Vitamin E and Silymarin Reduce Oxidative Tissue Damage during Gentamycin-Induced Nephrotoxicity
Abstract
:1. Introduction
2. Results
2.1. Physiological and Biochemical Status
2.2. Kidney Histopathology
2.3. Analysis of Hydroxyproline (Hyp), MDA and 8-OHdG in Kidney Tissue
2.4. Activity of Antioxidant Enzymes
2.5. Determination of Oxidative Protein Remodeling in Kidney Tissue
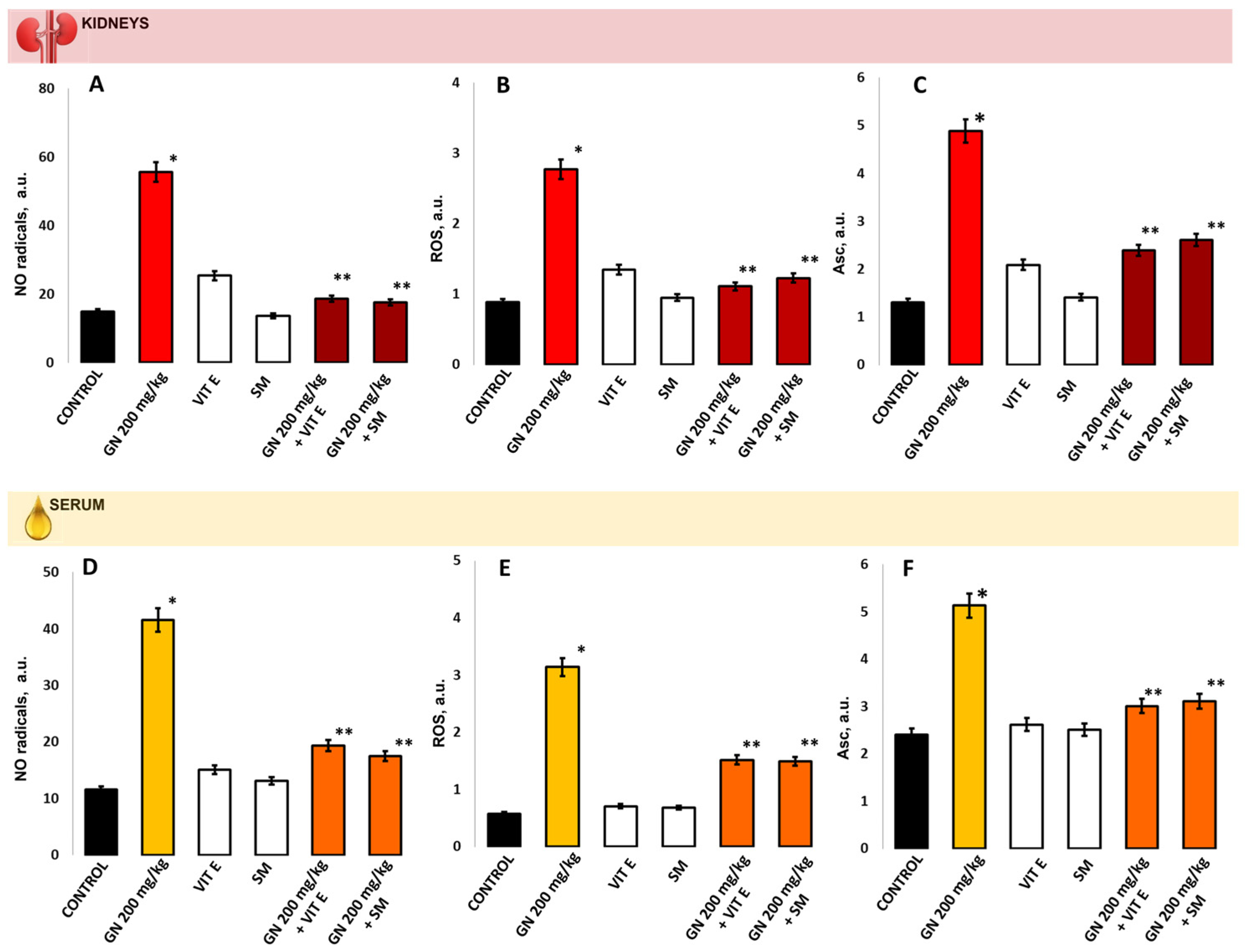
2.6. Parameters of Oxidative Damage in Kidney Tissue and Serum
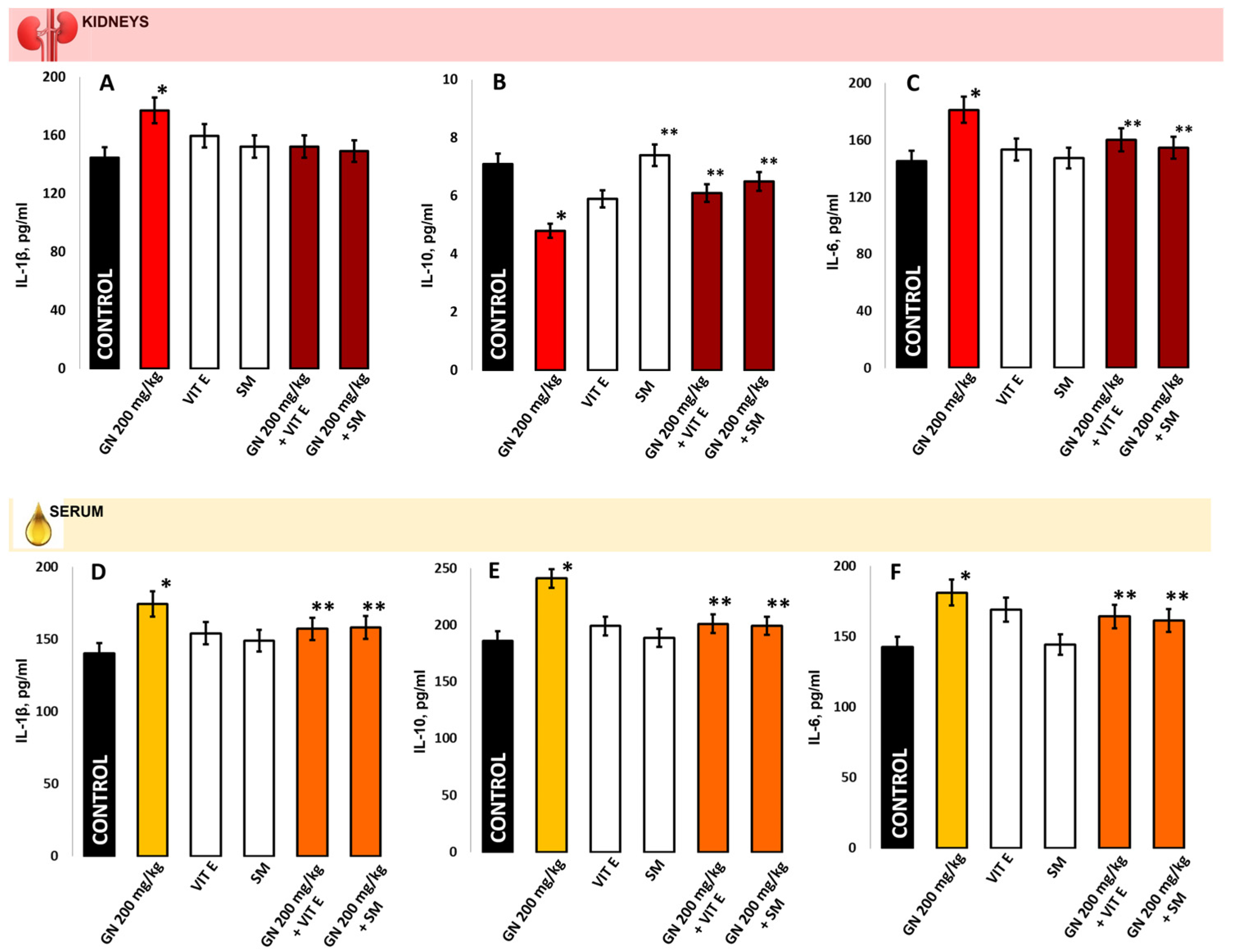
2.7. IL-1b, IL-6, IL-10 in Kidney Homogenate/Serum
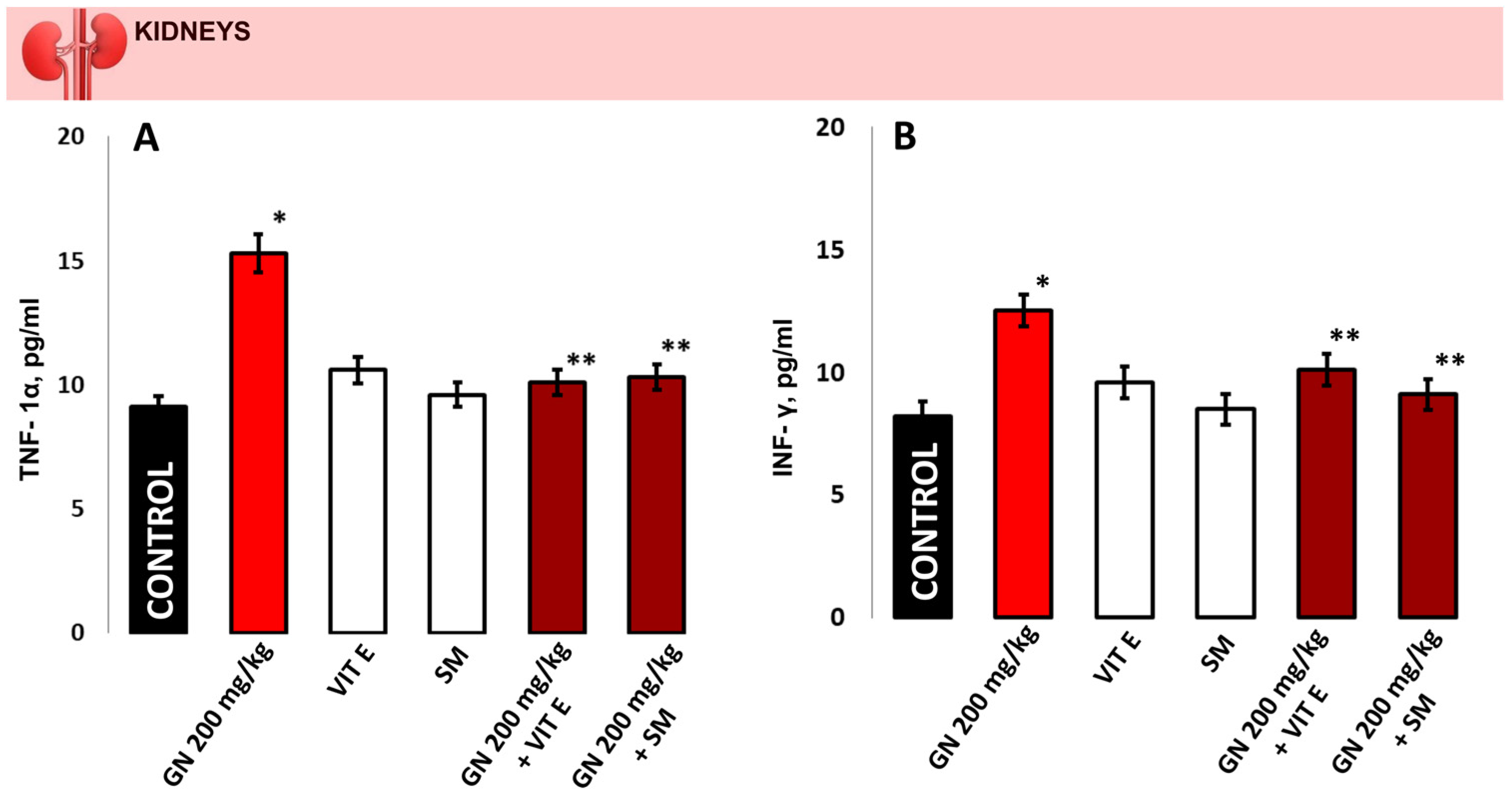
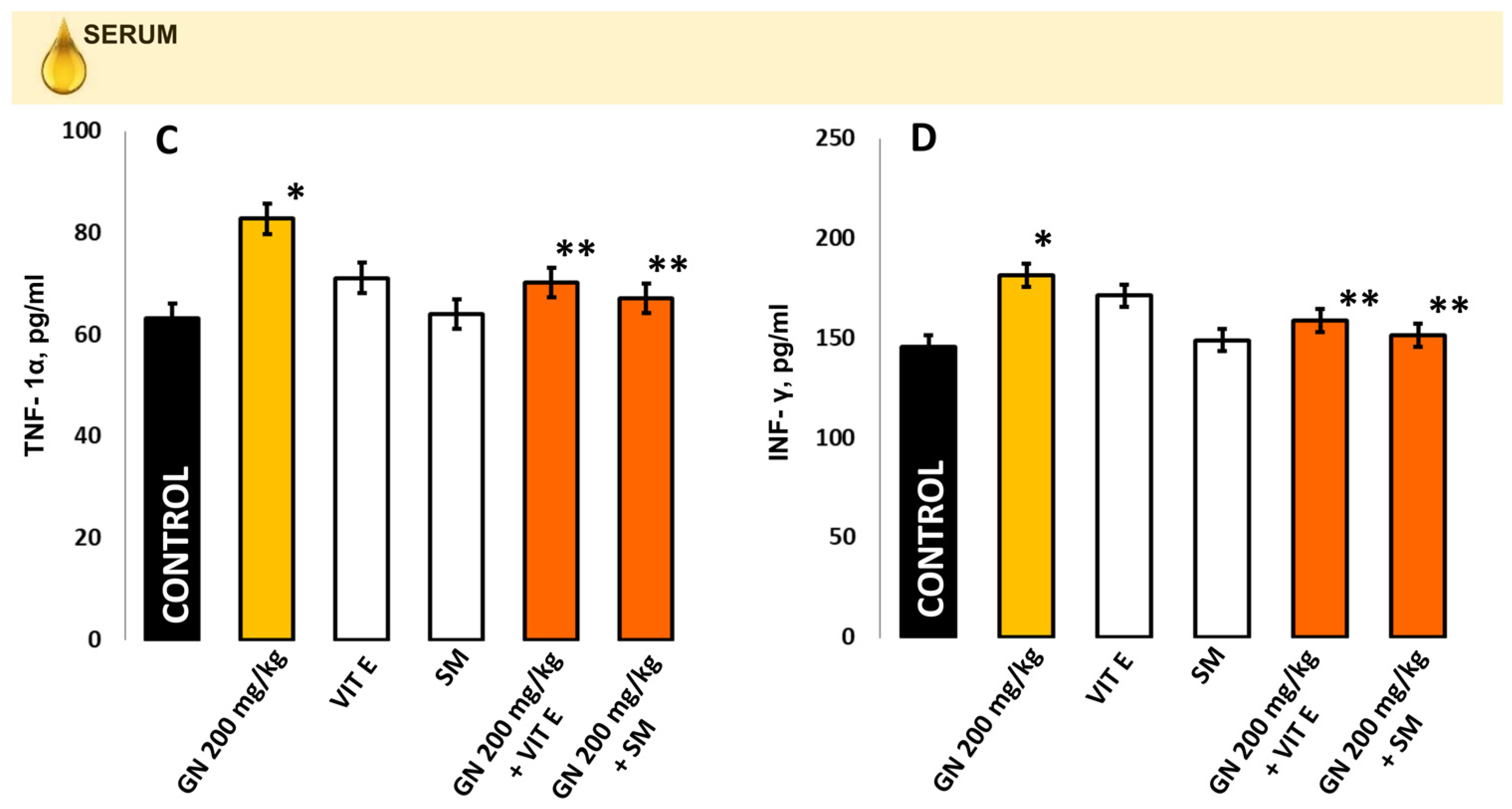
2.8. TNF-a and INF-γ Concentration
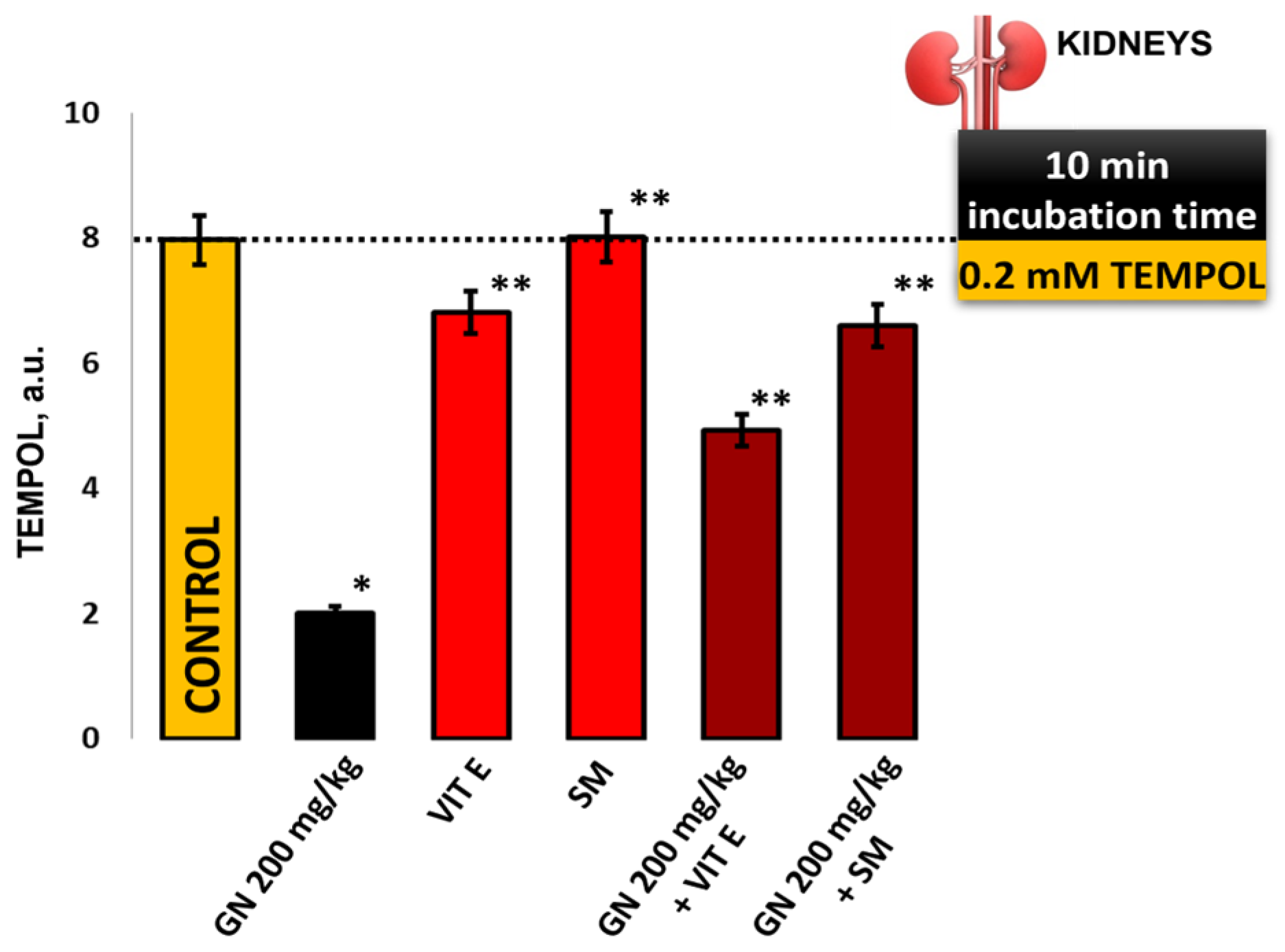
2.9. EPR Measurement of Oxidative Stress by 4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-Oxyl (TEMPOL)
3. Discussion
4. Materials and Methods
4.1. Plant Material and Drugs
4.2. Animals, Experimental Design, and Ethical Approval
4.3. Dissection Procedure
4.4. Histological Analysis for Visualization of Kidney Changes
4.5. Analysis of Hydroxyproline (Hyp) in Kidney Tissue
4.6. Determination of Glutathione S-Transferase (GST)
4.7. Protocol for Functional Markers Measurement of Kidney Damage
4.8. Electron Paramagnetic Resonance (EPR) Study
4.8.1. Evaluation of the ROS Product Levels
4.8.2. Evaluation of the •NO Radical Levels
4.8.3. TEMPOL
4.9. Immunoenzyme Assays
4.9.1. Determination of the Activity of Antioxidant Enzyme System and Products of Oxidation of Proteins and Lipids in the Kidney
4.9.2. Measurement of Proinflammatory Markers in Kidney Tissue and Blood
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- von Mässenhausen, A.; Tonnus, W.; Linkermann, A. Cell Death Pathways Drive Necroinflammation during Acute Kidney Injury. Nephron 2018, 140, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, X.; Xie, X.; Liu, Y.; Friedmann Angeli, J.P.; Lai, L. Activation of Glutathione Peroxidase 4 as a Novel Anti-inflammatory Strategy. Front. Pharmacol. 2018, 9, 1120. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Xie, M.; Kang, R.; Fan, Y.; Niu, X.; Wang, H.; Cao, L.; Tang, D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 2015, 34, 5617–5625. [Google Scholar] [CrossRef] [PubMed]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Martin-Sanchez, D.; Ruiz-Andres, O.; Poveda, J.; Carrasco, S.; Cannata-Ortiz, P.; Sanchez-Niño, M.D.; Ruiz Ortega, M.; Egido, J.; Linkermann, A.; Ortiz, A.; et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI. J. Am. Soc. Nephrol. 2017, 28, 218–229. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, D.; Zhao, L.; Li, W.; Han, J.; Hu, B.; Nie, G.; He, Y. Liproxstatin-1 Protects Hair Cell-Like HEI-OC1 Cells and Cochlear Hair Cells against Neomycin Ototoxicity. Oxid. Med. Cell. Longev. 2020, 24, 12065–12081. [Google Scholar] [CrossRef]
- Randjelovic, P.; Veljkovic, S.; Stojiljkovic, N.; Sokolovic, D.; Ilic, I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017, 24, 388–399. [Google Scholar] [CrossRef]
- Fan, B.Y.; Pang, Y.L.; Li, W.X.; Zhao, C.X.; Zhang, Y.; Wang, X.; Ning, G.Z.; Kong, X.H.; Liu, C.; Yao, X.; et al. Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 2021, 16, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Arora, R.; Chawla, R.; Sharma, J.; Dhaker, A.S.; Gupta, D.; Dubey, N.; Kumar, R.; Ivanov, V.; Gadjeva, V.; et al. Evaluation of silymarin as a promising radioprotector. Z. Naturforsch. C J. Biosci. 2010, 65, 337–346. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.; Liu, Y.; Graf, T.N.; Oberlies, N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J. Basic Med. Sci. 2011, 14, 308–317. [Google Scholar]
- Ivanov, V.; Slavova, V.; Georgieva, D.; Petrova-Tacheva, V.; Tolekova, A. Beneficial effects of silymarin against nephrotoxicity caused by some chemicals. Trakia J. Sci. 2021, 19, 178–184. [Google Scholar] [CrossRef]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of Reactive Oxygen and Nitrogen Species by Electron Paramagnetic Resonance (EPR) Technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron Paramagnetic Resonance Measurements of Reactive Oxygen Species by Cyclic Hydroxylamine Spin Probes. Antioxid. Redox Signal. 2018, 28, 1433–1443. [Google Scholar] [CrossRef]
- Babić, N.; Peyrot, F. Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry 2019, 5, 13. [Google Scholar] [CrossRef]
- Delgadillo-Valero, L.F.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. The Protective Role of Ozone Therapy in Kidney Disease: A Review. Life 2023, 13, 752. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Yang, W.; Wang, S.; Geng, C.; Guan, X. SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem. Biophys. Res. Commun. 2021, 561, 33–39. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, T.; Hadzhibozheva, P.; Karamalakova, Y.; Georgieva, E.; Perinkadakatt, F.; Ilinov, Z.; Petkov, K.; Ananiev, J. Therapeutic approach of glutathione/glutathione peroxidase-4 axis modulation in the light of ferroptosis. Pharmacia 2022, 69, 839–846. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Andia, A.A.; Liu, H.; Csuka, J.M.; Hurlocker, B.; Vaiana, C.A.; Heindel, D.W.; Zuckerman, D.S.; Bos, P.H.; Reznik, E.; et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 2018, 14, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Yang, Y.R.; Ma, W.X.; Wang, H.Y.; Fan, Q.W.; Wang, Y.Y.; Li, C.; Wang, J.; Hu, Z.M.; Wang, X.F.; et al. Epigallocatechin Gallate Attenuates Gentamicin-Induced Nephrotoxicity by Suppressing Apoptosis and Ferroptosis. Molecules 2022, 27, 8564. [Google Scholar] [CrossRef]
- Baltusnikiene, A.; Staneviciene, I.; Jansen, E. Beneficial and adverse effects of vitamin E on the kidney. Front. Physiol. 2023, 14, 1145216. [Google Scholar] [CrossRef]
- Galli, F.; Bonomini, M.; Bartolini, D.; Zatini, L.; Reboldi, G.; Marcantonini, G.; Gentile, G.; Sirolli, V.; Di Pietro, N. Vitamin E (Alpha-Tocopherol) Metabolism and Nutrition in Chronic Kidney Disease. Antioxidants 2022, 11, 989. [Google Scholar] [CrossRef]
- Sneddon, A.A.; Wu, H.C.; Farquharson, A.; Grant, I.; Arthur, J.R.; Rotondo, D.; Choe, S.N.; Wahle, K.W. Regulation of selenoprotein GPx4 expression and activity in human endothelial cells by fatty acids, cytokines and antioxidants. Atherosclerosis 2003, 171, 57–65. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Silva, C.A.; Barbieri, C.D.; Oliveira, G.M.; Zanetta, D.M.; Burdmann, E.A. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob. Agents Chemother. 2009, 53, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Wu, L.J.; Tashiro, S.I.; Onodera, S.; Uchiumi, F.; Ikejima, T. Activation of the SIRT1 pathway and modulation of the cell cycle were involved in silymarin’s protection against UV-induced A375-S2 cell apoptosis. J. Asian Nat. Prod. Res. 2007, 9, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Barbagallo, I.; Godos, J.; Lembo, V.; Currenti, W.; Cinà, D.; Avola, R.; D’Orazio, N.; Morisco, F.; Galvano, F.; et al. Silibinin Restores NAD⁺ Levels and Induces the SIRT1/AMPK Pathway in Non-Alcoholic Fatty Liver. Nutrients 2017, 9, 1086. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A. Colombo R Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Alshammari, G.M.; Ajarem, J.S.; Al-Zahrani, A.Y.; Alzuwaydi, A.; Eid, R.; Yahya, M.A. Isoliquiritigenin prevents Doxorubicin-induced hepatic damage in rats by upregulating and activating SIRT1. Biomed. Pharmacother. 2022, 146, 112594. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in mast cell-mediated immune responses: Anti-inflammatory and proinflammatory roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Cevey, Á.C.; Penas, F.N.; Alba Soto, C.D.; Mirkin, G.A.; Goren, N.B. IL-10/STAT3/SOCS3 axis is involved in the anti-inflammatory effect of benznidazole. Front. Immunol. 2019, 10, 1267. [Google Scholar] [CrossRef]
- Akbaribazm, M.; Goodarzi, N.; Rahimi, M.; Naseri, L.; Khazaei, M. Anti-inflammatory, anti-oxidative and anti-apoptotic effects of Heracleum persicum L. extract on rats with gentamicin-induced nephrotoxicity. Asian Pac. J. Trop. Biomed. 2021, 11, 47. [Google Scholar]
- Torricella, F.; Pierro, A.; Mileo, E.; Belle, V.; Bonucci, A. Nitroxide spin labels and EPR spectroscopy: A powerful association for protein dynamics studies. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140653. [Google Scholar] [CrossRef] [PubMed]
- Rančić, A.; Babić, N.; Orio, M.; Peyrot, F. Structural Features Governing the Metabolic Stability of Tetraethyl-Substituted Nitroxides in Rat Liver Microsomes. Antioxidants 2023, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Davis, R.M.; Mitchell, J.B.; Krishna, M.C. Nitroxides as cancer imaging agents. Anticancer Agents Med. Chem. 2011, 11, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as Antioxidants and Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Cao, P.; Ito, O.; Ito, D.; Rong, R.; Zheng, Y.; Kohzuki, M. Combination of Exercise Training and SOD Mimetic Tempol Enhances Upregulation of Nitric Oxide Synthase in the Kidney of Spontaneously Hypertensive Rats. Int. J. Hypertens. 2020, 2020, 2142740. [Google Scholar] [CrossRef]
- Chatterjee, P.K.; Cuzzocrea, S.; Brown, P.A.; Zacharowski, K.; Stewart, K.N.; Mota-Filipe, H.; Thiemermann, C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000, 58, 658–673. [Google Scholar] [CrossRef]
- Fujii, H.G.; Emoto, M.C.; Sato-Akaba, H. Brain Redox Imaging Using In Vivo Electron Paramagnetic Resonance Imaging and Nitroxide Imaging Probes. Magnetochemistry 2019, 5, 11. [Google Scholar] [CrossRef]
- Woessner, J.B. The determination of hidroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Phys. Part C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Iwamoto, N.; Lto, K. An application of Electron Paramagnetic Resonance to evaluate nitric oxide and its quenchers. J. Am. Soc. Nephrol. 1996, 7, 961–965. [Google Scholar] [CrossRef]
- Yokoyama, K.; Hashiba, K.; Wakabayashi, H.; Hashimoto, K.; Saton, K.; Kurihara, T.; Motohashi, N.; Sakagami, H. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by Tropolones. Anticancer Res. 2004, 24, 3917–3922. [Google Scholar]
- Georgieva, E.; Atanasov, V.; Kostandieva, R.; Tsoneva, V.; Mitev, M.; Arabadzhiev, G.; Yovchev, Y.; Karamalakova, Y.; Nikolova, G. Direct Application of 3-Maleimido-PROXYL for Proving Hypoalbuminemia in Cases of SARS-CoV-2 Infection: The Potential Diagnostic Method of Determining Albumin Instability and Oxidized Protein Level in Severe COVID-19. Int. J. Mol. Sci. 2023, 24, 5807. [Google Scholar] [CrossRef]
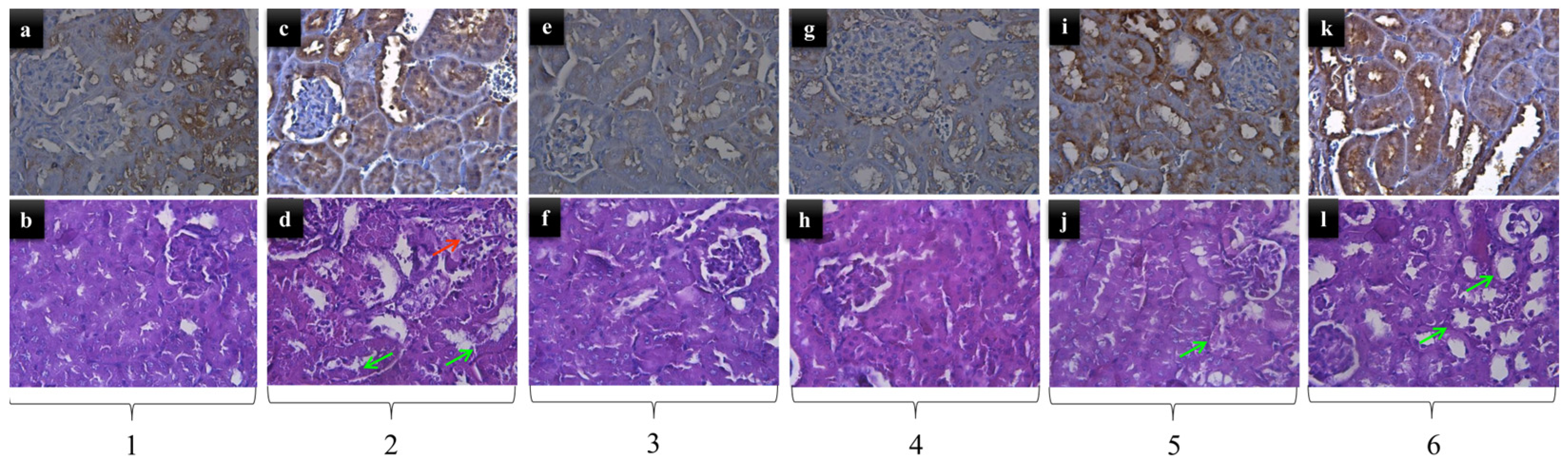
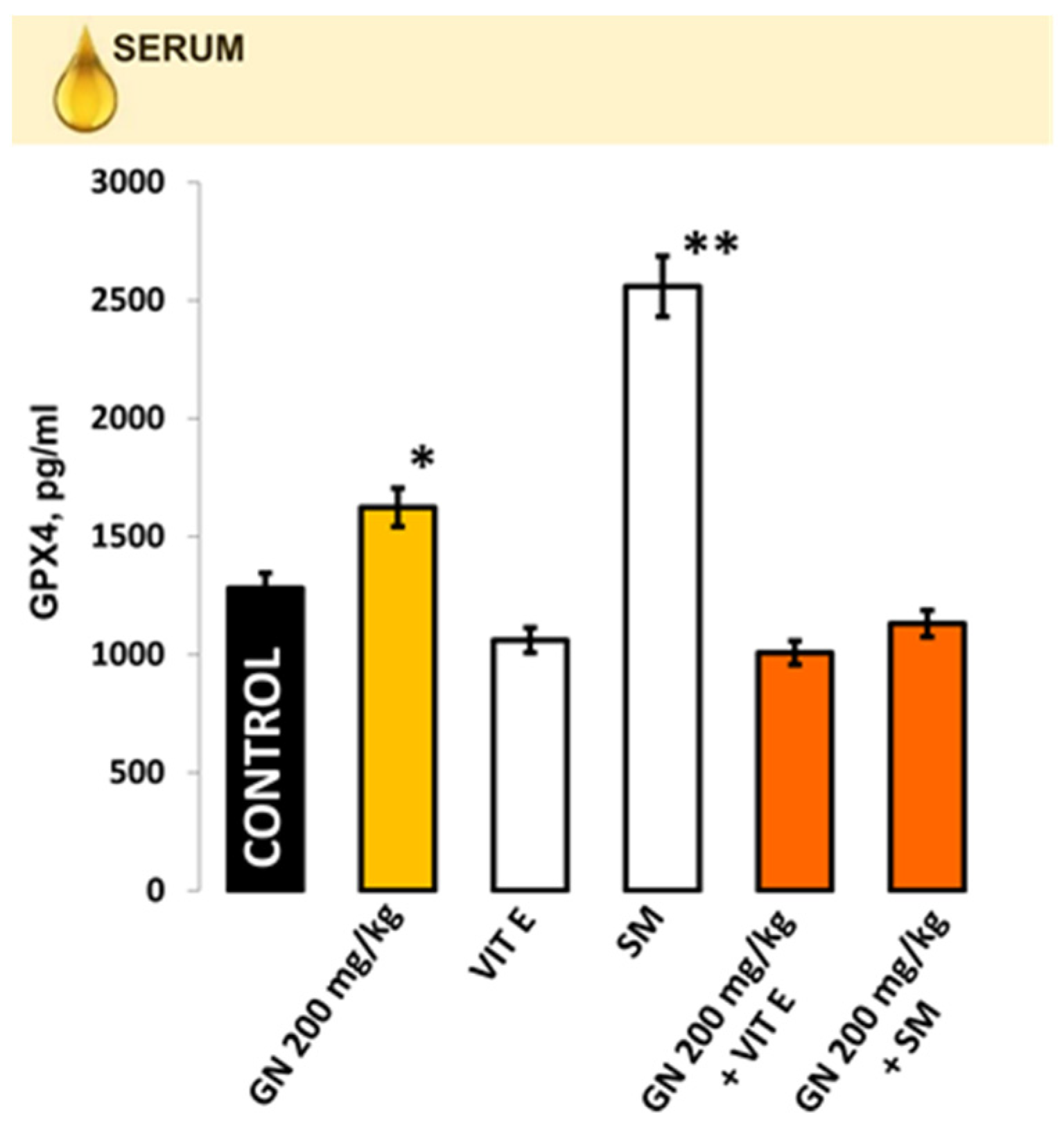

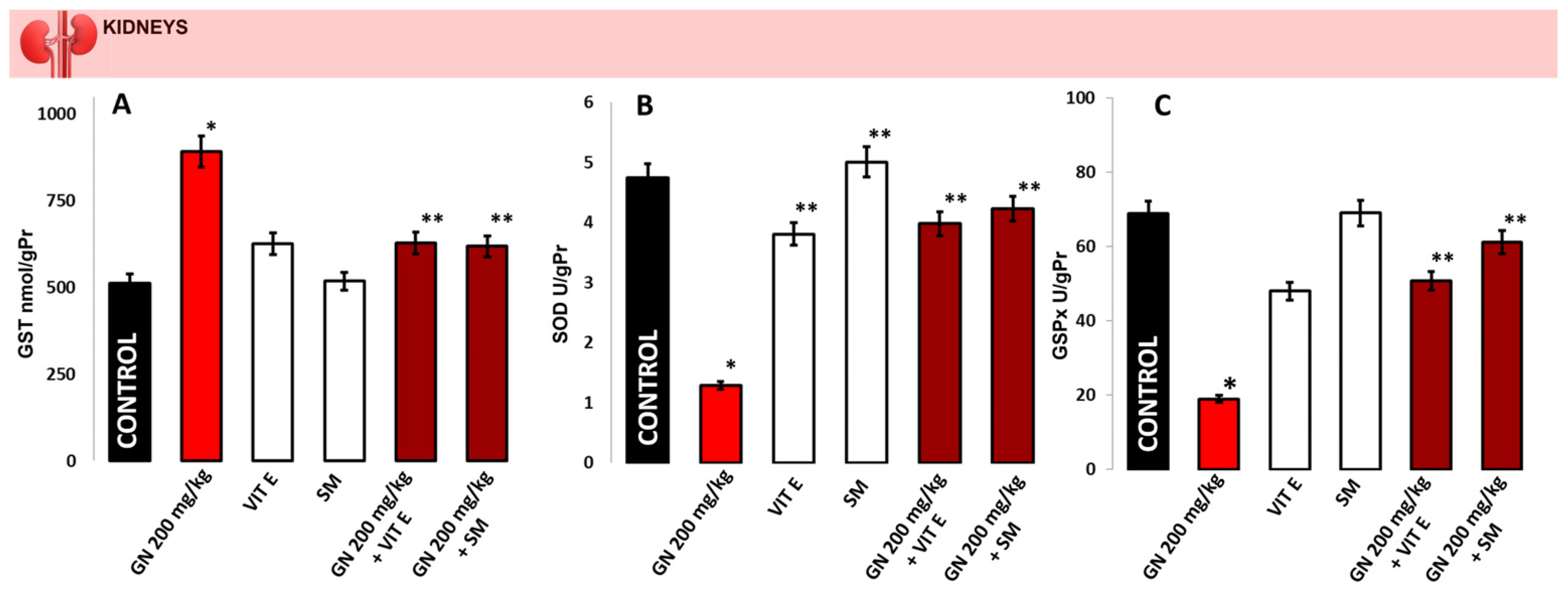
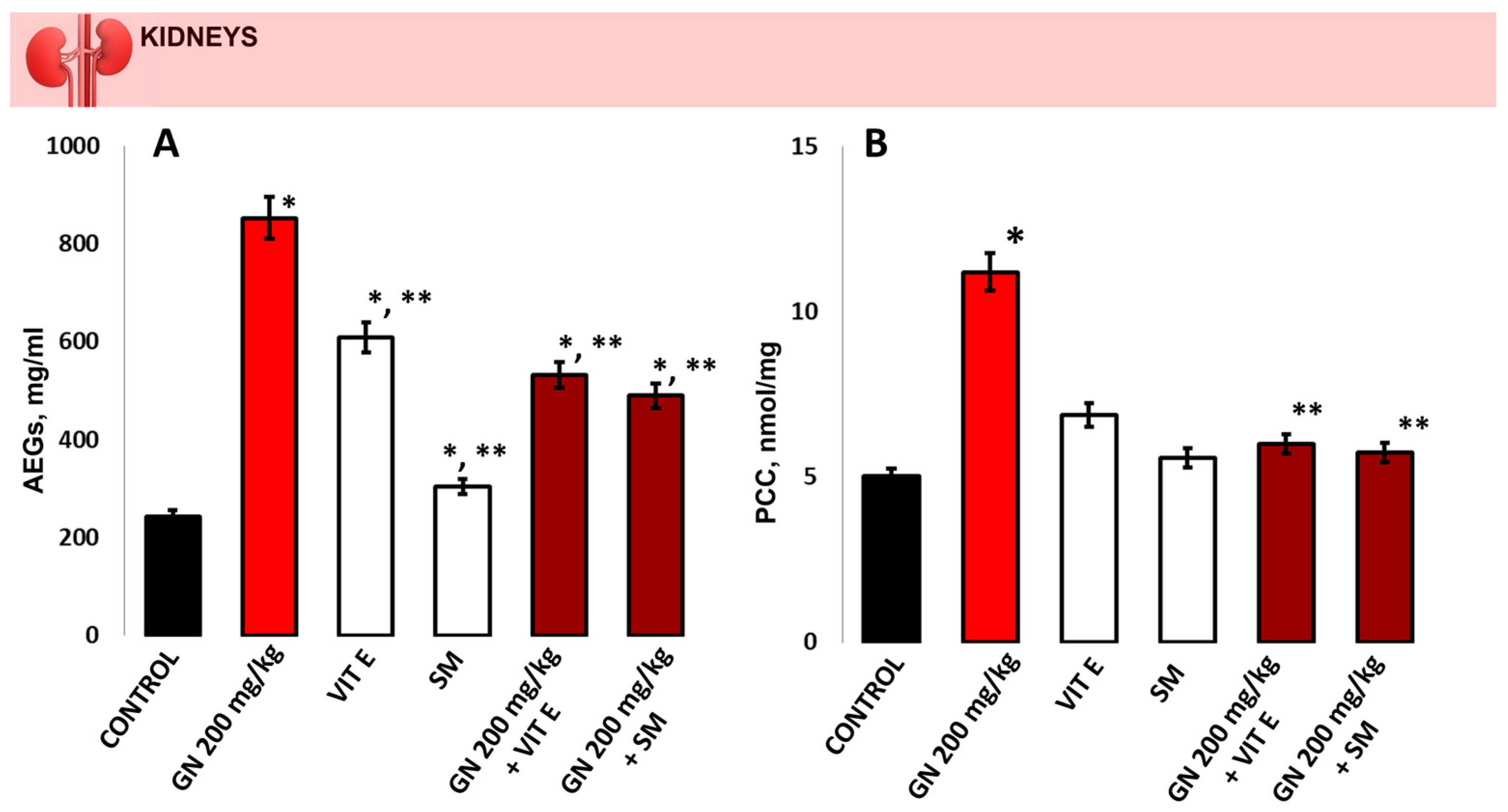
| Administration (n = 6) | Creatinine Umol/L | Urea mmol/L | Na+ mmol/L | K+ mmol/L |
|---|---|---|---|---|
| Control | 27.2 ± 3.2 | 7.5 ± 1.3 | 148.5 ± 5.1 | 5.3 ± 0.7 |
| GN 200 mg/kg | 37.1 ± 3.3 * | 12.6 ± 2.3 * | 142.4 ± 4.8 | 6.7 ± 1.2 |
| VIT E | 27.3 ± 2.8 | 6.8 ± 1.1 | 152.0 ± 5.2 | 5.4 ± 0.6 |
| SM | 26.0 ± 2.6 | 5.7 ± 0.9 | 149.1 ± 5.4 | 6.2 ± 0.8 |
| GN 200 mg/kg + VIT E | 22.1 ± 2.3 | 8.7 ± 1.3 ** | 151.2 ± 4.9 | 5.9 ± 0.8 |
| GN 200 mg/kg + SM | 24.2 ± 2.5 | 8.9 ± 1.3 ** | 149.3 ± 4.5 | 6.2 ± 1.1 |
| Groups (n = 6) | GPX4 | Degeneration | Necrosis | Inflammation | Hyperemia |
|---|---|---|---|---|---|
| Control | 3+ | 0 | 0 | 0 | 0 |
| GN 200 mg/kg | 2+ | 1 | 0 | 1 | 1 |
| VIT E | 1+ | 0 | 0 | 0 | 0 |
| SM | 2+ | 0 | 0 | 0 | 0 |
| GN 200 mg/kg + VIT E | 4+ | 0/1 | 0 | 0 | 0 |
| GN 200 mg/kg + SM | 3+ | 0/1 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, T.; Nikolova, G.; Dyakova, V.; Karamalakova, Y.; Georgieva, E.; Ananiev, J.; Ivanov, V.; Hadzhibozheva, P. Vitamin E and Silymarin Reduce Oxidative Tissue Damage during Gentamycin-Induced Nephrotoxicity. Pharmaceuticals 2023, 16, 1365. https://doi.org/10.3390/ph16101365
Georgiev T, Nikolova G, Dyakova V, Karamalakova Y, Georgieva E, Ananiev J, Ivanov V, Hadzhibozheva P. Vitamin E and Silymarin Reduce Oxidative Tissue Damage during Gentamycin-Induced Nephrotoxicity. Pharmaceuticals. 2023; 16(10):1365. https://doi.org/10.3390/ph16101365
Chicago/Turabian StyleGeorgiev, Tsvetelin, Galina Nikolova, Viktoriya Dyakova, Yanka Karamalakova, Ekaterina Georgieva, Julian Ananiev, Veselin Ivanov, and Petya Hadzhibozheva. 2023. "Vitamin E and Silymarin Reduce Oxidative Tissue Damage during Gentamycin-Induced Nephrotoxicity" Pharmaceuticals 16, no. 10: 1365. https://doi.org/10.3390/ph16101365
APA StyleGeorgiev, T., Nikolova, G., Dyakova, V., Karamalakova, Y., Georgieva, E., Ananiev, J., Ivanov, V., & Hadzhibozheva, P. (2023). Vitamin E and Silymarin Reduce Oxidative Tissue Damage during Gentamycin-Induced Nephrotoxicity. Pharmaceuticals, 16(10), 1365. https://doi.org/10.3390/ph16101365










