Distinct and Dynamic Changes in the Temporal Profiles of Neurotransmitters in Drosophila melanogaster Brain following Volatilized Cocaine or Methamphetamine Administrations
Abstract
:1. Introduction
2. Results
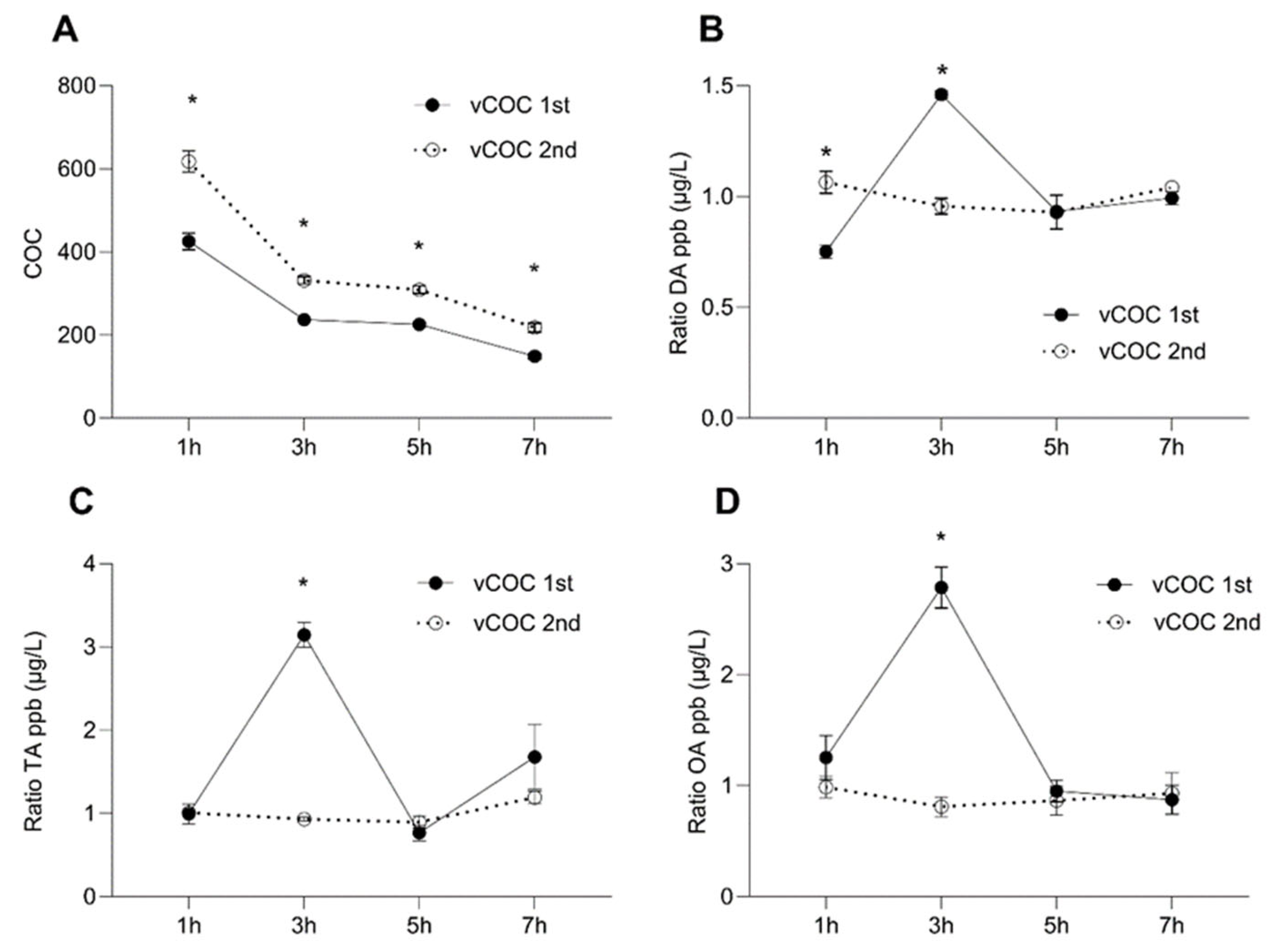
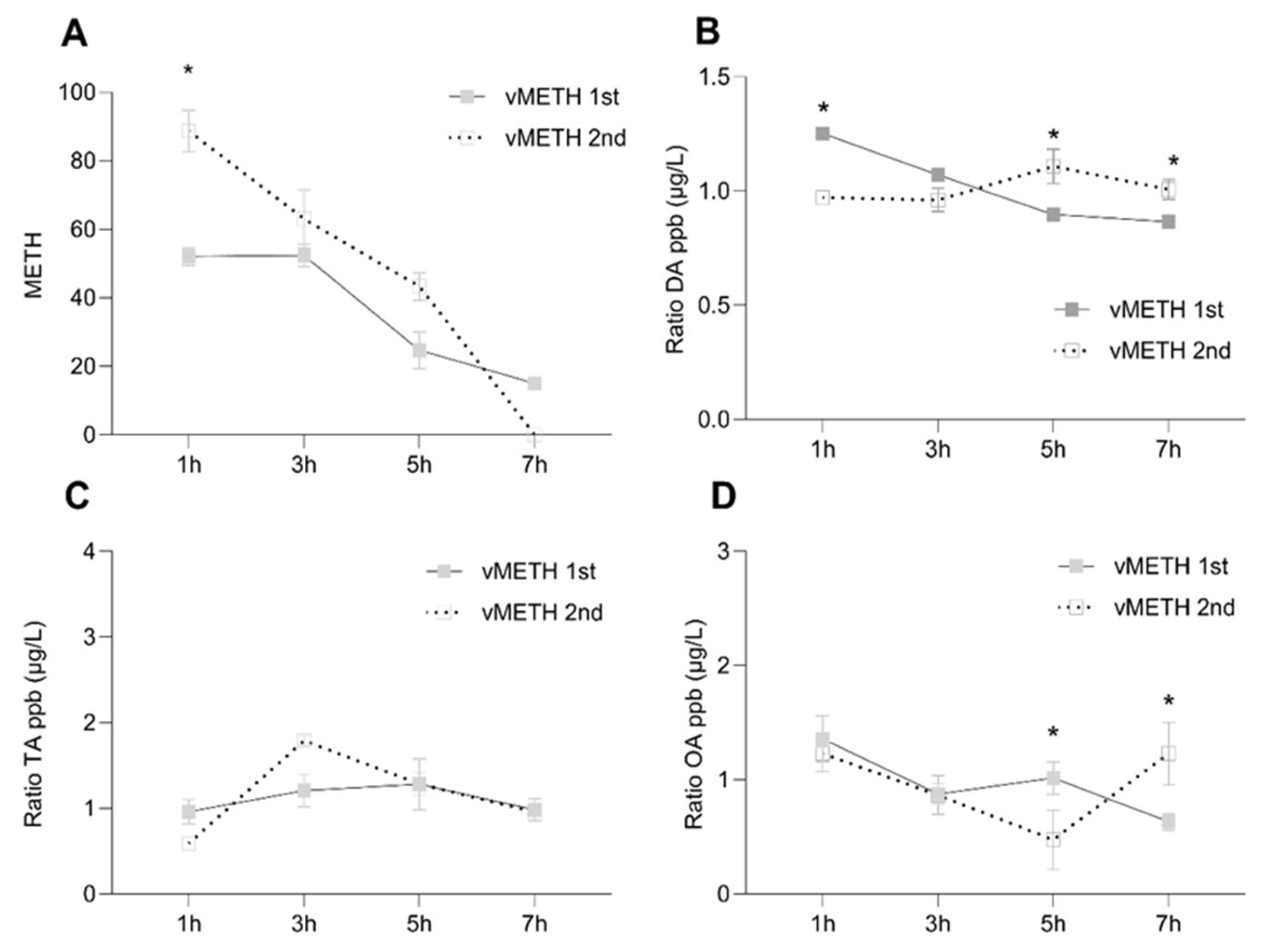
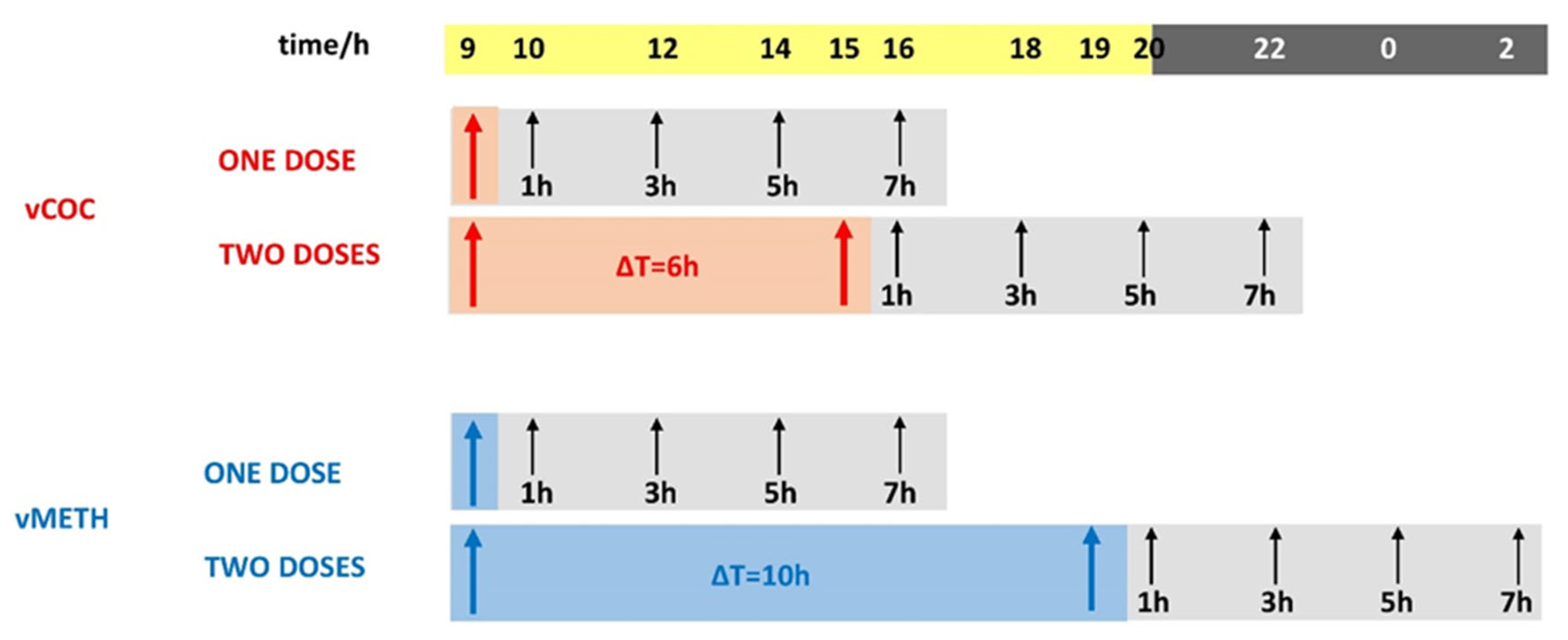
2.1. Distinct Temporal Profiles of Monoamine Concentrations after vCOC and vMETH Administrations
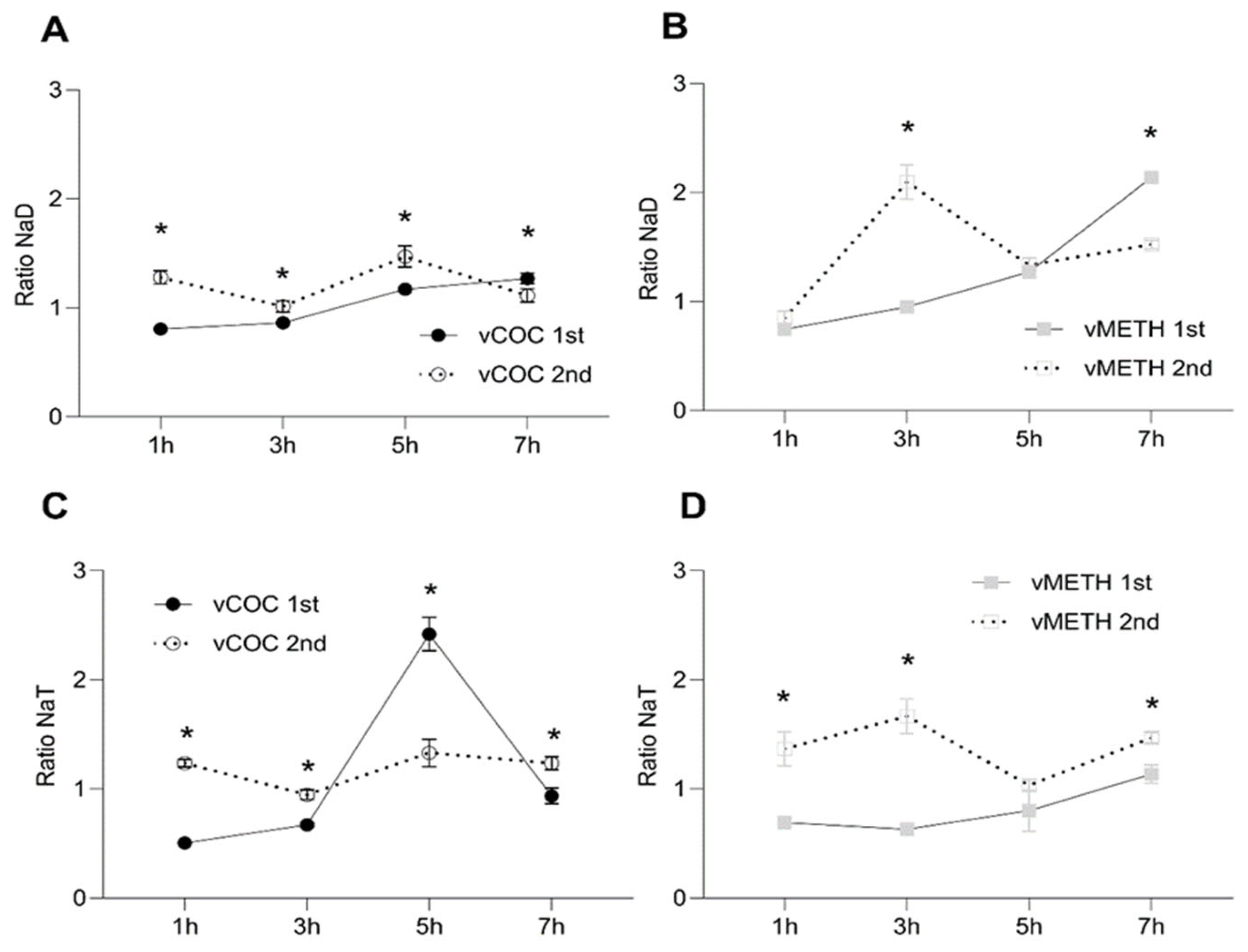
2.2. Temporal Profiles of Dopamine and Tyramine Metabolites after vCOC and vMETH Administrations
2.3. Temporal Change after vCOC and vMETH Exposure of the Excitatory and Inhibitory Neurotransmitters
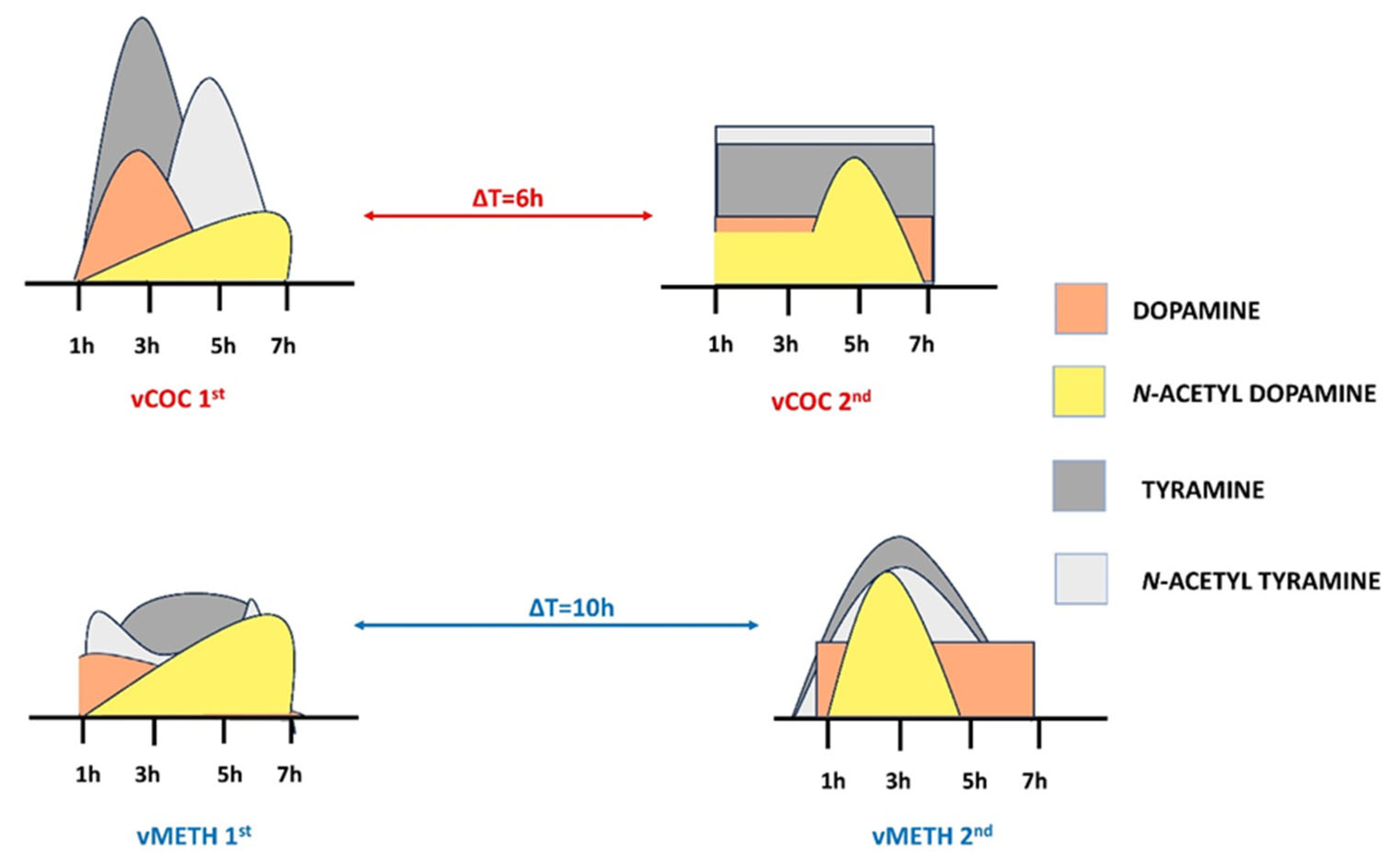
3. Discussion
4. Materials and Methods
4.1. Fly Strains
4.2. Psychostimulant Delivery Using FlyBong
4.3. Sampling and Sample Preparation
4.4. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
4.5. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchis-Segura, C.; Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict. Biol. 2006, 11, 2–38. [Google Scholar] [CrossRef] [PubMed]
- Steketee, J.D.; Kalivas, P.W. Drug Wanting: Behavioral Sensitization and Relapse to Drug-Seeking Behavior. Pharmacol. Rev. 2011, 63, 348–365. [Google Scholar] [CrossRef]
- Bravo, R.R.; Faria, A.C.; Brito-Da-Costa, A.M.; Carmo, H.; Mladěnka, P.; da Silva, D.D.; Remião, F.; The OEMONOM Researchers. Cocaine: An Updated Overview on Chemistry, Detection, Biokinetics, and Pharmacotoxicological Aspects including Abuse Pattern. Toxins 2022, 14, 278. [Google Scholar] [CrossRef]
- Hall, F.; Li, X.-F.; Randall-Thompson, J.; Sora, I.; Murphy, D.; Lesch, K.-P.; Caron, M.; Uhl, G. Cocaine-conditioned locomotion in dopamine transporter, norepinephrine transporter and 5-HT transporter knockout mice. Neuroscience 2009, 162, 870–880. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Li, Q.; Zhong, Y.; Chen, L.; Du, Y.; He, J.; Liao, L.; Xiong, K.; Yi, C.-X.; et al. The Main Molecular Mechanisms Underlying Methamphetamine- Induced Neurotoxicity and Implications for Pharmacological Treatment. Front. Mol. Neurosci. 2018, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Saad, L.; Zwiller, J.; Kalsbeek, A.; Anglard, P. Epigenetic Regulation of Circadian Clocks and Its Involvement in Drug Addiction. Genes 2021, 12, 1263. [Google Scholar] [CrossRef]
- Teague, C.D.; Nestler, E.J. Key transcription factors mediating cocaine-induced plasticity in the nucleus accumbens. Mol. Psychiatry 2022, 27, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.F.; Fulton, S.L.; Maze, I. Epigenetics of Drug Addiction. Cold Spring Harb. Perspect. Med. 2021, 11, a040253. [Google Scholar] [CrossRef]
- Highfill, C.A.; Baker, B.M.; Stevens, S.D.; Anholt, R.R.H.; Mackay, T.F.C. Genetics of cocaine and methamphetamine consumption and preference in Drosophila melanogaster. PLOS Genet. 2019, 15, e1007834. [Google Scholar] [CrossRef]
- Baker, B.M.; Carbone, M.A.; Huang, W.; Anholt, R.R.H.; Mackay, T.F.C. Genetic basis of variation in cocaine and methamphetamine consumption in outbred populations of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2021, 118, e2104131118. [Google Scholar] [CrossRef]
- Heberlein, U.; Tsai, L.T.-Y.; Kapfhamer, D.; Lasek, A.W. Drosophila, a genetic model system to study cocaine-related behaviors: A review with focus on LIM-only proteins. Neuropharmacology 2009, 56, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Denno, M.E.; Privman, E.; Venton, B.J. Analysis of Neurotransmitter Tissue Content of Drosophila melanogaster in Different Life Stages. ACS Chem. Neurosci. 2015, 6, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T. TYRAMINE AND OCTOPAMINE: Ruling Behavior and Metabolism. Annu. Rev. Èntomol. 2005, 50, 447–477. [Google Scholar] [CrossRef]
- Dempsey, D.R.; Jeffries, K.A.; Bond, J.D.; Carpenter, A.-M.; Rodriguez-Ospina, S.; Breydo, L.; Caswell, K.K.; Merkler, D.J. Mechanistic and Structural Analysis of Drosophila melanogaster Arylalkylamine N-Acetyltransferases. Biochemistry 2014, 53, 7777–7793. [Google Scholar] [CrossRef]
- Dempsey, D.R.; Carpenter, A.-M.; Ospina, S.R.; Merkler, D.J. Probing the chemical mechanism and critical regulatory amino acid residues of Drosophila melanogaster arylalkylamine N-acyltransferase like 2. Insect Biochem. Mol. Biol. 2015, 66, 1–12. [Google Scholar] [CrossRef]
- Kaun, K.R.; Devineni, A.V.; Heberlein, U. Drosophila melanogaster as a model to study drug addiction. Hum. Genet. 2012, 131, 959–975. [Google Scholar] [CrossRef]
- Philyaw, T.J.; Rothenfluh, A.; Titos, I. The Use of Drosophila to Understand Psychostimulant Responses. Biomedicines 2022, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Cummins-Beebee, P.N.; Chvilicek, M.M.; Rothenfluh, A. The Stage-Based Model of Addiction—Using Drosophila to Investigate Alcohol and Psychostimulant Responses. Int. J. Mol. Sci. 2023, 24, 10909. [Google Scholar] [CrossRef]
- McClung, C.; Hirsh, J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr. Biol. 1998, 8, 109–112. [Google Scholar] [CrossRef]
- Filošević, A.; Al-Samarai, S.; Waldowski, R.A. High Throughput Measurement of Locomotor Sensitization to Volatilized Cocaine in Drosophila melanogaster. Front. Mol. Neurosci. 2018, 11, 25. [Google Scholar] [CrossRef]
- Andretic, R.; Chaney, S.; Hirsh, J. Requirement of Circadian Genes for Cocaine Sensitization in Drosophila. Science 1999, 285, 1066–1068. [Google Scholar] [CrossRef]
- Bainton, R.J.; Tsai, L.T.-Y.; Singh, C.M.; Moore, M.S.; Neckameyer, W.S.; Heberlein, U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr. Biol. 2000, 10, 187–194. [Google Scholar] [CrossRef]
- Andretic, R.; van Swinderen, B.; Greenspan, R.J. Dopaminergic Modulation of Arousal in Drosophila. Curr. Biol. 2005, 15, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; Filošević, A.; Petrović, M.; Jović, K.; Waldowski, R.A. Locomotor sensitization modulates voluntary self-administration of methamphetamine in Drosophila melanogaster. Addict. Biol. 2020, 26, e12963. [Google Scholar] [CrossRef]
- Mendrek, A.; Blaha, C.D.; Phillips, A.G. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacol. 1998, 135, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Belin, D.; Economidou, D.; Pelloux, Y.; Dalley, J.; Robbins, T. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lon. B Biol. Sci. 2008, 363, 3125–3135. [Google Scholar] [CrossRef]
- Giorgi, O.; Piras, G.; Corda, M.G. The psychogenetically selected Roman high- and low-avoidance rat lines: A model to study the individual vulnerability to drug addiction. Neurosci. Biobehav. Rev. 2007, 31, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Das, D.; Pradhan, J.; Ahmed, R.; Laureano-Melo, R.; Dandapat, J. Epigenetic signature in neural plasticity: The journey so far and journey ahead. Heliyon 2022, 8, e12292. [Google Scholar] [CrossRef]
- Limanaqi, F.; Gambardella, S.; Biagioni, F.; Busceti, C.L.; Fornai, F. Epigenetic Effects Induced by Methamphetamine and Methamphetamine-Dependent Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 4982453. [Google Scholar] [CrossRef]
- Chvilicek, M.M.; Titos, I.; Rothenfluh, A. The Neurotransmitters Involved in Drosophila Alcohol-Induced Behaviors. Front. Behav. Neurosci. 2020, 14, 607700. [Google Scholar] [CrossRef] [PubMed]
- Makos, M.A.; Han, K.-A.; Heien, M.L.; Ewing, A.G. Using in Vivo Electrochemistry to Study the Physiological Effects of Cocaine and Other Stimulants on the Drosophila melanogaster Dopamine Transporter. ACS Chem. Neurosci. 2010, 1, 74–83. [Google Scholar] [CrossRef]
- Vujnović, A.F.; Rubinić, M.; Starčević, I.; Waldowski, R.A. Influence of Redox and Dopamine Regulation in Cocaine-Induced Phenotypes Using Drosophila. Antioxidants 2023, 12, 933. [Google Scholar] [CrossRef]
- Li, H.; Chaney, S.; Forte, M.; Hirsh, J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr. Biol. 2000, 10, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.; Hirsh, J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr. Biol. 1999, 9, 853–860. [Google Scholar] [CrossRef]
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef]
- Fumagalli, F.; Gainetdinov, R.R.; Valenzano, K.J.; Caron, M.G. Role of Dopamine Transporter in Methamphetamine-Induced Neurotoxicity: Evidence from Mice Lacking the Transporter. J. Neurosci. 1998, 18, 4861–4869. [Google Scholar] [CrossRef]
- Kume, K.; Kume, S.; Park, S.K.; Hirsh, J.; Jackson, F.R. Dopamine Is a Regulator of Arousal in the Fruit Fly. J. Neurosci. 2005, 25, 7377–7384. [Google Scholar] [CrossRef] [PubMed]
- Van Swinderen, B.; Andretic, R. Dopamine in Drosophila: Setting arousal thresholds in a miniature brain. Proc. R. Soc. B Boil. Sci. 2011, 278, 906–913. [Google Scholar] [CrossRef]
- Ueno, T.; Tomita, J.; Tanimoto, H.; Endo, K.; Ito, K.; Kume, S.; Kume, K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 2012, 15, 1516–1523. [Google Scholar] [CrossRef]
- Xie, T.; Ho, M.C.; Liu, Q.; Horiuchi, W.; Lin, C.-C.; Task, D.; Luan, H.; White, B.H.; Potter, C.J.; Wu, M.N. A Genetic Toolkit for Dissecting Dopamine Circuit Function in Drosophila. Cell Rep. 2018, 23, 652–665. [Google Scholar] [CrossRef]
- Landayan, D.; Wolf, F.W. Shared neurocircuitry underlying feeding and drugs of abuse in Drosophila. Biomed. J. 2015, 38, 496–509. [Google Scholar] [CrossRef]
- DiCarlo, G.E.; Aguilar, J.I.; Matthies, H.J.; Harrison, F.E.; Bundschuh, K.E.; West, A.; Hashemi, P.; Herborg, F.; Rickhag, M.; Chen, H.; et al. Autism-linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine-dependent behaviors. J. Clin. Investig. 2019, 129, 3407–3419. [Google Scholar] [CrossRef]
- Lowenstein, E.G.; Velazquez-Ulloa, N.A. A Fly’s Eye View of Natural and Drug Reward. Front. Physiol. 2018, 9, 407. [Google Scholar] [CrossRef]
- Underhill, S.M.; Hullihen, P.D.; Chen, J.; Fenollar-Ferrer, C.; Rizzo, M.A.; Ingram, S.L.; Amara, S.G. Amphetamines signal through intracellular TAAR1 receptors coupled to Gα13 and GαS in discrete subcellular domains. Mol. Psychiatry 2021, 26, 1208–1223. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Swanson, T.L.; Miner, N.B.; Eshleman, A.J.; Janowsky, A. Activation of Trace Amine-Associated Receptor 1 Stimulates an Antiapoptotic Signal Cascade via Extracellular Signal-Regulated Kinase 1/2. Mol. Pharmacol. 2019, 96, 493–504. [Google Scholar] [CrossRef]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2011, 116, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Seto, E.S. Dopamine Dynamics and Signaling in Drosophila: An Overview of Genes, Drugs and Behavioral Paradigms. Exp. Anim. 2014, 63, 107–119. [Google Scholar] [CrossRef]
- Konradi, C.; Heckers, S. Molecular aspects of glutamate dysregulation: Implications for schizophrenia and its treatment. Pharmacol. Ther. 2003, 97, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Lopes, E.F.; Cragg, S.J. Axonal Modulation of Striatal Dopamine Release by Local γ-Aminobutyric Acid (GABA) Signalling. Cells 2021, 10, 709. [Google Scholar] [CrossRef] [PubMed]
- Bogomolova, E.; Rauschenbach, I.; Adonyeva, N.; Alekseev, A.; Faddeeva, N.; Gruntenko, N. Dopamine down-regulates activity of alkaline phosphatase in Drosophila: The role of D2-like receptors. J. Insect Physiol. 2010, 56, 1155–1159. [Google Scholar] [CrossRef]
- Faust, K.; Gehrke, S.; Yang, Y.; Yang, L.; Beal, M.F.; Lu, B. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2009, 10, 109. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filošević Vujnović, A.; Saftić Martinović, L.; Medija, M.; Andretić Waldowski, R. Distinct and Dynamic Changes in the Temporal Profiles of Neurotransmitters in Drosophila melanogaster Brain following Volatilized Cocaine or Methamphetamine Administrations. Pharmaceuticals 2023, 16, 1489. https://doi.org/10.3390/ph16101489
Filošević Vujnović A, Saftić Martinović L, Medija M, Andretić Waldowski R. Distinct and Dynamic Changes in the Temporal Profiles of Neurotransmitters in Drosophila melanogaster Brain following Volatilized Cocaine or Methamphetamine Administrations. Pharmaceuticals. 2023; 16(10):1489. https://doi.org/10.3390/ph16101489
Chicago/Turabian StyleFilošević Vujnović, Ana, Lara Saftić Martinović, Marta Medija, and Rozi Andretić Waldowski. 2023. "Distinct and Dynamic Changes in the Temporal Profiles of Neurotransmitters in Drosophila melanogaster Brain following Volatilized Cocaine or Methamphetamine Administrations" Pharmaceuticals 16, no. 10: 1489. https://doi.org/10.3390/ph16101489
APA StyleFilošević Vujnović, A., Saftić Martinović, L., Medija, M., & Andretić Waldowski, R. (2023). Distinct and Dynamic Changes in the Temporal Profiles of Neurotransmitters in Drosophila melanogaster Brain following Volatilized Cocaine or Methamphetamine Administrations. Pharmaceuticals, 16(10), 1489. https://doi.org/10.3390/ph16101489






