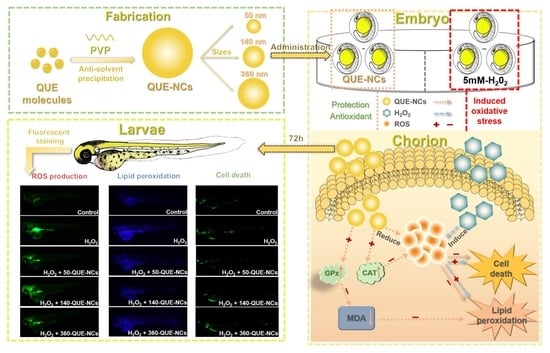
Antioxidant Effects of Quercetin Nanocrystals in Nanosuspension against Hydrogen Peroxide-Induced Oxidative Stress in a Zebrafish Model
Abstract
1. Introduction
2. Results and Discussion
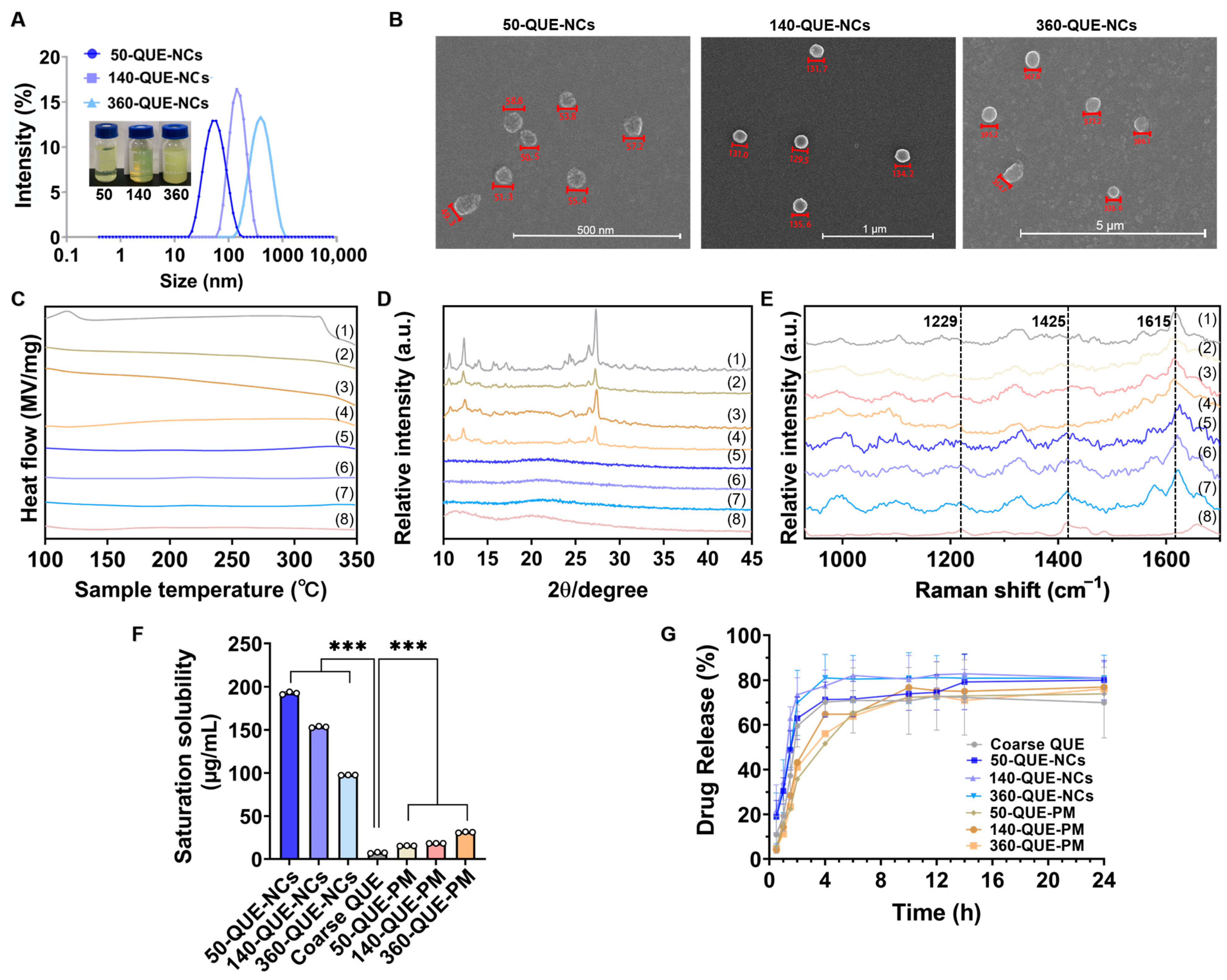
2.1. Characterization of QUE-NCs
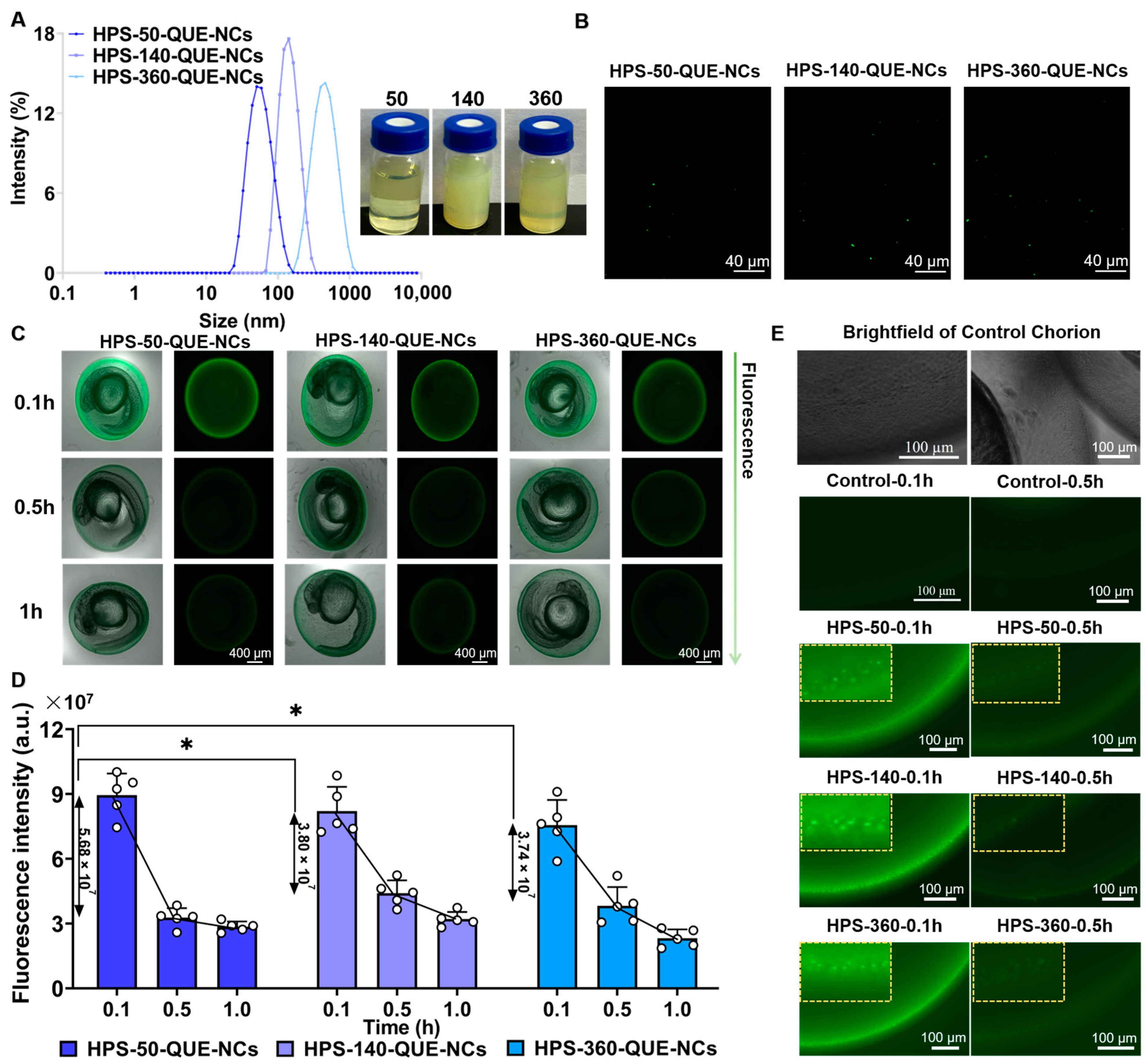
2.1.1. Size, Morphology, and Stability of QUE-NCs
2.1.2. Solid State Characterization of QUE-NCs
2.2. Solubility Study
2.3. In Vitro Drug Release Study
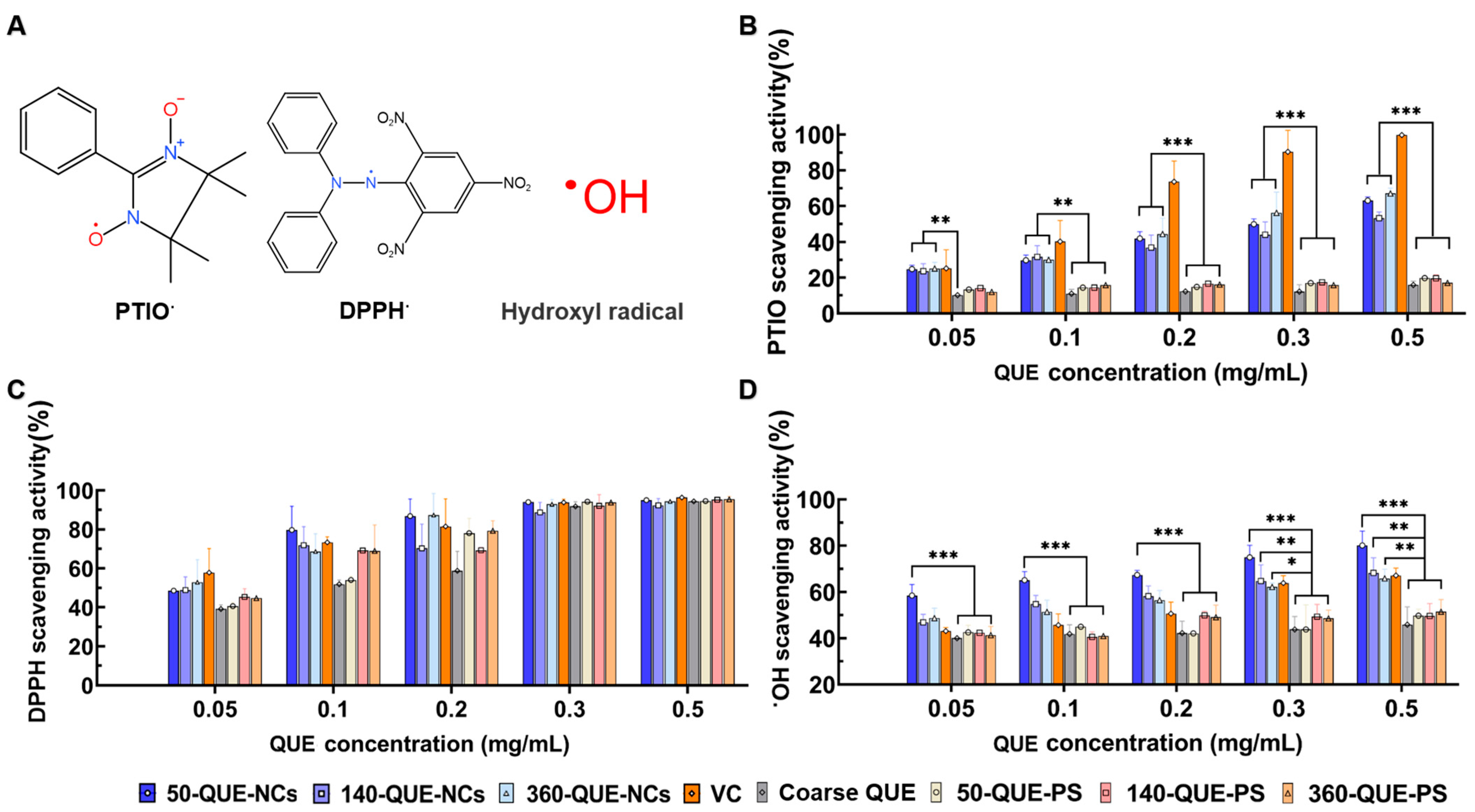
2.4. The Radical Scavenging Ability of QUE-NCs In Vitro
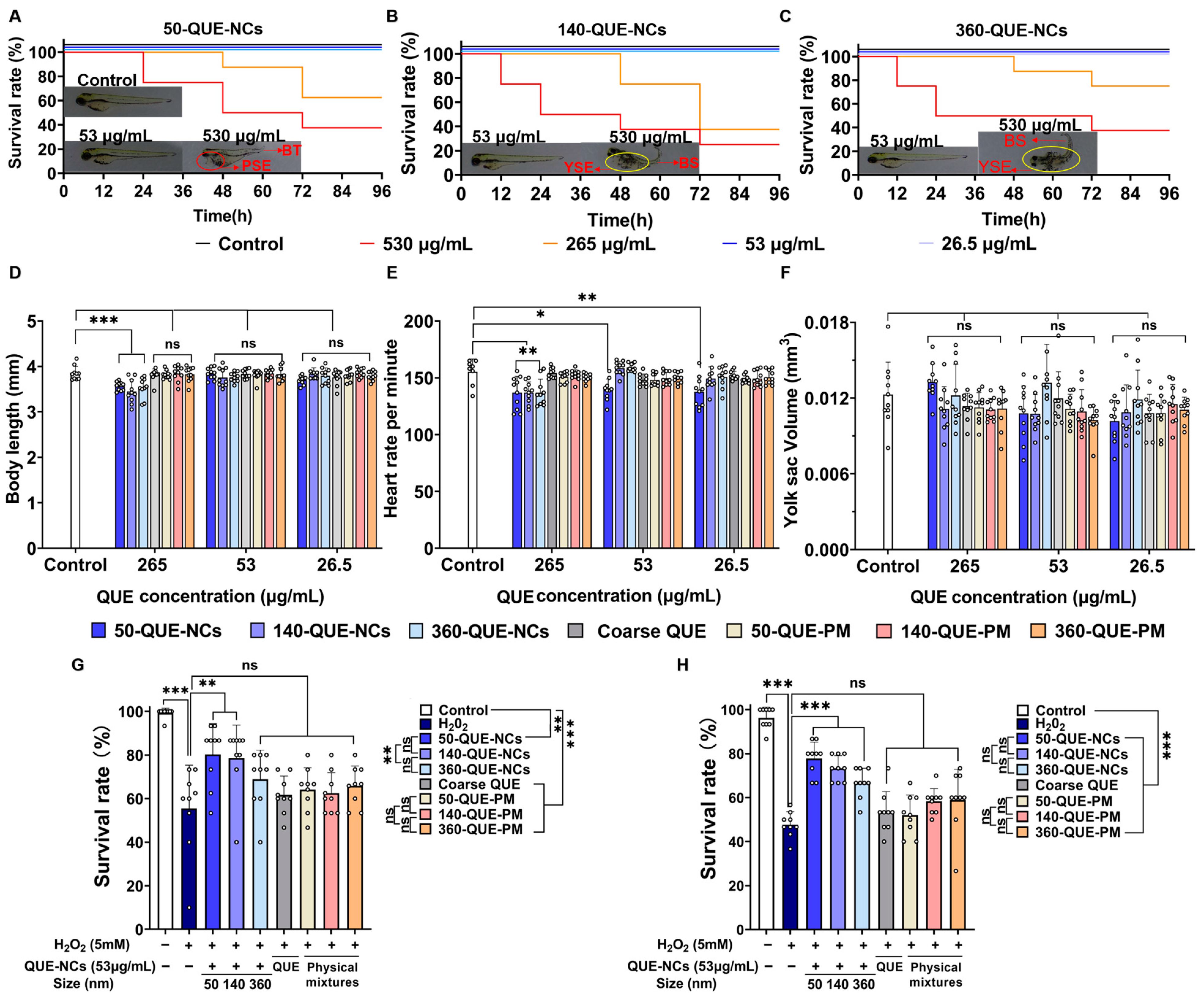
2.5. Effects of QUE-NCs in Zebrafish In Vivo
2.5.1. Toxicity of QUE-NCs on the Development of Zebrafish Embryos
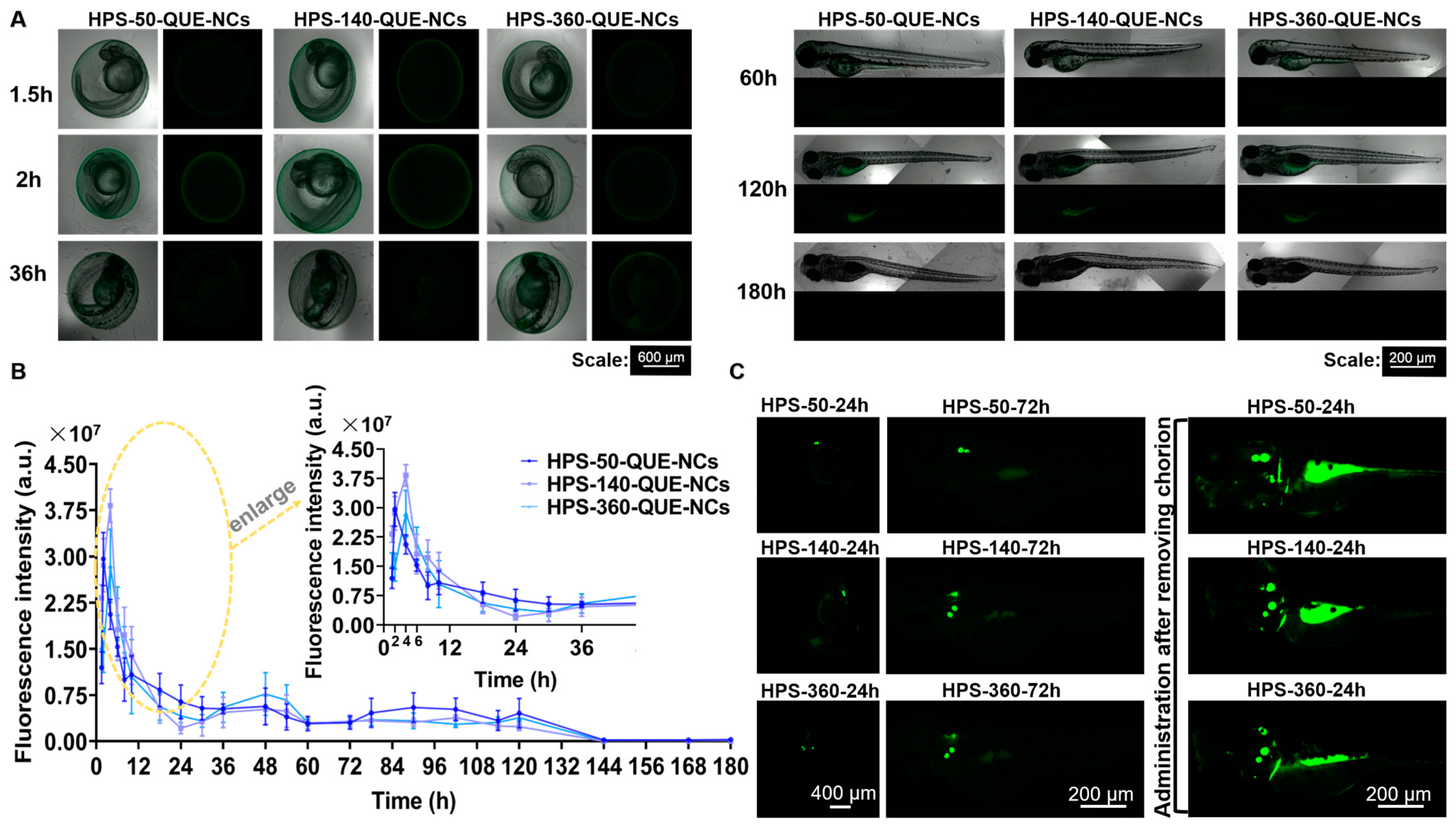
2.5.2. Transport of QUE-NCs in Zebrafish Embryos In Vivo
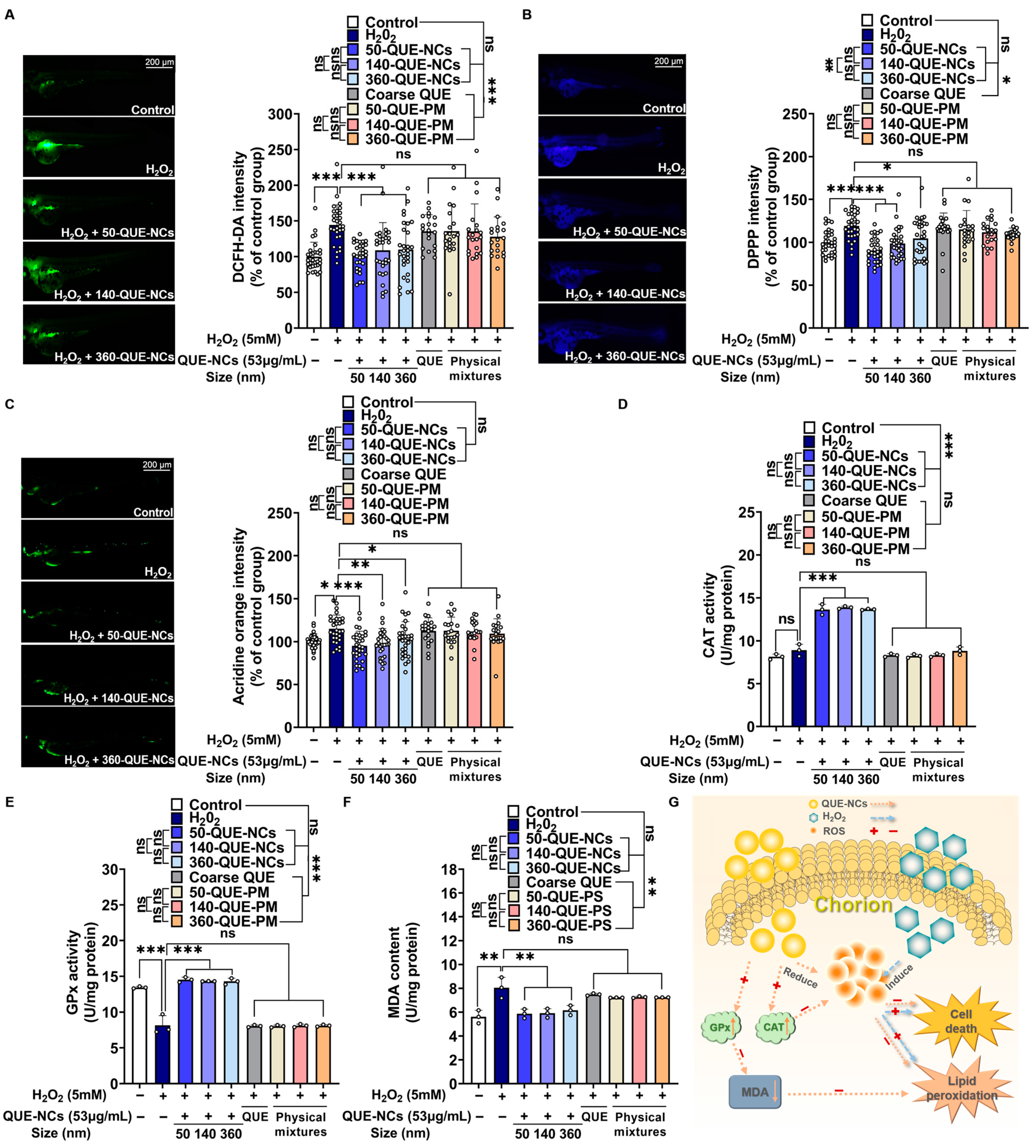
2.5.3. Inhibitory Effects of QUE-NCs on ROS Generation, Lipid Peroxidation, and Cell Death in Zebrafish Embryos
2.5.4. Effects of QUE-NCs on the Activity of Antioxidant Enzymes in Zebrafish
3. Materials and Methods
3.1. Materials
3.2. Method of Fabricating QUE-NCs of Different Particle Sizes
3.3. Fabrication of QUE-NCs
3.3.1. Investigation of the Size, Morphology, and Stability of QUE-NCs
3.3.2. Differential Scanning Calorimetry
3.3.3. X-ray Powder Diffraction
3.3.4. Raman Spectra
3.4. Solubility Determination
3.5. In Vitro Drug Release
3.6. Antioxidant Study of QUE-NCs In Vitro
3.6.1. PTIO Radical Scavenging Assay
3.6.2. DPPH Radical Scavenging Assay
3.6.3. Hydroxyl Radical Scavenging Assay
3.7. Toxicity Tests of QUE-NCs on the Development of Zebrafish Embryos In Vivo
3.8. Transport of QUE-NCs in Zebrafish Embryos In Vivo
3.9. Effects of QUE-NCs on Antioxidant Capacity by H2O2-Induced Oxidative Stress in a Zebrafish Model
3.9.1. Estimation of the Effects of QUE-NCs on ROS Generation, Lipid Peroxidation, and Cell Death in Zebrafish Embryos
3.9.2. Estimation of the Effects of QUE-NCs on the Activity of Antioxidant Enzymes in Zebrafish
3.10. HPLC Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIE | Aggregation-induced emission |
| AO | Acridine orange |
| CAT | Catalase |
| DCF | 2,7-dichlorofluorescein |
| DCFH | 2,7-dichlorodihydrofluorescein |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein Diacetate |
| DLS | Dynamic light scattering |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DPPP | 1,3-bis (diphenylphosphino) propane |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HPS | 1,1,2,3,4,5-hexaphenylsilacyclopenta-2,4-diene |
| Hpf | Hours post-fertilization |
| NCs | Nanocrystals |
| PTIO | 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide |
| PVP | Polyvinyl pyrrolidone |
| QUE | Quercetin |
| QUE-NCs | Quercetin nanocrystals |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| VC | Vitamin C |
| 50-QUE-NCs | 50 nm quercetin-nanocrystals |
| 140-QUE-NCs | 140 nm quercetin-nanocrystals |
| 360-QUE-NCs | 360 nm quercetin-nanocrystals |
| 50-QUE-PM | 50 nm quercetin-physical mixture |
| 140-QUE-PM | 140 nm quercetin-physical mixture |
| 360-QUE-PM | 360 nm quercetin-physical mixture |
References
- Liu, D.; Xu, Y. p53, oxidative stress, and aging. Antioxid. Redox Signal. 2011, 15, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical applications. Asian J. Pharm. Sci. 2018, 13, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Ghassam, B.J.; Nayaka, S.C.; Kini, K.R.; Prakash, H.S. Antioxidant and Neuroprotective Activities of Hyptis suaveolens (L.) Poit. against Oxidative Stress-Induced Neurotoxicity. Cell. Mol. Neurobiol. 2014, 34, 323–331. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B. Antioxidants of natural plant origins: From sources to food industry applications. Adv. Food Nutr. Res. 2019, 24, 4132. [Google Scholar] [CrossRef]
- Lee, Y.L.; Park, S.M.; Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.-H.; Lee, C.-H.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, R.; Huang, H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 692–701. [Google Scholar] [CrossRef]
- Petersen, B.; Egert, S.; Bosy-Westphal, A.; Müller, M.J.; Wolffram, S.; Hubbermann, E.M.; Rimbach, G.; Schwarz, K. Bioavailability of quercetin in humans and the influence of food matrix comparing quercetin capsules and different apple sources. Food Res. Int. 2016, 88, 159–165. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. Fabrication of quercetin-loaded biopolymer films as functional packaging materials. ACS Appl. Polym. Mater. 2021, 3, 2131–2137. [Google Scholar] [CrossRef]
- Pant, N.; Wairkar, S. Topical nanocrystals of bioflavonoids: A new technology platform for skin ailments. Int. J. Pharm. 2022, 619, 121707. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Manca, M.L.; Lai, F.; Pireddu, R.; Valenti, D.; Schlich, M.; Pini, E.; Ailuno, G.; Fadda, A.M.; Sinico, C. Impact of nanosizing on dermal delivery and antioxidant activity of quercetin nanocrystals. J. Drug Deliv. Sci. Technol. 2020, 55, 101482. [Google Scholar] [CrossRef]
- Ude, V.C.; Brown, D.M.; Viale, L.; Kanase, N.; Stone, V.; Johnston, H.J. Impact of copper oxide nanomaterials on differentiated and undifferentiated Caco-2 intestinal epithelial cells; assessment of cytotoxicity, barrier integrity, cytokine production and nanomaterial penetration. Part. Fibre Toxicol. 2017, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Bowman, T.V.; Zon, L.I. Swimming into the future of drug discovery: In vivo chemical screens in zebrafish. ACS Chem. Biol. 2010, 5, 159–161. [Google Scholar] [CrossRef]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Endo, Y.; Muraki, K.; Fuse, Y.; Kobayashi, M. Evaluation of antioxidant activity of spice-derived phytochemicals using zebrafish. Int. J. Mol. Sci. 2020, 21, 1109. [Google Scholar] [CrossRef]
- Kang, M.-C.; Cha, S.H.; Wijesinghe, W.; Kang, S.-M.; Lee, S.-H.; Kim, E.-A.; Song, C.B.; Jeon, Y.-J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, S.; Xiong, Z. Protective effect of polysaccharide from Ligusticum chuanxiong hort against H2O2-induced toxicity in zebrafish embryo. Carbohydr. Polym. 2019, 221, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, X.; Sun, J.; Yu, F.; Zhang, H.; Gao, J.; Zheng, A. Size-dependent biological effects of quercetin nanocrystals. Molecules 2019, 24, 1438. [Google Scholar] [CrossRef] [PubMed]
- Van Pomeren, M.; Brun, N.R.; Peijnenburg, W.; Vijver, M.G. Exploring uptake and biodistribution of polystyrene (nano) particles in zebrafish embryos at different developmental stages. Aquat. Toxicol. 2017, 190, 40–45. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, A.M.; Khan, L.U.; da Silva, G.H.; Ospina, C.A.; Alves, O.L.; de Castro, V.L.; Martinez, D.S.T. Graphene oxide-silver nanoparticle hybrid material: An integrated nanosafety study in zebrafish embryos. Ecotoxicol. Environ. Saf. 2021, 209, 111776. [Google Scholar] [CrossRef]
- Chen, Y.; Jihad, A.; Hussam, F.; Al-Abdeen, S.H.Z.; Hussein, J.M.; Adhab, Z.H.; Alzahraa, Z.H.A.; Ahmad, I.; Fatolahi, L.; Janani, B.J. A facile preparation method for efficiency a novel LaNiO3/SrCeO3 (p-n type) heterojunction catalyst in photocatalytic activities, bactericidal assessment and dopamine detection. Surf. Interfaces 2023, 38, 102830. [Google Scholar] [CrossRef]
- Liang, H.; Zou, F.; Liu, Q.; Wang, B.; Fu, L.; Liang, X.; Liu, J.; Liu, Q. Nanocrystal-loaded liposome for targeted delivery of poorly water-soluble antitumor drugs with high drug loading and stability towards efficient cancer therapy. Int. J. Pharm. 2021, 599, 120418. [Google Scholar] [CrossRef]
- Tzeng, C.-W.; Yen, F.-L.; Wu, T.-H.; Ko, H.-H.; Lee, C.-W.; Tzeng, W.-S.; Lin, C.-C. Enhancement of Dissolution and Antioxidant Activity of Kaempferol Using a Nanoparticle Engineering Process. J. Agric. Food Chem. 2011, 59, 5073–5080. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Obeid, R.A.; Abdullah, K.; Mohammed, S.; Kadhim, A.J.; Ramadan, M.F.; Hussien, B.M.; Alkahtani, A.; Ali, F.A.; Alkhathami, A.G.; et al. A facile construction of NiV2O6/CeO2 nano-heterojunction for photo-operated process in water remediation reaction, antibacterial studies, and detection of D-Amino acid in peroxidase system. Surf. Interfaces 2023, 40, 102970. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Gao, L.; Zhang, Y.; Yi, J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018, 261, 283–291. [Google Scholar] [CrossRef]
- Teslova, T.; Corredor, C.; Livingstone, R.; Spataru, T.; Birke, R.L.; Lombardi, J.R.; Cañamares, M.V.; Leona, M. Raman and surface-enhanced Raman spectra of flavone and several hydroxy derivatives. J. Raman Spectrosc. 2007, 38, 802–818. [Google Scholar] [CrossRef]
- Cakar, S.; Özacar, M. The effect of iron complexes of quercetin on dye-sensitized solar cell efficiency. J. Photochem. Photobiol. A Chem. 2017, 346, 512–522. [Google Scholar] [CrossRef]
- Dendisová, M.; Palounek, D.; Švecová, M.; Prokopec, V. SERS study of fluorescent and non-fluorescent flavonoids: What is the role of excitation wavelength on SERS optical response? Chem. Pap. 2019, 73, 2945–2953. [Google Scholar] [CrossRef]
- Kommavarapu, P.; Maruthapillai, A.; Palanisamy, K.; Sunkara, M. Preparation and characterization of rilpivirine solid dispersions with the application of enhanced solubility and dissolution rate. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 71–79. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Dai, B.; Tang, X.; Che, Z.; Hu, F.; Shen, C.; Wu, W.; Shen, B.; Yuan, H. Fabrication and In Vitro/Vivo Evaluation of Drug Nanocrystals Self-Stabilized Pickering Emulsion for Oral Delivery of Quercetin. Pharmaceutics 2022, 14, 897. [Google Scholar] [CrossRef]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef]
- Li, X. 2-Phenyl-4, 4, 5, 5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure–activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, N.; Xie, Y.; Zhao, Y.; Song, X.; Zhong, W. Antioxidant and anti-aging activities of mycelial polysaccharides from Lepista sordida. Int. J. Biol. Macromol. 2013, 60, 355–359. [Google Scholar] [CrossRef]
- Carmignani, A.; Battaglini, M.; Sinibaldi, E.; Marino, A.; Vighetto, V.; Cauda, V.; Ciofani, G. In vitro and ex vivo investigation of the effects of polydopamine nanoparticle size on their antioxidant and photothermal properties: Implications for biomedical applications. ACS Appl. Nano Mater. 2022, 5, 1702–1713. [Google Scholar] [CrossRef]
- Bartekova, M.; Radosinska, J.; Jelemensky, M.; Dhalla, N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018, 23, 733–758. [Google Scholar] [CrossRef] [PubMed]
- Manabe, M.; Tatarazako, N.; Kinoshita, M. Uptake, excretion and toxicity of nano-sized latex particles on medaka (Oryzias latipes) embryos and larvae. Aquat. Toxicol. 2011, 105, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Dharmar, M.; Krishnan, N.; Thangavel, M.; Krishnan, M.; Namasivayam, E. Erythrosine Induces Teratogenic Effects via Activation of ROS Biogenesis in Zebrafish Embryos. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 763–771. [Google Scholar] [CrossRef]
- Park, J.; Hong, T.; An, G.; Park, H.; Song, G.; Lim, W. Triadimenol promotes the production of reactive oxygen species and apoptosis with cardiotoxicity and developmental abnormalities in zebrafish. Sci. Total Environ. 2023, 862, 160761. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Rev. 2019, 151, 152–168. [Google Scholar] [CrossRef]
- Poór, M.; Boda, G.; Kunsági-Máté, S.; Needs, P.W.; Kroon, P.A.; Lemli, B. Fluorescence spectroscopic evaluation of the interactions of quercetin, isorhamnetin, and quercetin-3 ‘-sulfate with different albumins. J. Lumin. 2018, 194, 156–163. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, H.; Xue, X.; Chen, Y.; He, Z.; Yu, Z.; Zhang, L.; Miao, X. Dual Antimelanogenic Effect of Nicotinamide-Stabilized Phloretin Nanocrystals in Larval Zebrafish. Pharmaceutics 2022, 14, 1825. [Google Scholar] [CrossRef]
- Li, Y.; Miao, X.; Chen, T.; Yi, X.; Wang, R.; Zhao, H.; Lee, S.M.-Y.; Wang, X.; Zheng, Y. Zebrafish as a visual and dynamic model to study the transport of nanosized drug delivery systems across the biological barriers. Colloids Surf. B Biointerfaces 2017, 156, 227–235. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Hotta, A.; Ido, H.; Kawai, Y.; Moon, J.-H.; Sekido, K.; Hayashi, H.; Inakuma, T.; Terao, J. Antioxidant capacity of albumin-bound quercetin metabolites after onion consumption in humans. J. Med. Investig. 2007, 54, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, H.; Huang, W.; Ma, W.; Lu, Y.; Yan, R. Enzymatic acylation improves the stability and bioactivity of lutein: Protective effects of acylated lutein derivatives on L-O2 cells upon H2O2-induced oxidative stress. Food Chem. 2023, 410, 135393. [Google Scholar] [CrossRef]
- Félix, L.M.; Vidal, A.M.; Serafim, C.; Valentim, A.M.; Antunes, L.M.; Campos, S.; Matos, M.; Monteiro, S.M.; Coimbra, A.M. Ketamine-induced oxidative stress at different developmental stages of zebrafish (Danio rerio) embryos. RSC Adv. 2016, 6, 61254–61266. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Y.; Deng, M.; Liu, Y.; Wang, S.; He, X.; Allaire-Leung, M.; Wan, J.; Zou, Y.; Yang, C.; et al. Tetracycline antibiotics as PI3K inhibitors in the Nrf2-mediated regulation of antioxidative stress in zebrafish larvae. Chemosphere 2019, 226, 696–703. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Chen, L.; Sun, L.; Liu, Z.; Wang, H.; Xu, C. Protection afforded by quercetin against H2O2-induced apoptosis on PC12 cells via activating PI3K/Akt signal pathway. J. Recept. Signal Transduct. 2016, 36, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-S.; Kwon, S.; Ban, K.; Hong, Y.K.; Ahn, C.; Sung, J.-S.; Choi, I. Regulation of the intracellular ROS level is critical for the antiproliferative effect of quercetin in the hepatocellular carcinoma cell line HepG2. Nutr. Cancer 2019, 71, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, M.; Alak, G.; Atamanalp, M. The effects of n-butanol on oxidative stress and apoptosis in zebra fish (Danio rerio) larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 227, 108636. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.A.; Lindsay, C.B.; Quintanilla, R.A.; Carvajal, F.J.; Cerpa, W.; Inestrosa, N.C. Quercetin exerts differential neuroprotective effects against H2O2 and Aβ aggregates in hippocampal neurons: The role of mitochondria. Mol. Neurobiol. 2017, 54, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, B.; Deng, Y.; Li, T.; Tian, Q.; Yuan, Z.; Ma, L.; Cheng, C.; Guo, Q.; Qiu, L. Biocatalytic and antioxidant nanostructures for ROS scavenging and biotherapeutics. Adv. Funct. Mater. 2021, 31, 2101804. [Google Scholar] [CrossRef]
- Adedara, I.A.; Ego, V.C.; Subair, T.I.; Oyediran, O.; Farombi, E.O. Quercetin improves neurobehavioral performance through restoration of brain antioxidant status and acetylcholinesterase activity in manganese-treated rats. Neurochem. Res. 2017, 42, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Rojas, J.M.; Gavilán, H.; del Dedo, V.; Lorente-Sorolla, E.; Sanz-Ortega, L.; da Silva, G.B.; Costo, R.; Perez-Yagüe, S.; Talelli, M.; Marciello, M.; et al. Time-course assessment of the aggregation and metabolization of magnetic nanoparticles. Acta Biomater. 2017, 58, 181–195. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M.-K.; Li, K.; Hu, C.; Lu, M.-H.; Situ, J. Eupafolin nanoparticle improves acute renal injury induced by LPS through inhibiting ROS and inflammation. Biomed. Pharmacother. 2017, 85, 704–711. [Google Scholar] [CrossRef]
- Aldhalmi, A.K.; Alkhayyat, S.; Albahadly, W.K.Y.; Jawad, M.A.; Alsaraf, K.M.; Muedii, Z.A.-H.R.; Ali, F.A.; Ahmed, M.; Asiri, M.; Al-Fatolahi, L.; et al. A novel fabricate of iron and nickel-introduced bimetallic MOFs for quickly catalytic degradation via the peroxymonosulfate, antibacterial efficiency, and cytotoxicity assay. Inorg. Chem. Commun. 2023, 153, 110823. [Google Scholar] [CrossRef]
- Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Eswaramoorthy, R.; Verma, M.; Varma, R.S.; Janani, B.J. Highly-impressive performances of novel NiCo2O4/Bi2O3/Ag2ZrO3 nanocomposites in photocatalysis system, removal pathway, toxicity estimation, and antibacterial activities. J. Taiwan Inst. Chem. Eng. 2023, 149, 105004. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, W.; Tian, S. Artificial neural network optimization of Althaea rosea seeds polysaccharides and its antioxidant activity. Int. J. Biol. Macromol. 2014, 70, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Shashidhara, S.; Kumar, M.M.; Sridhara, B.Y. Effect of Luffa echinata on lipid peroxidation and free radical scavenging activity. J. Pharm. Pharmacol. 2000, 52, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Bhongale, C.J.; Chang, C.-W.; Diau, E.W.-G.; Hsu, C.-S.; Dong, Y.; Tang, B.-Z. Formation of nanostructures of hexaphenylsilole with enhanced color-tunable emissions. Chem. Phys. Lett. 2006, 419, 444–449. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, S.-Y.; Kim, E.-A.; Lee, J.-H.; Kim, Y.-S.; Yu, S.-K.; Chae, J.B.; Choe, I.-H.; Cho, J.H.; Jeon, Y.-J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kang, M.-C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.-H. Antioxidant effects of turmeric leaf extract against hydrogen peroxide-induced oxidative stress in vitro in vero cells and in vivo in zebrafish. Antioxidants 2021, 10, 112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xue, X.; Miao, X. Antioxidant Effects of Quercetin Nanocrystals in Nanosuspension against Hydrogen Peroxide-Induced Oxidative Stress in a Zebrafish Model. Pharmaceuticals 2023, 16, 1209. https://doi.org/10.3390/ph16091209
Wang J, Xue X, Miao X. Antioxidant Effects of Quercetin Nanocrystals in Nanosuspension against Hydrogen Peroxide-Induced Oxidative Stress in a Zebrafish Model. Pharmaceuticals. 2023; 16(9):1209. https://doi.org/10.3390/ph16091209
Chicago/Turabian StyleWang, Junjie, Xinyue Xue, and Xiaoqing Miao. 2023. "Antioxidant Effects of Quercetin Nanocrystals in Nanosuspension against Hydrogen Peroxide-Induced Oxidative Stress in a Zebrafish Model" Pharmaceuticals 16, no. 9: 1209. https://doi.org/10.3390/ph16091209
APA StyleWang, J., Xue, X., & Miao, X. (2023). Antioxidant Effects of Quercetin Nanocrystals in Nanosuspension against Hydrogen Peroxide-Induced Oxidative Stress in a Zebrafish Model. Pharmaceuticals, 16(9), 1209. https://doi.org/10.3390/ph16091209