2021, 13, 1984104. [Google Scholar] [CrossRef]
- Bahar-Tokman, H.; Demirci, M.; Keskin, F.; Cagatay, P.; Taner, Z.; Ozturk-Bakar, Y.; Ozyazar, M.; Kiraz, N.; Kocazeybek, B. Firmicutes/Bacteroidetes Ratio in the Gut Microbiota and IL-1β, IL-6, IL-8, TLR2, TLR4, TLR5 Gene Expressions in Type 2 Diabetes. Clin. Lab. 2022, 68, 1903–1910. [Google Scholar] [CrossRef]
- Fassatoui, M.; Lopez-Siles, M.; Díaz-Rizzolo, D.A.; Jmel, H.; Naouali, C.; Abdessalem, G.; Chikhaoui, A.; Nadal, B.; Jamoussi, H.; Abid, A.; et al. Gut Microbiota Imbalances in Tunisian Participants with Type 1 and Type 2 Diabetes Mellitus. Biosci. Rep. 2019, 39, BSR20182348. [Google Scholar] [CrossRef]
- Demirci, M.; Bahar Tokman, H.; Taner, Z.; Keskin, F.E.; Çağatay, P.; Ozturk Bakar, Y.; Özyazar, M.; Kiraz, N.; Kocazeybek, B.S. Bacteroidetes and Firmicutes Levels in Gut Microbiota and Effects of Hosts TLR2/TLR4 Gene Expression Levels in Adult Type 1 Diabetes Patients in Istanbul, Turkey. J. Diabetes Complicat. 2020, 34, 107449. [Google Scholar] [CrossRef]
- Lin, J.; Wen, J.; Xiao, N.; Cai, Y.T.; Xiao, J.; Dai, W.; Chen, J.P.; Zeng, K.W.; Liu, F.; Du, B.; et al. Anti-Diabetic and Gut Microbiota Modulation Effects of Sacha Inchi (Plukenetia volubilis L.) Leaf Extract in Streptozotocin-Induced Type 1 Diabetic Mice. J. Sci. Food Agric. 2022, 102, 4304–4312. [Google Scholar] [CrossRef]
- Alayón, A.N.; Ortega Avila, J.G.; Echeverri Jiménez, I. Carbohydrate Metabolism and Gene Expression of Sirtuin 1 in Healthy Subjects after Sacha Inchi Oil Supplementation: A Randomized Trial. Food Funct. 2018, 9, 1570–1577. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.; Yang, Y. Sirtuins in Glucose and Lipid Metabolism. Oncotarget 2017, 8, 1845–1859. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, X.; Chen, H.-Z. Sirtuins and Insulin Resistance. Front. Endocrinol. 2018, 9, 748. [Google Scholar] [CrossRef]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, B.S. Mechanisms of Diabetes-Induced Liver Damage: The Role of Oxidative Stress and Inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Son, J.S.; Choi, S.; Lee, G.; Jeong, S.-M.; Kim, S.M.; Kim, K.; Yun, J.M.; Park, S.M. Blood Pressure Change from Normal to 2017 ACC/AHA Defined Stage 1 Hypertension and Cardiovascular Risk. J. Clin. Med. 2019, 8, 820. [Google Scholar] [CrossRef]
- Losurdo, P.; Grillo, A.; Panizon, E.; Cappellari, G.G.; Fabris, B.; Bardelli, M.; Biolo, G.; Zanetti, M.; Carretta, R. Supplementation of Omega-3 Polyunsaturated Fatty Acids Prevents Increase in Arterial Stiffness After Experimental Menopause. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 114–120. [Google Scholar] [CrossRef]
- Spector, A.A.; Kim, H.-Y. Discovery of Essential Fatty Acids. J. Lipid Res. 2015, 56, 11–21. [Google Scholar] [CrossRef]
- Bentsen, H. Dietary Polyunsaturated Fatty Acids, Brain Function and Mental Health. Microb. Ecol. Health Dis. 2017, 28, 1281916. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Zhu, Y.; Zhang, X. Omega-3 Polyunsaturated Fatty Acids: Versatile Roles in Blood Pressure Regulation. Antioxid. Redox Signal. 2021, 34, 800–810. [Google Scholar] [CrossRef]
- Ferrari, R. RAAS Inhibition and Mortality in Hypertension. Glob. Cardiol. Sci. Pract. 2013, 2013, 34. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Campos, D. Enzyme-Assisted Hydrolysates from Sacha Inchi (Plukenetia Volubilis) Protein with in Vitro Antioxidant and Antihypertensive Properties. J. Food Process. Preserv. 2020, 44, e14969. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.P.V. Antihypertensive Effect of Caffeic Acid and Its Analogs through Dual Renin–Angiotensin–Aldosterone System Inhibition. Eur. J. Pharmacol. 2014, 730, 125–132. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Leenen, F.H.H.; Chen, L.; Golovina, V.A.; Hamlyn, J.M.; Pallone, T.L.; Van Huysse, J.W.; Zhang, J.; Wier, W.G. How NaCl Raises Blood Pressure: A New Paradigm for the Pathogenesis of Salt-Dependent Hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H1031–H1049. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Gluvic, Z.; Banjac, K.; Rizzo, M.; Isenovic, E.R. The Na+/K+-ATPase: A Potential Therapeutic Target in Cardiometabolic Diseases. Front. Endocrinol. 2023, 14, 1150171. [Google Scholar] [CrossRef]
- Li, P.; Cai, X.; Xiao, N.; Ma, X.; Zeng, L.; Zhang, L.-H.; Xie, L.; Du, B. Sacha Inchi (Plukenetia volubilis L.) Shell Extract Alleviates Hypertension in Association with the Regulation of Gut Microbiota. Food Funct. 2020, 11, 8051–8067. [Google Scholar] [CrossRef]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial Hypertension—Oxidative Stress and Inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef]
- Lamb, F.S.; Choi, H.; Miller, M.R.; Stark, R.J. TNFα and Reactive Oxygen Signaling in Vascular Smooth Muscle Cells in Hypertension and Atherosclerosis. Am. J. Hypertens. 2020, 33, 902–913. [Google Scholar] [CrossRef]
- Rybalkin, S.D.; Rybalkina, I.G.; Fei, R.; Hofmann, F.; Beavo, J.A. Regulation of CGMP-Specific Phosphodiesterase (PDE5) Phosphorylation in Smooth Muscle Cells. J. Biol. Chem. 2002, 277, 3310–3317. [Google Scholar] [CrossRef]
- Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C.; Rochette, L. Iron, Oxidative Stress, and Redox Signaling in the Cardiovascular System. Mol. Nutr. Food Res. 2014, 58, 1721–1738. [Google Scholar] [CrossRef]
- Ugusman, A.; Kumar, J.; Aminuddin, A. Endothelial Function and Dysfunction: Impact of Sodium-Glucose Cotransporter 2 Inhibitors. Pharmacol. Ther. 2021, 224, 107832. [Google Scholar] [CrossRef]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial Dysfunction, Endothelial Nitric Oxide Bioavailability, Tetrahydrobiopterin, and 5-Methyltetrahydrofolate in Cardiovascular Disease. Where Are We with Therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef]
- Al Samarraie, A.; Pichette, M.; Rousseau, G. Role of the Gut Microbiome in the Development of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 5420. [Google Scholar] [CrossRef]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sun, Y.; Zhou, X.; Si, W.; Liu, J.; Li, M.; Wu, M. Regulatory Effect of Gut Microbes on Blood Pressure. Anim. Models Exp. Med. 2022, 5, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Mhd Rodzi, N.A.R.; Lee, L.K. Sacha Inchi (Plukenetia volubilis L.): Recent Insight on Phytochemistry, Pharmacology, Organoleptic, Safety and Toxicity Perspectives. Heliyon 2022, 8, e10572. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers Impairing Mineral Bioaccessibility and Bioavailability in Plant-Based Foods and the Perspectives for Food Processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef]
- Srichamnong, W.; Ting, P.; Pitchakarn, P.; Nuchuchua, O.; Temviriyanukul, P. Safety Assessment of Plukenetia volubilis (Inca Peanut) Seeds, Leaves, and Their Products. Food Sci. Nutr. 2018, 6, 962–969. [Google Scholar] [CrossRef]
- Rodeiro, I.; Remirez, D.; Flores, D. Sacha Inchi (Plukenetia volubilis L.) Powder: Acute Toxicity, 90 Days Oral Toxicity Study and Micronucleus Assay in Rodents. J. Pharm. Pharmacogn. Res. 2018, 6, 17–26. [Google Scholar]
- Gorriti, A.; Arroyo, J.; Quispe, F.; Cisneros, B.; Condorhuamán, M.; Almora, Y.; Chumpitaz, V. Oral Toxicity at 60-Days of Sacha Inchi Oil (Plukenetia volubilis L.) and Linseed (Linum usitatissimum L.), and Determination of Lethal Dose 50 in Rodents. Rev. Peru. Med. Exp. Salud Publica 2010, 27, 352–360. [Google Scholar] [CrossRef]
- Bueso, A.; Rodriguez-Perez, R.; Rodriguez, M.; Dionicio, J.; Perez-Pimiento, A.; Caballero, M.L. Occupational Allergic Rhinoconjunctivitis and Bronchial Asthma Induced by Plukenetia volubilis Seeds. Occup. Environ. Med. 2010, 67, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, S.S.; Barber, K.E.; Kishk, O.A.; Mays, A.A.; Jones, S.R.; Turner, A.L.; Riche, D.M. Effect of Fish Oil Supplement Administration Method on Tolerability and Adherence: A Randomized Pilot Clinical Trial. Pilot Feasibility Stud. 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil | 5 or 10 mL for 16 weeks | Healthy adults | ↓ TC and LDLc ↑ HDLc | [48] |
| SI oil | 15 mL | Metabolically healthy and unhealthy men given high-fat meal | ↓ postprandial TC and IL-6 in metabolically healthy men ↓ postprandial IL-6 in metabolically unhealthy men | [49] |
| SI oil | 5 or 10 mL (contains 2 g or 4 g ω-3/day) for 16 weeks | Hypercholesterolemic patients | ↓ TC and LDLc ↑ HDLc | [47] |
| SI emulsion oil | 2.5 mL (contains 0.2 g or 0.5 g ω-3/day) for 8 weeks | Obese rats | ↓ TC, TG, and LDLc ↑ HDLc | [46] |
| SI oil | 0.5–1.5 mL/kg for 8 weeks | Obese rats | ↓ TC, TG, and LDLc Reverse gut microbiota dysbiosis and metabolome Improve bile acid compositions | [59] |
| SI nutshell, baby nut and leaf hot water extracts SI nut oil | 125 µg/mL | In vitro enzyme inhibitory assays | Only SI nutshell hot water extract ↓ HMG-CoA reductase and cholesterol esterase activities | [53] |
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil emulsion | 2.5 mL (contains 0.25 g and 0.5 g ω-3/day) for 8 weeks | Obese rats | ↓ MDA and AOPP ↑ catalase activity ↓ IL-6 and TNF-α ↓ leptin ↑ adiponectin ↑ PPAR-α | [46] |
| SI oil | 0.5–1.5 mL/kg for 8 weeks | Obese rats | ↓ mean adipocyte size ↓ hepatic steatosis, hepatic lipase activity and inflammation ↓ PGS1 expression ↑ lipolysis | [59] |
| SI meal-derived peptides | 0.1–0.5 mM | In vitro enzyme inhibitory assay Oleic acid-induced HepG2 cells | ↓ pancreatic lipase activity ↓ intracellular fat accumulation and ROS levels in HepG2 cells | [68] |
| SI husk aqueous ethanol extract | 0.4 mg/mL | In vitro enzyme inhibitory assay | ↓ lipase activity | [70] |
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil | 15 mL | Healthy adults given high-fat breakfast | ↓ postprandial hyperglycemia ↑ insulin sensitivity ↑ SIRT-1 expression in healthy adults with higher baseline triglycerides and glycemic response | [108] |
| SI leaves water extract | 400 mg/kg for 6 weeks | Type 1 diabetic rats | ↓ FBS ↑ insulin sensitivity and glucose tolerance ↓ gut microbiota dysbiosis | [107] |
| SI oil | 0.5–2 mL/kg for 5 weeks | Type 2 diabetic rats | ↓ FBS ↑ insulin sensitivity indices and glucose tolerance ↑ IRS-1 and Akt ↓ IR-β ↓ G-6-Pase and PCK-1 activities ↑ hepatic glycogen content ↓ AST and ALT ↓ MDA ↑ SOD, CAT and GPX activities ↓TNF-α and IL-6 | [98] |
| SI husk and shell aqueous ethanol extract | 0.025 mg/mL | In vitro enzyme inhibitory assays | ↓ α-glucosidase and α-amylase activities | [70] |
| SI essential oil | 25 µg/mL | In vitro enzyme inhibitory assays | ↓ α-amylase activity | [86] |
| SI meal-derived peptides | 0.25–0.5 mM | In vitro enzyme inhibitory assay Palmitic acid-induced insulin resistant HepG2 cells | ↓ DPP-IV activity ↑ glucose consumption by HepG2 cells | [91] |
| SI Preparation | Dose | Experimental Model | Outcomes | Reference |
|---|---|---|---|---|
| SI oil | 10 or 15 mL for 4 months | Healthy adults | ↓ SBP and DBP ↓ LDLc | [48] |
| SI shell extract | 400 mg/kg for 51 days | SHR and WKY rats on high-salt diet | ↓ SBP ↓ LTCC expression ↑ Na+/K+-ATPase expression Restored calcium and sodium homeostasis ↓ MDA ↑ SOD and GSH ↑ eNOS expression ↑ NO ↑ 5-MTHF Reshaped gut microbiota and metabolome, ↑ prevalence of Roseburia and dihydrofolic acid Normalized F/B ratio | [122] |
| SI protein hydrolysates | 98 µg/mL | In vitro enzyme inhibitory assay | ↓ ACE activity | [118] |
| SI husk and shell aqueous ethanol extract | 0.013 mg/mL | In vitro enzyme inhibitory assay | ↓ ACE activity | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Rahman, I.Z.; Nor Hisam, N.S.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Ugusman, A. Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review. Pharmaceuticals 2023, 16, 1588. https://doi.org/10.3390/ph16111588
Abd Rahman IZ, Nor Hisam NS, Aminuddin A, Hamid AA, Kumar J, Ugusman A. Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review. Pharmaceuticals. 2023; 16(11):1588. https://doi.org/10.3390/ph16111588
Chicago/Turabian StyleAbd Rahman, Izzat Zulhilmi, Nur Syahidah Nor Hisam, Amilia Aminuddin, Adila A. Hamid, Jaya Kumar, and Azizah Ugusman. 2023. "Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review" Pharmaceuticals 16, no. 11: 1588. https://doi.org/10.3390/ph16111588
APA StyleAbd Rahman, I. Z., Nor Hisam, N. S., Aminuddin, A., Hamid, A. A., Kumar, J., & Ugusman, A. (2023). Evaluating the Potential of Plukenetia volubilis Linneo (Sacha Inchi) in Alleviating Cardiovascular Disease Risk Factors: A Mini Review. Pharmaceuticals, 16(11), 1588. https://doi.org/10.3390/ph16111588





