Development and Evaluation of Solid Dispersion-Based Sublingual Films of Nisoldipine
Abstract
1. Introduction
2. Results
2.1. Phase Solubility Studies of the Carrier
2.2. Evaluation of Sublingual Films
2.2.1. Bright Field Microscopy
2.2.2. Compatibility Studies
2.2.3. Weight Variation
2.2.4. Thickness Measurement
2.2.5. Drug Content
2.2.6. Folding Endurance
2.2.7. Surface pH
2.2.8. Tack Test
2.2.9. Differential Scanning Calorimetry
2.2.10. X-ray Diffraction
2.2.11. Disintegration Time
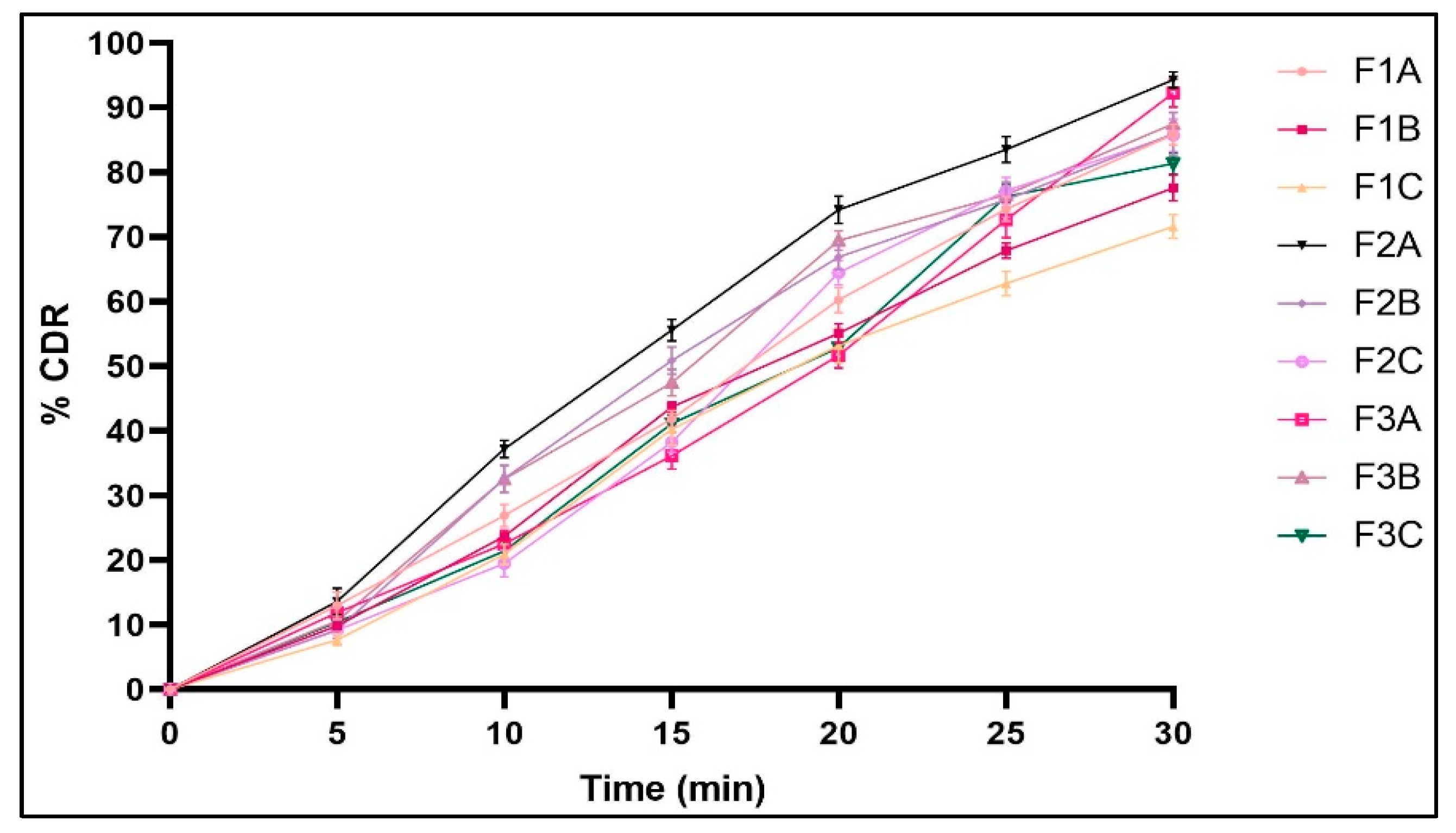
2.2.12. In Vitro Dissolution Studies
2.2.13. Ex Vivo Mucoadhesion Strength
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Phase Solubility Studies
4.3. Preparation of Solid Dispersion
4.4. Preparation of Sublingual Films
4.5. Evaluation of Sublingual Films
4.5.1. Bright-Field Microscopy
4.5.2. Compatibility Studies
4.5.3. Weight Variation
4.5.4. Thickness Measurements
4.5.5. Drug Content Uniformity
4.5.6. Folding Endurance
4.5.7. Surface pH Measurement
4.5.8. Tack Test
4.5.9. Differential Scanning Calorimetry
4.5.10. X-ray Diffraction
4.5.11. Disintegration Test
4.5.12. In Vitro Dissolution Studies
4.5.13. Ex Vivo Mucoadhesion Strength
4.5.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jana, U.; Mohanty, A.K.; Pal, S.L.; Manna, P.K.; Mohanta, G.P. Felodipine loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vivo toxicity study. Nano. Converg. 2014, 1, 31. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Tanriover, C.; Ucku, D.; Laffin, L. Future treatments in hypertension: Can we meet the unmet needs of patients? Eur. J. Intern. Med. 2023, 115, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mohanty, S.; Ramadoss, A. Functionality of flexible pressure sensors in cardiovascular health monitoring: A review. ACS Sens. 2022, 7, 2495–2520. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Forman, D.E.; Wang, T.Y.; Chikwe, J.; Kunadian, V.; Rich, M.W.; Young, B.A.; Page, R.L.; DeVon, H.A.; Alexander, K.P. Management of acute coronary syndrome in the older adult population: A scientific statement from the American Heart Association. Circulation 2023, 147, 32–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Garg, T.; Goyal, A.K.; Rath, G. Development, optimization, and characterization of polymeric electrospun nanofiber: A new attempt in sublingual delivery of nicorandil for the management of angina pectoris. Artif. Cells. Nanomed. Biotechnol. 2016, 44, 1498–1507. [Google Scholar] [CrossRef]
- Shipp, L.; Liu, F.; Kerai-Varsani, L.; Okwuosa, T.C. Buccal films: A review of therapeutic opportunities, formulations & relevant evaluation approaches. J. Control. Release 2022, 352, 1071–1092. [Google Scholar] [CrossRef]
- Reddy, L.H.; Ghosh, B. Fast dissolving drug delivery systems: A review of the literature. Indian J. Pharm. Sci. 2002, 64, 331–336. [Google Scholar]
- Labeling, F.P.; Sular®. US Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER); US Government Printing Office: Washington, DC, USA, 2000.
- Vogt, A.; Kreuzer, H. Hemodynamic effects of nisoldipine in chronic congestive heart failure. Arzneimittelforschung 1983, 33, 877–879. [Google Scholar]
- Chavan, R.B.; Lodagekar, A.; Yadav, B.; Shastri, N.R. Amorphous solid dispersion of nisoldipine by solvent evaporation technique: Preparation, characterization, in vitro, in vivo evaluation, and scale up feasibility study. Drug Deliv. Transl. Res. 2020, 10, 903–918. [Google Scholar] [CrossRef]
- Patel, K.A.; Bhatt, M.H.; Hirani, R.V.; Patel, V.A.; Patel, V.N.; Shah, G.B.; Chorawala, M.R. Assessment of potential drug–drug interactions among outpatients in a tertiary care hospital: Focusing on the role of P-glycoprotein and CYP3A4 (retrospective observational study). Heliyon 2022, 8, 11278. [Google Scholar] [CrossRef]
- De Boer, A.G.; De Leede, L.G.J.; Breimer, D.D. Drug Absorption by oral and sublingual routes. Br. J. Anaesth. 1984, 56, 69–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El Maghraby, G.M.; Elsergany, R.N. Fast disintegrating tablets of nisoldipine for intra-oral administration. Pharm. Dev. Technol. 2014, 19, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Jansten, G.M.; Robinson, J.R. Sustained and Controlled Drug-delivery systems. In Modern Pharmaceutics; Banker, G.S., Rhodes, C.T., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 501–528. [Google Scholar]
- Heinig, R. Clinical pharmacokinetics of nisoldipine coat-core. Clin. Pharmacokinet. 1998, 35, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Hassnain, F.; Bashir, S.; Asad, M.; Nazir, I.; Qamar, S.; Imran, M. Formulation and characterization of solid dispersion of nisoldipine by solvent evaporation method. J. Pharm. Altern. Med. 2012, 2, 21–28. [Google Scholar]
- Zhang, K.; Yu, H.; Luo, Q.; Yang, S.; Lin, X.; Zhang, Y.; Tian, B.; Tang, X. Increased dissolution and oral absorption of itraconazole/soluplus extrudate compared with itraconazole nanosuspension. Eur. J. Pharm. Biopharm. 2013, 85, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yan, J.; Ren, L.; Xue, M.; Yuan, Z.; Wang, T.; Yan, Z.; Yin, L.; Yang, L.; Qin, C. Preparation and evaluation of orally disintegrating film containing donepezil for Alzheimer disease. J. Drug. Deliv. Sci. Technol. 2019, 54, 101321. [Google Scholar] [CrossRef]
- Prasanthi, N.L.; Krishna, C.S.; Gupta, M.E.; Manikiran, S.S.; Rao, N.R. Design and development of sublingual fast dissolving films for an antiasthmatic drug. Der Pharm. Lett. 2011, 3, 382–395. [Google Scholar]
- Karki, S.; Kim, H.; Na, S.J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Swartwout, R.; Hoerantner, M.T.; Bulović, V. Scalable deposition methods for large-area production of perovskite thin films. Energy Environ. Mater. 2019, 2, 119–145. [Google Scholar] [CrossRef]
- Vasanthan, M.; Narayanasamy, D. Development of fast dissolving tablets of nisoldipine by solid dispersion technology using poloxamer 407 and poloxamer 188. J. Young. Pharm. 2016, 8, 341–349. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Dave, R.H. Investigating the effect of molecular weight of polyvinylpyrrolidone and hydroxypropyl methyl cellulose as potential antiprecipitants on supersaturated drug solutions and formulations using weakly acidic drug: Indomethacin. Int. J. Pharm. Sci. Res. 2016, 1, 73931. [Google Scholar] [CrossRef]
- Olechno, K.; Basa, A.; Winnicka, K. Success depends on your backbone- About the use of polymers as essential materials forming orodispersible films. Materials 2021, 27, 144872. [Google Scholar] [CrossRef]
- Chonkar, A.D.; Bhagawati, S.T.; Udupa, N. An overview on fast dissolving oral films. Asian J. Pharm. Technol. 2015, 5, 129–137. [Google Scholar] [CrossRef]
- Padamwar, P.A.; Phasate, P.P. Formulation and evaluation of fast dissolving oral film of bisoprololfumarate. Int. J. Pharm. Sci. Res. 2015, 6, 135–142. [Google Scholar]
- Kyaw, O.M.; Mandal, U.K.; Chatterjee, B. Polymeric behavior evaluation of PVP K30-poloxamer binary carrier for solid dispersed nisoldipine by experimental design. Pharm. Dev. Technol. 2017, 22, 2–12. [Google Scholar] [CrossRef]
- Marsac, P.J.; Li, T.; Taylor, L.S. Estimation of drug–polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm. Res. 2009, 26, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Krstic, M.; Djuris, J.; Petrovic, O.; Lazarevic, N.; Cvijic, S.; Ibric, S. Application of the melt granulation technique in development of lipid matrix tablets with immediate release of carbamazepine. J. Drug. Deliv. Sci. Technol. 2017, 39, 467–474. [Google Scholar] [CrossRef]
- Gurny, R.; Meyer, J.M.; Peppas, N.A. Bioadhesive intraoral release systems: Design, testing and analysis. Biomaterials 1984, 5, 336–340. [Google Scholar] [CrossRef]
- Dahiya, M.; Saha, S.; Shahiwala, A.F. A review on mouth dissolving films. Curr. Drug Deliv. 2009, 6, 469–476. [Google Scholar] [CrossRef]
- Bhyan, B.; Jangra, S.; Kaur, M.; Singh, H. Orally fast dissolving films: Innovations in formulation and technology. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 9–15. [Google Scholar]
- Higuchi, T.K. Phase-solubility techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–212. [Google Scholar]
- Vats, S.K.; Gupta, R.N.; Ramaraju, K.; Singh, R. Design and statistical evaluation of a multiunit delivery system containing Nisoldipine-Soluplusâ® solid dispersion for hypertension chronotherapy. Int. J. Pharm. Sci. 2016, 8, 170–177. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Singh, R.; Gupta, R.N. Development of a single core osmotic tablet containing thermodynamically stable solid dispersion of nisoldipine. Indian J. Pharm. Sci. 2017, 79, 740–750. [Google Scholar] [CrossRef]
- Ali, M.S.; Vijendar, C.; Kumar, S.D.; Krishnaveni, J. Formulation and evaluation of fast dissolving oral films of diazepam. J. Pharmacovigil. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Bhupinder, B.; Sarita, J. Formulation and evaluation of fast dissolving sublingual films of Rizatriptan Benzoate. Int. J. Drug. Dev. Res. 2012, 4, 133–143. [Google Scholar]
- Prajapati, S.T.; Patel, M.V.; Patel, C.N. Preparation and evaluation of sublingual tablets of zolmitriptan. Int. J. Pharm. Investig. 2014, 4, 27–31. [Google Scholar] [CrossRef]
- Echanur, V.A.; Matadh, A.V.; Pragathi, S.G.; Sarasija, S.; Thean, Y.; Badruddoza, A.Z.; Shah, J.; Kulkarni, V.; Ajjarapu, S.; Reena, N.M.; et al. Continuous manufacturing of oil in water (O/W) emulgel by extrusion process. AAPS PharmSciTech. 2023, 24, 76. [Google Scholar] [CrossRef]
- Krishnamoorthy, B.; Habibur, R.S.M.; Tamil, S.N.; Hari, P.R.; Rajkumar, M.; Siva, S.M.; Vamshikrishna, K.; Gregory, M.; Vijayaraghavan, C. Design, formulation, in vitro, in vivo, and pharmacokinetic evaluation of nisoldipine-loaded self-nanoemulsifying drug delivery system. J. Nanopart. Res. 2015, 17, 34. [Google Scholar] [CrossRef]
- Londhe, V.; Shirsat, R. Formulation and characterization of fast-dissolving sublingual film of iloperidone using Box–Behnken design for enhancement of oral bioavailability. AAPS PharmSciTech. 2018, 19, 1392–1400. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, W.; Li, G.; Fu, Y.; Li, Z.; Qin, M.; Yuan, Z. Highly strong luminescent chiral nematic cellulose nanocrystal/PEI composites for anticounterfeiting. Chem. Eng. J. 2022, 430, 132780. [Google Scholar] [CrossRef]
- Mushtaque, M.; Muhammad, I.N.; Hassan, S.M.; Ali, A.; Masood, R. Development and pharmaceutical evaluation of oral fast dissolving thin film of escitalopram: A patient friendly dosage form. Pak. J. Pharm. Sci. 2020, 1, 183–189. [Google Scholar] [CrossRef]
- Wadetwar, R.N.; Ali, F.; Kanojiya, P.R. Formulation and evaluation of fast dissolving sublingual film of paroxetine hydrochloride for treatment of depression. Asian. J. Pharm. Clin. Res. 2019, 12, 126–132. [Google Scholar] [CrossRef]
- Moreira, L.G.; Zhang, X.; Muller, C.; Hensel, R.; Arzt, E. Film-Terminated fibrillar microstructures with improved adhesion on skin-like surfaces. ACS Appl. Mater. Interfaces 2022, 14, 46239–46251. [Google Scholar] [CrossRef]
- Zaki, D.Y.; Safwat, E.M.; Nagi, S.M.; Salem, H.N.; Hamdy, T.M.; Moharam, L.M.; Hassan, M.L.; Hamzawy, E.M. A novel dental re-mineralizing blend of hydroxyethyl-cellulose and cellulose nanofibers oral film loaded with nepheline apatite glass: Preparation, characterization and in vitro evaluation of re-mineralizing effect. Carbohydr. Polym. Technol. Appl. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Passi, I.; Salwan, S.; Ganti, S.S.; Kumar, B. Differential scanning calorimetry has emerged as a key analytical tool in the thermal analysis of pharmaceutical formulations. Curr. Pharm. Des. 2022, 28, 3082–3084. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Deokar, A.B.; Vilegave, K.V.; Morani, D.O.; Dhadde, S.B. Design, evaluation and characterization of rapidly dissolving oral strips of metoprolol succinate. J. Pharm. Analy. Insights. 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Tarani, E.; Arvanitidis, I.; Christofilos, D.; Bikiaris, D.N.; Chrissafis, K.; Vourlias, G. Calculation of the degree of crystallinity of HDPE/GNPs nanocomposites by using various experimental techniques: A comparative study. J. Mater. Sci. 2023, 58, 1621–1639. [Google Scholar] [CrossRef]
- Lai, K.L.; Fang, Y.; Han, H.; Li, Q.; Zhang, S.; Li, H.Y.; Chow, S.F.; Lam, T.N.; Lee, W.Y. Orally-dissolving film for sublingual and buccal delivery of ropinirole. Colloids Surf. B Biointerfaces 2018, 163, 9–18. [Google Scholar] [CrossRef]
- Aleem, O.; Kuchekar, B.; Pore, Y.; Late, S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharm. Biomed. Anal. 2008, 47, 535–540. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DD Solver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Adrover, A.; Pedacchia, A.; Petralito, S.; Spera, R. In vitro dissolution testing of oral thin films: A comparison between USP 1, USP 2 apparatuses and a new millifluidic flow-through device. Chem. Eng. Res. Des. 2015, 95, 173–178. [Google Scholar] [CrossRef]
- Bonsu, M.A.; Ofori-Kwakye, K.; Kipo, S.L.; Boakye-Gyasi, M.E.; Fosu, M.A. Development of oral dissolvable films of diclofenac sodium for osteoarthritis using Albizia and Khaya gums as hydrophilic film formers. J. Drug Deliv. 2016, 2016, 6459280. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Asadi, R.; Jelvehgari, M. Development and characterization of sublingual films for enhanced bioavailability of selegiline hydrochloride. Ther. Deliv. 2021, 12, 159–174. [Google Scholar] [CrossRef] [PubMed]






| % of Carrier | 0 | 2 | 4 | 6 | 8 | 10 |
|---|---|---|---|---|---|---|
| Nisoldipine Dissolved (mg/mL), Mean ± SD | ||||||
| Soluplus® | 0.25 ± 0.02 | 1.57 ± 0.14 | 3.62 ± 0.21 | 4.12 ± 0.31 | 5.65 ± 0.34 | 8.01 ± 0.31 |
| Kolliphor RH40 | 0.25 ± 0.02 | 1.04 ± 0.22 | 2.47 ± 0.32 | 3.28 ± 0.38 | 4.12 ± 0.42 | 5.06 ± 0.35 |
| PVP K30 | 0.25 ± 0.02 | 1.31 ± 0.18 | 3.01 ± 0.26 | 4.40 ± 0.29 | 5.11 ± 0.31 | 6.10 ± 0.25 |
| Characteristic Vibrational Bands (cm−1) | Functional Groups | Band Positions Observed in Nisoldipine (cm−1) | Band Positions Observed in Formulation (cm−1) |
|---|---|---|---|
| 3000–2800 | N-H (Stretching) | 2967.91 | 2968.87 |
| 1725–1705 | C=O (Stretching) | 1707.66 | 1706.69 |
| 1662–1626 | C=C (Stretching) | 1655.59 | 1656.55 |
| 1205–1124 | C-O (Stretching) | 1187.94 | 1148.4 |
| Formulations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | F1A | F1B | F1C | F2A | F2B | F2C | F3A | F3B | F3C |
| Weight (mg) | 38.58 ± 2.06 | 53.57 ± 2.98 | 75.15 ± 3.39 | 25.67 ± 2.82 | 39.01 ± 1.71 | 60.41 ± 1.74 | 33.04 ± 1.91 | 45.32 ± 1.28 | 65.91 ± 2.07 |
| Thickness (mm) | 0.36 ± 0.01 | 0.47 ± 0.01 | 0.61 ± 0.02 | 0.29 ± 0.02 | 0.40 ± 0.02 | 0.52 ± 0.01 | 0.31 ± 0.02 | 0.43 ± 0.01 | 0.56 ± 0.01 |
| Tack test | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Non-tacky |
| Folding endurance (times) | 144.66 ± 5.5 | 120.33 ± 4.04 | 92.66 ± 5.03 | 168.66 ± 4.50 | 134.33 ± 2.51 | 98.66 ± 5.03 | 173.33 ± 4.50 | 141.33 ± 3.05 | 113.33 ± 6.02 |
| Surface pH | 6.66 ± 0.05 | 6.43 ± 0.11 | 6.26 ± 0.05 | 6.66 ± 0.11 | 6.53 ± 0.11 | 6.33 ± 0.05 | 6.53 ± 0.11 | 6.43 ± 0.05 | 6.23 ± 0.05 |
| Disintegration time (Sec) | 51.33 ± 2.51 | 58.66 ± 2.51 | 66.66 ± 3.21 | 28.66 ± 3.05 | 41.33 ± 3.51 | 48.66 ± 2.51 | 35.66 ± 3.05 | 47.66 ± 2.08 | 55.33 ± 3.51 |
| Drug content (%) | 86.72 ± 2.25 | 77.64 ± 2.38 | 71.57 ± 2.76 | 96.79 ± 0.63 | 93.71 ± 0.55 | 90.69 ± 0.88 | 95.13 ± 1.05 | 92.46 ± 0.48 | 89.44 ± 0.81 |
| % Drug release (30 Sec) | 85.80 ± 1.62 | 77.57 ± 2.0 | 71.57 ± 1.84 | 94.24 ± 1.22 | 89.93 ± 3.33 | 85.70 ± 2.48 | 91.19 ± 2.20 | 87.46 ± 1.79 | 82.31 ± 1.68 |
| Sample | Sample Wt (mg) | Quantity of Drug (mg) | Melting Point | Observed | Sample |
|---|---|---|---|---|---|
| Nisoldipine | 250 | 0.33 | 151.24 °C | 160.82 | - |
| Physical mixture | 250 | 0.33 | 150.28 °C | 17.61 | 11.15% |
| F2A | 250 | 0.33 | 139.21 °C | 36.71 | 0.21% |
| Ingredients | F1A | F1B | F1C | F2A | F2B | F2C | F3A | F3B | F3C |
|---|---|---|---|---|---|---|---|---|---|
| NISSD * | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| HPC EF (mg) | 200 | 300 | 400 | - | - | - | - | - | - |
| HPMC E5 (mg) | - | - | - | 200 | 300 | 400 | - | - | - |
| HPMC E15 (mg) | - | - | - | - | - | - | 200 | 300 | 400 |
| PEG 400 (mL) | 0.20 | 0.30 | 0.40 | 0.20 | 0.30 | 0.40 | 0.20 | 0.30 | 0.40 |
| Ethanol (mL) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamhoom, Y.; Sharma, A.; Nanjappa, S.H.; Kumar, A.; Alshishani, A.; Ahmed, M.M.; Farhana, S.A.; Rahamathulla, M. Development and Evaluation of Solid Dispersion-Based Sublingual Films of Nisoldipine. Pharmaceuticals 2023, 16, 1589. https://doi.org/10.3390/ph16111589
Alhamhoom Y, Sharma A, Nanjappa SH, Kumar A, Alshishani A, Ahmed MM, Farhana SA, Rahamathulla M. Development and Evaluation of Solid Dispersion-Based Sublingual Films of Nisoldipine. Pharmaceuticals. 2023; 16(11):1589. https://doi.org/10.3390/ph16111589
Chicago/Turabian StyleAlhamhoom, Yahya, Abhay Sharma, Shivakumar Hagalavadi Nanjappa, Avichal Kumar, Anas Alshishani, Mohammed Muqtader Ahmed, Syeda Ayesha Farhana, and Mohamed Rahamathulla. 2023. "Development and Evaluation of Solid Dispersion-Based Sublingual Films of Nisoldipine" Pharmaceuticals 16, no. 11: 1589. https://doi.org/10.3390/ph16111589
APA StyleAlhamhoom, Y., Sharma, A., Nanjappa, S. H., Kumar, A., Alshishani, A., Ahmed, M. M., Farhana, S. A., & Rahamathulla, M. (2023). Development and Evaluation of Solid Dispersion-Based Sublingual Films of Nisoldipine. Pharmaceuticals, 16(11), 1589. https://doi.org/10.3390/ph16111589






