Euphorbia royleana Boiss Derived Silver Nanoparticles and Their Applications as a Nanotherapeutic Agent to Control Microbial and Oxidative Stress-Originated Diseases
Abstract
:1. Introduction
2. Results
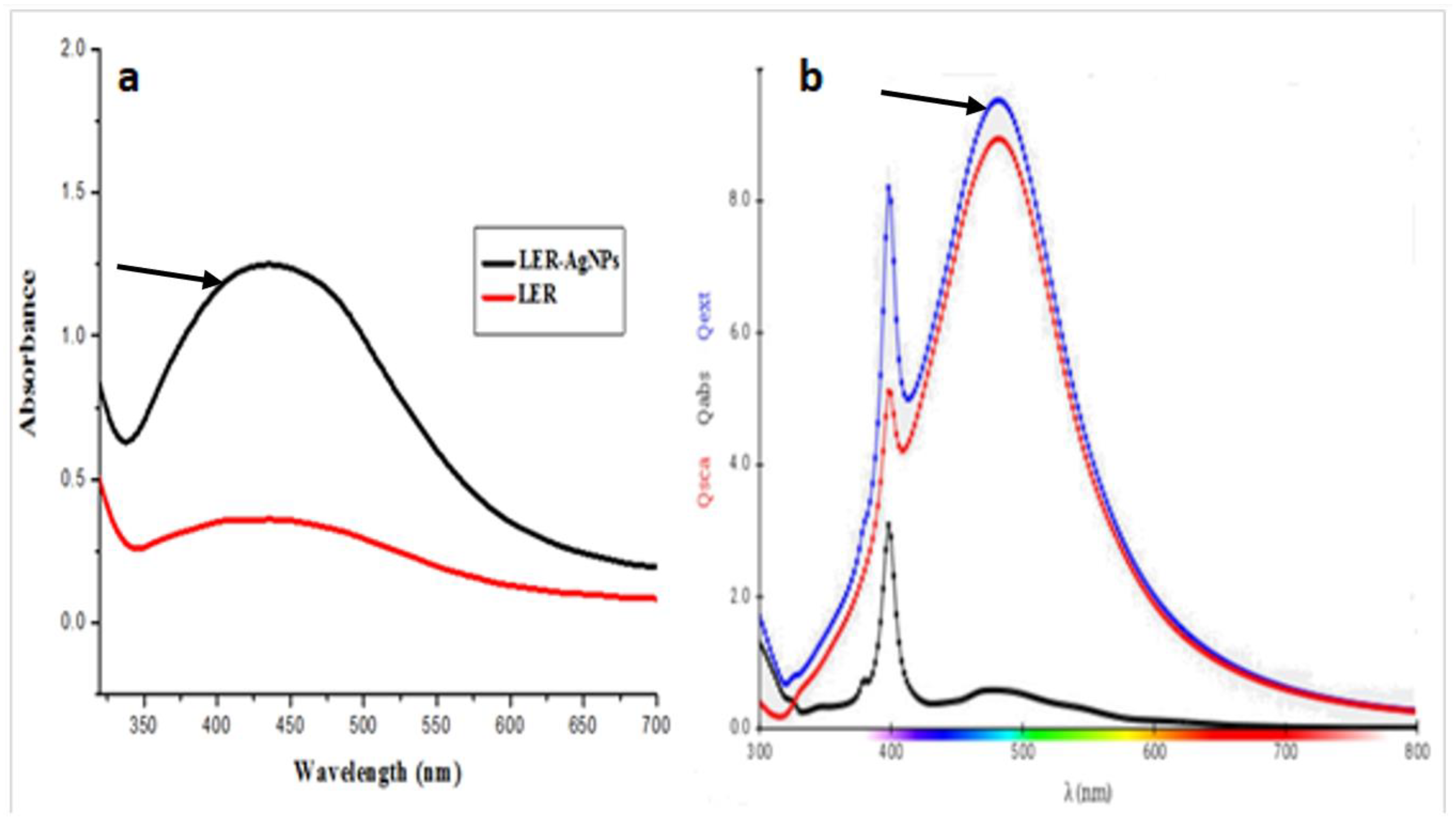
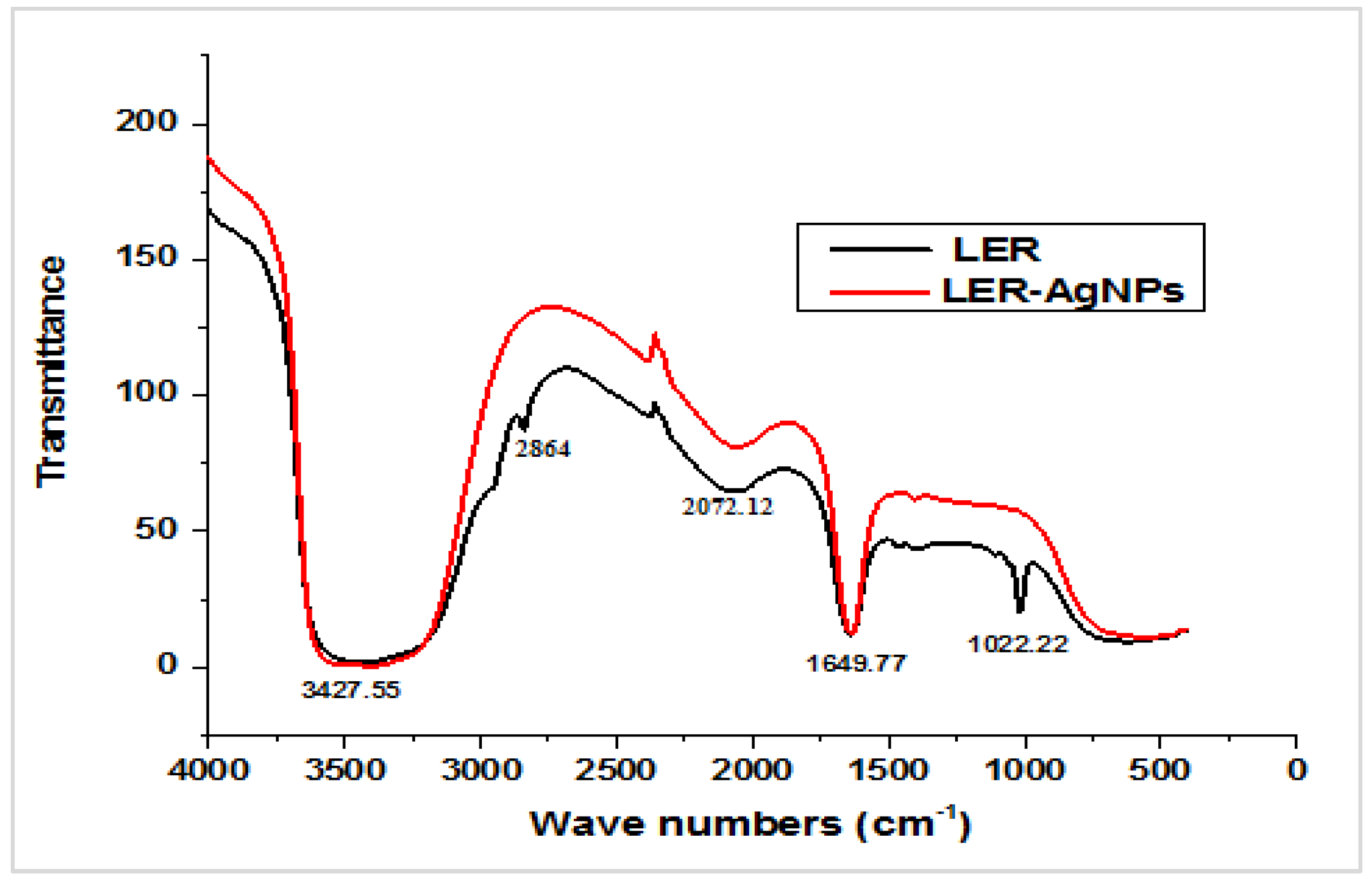
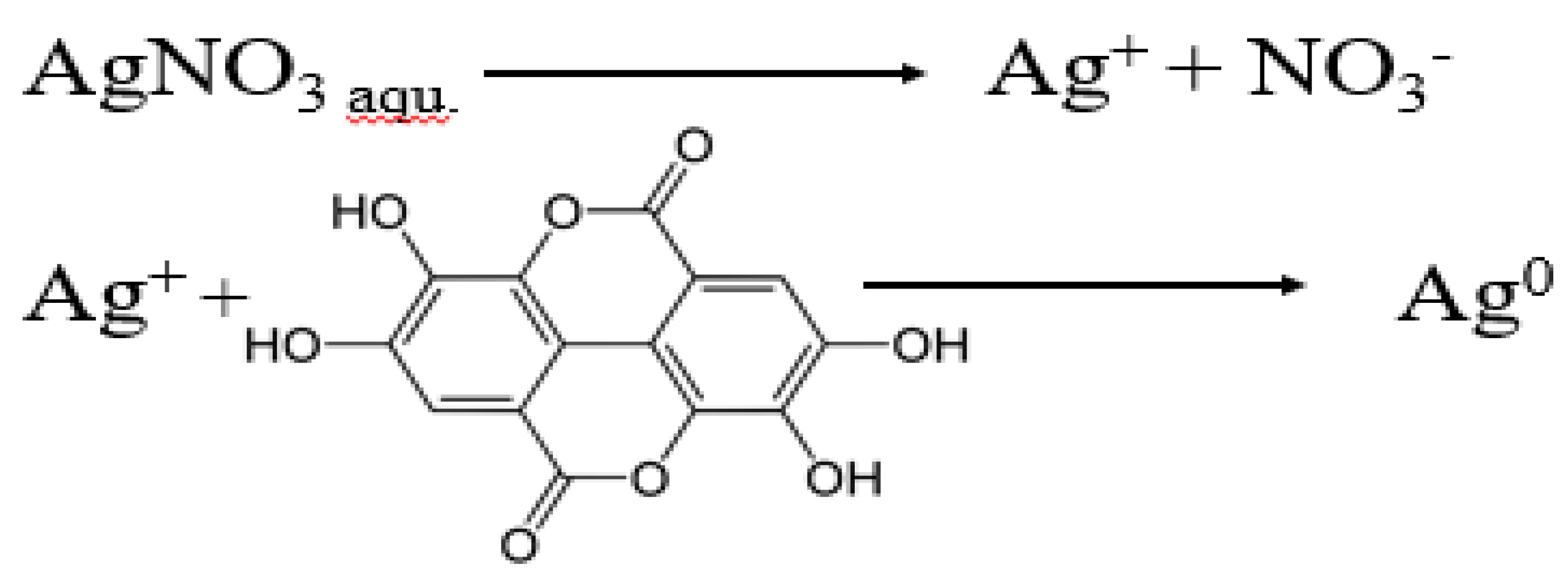
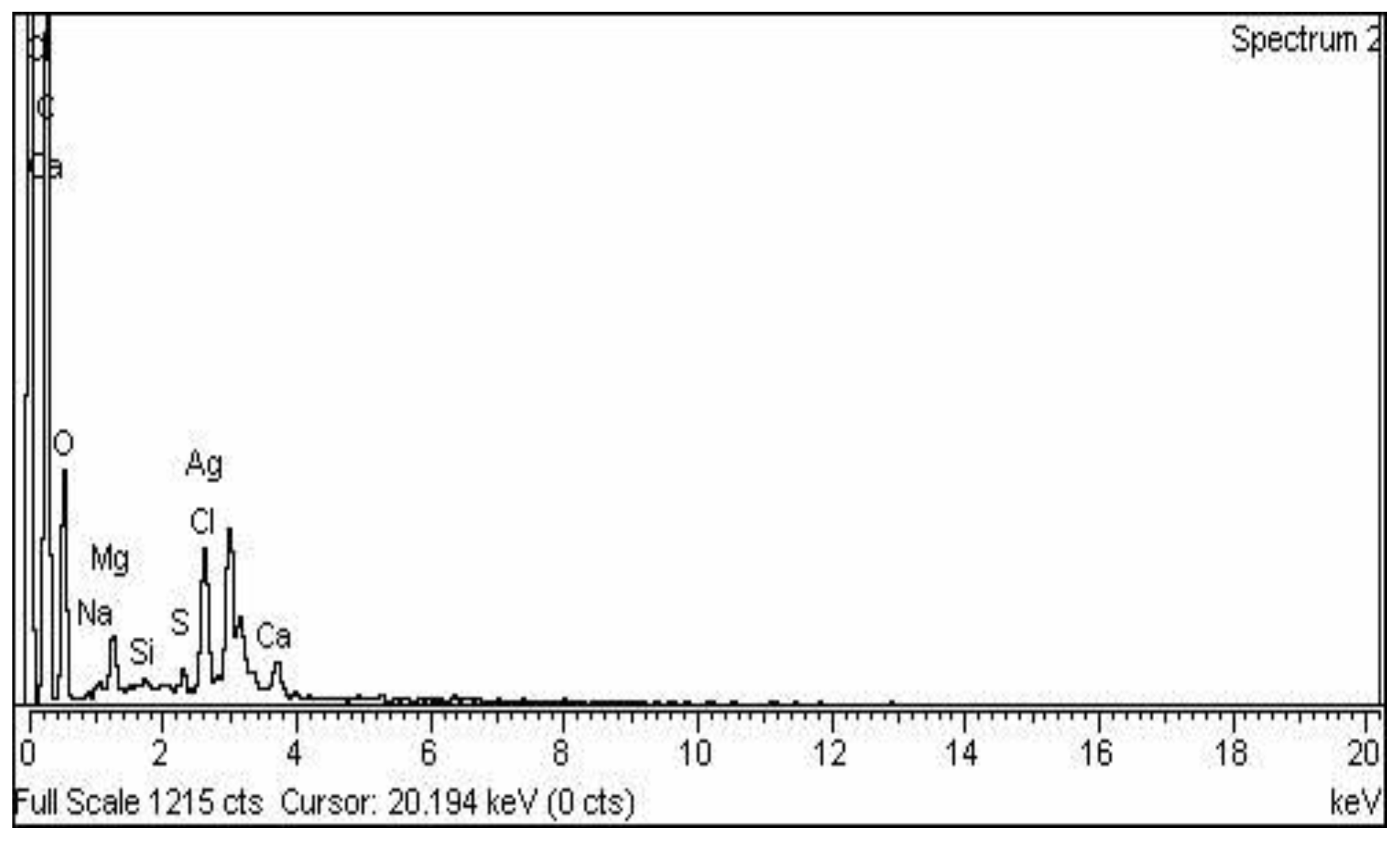
2.1. Nanoparticle Synthesis and Characterization
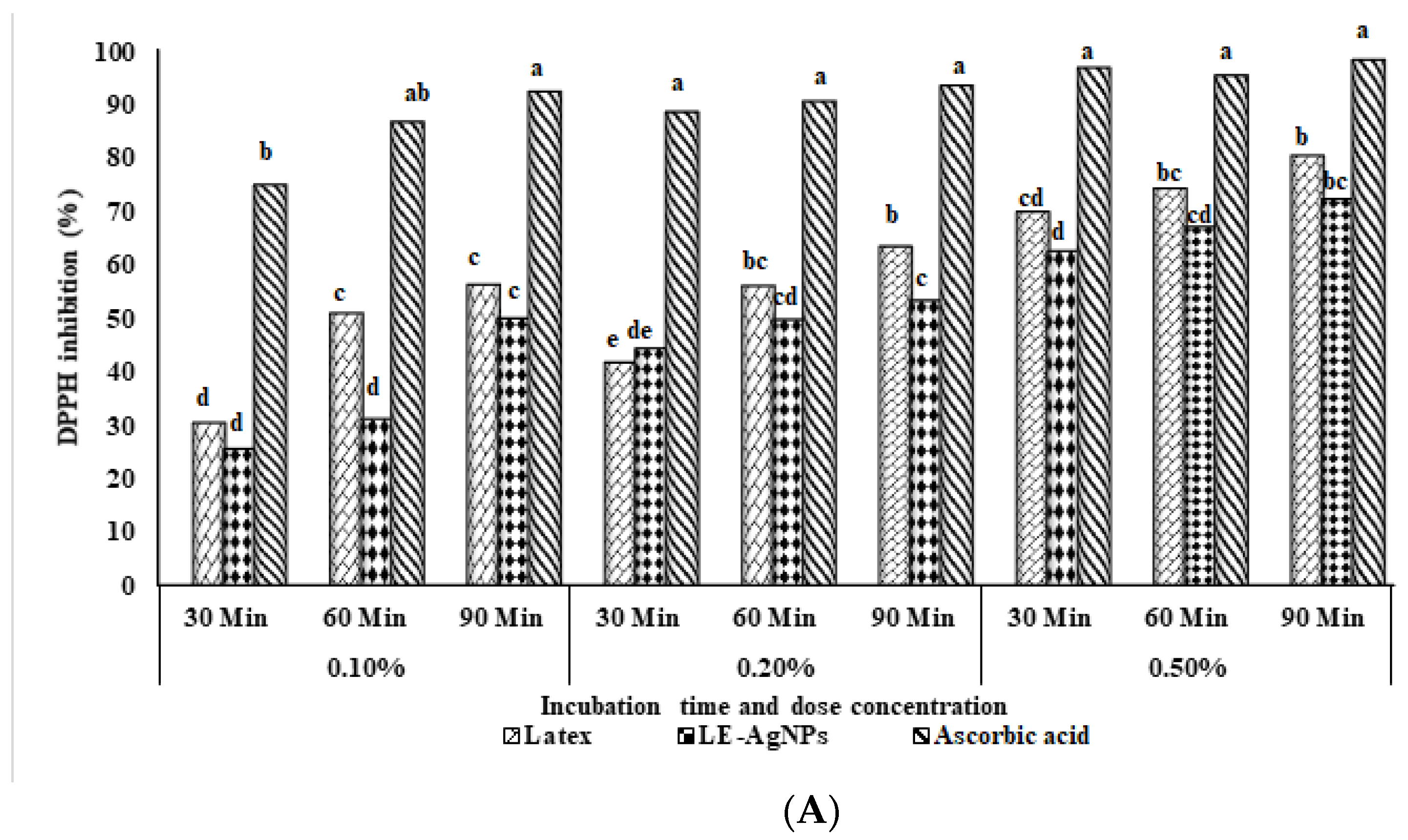
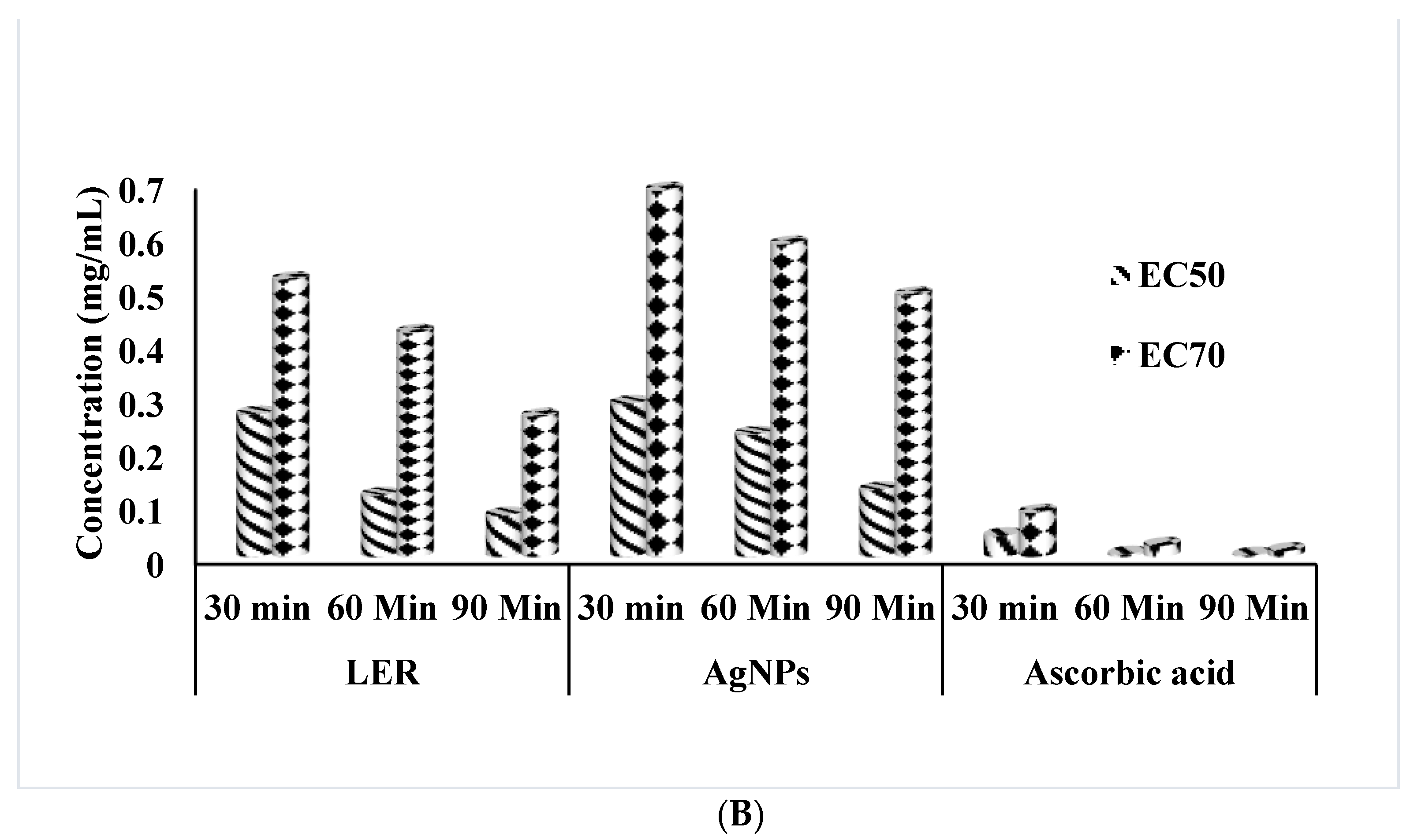
2.2. DPPH Scavenging Activity
2.3. Hydroxyl Scavenging Potential
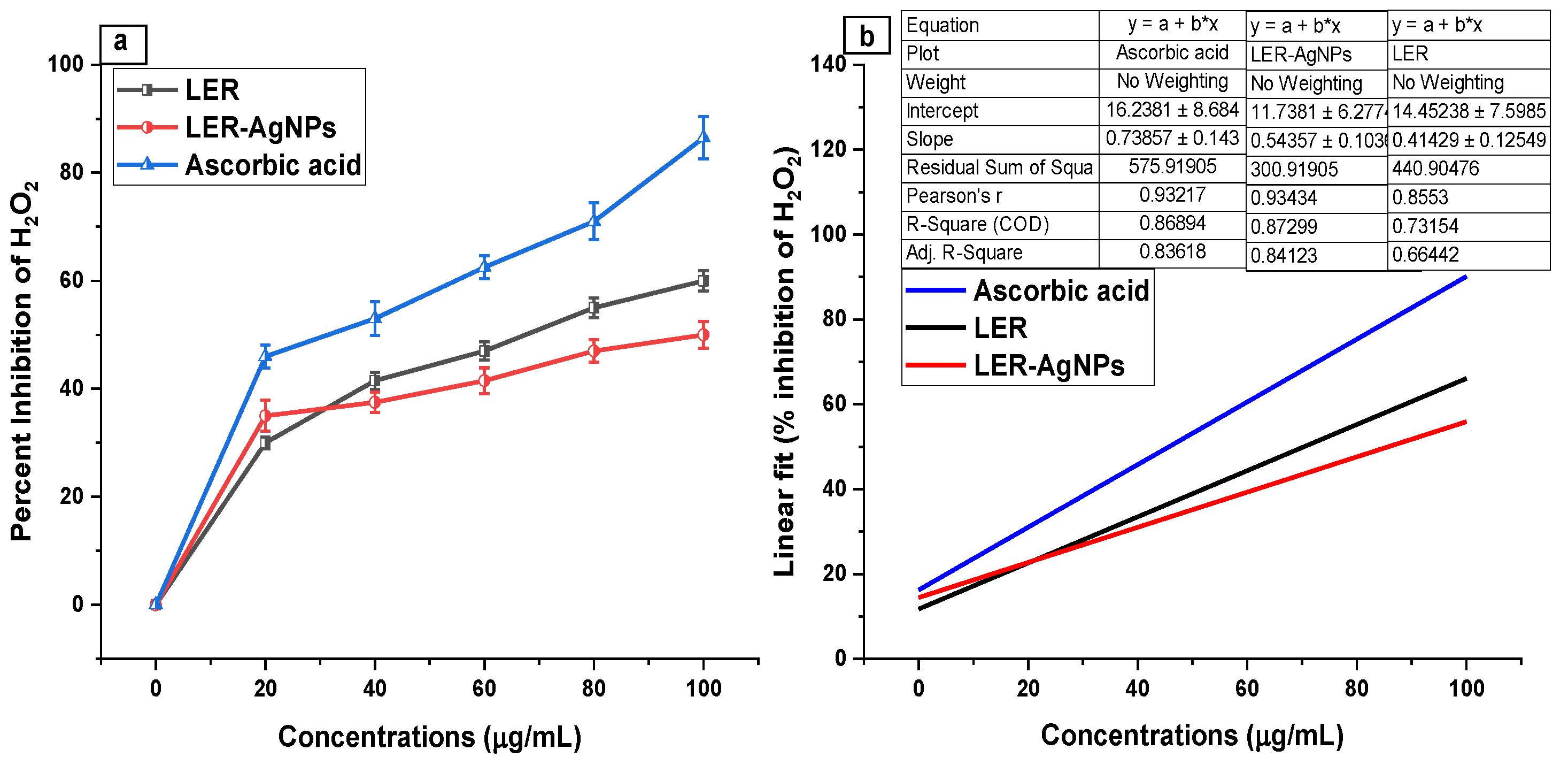
2.4. Hydrogen Peroxide Scavenging
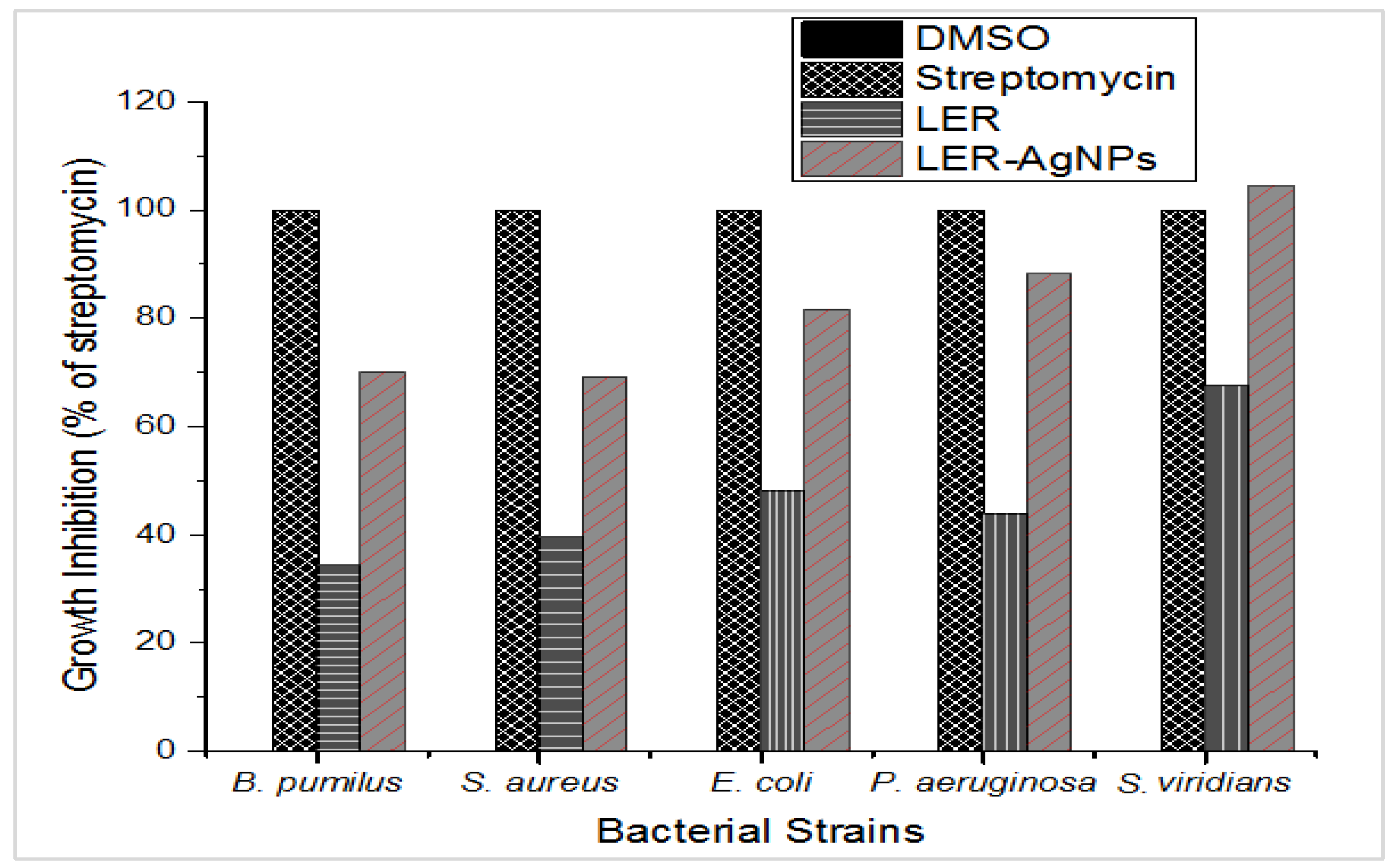
2.5. Antibacterial Activity
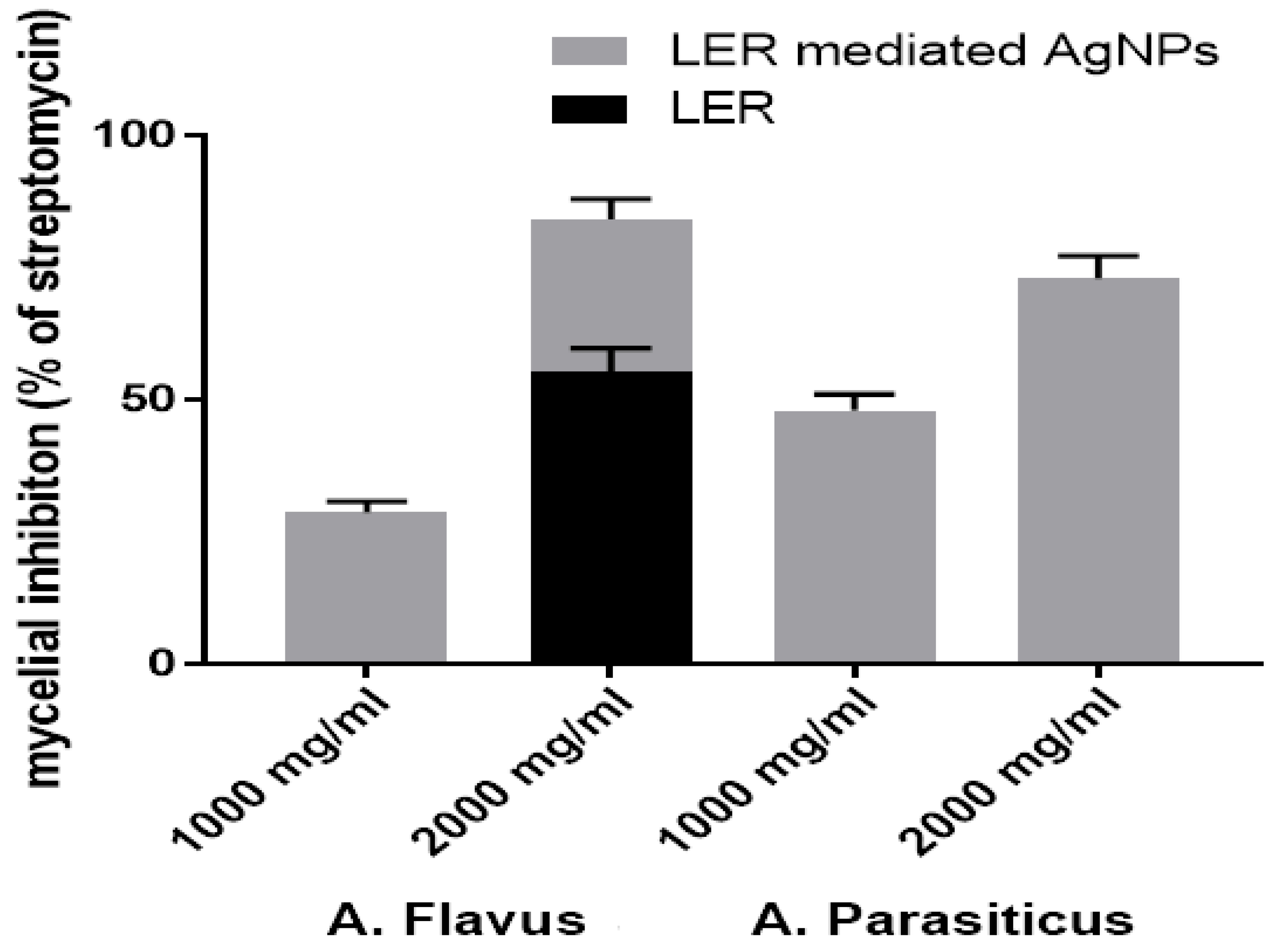
2.6. Antifungal Activity
2.7. Hepatoprotective Activity
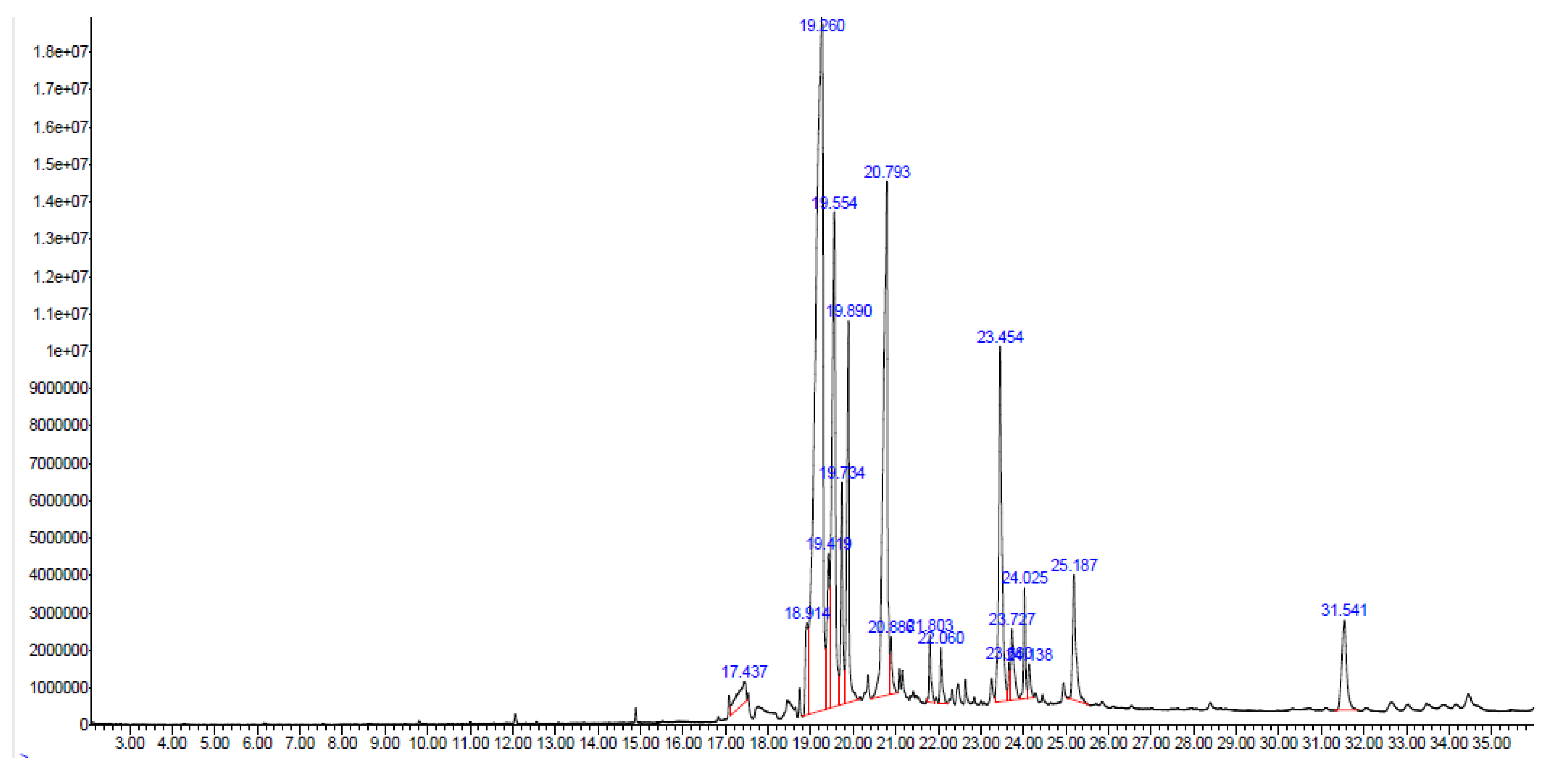
2.8. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3. Discussion
4. Material and Methods
4.1. Preparation of Plant Extract
4.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.3. Synthesis of Silver Nanoparticles (AgNPs)
4.4. Characterization of AgNPs
4.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
4.4.2. UV-Vis Spectrophotometry
4.4.3. X-ray Diffraction (XRD)
- D = average crystalline domain size perpendicular to reflecting planes,
- k = shape factor,
- λ = X-ray wavelength,
- β = FWHM (full width at the half maximum),
- and θ = the diffraction angle.
4.4.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX)
4.4.5. Dynamic Light Scattering Spectroscopy and Zeta Potential
4.5. Antioxidant Potential
4.5.1. DPPH Scavenging Assay
4.5.2. Hydroxyl (•OH) Scavenging Assay
4.5.3. Hydrogen Peroxide (H2O2) Scavenging Assay
4.6. Antibacterial Potential
4.7. Antifungal Potential
4.8. Hepatoprotective Activity
4.9. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, R.; Bibi, S.; Khan, M.N.; Al Mohaimeed, A.M.; Naz, Q.; Kamal, A. Application of Bio-Inspired Gold Nanoparticles as Advanced Nanomaterial in Halt Nociceptive Pathway and Hepatotoxicity via Triggering Antioxidation System. Catalysts 2023, 13, 786. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, Z.; Afroz, H. Prospects and applications of nanobiotechnology: A medical perspective. J. Nanobiotechnology 2012, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, T.; Ullah, R.; Khan, M.N.; Nazish, M.; Almutairi, S.M.; Rasheed, R.A. Seed Priming with Glutamic-Acid-Functionalized Iron Nanoparticles Modulating Response of Vigna radiata (L.) R. Wilczek (Mung Bean) to Induce Osmotic Stress. Micromachines 2023, 14, 736. [Google Scholar] [CrossRef]
- Beyene, H.D.; Werkneh, A.A.; Bezabh, H.K.; Ambaye, T.G. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 2017, 13, 18–23. [Google Scholar]
- Gautam, P.K.; Shivalkar, S.; Samanta, S.K. Environmentally benign synthesis of nanocatalysts: Recent advancements and applications. Handb. Nanomater. Nanocomposites Energy Environ. Appl. 2021, 1163–1181. [Google Scholar]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-capped nanoapatites amplify osmotic stress tolerance in Zea mays L. by conserving photosynthetic pigments, osmolytes biosynthesis and antioxidant biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef]
- Annu, A.A.; Ahmed, S. Green synthesis of metal, metal oxide nanoparticles, and their various applications. Handb. Ecomater. 2018, 2018, 1–45. [Google Scholar]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostructure Chem. 2018, 8, 369–391. [Google Scholar]
- Kumari, A.; Dhatwalia, J.; Thakur, S.; Radhakrishnan, A.; Chauhan, A.; Chandan, G.; Choi, B.H. Antioxidant, antimicrobial, and cytotoxic potential of Euphorbia royleana extract-mediated silver and copper oxide nanoparticles. Chem. Pap. 2023, 77, 4643–4657. [Google Scholar] [CrossRef]
- Song, J.Y.; Jang, H.-K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rani, S.; Rana, J. Traditionally used common fibre plants in outer siraj area, Himachal Pradesh. Indian J. Nat. Prod. Resour. (IJNPR) [Former. Nat. Prod. Radiance (NPR)] 2015, 5, 190–194. [Google Scholar]
- Bhattarai, S.B.S.; Tamang, R.T.R. Medicinal and aromatic plants: A synopsis of Makawanpur district, central Nepal. Int. J. Indig. Herbs Drugs 2017, 6–15. [Google Scholar]
- Akram, M.; Iqbal, N.; Aqeel, M.; Khalid, N.; Alamri, S.; Hashem, M.; Abrar, M.; Manan, A.; Islam, W.; Noman, A. Exploration of medicinal phyto-diversity of the semi-arid area in Punjab province, Pakistan. JAPS: J. Anim. Plant Sci. 2020, 30, 1442–1464. [Google Scholar]
- Kushwaha, S.; Semwal, B.; Upadhayay, P.K.; Yadav, H.N.; Vishwakarma, V.K. Hepatoprotective activity of ethanolic extract of Euphorbia royleana linn in carbon tetrachloride treated guinea pig. Innov. Pharm. Pharmacother. 2017, 4, 22–32. [Google Scholar]
- Kumar, K.S.; Ravindra, N.; Dara, H. Evaluation of Analgesics and Anti-inflammatory Activity of Polyherbal Formulation Containing Some Indigenous Medicinal Plants. Bull. Environ. Pharmacol. Life Sci 2020, 9, 69–80. [Google Scholar]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K. Euphorbia-derived natural products with potential for use in health maintenance. Biomolecules 2019, 9, 337. [Google Scholar]
- Shah, N.A.; Ullah, S.; Nafees, M.; Khan, M.N. Exogenous Effect of Sugar Beet Extract On Physio-biochemical Traits of Hordeum vulagre L. Under Induced Salinity Stress. Gesunde Pflanz. 2023, 1–13. [Google Scholar]
- Reynoso-García, P.J.; Güizado-Rodríguez, M.; Barba, V.; Ramos-Ortiz, G.; Martínez-Gutiérrez, H. Stabilization of silver nanoparticles with a dithiocarbamate ligand and formation of nanocomposites by combination with polythiophene derivative nanoparticles. Adv. Condens. Matter Phys. 2018, 2018, 4376051. [Google Scholar] [CrossRef]
- Soliman, H.; Elsayed, A.; Dyaa, A. Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egypt. J. Basic Appl. Sci. 2018, 5, 228–233. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hussein, G.; Brza, M.; Mohammed, S.J.; Abdulwahid, R.T.; Raza Saeed, S.; Hassanzadeh, A. Fabrication of interconnected plasmonic spherical silver nanoparticles with enhanced localized surface plasmon resonance (LSPR) peaks using quince leaf extract solution. Nanomaterials 2019, 9, 1557. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Li, C.; Nabeel, F.; Khalid, M.; Iqbal, H.M. Catalytic potential of bio-synthesized silver nanoparticles using Convolvulus arvensis extract for the degradation of environmental pollutants. J. Photochem. Photobiol. B Biol. 2018, 181, 44–52. [Google Scholar] [CrossRef]
- Ardani, H.; Imawan, C.; Handayani, W.; Djuhana, D.; Harmoko, A.; Fauzia, V. Enhancement of the stability of silver nanoparticles synthesized using aqueous extract of Diospyros discolor Willd. leaves using polyvinyl alcohol. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 26–27 July 2016; p. 012056. [Google Scholar]
- Ullah, S.; Khalid, R.; Rehman, M.F.; Irfan, M.I.; Abbas, A.; Alhoshani, A.; Anwar, F.; Amin, H. Biosynthesis of phyto-functionalized silver nanoparticles using olive fruit extract and evaluation of their antibacterial and antioxidant properties. Front. Chem. 2023, 11, 1202252. [Google Scholar] [CrossRef]
- Gul, R.; Jan, S.U.; Faridullah, S.; Sherani, S.; Jahan, N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci. World J. 2017, 2017, 5873648. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; ud Din, S.; Muhammad, Z.; Shah, S.; Jan, S.A. Biological efficacy of phyto-synthetic silver nanoparticles using ethanol extract of Euphorbia wallichii Hook Rhizome as bio-reductant and surfactant. Trop. J. Pharm. Res. 2018, 17, 1903–1909. [Google Scholar] [CrossRef]
- Eswaran, A.; Muthukrishnan, S.; Mathaiyan, M.; Pradeepkumar, S.; Mari, K.R.; Manogaran, P. Green synthesis, characterization and hepatoprotective activity of silver nanoparticles synthesized from pre-formulated Liv-Pro-08 poly-herbal formulation. Appl. Nanosci. 2021, 13, 2315–2327. [Google Scholar]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B: Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, U.G.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar]
- Arif, M.; Ullah, R.; Ahmad, M.; Ali, A.; Ullah, Z.; Ali, M.; Al-Joufi, F.A.; Zahoor, M.; Sher, H. Green synthesis of silver nanoparticles using Euphorbia wallichii leaf extract: Its antibacterial action against citrus canker causal agent and antioxidant potential. Molecules 2022, 27, 3525. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, J.-X.; Wei, Z.-B.; Sun, H.; Li, L.; Zhu, J.-Y.; Xia, G.-Q.; Zang, H. Phytochemical Analysis, Antioxidant Activities In Vitro and In Vivo, and Theoretical Calculation of Different Extracts of Euphorbia fischeriana. Molecules 2023, 28, 5172. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Ikram, M.; Farooq, F.; Sultana, T.; Mashwani, Z.-u.-R.; Raja, N.I. Biogenesis of silver nanoparticles to treat cancer, diabetes, and microbial infections: A mechanistic overview. Appl. Microbiol. Biotechnol. 2021, 105, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Zhang, Y.; Lu, Z.W.; Lebrun, R.; Gontero, B.; Li, W. Interaction between silver nanoparticles and two dehydrogenases: Role of thiol groups. Small 2019, 15, 1900860. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Rafiq, K.; Shaheen, N.; Shah, M.H. Evaluation of antioxidant activities and essential/toxicmetal levels and their health risk assessment in citrus fruits from Pakistan. Environ. Monit. Assess. 2019, 191, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Azeez, M.A.; Yekeen, T.A.; Akinboro, A. Evaluation of some biosynthesized silver nanoparticles for biomedical applications: Hydrogen peroxide scavenging, anticoagulant and thrombolytic activities. J. Clust. Sci. 2017, 28, 1379–1392. [Google Scholar] [CrossRef]
- Fatemi, M.; Shomali, T.; Nazifi, S.; Fazeli, M. Optimization, Characterization and In Vivo Hepatoprotective Effects of Gold Nanoparticles Biosynthesized by Eryngium bungei Boiss. Hydro-Alcoholic Extract. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4170–4179. [Google Scholar] [CrossRef]
- Elfaky, M.A.; Sirwi, A.; Ismail, S.H.; Awad, H.H.; Gad, S.S. Hepatoprotective effect of silver nanoparticles at two different particle sizes: Comparative study with and without silymarin. Curr. Issues Mol. Biol. 2022, 44, 2923–2938. [Google Scholar] [CrossRef]
- Zhang, H.; Jacob, J.A.; Jiang, Z.; Xu, S.; Sun, K.; Zhong, Z.; Varadharaju, N.; Shanmugam, A. Hepatoprotective effect of silver nanoparticles synthesized using aqueous leaf extract of Rhizophora apiculata. Int. J. Nanomed. 2019, 2019, 3517–3524. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, H.; Su, X.; Fang, Z.; Dong, Z.; Yu, C.; Luo, K. Role of elevated liver transaminase levels in the diagnosis of liver injury after blunt abdominal trauma. Exp. Ther. Med. 2012, 4, 255–260. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxidative Med. Cell. Longev. 2016, 2016, 3529149. [Google Scholar] [CrossRef]
- Baran, M.F.; Keskin, C.; Baran, A.; Hatipoğlu, A.; Yildiztekin, M.; Küçükaydin, S.; Kurt, K.; Hoşgören, H.; Sarker, M.M.R.; Sufianov, A. Green Synthesis of Silver Nanoparticles from Allium cepa L. Peel Extract, Their Antioxidant, Antipathogenic, and Anticholinesterase Activity. Molecules 2023, 28, 2310. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Ullah, R.; Muhammad, Z.; Khan, M.N.; Kakar, H.A.; Kaplan, A.; Okla, M.K.; Saleh, I.A.; Kamal, A.; Abdullah, A. Kinetin Capped Zinc Oxide Nanoparticles Improve Plant Growth and Ameliorate Resistivity to Polyethylene Glycol (PEG)-Induced Drought Stress in Vigna radiata (L.) R. Wilczek (Mung Bean). Molecules 2023, 28, 5059. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, W.-Y.; Wu, M.-F.; Wang, Y.-M.; Zhu, W.-Y.; Chi, C.-F.; Wang, B. Eighteen Novel Bioactive Peptides from Monkfish (Lophius litulon) Swim Bladders: Production, Identification, Antioxidant Activity, and Stability. Mar. Drugs 2023, 21, 169. [Google Scholar] [CrossRef]
- Marchese, E.; Orlandi, V.; Turrini, F.; Romeo, I.; Boggia, R.; Alcaro, S.; Costa, G. In Silico and In Vitro Study of Antioxidant Potential of Urolithins. Antioxidants 2023, 12, 697. [Google Scholar] [CrossRef]
- Petraglia, T.; Latronico, T.; Fanigliulo, A.; Crescenzi, A.; Liuzzi, G.M.; Rossano, R. Antioxidant activity of polysaccharides from the edible mushroom Pleurotus eryngii. Molecules 2023, 28, 2176. [Google Scholar] [CrossRef]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013, 141, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, R.; Banan-MwineDaliri, E.; Oh, D.-H. Screening for Antioxidant Activity: Hydrogen Peroxide Scavenging Assay. Methods Actinobacteriology 2022, 461–462. [Google Scholar]
- Shah, W.; Ullah, S.; Ali, S.; Idrees, M.; Khan, M.N.; Ali, K.; Khan, A.; Ali, M.; Younas, F. Effect of exogenous alpha-tocopherol on physio-biochemical attributes and agronomic performance of lentil (Lens culinaris Medik.) under drought stress. PLoS ONE 2021, 16, e0248200. [Google Scholar] [CrossRef] [PubMed]
- Hendi, N.K.; Naher, H.S.; Al-Charrakh, A.H. In vitro antibacterial and antifungal activity of Iraqi propolis. J. Med. Plants Res 2011, 5, 5058–5066. [Google Scholar]
- Ilhan, S.; Savaroğlu, F.; Çolak, F. Antibacterial and Antifungal Activity of Corchorus olitorius L.(Molokhia) Extracts. Int. J. Nat. Eng. Sci. 2007, 1, 59–61. [Google Scholar]
- Hamayun, M.; Khan, N.; Khan, M.N.; Qadir, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Rehman, K.U.; Lee, I.-J. Antimicrobial and plant growth-promoting activities of bacterial endophytes isolated from Calotropis procera (Ait.) WT Aiton. Biocell 2021, 45, 363. [Google Scholar] [CrossRef]





| Treatments | ALP (IU/L) | AST (IU/L) | ALT (IU/L) | Bilirubin (g/dL) | Protein (g/dL) |
|---|---|---|---|---|---|
| Saline 10 mL/Kg | 144 | 62 | 53 | 0.51 | 4.49 |
| PCM 1 g/kg | 238 | 129 | 113 | 1.29 | 1.76 |
| Silymarin 10 mg/kg | 164 | 74 | 67 | 0.68 | 3.08 |
| LER-AgNPs 100 mg/kg | 208 | 112 | 91 | 0.97 | 1.87 |
| LER-AgNPs 200 mg/kg | 192 | 96 | 86 | 0.94 | 2.08 |
| LER-AgNPs 300 mg/kg | 187 | 92 | 79 | 0.86 | 2.81 |
| S. No | RT (min) | Area % | Compound | Structure | Mass Spectra | Molecular Formula/Molar Mass |
|---|---|---|---|---|---|---|
| 1. | 17.437 | 1.41 | n-Hexadecanoic acid |  |  | CH3(CH2)14COOH/256.4241 |
| 2. | 18.914 | 1.62 | Methyl 9-cis,11-trans-octadecadienoate |  |  | C19H34O2/294.472 |
| 3. | 19.260 | 36.86 | 6-Octadecenoic acid |  |  | C18H34O2/282.5 |
| 4. | 19.419 | 2.70 | Octadecanoic acid |  |  | CH3(CH2)16COOH/284.5 |
| 5. | 19.554 | 12.37 | (9E,11E)-Octadecadienoic acid |  |  | C18H32O2/280.4 |
| 6. | 19.734 | 2.99 | 10E,12Z-Octadecadienoic acid |  |  | C18H32O2/280.4 |
| 7. | 19.890 | 6.33 | 9,12-Octadecadienoic acid (Z,Z)- |  | 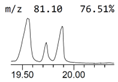 | C18H32O2/280.4 |
| 8. | 20.793 | 15.26 | Ricinoleic acid | 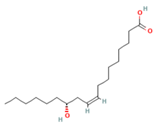 | 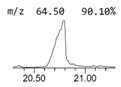 | C18H34O3/298.5 |
| 9. | 20.886 | 0.85 | cis-13-Eicosenoic acid |  | 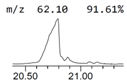 | C20H38O2/310.5 |
| 10. | 21.803 | 0.85 | 9-Octadecenoic acid (Z)-, oxiranylmethyl ester |  |  | C21H38O3/338.5 |
| 11. | 22.060 | 0.85 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 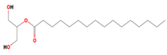 |  | C19H38O4/330.5 |
| 12. | 23.454 | 7.89 | 9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester |  |  | C21H40O4/356.5 |
| 13. | 23.660 | 0.44 | Octadecanoic acid, 2,3-dihydroxypropyl ester |  |  | C21H42O4/358.5 |
| 14. | 23.727 | 1.63 | 9,12-Octadecadienoic acid (Z,Z)-, 2,3-dihydroxypropyl ester |  |  | C21H28O4/354.5 |
| 15. | 24.025 | 1.37 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester |  |  | C24H38O4/390.6 |
| 16. | 24.138 | 0.55 | 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester |  |  | C21H38O4/354.5 |
| 17. | 25.187 | 2.95 | Bicyclo [5.3.1]undecan-11-one |  |  | C11H18O/166.26 |
| 18. | 31.541 | 3.05 | Obtusifoliol |  |  | C30H50O/426.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Jan, S.A.; Khan, M.N.; Nazish, M.; Kamal, A.; Kaplan, A.; Yehia, H.M.; Alarjani, K.M.; Alkasir, R.; Zaman, W. Euphorbia royleana Boiss Derived Silver Nanoparticles and Their Applications as a Nanotherapeutic Agent to Control Microbial and Oxidative Stress-Originated Diseases. Pharmaceuticals 2023, 16, 1413. https://doi.org/10.3390/ph16101413
Ullah R, Jan SA, Khan MN, Nazish M, Kamal A, Kaplan A, Yehia HM, Alarjani KM, Alkasir R, Zaman W. Euphorbia royleana Boiss Derived Silver Nanoparticles and Their Applications as a Nanotherapeutic Agent to Control Microbial and Oxidative Stress-Originated Diseases. Pharmaceuticals. 2023; 16(10):1413. https://doi.org/10.3390/ph16101413
Chicago/Turabian StyleUllah, Rehman, Saiqa Afriq Jan, Muhammad Nauman Khan, Moona Nazish, Asif Kamal, Alevcan Kaplan, Hany M. Yehia, Khaloud Mohammed Alarjani, Rashad Alkasir, and Wajid Zaman. 2023. "Euphorbia royleana Boiss Derived Silver Nanoparticles and Their Applications as a Nanotherapeutic Agent to Control Microbial and Oxidative Stress-Originated Diseases" Pharmaceuticals 16, no. 10: 1413. https://doi.org/10.3390/ph16101413
APA StyleUllah, R., Jan, S. A., Khan, M. N., Nazish, M., Kamal, A., Kaplan, A., Yehia, H. M., Alarjani, K. M., Alkasir, R., & Zaman, W. (2023). Euphorbia royleana Boiss Derived Silver Nanoparticles and Their Applications as a Nanotherapeutic Agent to Control Microbial and Oxidative Stress-Originated Diseases. Pharmaceuticals, 16(10), 1413. https://doi.org/10.3390/ph16101413









