Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of Suregada Genus: A Review
Abstract
:1. Introduction
2. Literature Search Strategy
3. Medicinal Uses of Suregada Species
3.1. Suregada adenophora Baill.
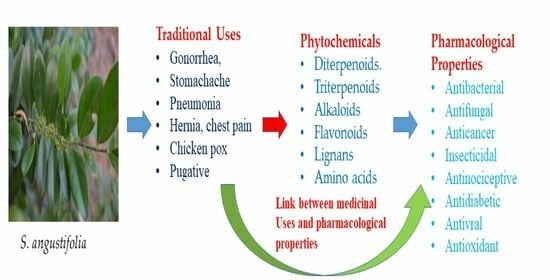
3.2. Suregada angustifolia (Müll.Arg.) Airy Shaw
3.3. Suregada boiviniana Baill. (Suregada boiviniana var. boiviniana)
3.4. Suregada decidua Radcl.-Sm.
3.5. Suregada lanceolata (Willd.) Kuntze
3.6. Suregada lithoxyla (Pax & K.Hoffm.) Croizat
3.7. Suregada multiflora (A. Juss.) Baill.
3.8. Suregada procera (Prain) Croizat
3.9. Suregada zanzibariensis Baill.
| Plant | Plant Part | Uses | References |
|---|---|---|---|
| S. adenophora | Unspecified | Purgative | [8] |
| S. angustifolia | Stem bark | Skin infections, toothache | [9] |
| S. boiviniana | Unspecified | Purgative | [10] |
| Leaves | Headaches and cold, dysentery, malaria, placenta apposition and epilepsy | [10,11] | |
| S. decidua | Fresh sap | Wounds | [8] |
| S. lanceolata/S. angustifolia | Shrub | Astringent | [12] |
| Whole plant | Skin diseases, worms, weakness, blood vomiting, piles, toothache | [13] | |
| S. lithoxylia | Wood | Poles, fuel, spoons, tool handles | [14] |
| S. multiflora | Wood | Eczema, venereal diseases, and pyrexia | [17] |
| Roots | Lymphatic disorders, skin diseases | ||
| Bark | Lymphatic disorder, hepatitis, skin diseases, fungal infection, venereal diseases, and leprosy | ||
| Whole Plant | Pneumonia, fever, poisonous effects, stomach disorder, squint eye, and gum disease | ||
| S. procera | Wood | Poles, handles, and firewood | [14] |
| Tree | Ornaments and shade | ||
| Stem | Hemorrhoids, gonorrhea | [24] | |
| S. zanzibariensis | Leaves | Asthma, malaria, skin diseases, dysentery, vaginal candidiasis and abdominal pains | [21,26,27,29] |
| Roots and stem bark | Ankylostomiasis | [25,29] | |
| Roots | Gonorrhoea, chest pain, stomach ache, hernia, chicken pox, schistosomiasis, body swelling, pneumonia and purgative | [14,25] | |
| Wood | Building poles, tool handles, withies, fuel and spoons | [14] |
4. Phytochemical Composition of the Suregada Genus
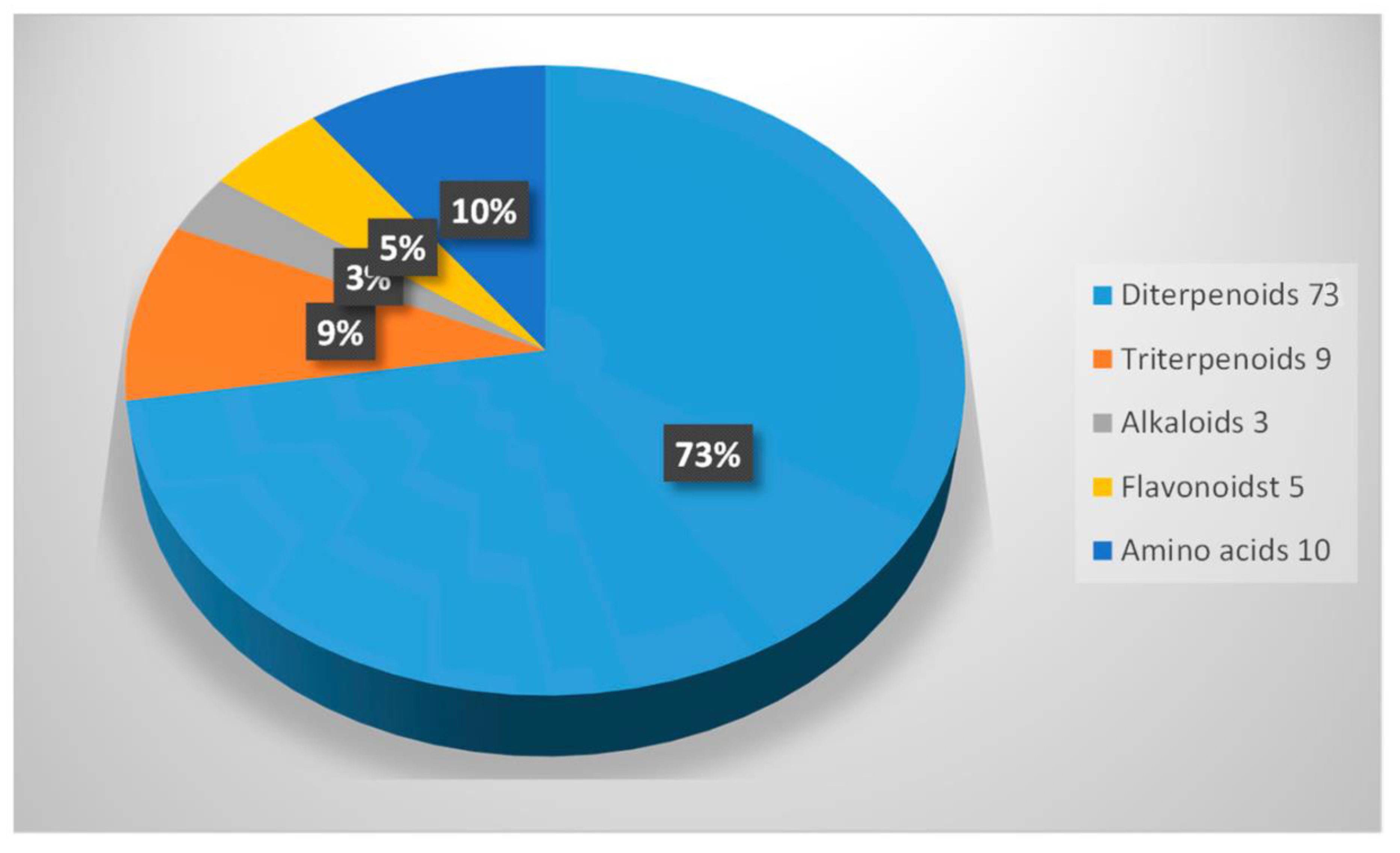
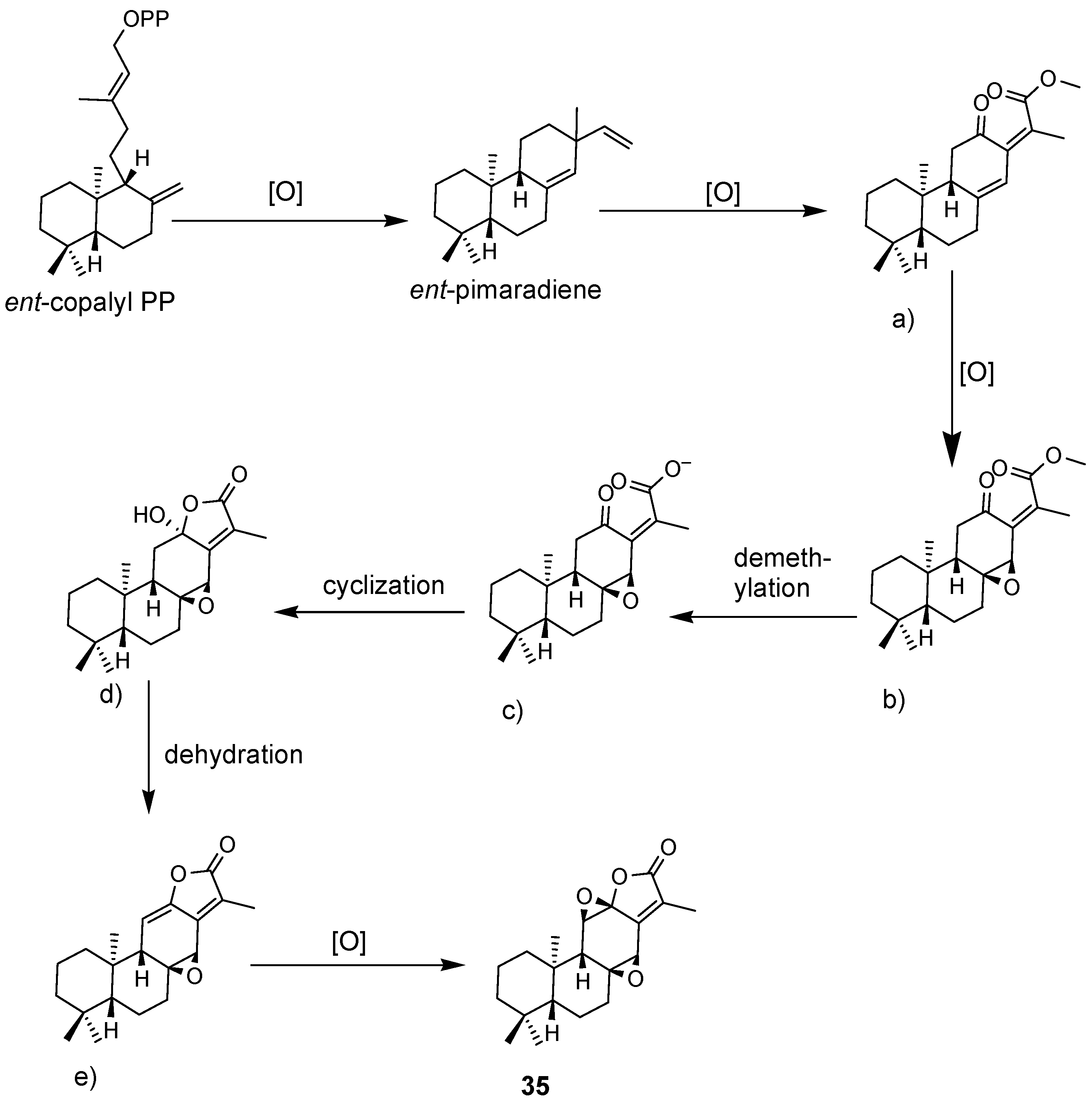
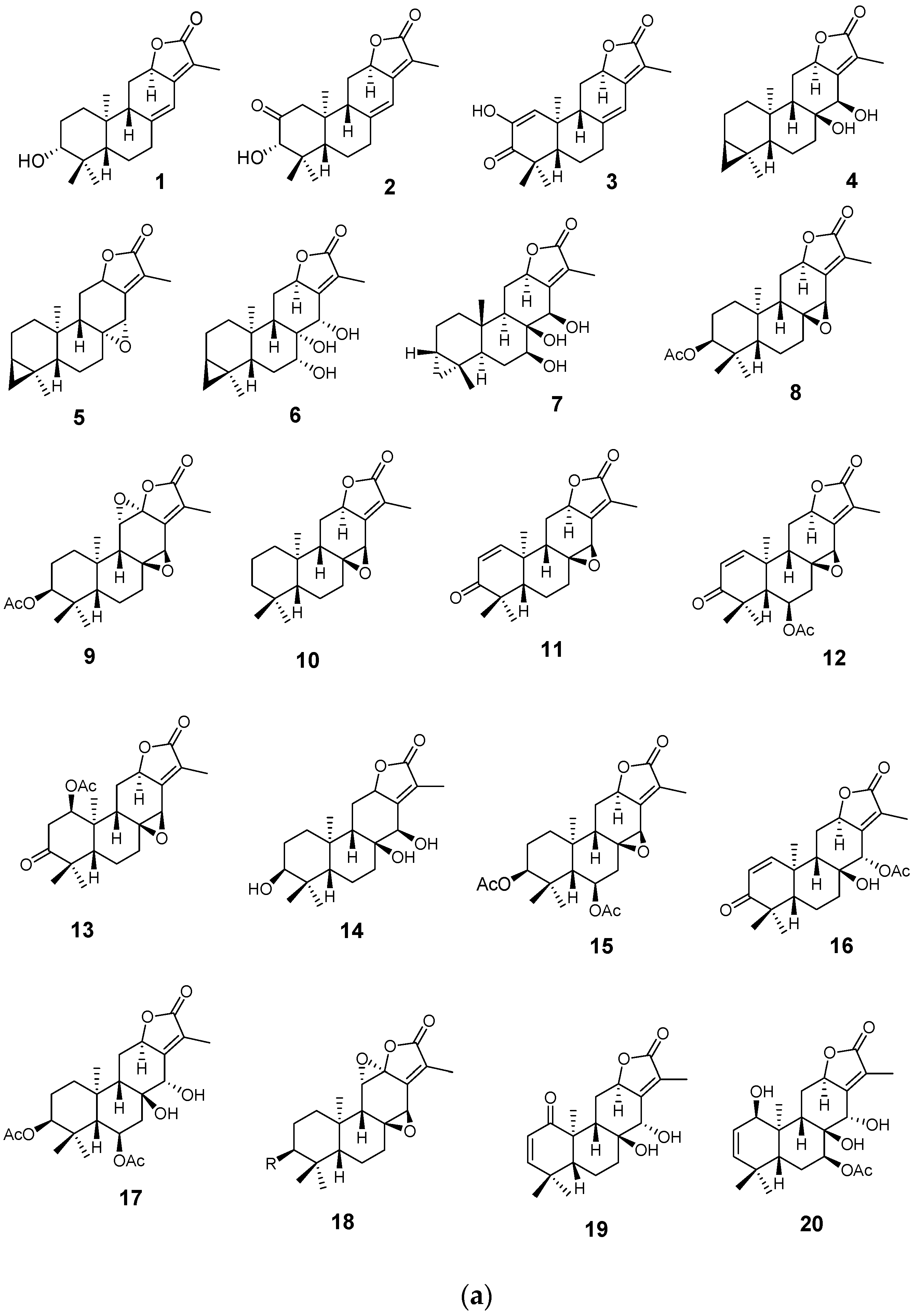
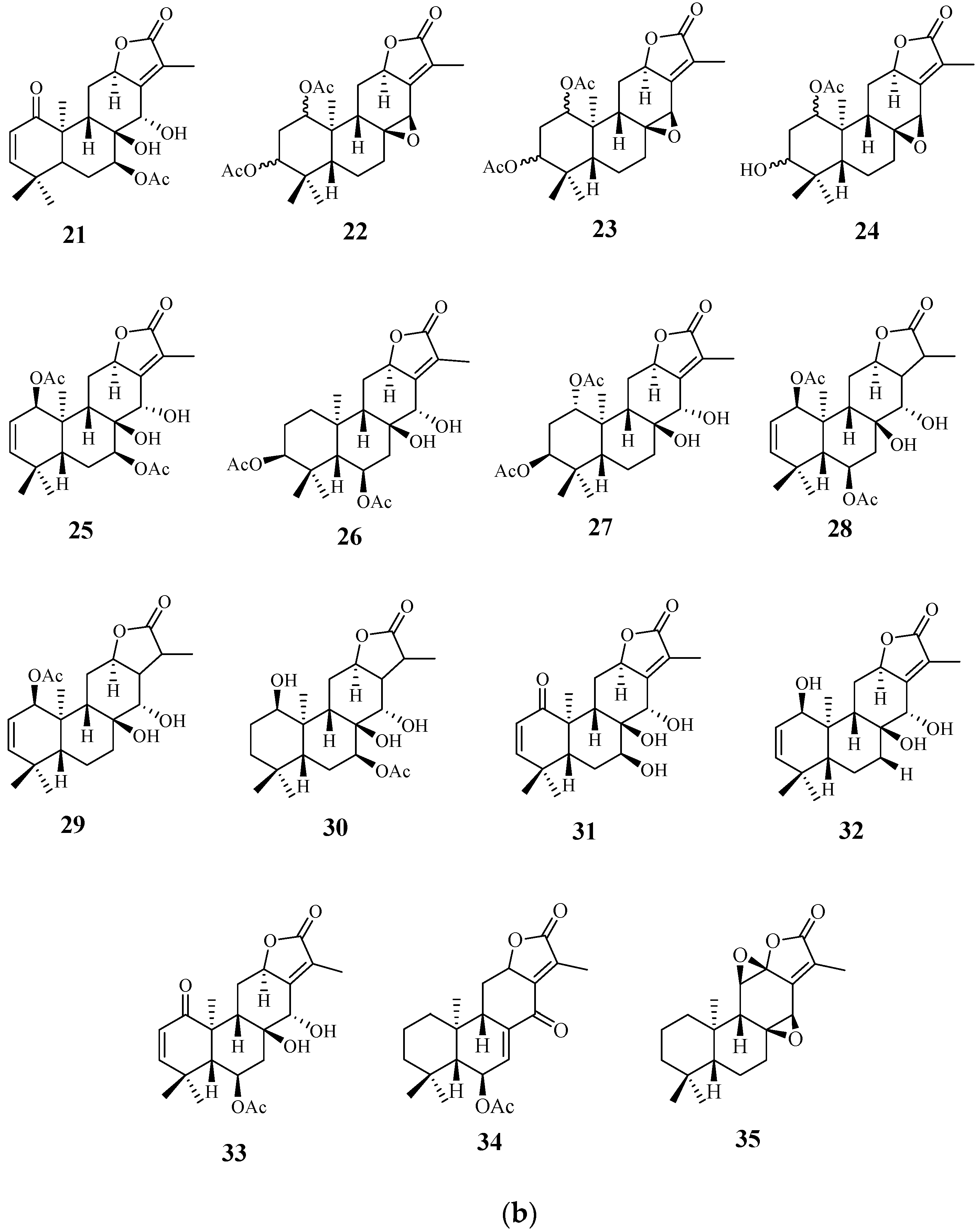
4.1. Diterpenoids
4.2. Abiatane-Type Diterpenoids
4.3. Kaurene-Type Diterpenoids
4.4. Ent-Pimarane Diterpenoids
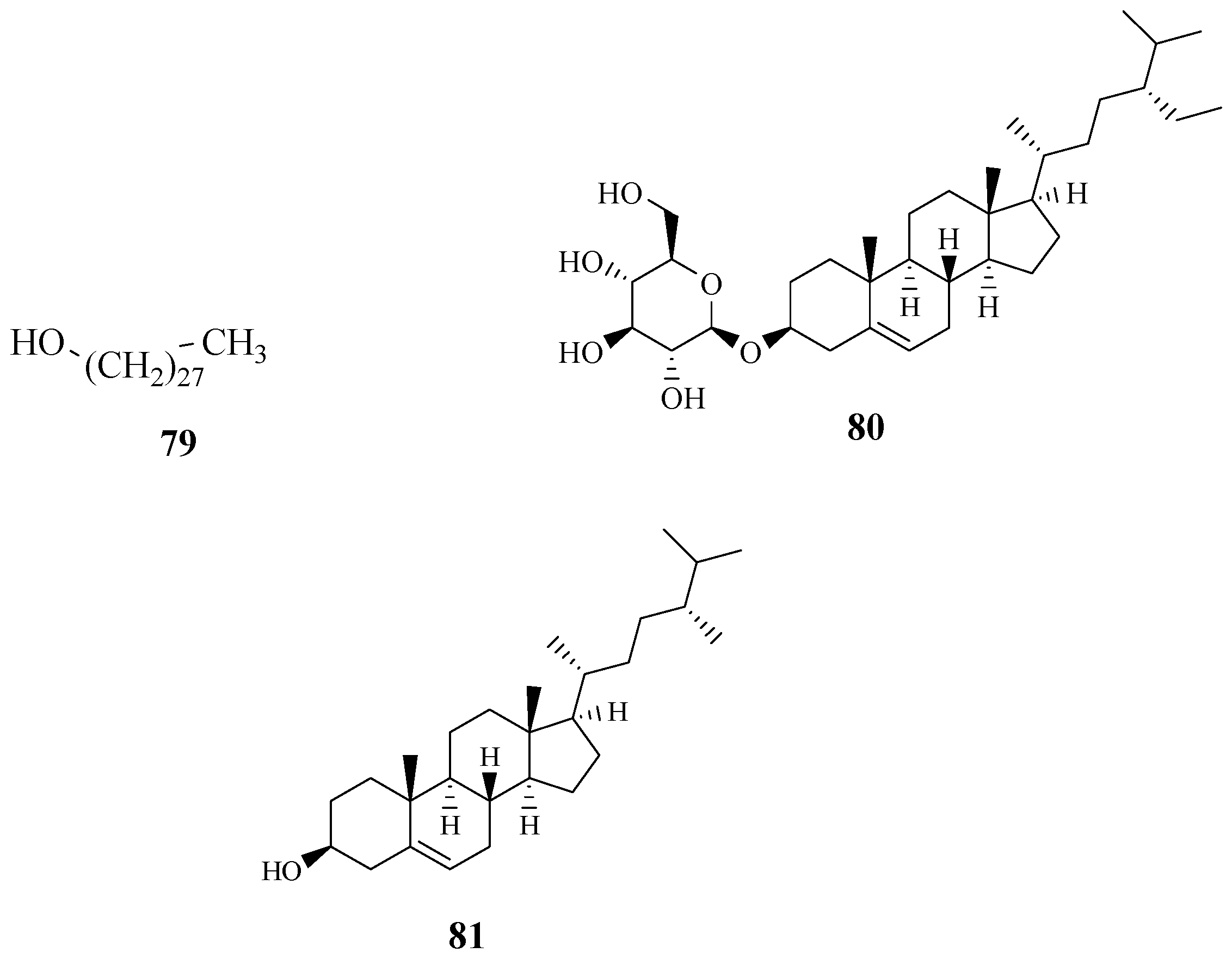
4.5. Triterpenoids
4.6. Fatty Alcohol, Steroidal Glycoside and Phytosterol
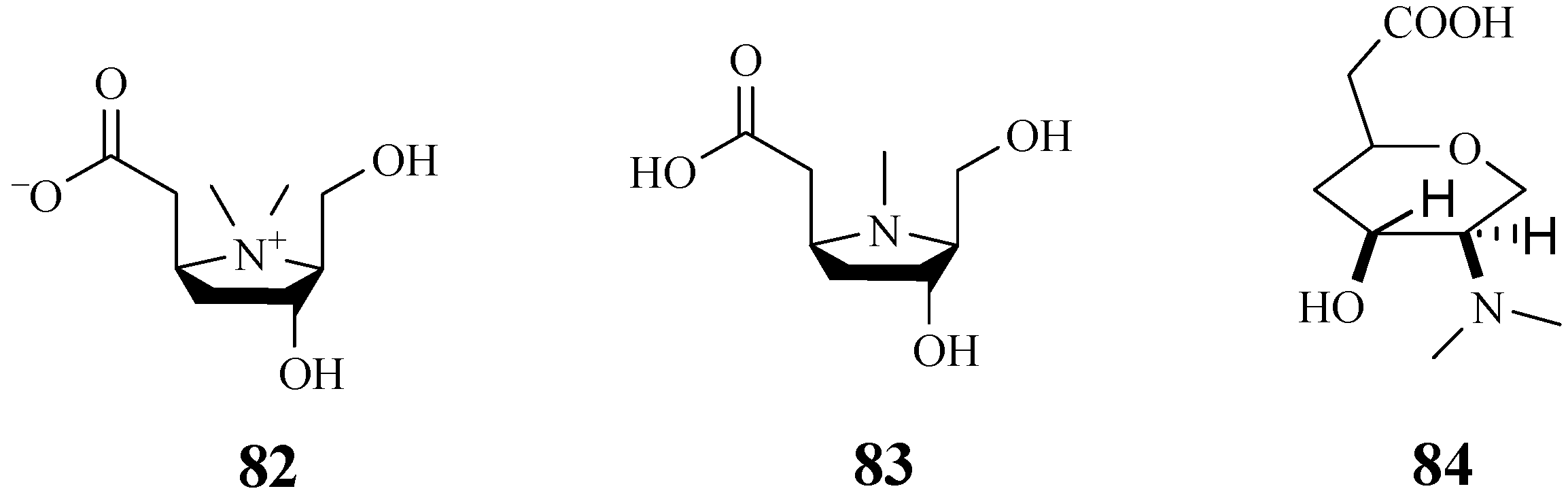
4.7. Alkaloids
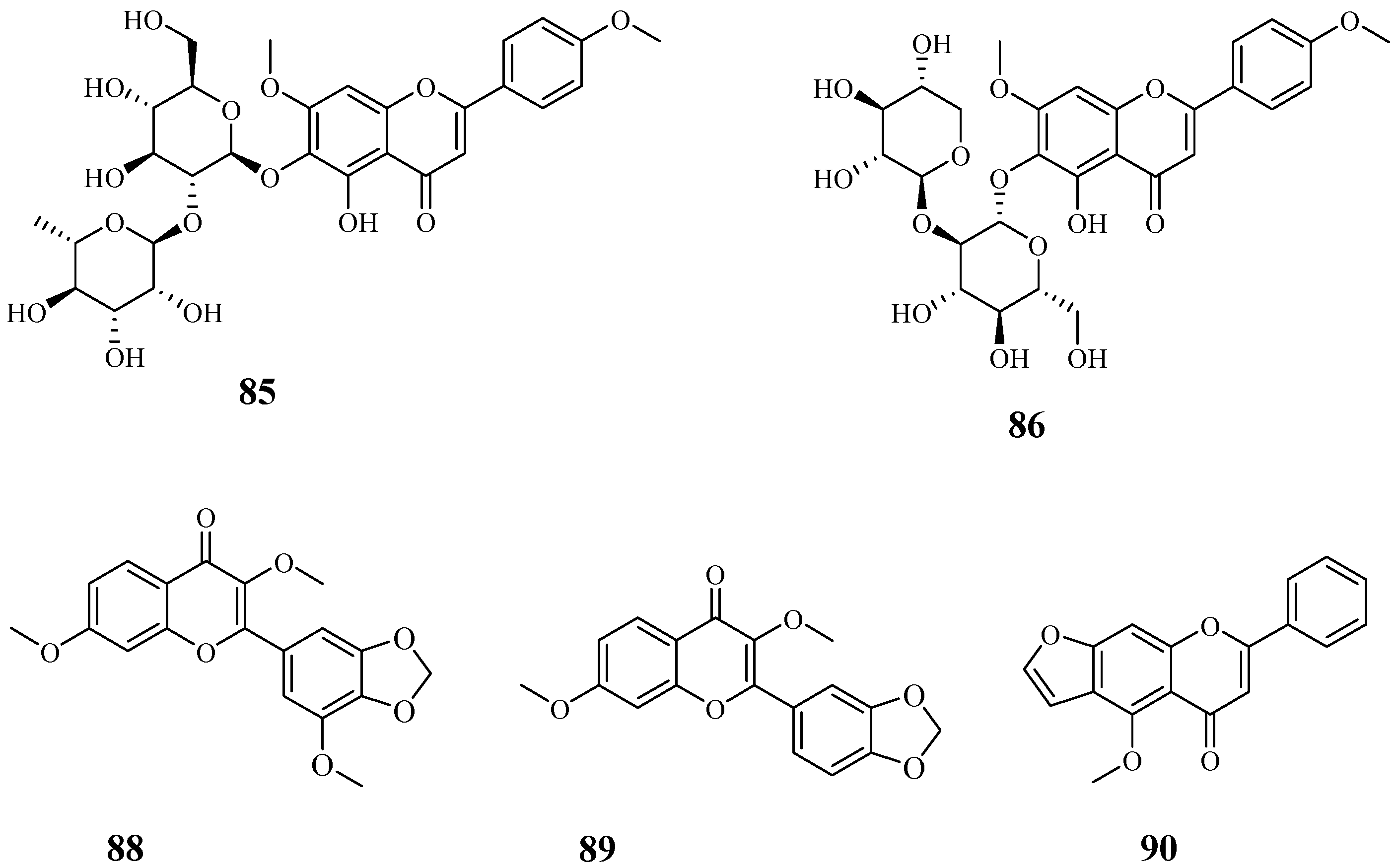
4.8. Flavonoids
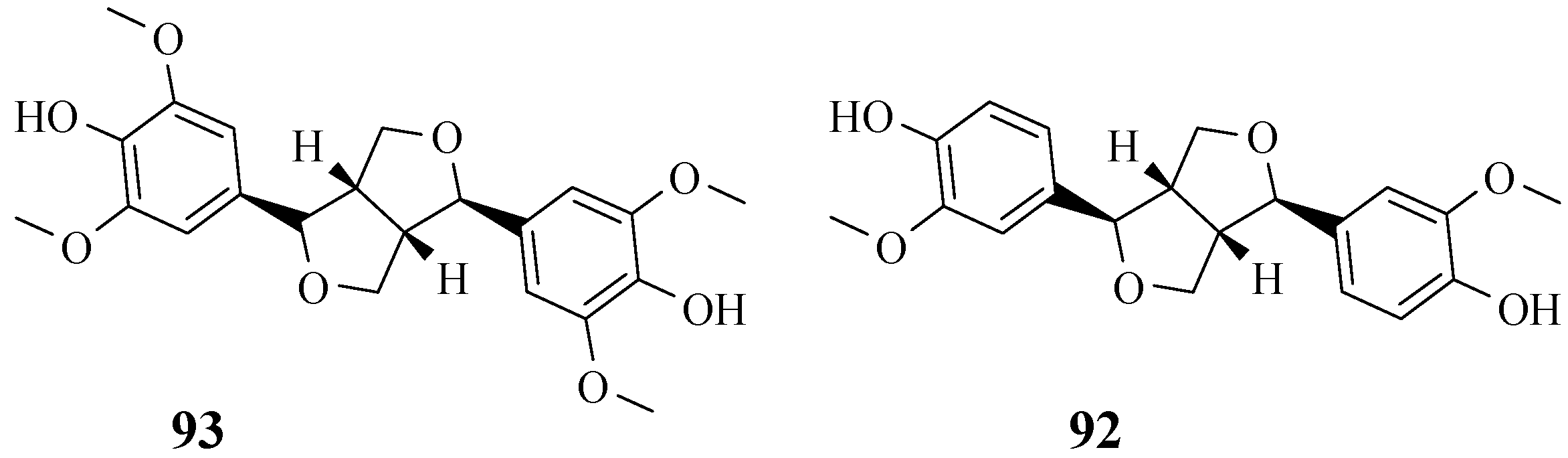
4.9. Lignans
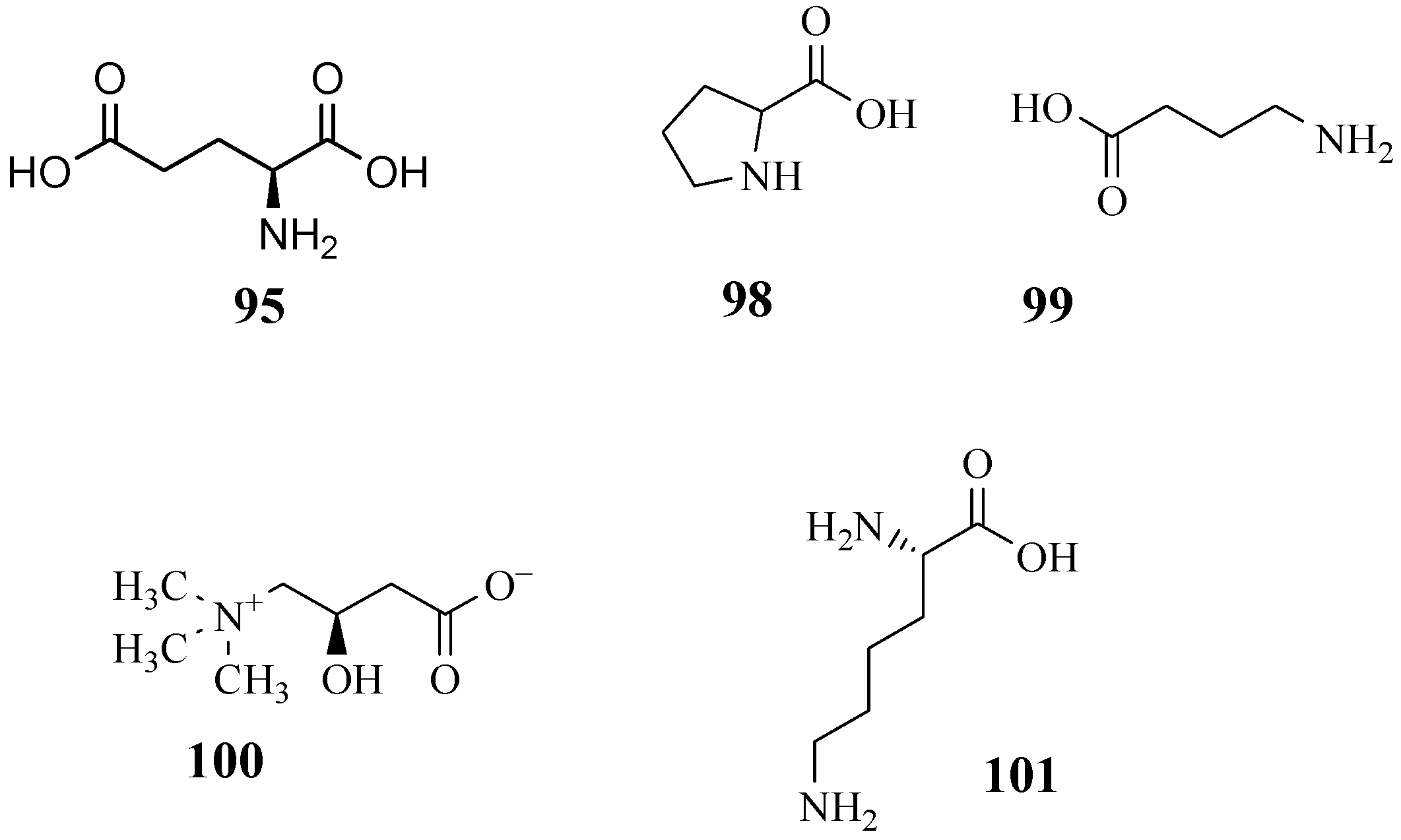
4.10. Amino Acids
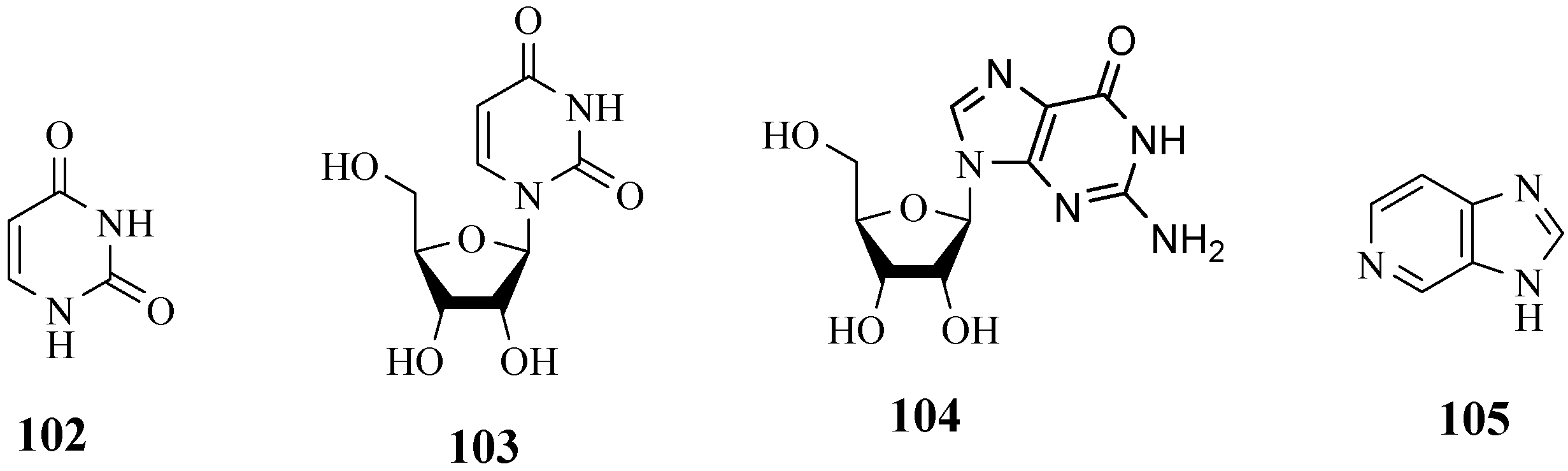
4.11. Pyrimidines, Purine Nucleoside and Pyridines
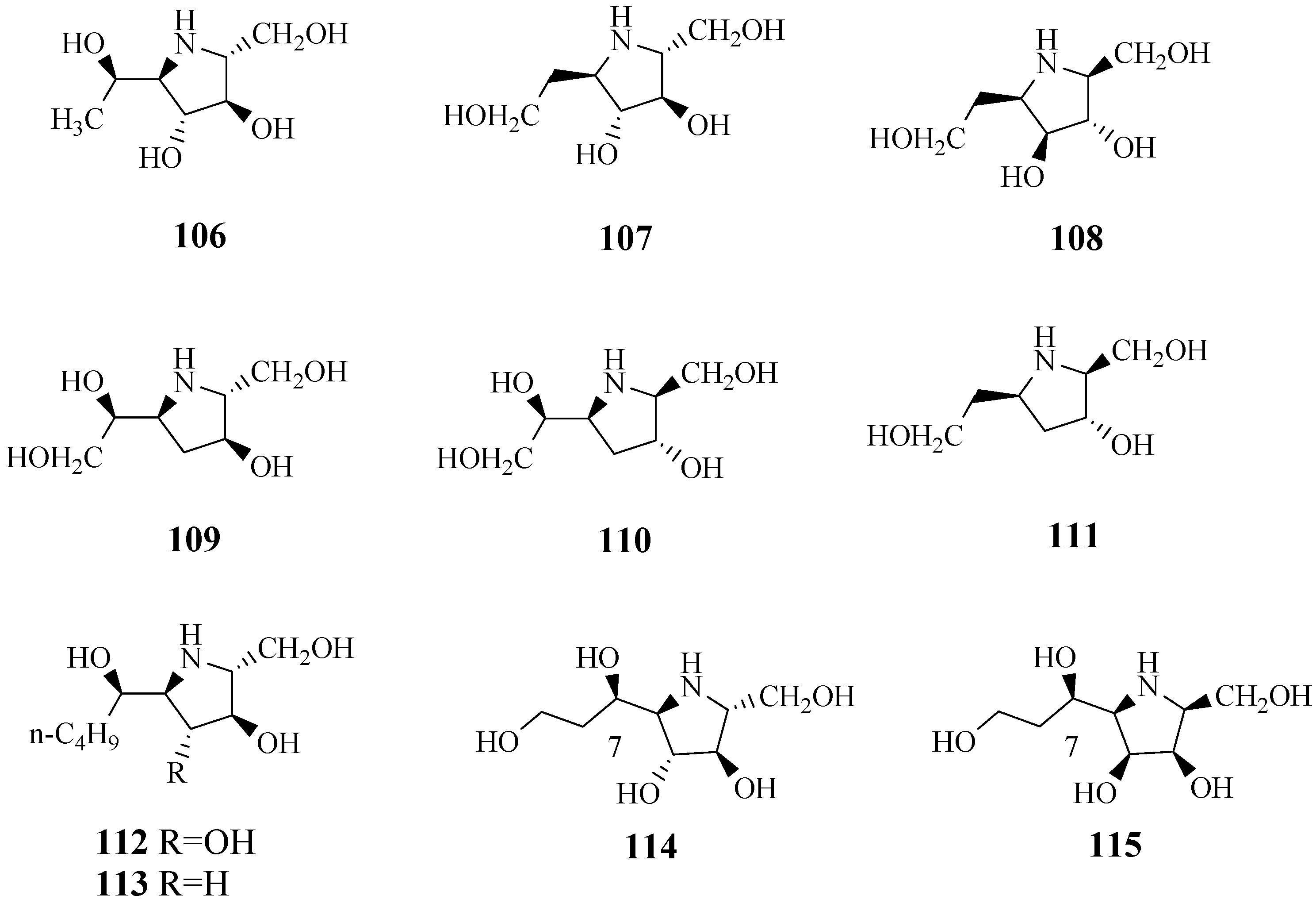
4.12. Pyrrolidine-Type Iminosugars
5. Pharmacological Activities of the Suregada Genus
5.1. Antibacterial and Antimicrobial Activity
5.2. Insecticidal Activity
5.3. Antifungal Activity
5.4. Anticancer Activity
5.5. Antinociceptive Activity
5.6. Antidiabetic Activity
5.7. Antiviral Activity
5.8. Cytotoxicity
5.9. Anti-Inflammatory
5.10. Antioxidant
5.11. Anti-Allergic Activity
5.12. Antileishmanial Activity
5.13. Antiplasmodial Activity
| Plant Species | Plant Part | Extract | Activity | Effect | References |
|---|---|---|---|---|---|
| S. aequorea | Unspecified | Dichloromethane | Anticancer | Exhibited activity against different human cancer cells with the IC50 of <20 µg·mL−1. | [35] |
| S. angustifolia | Stem bark | Methanol | Antibacterial | Revealed zone inhibition of S. aureus (32 mm), Enterobacter aerogenes (26 mm), E. coli (26 mm), and P. vulgaris (25 mm) at 20 mg·mL−1. | [9] |
| Chloroform | Showed zone inhibition of A. hydrophila (40 mm), K. pneumonia (37 mm), S. Aureus (32 mm), and E. coli (40 mm) at 20 mg·mL−1. | ||||
| Hexane | Exhibited zone inhibition of A. hydrophila (29 mm), K. pneumonia (29 mm), P. vulgaris (25 mm), V. vulnificus (25 mm), V. parahaemolyticus (25 mm), V. cholera (25 mm) at 20 mg·mL−1. | ||||
| S. glomerulata | Leaves | Water | Antidiabetic | Potent inhibition against α-glucosidase with the IC50 of 2.29 µg·mL−1. | [49] |
| S. multiflora | Roots | Methanol: Ethyl acetate (1:9) | Insecticidal activity | Revealed 100% mortality rate of Tribolium castaneum | [61] |
| Leaves | Showed a 40% mortality rate of Tribolium castaneum | ||||
| Antibacterial and Antimicrobial | Revealed mild activity against E. coli, Sh. Flexneri, B. subtilis, S. aureus, and P. aeruginosa with the of zone inhibition range of 5 ± 0.10 to 6 ± 0.13 mm. | [7] | |||
| Roots | Exhibited significant activity against E. coli with zone inhibition of 13 ± 0.01 mm and MIC of 0.625 mg·mL−1. | [61] | |||
| Stem bark | Unspecified | Inflammatory activity | Exhibited NO inhibitory effect with the IC50 of 8.6 µg·mL−1. | [17] | |
| S. procera | Unspecified | Ethanol | Anti-leishmanial activity | Exhibited strong activity, IC50 value ≤ 10 µg·mL−1 | [76] |
| S. zanzibariensis | Leaves | Methanol | Anti-plasmodial activity | Revealed good anti-plasmodial properties against Plasmodium falciparum strains (W2 and D6) with IC50 of 4.66 ± 0.22 µg·mL−1 and 1.82 ± 0.07 µg·mL−1. | [74] |
| Showed anti-plasmodial activity (1.5 µg·mL−1) against Plasmodium falciparum ENT36 and K67 with the IC50 of 1.5 µg·mL−1. | [71] | ||||
| Aqueous extract | Anti-leishmanial activity | Revealed anti-leishmanial activity on Leishmanial major promastigotes and amastigotes with a mortality percentage of 40.5 ± 1.99%. | [74] | ||
| Methanol extracts | Possessed good anti-leishmanial activity on Leishmania major amastigotes with a mortality percentage of 28.0 ± 2.11%. | ||||
| Revealed substantial differences in the production of NO by macrophages infected with Leishmania major amastigotes (6.6 ± 0.63 μM). | |||||
| Aqueous extracts | Showed a significant difference in the production of nitric oxide by macrophages infected with Leishmania major amastigotes (4.0 ± 0.56 μM). | ||||
| In vitro cytotoxicity | Low toxicity was observed against HELF cells with a cytotoxic concentration of 50% (CC50) value > 20 µg·mL−1. | ||||
| Methanol extracts | Showed low toxicity against HELF cells with the CC50 > 20 µg·mL−1. | [78] | |||
| Stem bark | Dichloromethane/methanol | Anticancer | Showed potent anticancer activity against TK10 with the TGI and GI50 of 0.60 µg·mL−1 and 0.26 µg·mL−1. | [50] | |
| Revealed anticancer activity against UACC62 with the TGI and GI50 0.54 µg·mL−1 for and 0.25 µg·mL−1. | [50] | ||||
| Showed anticancer activity against MCF7 with the TGI and GI50 5.27 µg·mL−1 and 0.81 µg·mL−1. |
6. Comparison of Ethnomedicinal Uses with the Pharmacological Uses
- The methanol and hexane extracts of S. anguistifolia, showed a maximum antibacterial effect against E. coli, A. hydrophila, and K. pneumonia. A similar activity was observed in S. aureus in chloroform and methanol extracts of S. anguistifolia stem bark. Staphylococcus aureus bacteria cause toothache and skin infections [9]. The pharmacological results from this plant species support the claims of traditional medicinal uses of S. anguistifolia, where Indian people in Kanis utilize it to treat skin infections and toothache [9].
- The wood of S. multiflora was reported to treat pyrexia, eczema, and venereal diseases, and the roots are utilized to treat lymphatic disorders and skin infections [17]. In Thailand, S. multiflora is utilized to treat skin diseases and inflammation [17]. Various solvent extracts from S. multliflora were screened for antibacterial and antimicrobial activity and exhibited effects against B. subtilis, P. aeruginosa, Shi. flexineri, S. aureus, and E. coli [62]. S. aureus is responsible for skin infections, gum diseases, eczema, and pyrexia (fever). The bacteria P. aeruginosa is responsible for lymphatic disorders, which can include swelling. E. coli and shi. flexineri responsible for pyrexia (fever) [76]. Helioscopinolide A (1) and epifriedelinol (75) isolated from S. multiflora revealed antibacterial activity that further substantiates the traditional uses claims of S. multiflora. Epifriedelinol (75) exhibited the highest zone inhibition against S. aureus. S. aureus is the bacteria responsible for skin infection, eczema, and gum diseases [57,59,71]. Suregadolide (4) isolated from S. multiflora showed antifungal activity, which confirms the claims of the traditional uses of the plant being utilized for treating fungal infections and skin disease [34].
- In some regions, the granule products of this species can be prepared, which acts as a powerful organic herbicide [19]. When tested for insecticidal activity, the (1:9) methanol: ethyl acetate root extract of S. multiflora revealed a mortality rate of 100% for Tribolium castaneum at 50 mg·mL−1 dose in 12 h [61]. Furthermore, the dichloromethane extract of S. multiflora stem showed partial antibacterial activity with zone inhibition of 3 mm against X. campestris [55]. S. multiflora revealed an antibacterial activity against X. campestris, which is responsible for plant diseases and insecticidal activity, which confirms the claims that the granules of S. multiflora act as organic herbicide [55,78].
- In Thailand, S. multiflora is used to treat inflammation [18]. The stem bark of S. multiflora showed anti-inflammatory properties against lipopolysaccharide (LPS)-induced nitric oxide (NO) and prostaglandin E (2) (PGE(2)) releases in RAW264.7 cells, and the anti-inflammatory mechanism on mRNA expression was carried out [17].
- A mixture of S. multiflorum is mixed with other herbs it is used as an anticancer recipe [17]. Helioscopinolide A (5) and gelomulide E (12) isolated from S. multiflora showed anticancer activity against various types of cancer, namely, leukemia (CCRF CEM), leukemia (SR), leukemia (K-562), breast (MD-MB-435), and colon (HTC-15) which supports the claims of S. multiflora being used traditionally in the anticancer recipe.
- S. zanzibariensis leaves are utilized to treat malaria. The leaf extract of S. zanzibariensis revealed high anti-plasmodial activity with the IC50 value of1.5 µg·mL−1) against Plasmodium falciparum K67 and ENT36 [77].
- Giriama and Duruma people use a root decoction to treat body swelling [28]. Tanzanian people used the stem bark and root extract of S. zanzibariensis to treat ankylostomiasis caused by parasitic hookworms [25]. Nitrogen production macrophages infected with amastigotes of Leishmania major treated with the methanol and aqueous extracts of S. zanzibariensis showed significant Nitric oxide concentration at 4.0 ± 0.56 and 6.6 ± 0.63 µg·mL−1 at 1000 µg·mL−1 [74]. The potent activity of this species against Leishmania, a parasitic disease, supports the claim that S. zanzibariensis is used to treat ankylostomiasis and body swelling. Simiarenol (81) isolated from S. zanzibariensis exhibited noticeable antinociceptive properties with the ID50 of 18.87 (14.6–24.4) mmol·kg−1 [71,72]. The activity of Simiarenol validates the claims of S. zanziberiensis being traditionally used for chest and abdominal pains.
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Lists of Abbreviations
| A2780 | Human ovarian cancer cell line |
| Aca-109 | Human oesophageal carcinoma cell line |
| ATP | Adenosine triphosphate |
| Bax | pro-apoptosis gene |
| B16F10 | Mouse melanoma cell line |
| Bcl-2 | Anti-apoptosis gene |
| Bel 7402 | Human liver cancer cell |
| BGC 823 | Human stomach cancer cell |
| BT474 | Breast cancer cell line |
| CC50 | 50% cytotoxic concentration |
| CCRF-CEM | Human leukemic lymphoblast cell line |
| COX-2 | Cyclooxygenase-2 |
| CRC | Colorectal cancer cell line |
| D6 | chloroquine-sensitive |
| DCM | Dichloromethane |
| DNA | Deoxyribonucleic acid |
| DRC | Democratic Republic of Congo |
| ENT36 | chloroquine-resistant |
| ERK | Extracellular signal-regulated kinase |
| GAP3 | Glycosylation-associated protein |
| GI50 | (50% growth inhibition) |
| Glut1, Glut3 and Glut4 | Glucose transporter genes |
| K-562 | Human chronic myeloid leukemia cell line |
| K67 | chloroquine-resistant |
| H37Ra | Mycobacterium tuberculosis |
| HCT-8 | Human colon cancer cell line |
| HTC-15 | Human colon cancer cell line |
| HeLa | Human cervical cancer cell line |
| HELF | Human embryonic lung fibroblast |
| HepG2 | (hepatocellular carcinoma) Liver cancer cell line |
| Hk2 | Hexokinase 2 |
| HIV | Human Immunodeficiency Virus |
| HSV | Herpes simplex virus |
| HT29 | Human colon cancer cell line |
| IC12 | Minimum concentration that gives an inhibition zone of 12 mm diameter |
| IC50 | Half-maximal inhibitory concentration |
| iNOS | Inducible nitric oxide synthase |
| Ldha | Lactate dehydrogenase-A |
| LPS | Lipopolysaccharide |
| MCF7 | human breast cancer cell line |
| MDA-MB-231 | Epithelial, human breast cancer cell line |
| MDA-MB435 | Epithelial, human breast cancer cell line |
| MIC | Minimal inhibitory concentration |
| mRNA | Messenger Ribonucleic acid |
| NCI | National Cancer Institute |
| NCI-H460 | Non-small cell lung cancer |
| NCI-H490 | Lung cancer cell line |
| NO | Nitric oxide |
| NRF | National Research Foundation |
| PGE(2) | Prostaglandin E(2) |
| RAW 264.7 | Mouse macrophage cell line |
| ROS | Reactive oxygen species |
| SKBR3 | Breast cancer cell line |
| SR | Spontaneous remission leukemia cell line |
| SW620 | Human colon cancer cell line |
| TGI | Total growth inhibition |
| TK10 | Renal cancer |
| UACC62 | Melanoma cell line |
| U937 | Human leukemic cell line |
| W2 | chloroquine-resistant |
| WHO | World Health Organisation |
| µg/mL | Micrograms per milliliter |
| µmol/L | Micromole per litre |
| µg/spot | Micrograms per spot |
References
- Plants of the World Online (Continuously Updated). Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 11 August 2022).
- Radcliffe-Smith, A. Genera Euphorbiacearum; Royal Botanic Gardens, Kew: Richmond, UK, 2001. [Google Scholar]
- Radcliffe-Smith, A.; Hoffmann, P.; Ranaivojaona, R.; Ralimanan, A.H. Suregada celastroides Radcl.-Sm. & Petra Hoffm. (Euphorbiaceae), a new species from eastern Madagascar. Kew Bull. 2003, 58, 965–970. [Google Scholar]
- Wurdack, K.J.; Hoffmann, P.; Chase, M.W. Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid rbcL and trnL-F DNA sequences. Am. J. Bot. 2005, 92, 1397–1420. [Google Scholar] [CrossRef] [PubMed]
- Léonard, J. Notulae Systematicae XXIV. Note sur les genres Suregada Roxb. Ex Rottl. et Gelonium Roxb. Ex Willd. (Euphorbiaceae). Bull. Jard. Bot. L’état Brux. 1958, 28, 443–450. [Google Scholar] [CrossRef]
- Darbyshire, I.; Kordofani, M.; Farag, I. The Plants of Sudan and South Sudan: An Annotated Checklist; Royal Botanic Gardens, Kew: Richmond, UK, 2015. [Google Scholar]
- African Plant Database (Version 3.4.0). Conservatoire et Jardin Botaniques de la Ville de Genève and South African National Biodiversity Institute, Pretoria. Available online: http://africanplantdatabase.ch (accessed on 20 August 2022).
- Govaerts, R.; Frodin, D.G.; Radcliffe-Smith, A. World Checklist and Bibliography of Euphorbiaceae (and Pandaceae) 1-4: 1-1622; The Board of Trustees of the Royal Botanic Gardens, Kew: Richmond, UK, 2000. [Google Scholar]
- Venkatesan, M.; Viswanathan, M.B.; Ramesh, N.; Lakshmanaperumalsamy, P. Antibacterial potential from Indian Suregada angustifolia. J. Ethnopharmacol. 2005, 93, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Norscia, I.; Borgognini-Tarl, S.M. Ethnobotanical reputation of plant species from two forests of Madagascar: A preliminary investigation. S. Afr. J. Bot. 2006, 72, 656–660. [Google Scholar] [CrossRef]
- Razafindraibe, M.; Kuhlman, A.R.; Rabarison, H.; Rakotoarimanana, V.; Rajeriarison, C.; Rakotoarivelo, N.; Randrianarivony, T.; Rakotoarivony, F.; Ludovic, R.; Randrianasolo, A.; et al. Medicinal plants used by women from Agnalazaha littoral forest (Southeastern Madagascar). J. Ethnobiol. Ethnomedicine 2013, 9, 1–13. [Google Scholar] [CrossRef]
- Reddy, A.M.; Babu, M.V.S.; Rao, R.R. Ethnobotanical study of traditional herbal plants used by local people of Seshachalam Biosphere Reserve in Eastern Ghats, India. Herba Pol. 2019, 65, 40–54. [Google Scholar] [CrossRef]
- Pulladiah, T.; Krishnamurthy, K.V.; Bahadur, B. Ethnobotany of India, Volume 2: Western Ghats and West Coast of Peninsular India; Apple Academic Press, Inc.: Palm Bay, FL, USA, 2017; p. 184. [Google Scholar]
- Lovett, J.C.; Ruffo, C.K.; Gereau, R.E. Field Guide to the Moist Forest Trees of Tanzania; The Society for Environmental Exploration: London, UK, 2005; p. 97. [Google Scholar]
- Choudhary, M.I.; Gondal, H.Y.; Abbaskha, A.; Jahan, I.A.; Parvea, M.; Nahar, N.; Rahman, A.U. Revisiting diterpene lactones of Suregada multiflora. Tetrahedron 2004, 60, 7933–7941. [Google Scholar] [CrossRef]
- Jahan, I.A.; Nahar, N.; Mosihuzzaman, M.; Shaheen, F.; Rahman, A.U.; Choudhary, M.I. Six new diterpenoids from Suregada multiflora. J. Nat. Prod. 2004, 67, 1789–1795. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Subhadhirasakul, S.; Cheenpracha, S.; Yodsaoue, O.; Ponglimanont, C.; Karalai, C. Anti-inflammatory principles of Suregada multiflora against nitric oxide and prostaglandin E2 releases. J. Ethnopharmacol. 2011, 133, 63–66. [Google Scholar] [CrossRef]
- Patil, A.S. Ethnomedicinal uses of some plants used by Gond tribe of Gadchiroli district, Maharashtra. Indian J. Nat. Prod. Resour. 2016, 7, 56–62. [Google Scholar]
- Kumar, S.; Pandey, A.K.; Thakur, J.R. Plant extracts used as organic pesticides. J. Med. Plants Stud. 2017, 5, 117–120. [Google Scholar]
- Pal, D.C.; Sharma, S.K.; Das, M.; Sahu, R.K. Evaluation of hepatoprotective and in vitro antioxidant activity of Suregada multiflora (Bark) in Swiss Albino mice. Int. J. Pharm. Sci. 2011, 3, 127–130. [Google Scholar]
- Hartley, T.G. The ethnobotany of the Northern Cheyenne Indians of Montana. J. Ethnopharmacol. 2001, 74, 141–156. [Google Scholar]
- Jamaluddin, M.D.; Sayeed, A.; Khaleque, M.A.; Uddin, M.J. Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot. Res. Appl. 2015, 14, 119–133. [Google Scholar]
- Russof, L.L.; Dissanayake, M.D.M.S.; Amarasinghe, M.D.; Fonseka, D.M. Evaluation of selected forest tree species in the dry zone of Sri Lanka for wood strength properties. J. Trop. For. Sci. 2003, 15, 483–490. [Google Scholar]
- Fassil, A.; Gashaw, G. An ethnobotanical study of medicinal plants in chiro district, West Hararghe, Ethiopia. Afr. J. Plant Sci. 2019, 13, 309–323. [Google Scholar]
- Schmelzer, G.H.; Wageningen, A.G.F. Plant Resources of Tropical Africa; PROTA Foundation-Backhuys—CTA PROTA: Wageningen, The Netherlands, 2008; Volume 11, p. 582. [Google Scholar]
- Runyoro, D.K.B.; Ngassapa, O.D.; Matee, M.I.N.; Joseph, C.C.; Moshi, M.J. Medicinal plants used by Tanzanian traditional healers in the management of Candida infections. J. Ethnopharmacol. 2006, 106, 158–165. [Google Scholar] [CrossRef]
- Hedberg, I.; Hedberg, O.; Madati, P.J.; Mshigeni, K.E.; Mshiu, E.N.; Samuelsson, G. Inventory of plants used in traditional medicine in Tanzania. Part II. Plants of the families Dilleniaceae–Opiliaceae. J. Ethnopharmacol. 1983, 9, 105–127. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; E. & S. Livingstone: London, UK, 1962. [Google Scholar]
- Innocent, E.; Joseph, C.C.; Gikonyo, N.K.; Nkunya, M.H.; Hassanali, A. Constituents of the essential oil of Suregada zanzibariensis leaves are repellent to the mosquito, Anopheles gambiae s.s. J. Insect Sci. 2010, 2010, 10–57. [Google Scholar]
- Monaco Encyclopaedia Suregada multiflora; Monaco Nature Encyclopedia: Monte Carlo, Monaco, 2018.
- Useful Tropical Plants. Available online: www.theferns.info (accessed on 15 August 2023).
- Suregada lanceolata-South Indian Suregada. Available online: www.flowersofindia.net (accessed on 18 August 2023).
- Available online: https://casabio.org/taxa (accessed on 5 August 2023).
- Jahan, I.A.; Nahar, N.; Mosihuzzaman, M.; Shaheen, F.; Parween, Z.; Rahman, A.U.; Choudhary, M.I. Novel diterpene lactones from Suregada multiflora. J. Nat. Prod. 2002, 65, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Chang, F.R.; Hsieh, P.W.; Chiang, M.Y.; Wu, C.C.; Huang, Z.Y.; Lan, Y.H.; Chen, M.; Lee, K.H. Cytotoxic ent-abietane diterpenes from Gelonium aequoreum. Phytochemistry 2008, 69, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Cheenpracha, S.; Yodsoue, O.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Tewtrakul, S.; Kanjana-Opas, A. Potential anti-allergic ent-kaurene diterpenes from the bark of Suregada multiflora. Phytochemistry 2006, 67, 2630–2634. [Google Scholar] [CrossRef] [PubMed]
- Gondal, H.Y.; Choudhary, M.I. New Diterpene Lactone from the leaves of Suregada multiflora. Chem. Nat. Compd. 2016, 52, 421–423. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2015, 32, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Wu, Q.C.; Tang, Y.P.; Ding, A.W.; You, F.Q.; Zhang, L.; Duan, J.A. 13C-NMR data of three important diterpenes isolated from Euphorbia species. Molecules 2009, 14, 4454–4475. [Google Scholar] [CrossRef]
- Dewick, M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; ISBN 978-0-47074-168-9. [Google Scholar]
- Zhang, J.; He, J.; Wang, X.X.; Shi, Y.X.; Zhang, N.; Ma, B.Z.; Zhang, W.K.; Xu, J.K. Ent-abietane diterpenoids and their probable biogenetic precursors from the roots of Euphorbia fischeriana. RSC Adv. 2017, 7, 55859–55865. [Google Scholar] [CrossRef]
- Sandjo, L.P.; Kuete, V. Diterpenoids from the Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Elsevier: Amsterdam, The Netherlands, 2013; pp. 105–121. [Google Scholar]
- Rubinger, M.M.M.; Castelo-Branco, P.d.A.; Guilardi, S.; Souza, E.M.R.; Gambardella, M.T.d.o.P.; Borges, E.E.L.; Ferreira-Alves, D.L.; Pilo-Veloso, D. Preparation, X-ray structural studies, and plant growth regulatory activity of methyl 6α,7β-thiocarbonyldioxyvouacapan-17β-oate. J. Braz. Chem. Soc. 2004, 15, 219–223. [Google Scholar] [CrossRef]
- Sakan, F.; Iwashita, T.; Hamanaka, N. Structures of Metasequoic Acid A and B. Chem. Lett. 1988, 17, 123–126. [Google Scholar] [CrossRef]
- Kalenga, T.M.; Mollel, J.T.; Said, J.; Orthaber, A.; Ward, J.S.; Atilaw, Y.; Umereweneza, D.; Ndoile, M.M.; Munissi, J.J.E.; Rissanen, K.; et al. Modified ent-Abietane ditepenoids from the leaves of Suregada zanzibariesis. J. Nat. Prod. 2022, 85, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Gondal, H.Y.; Nisar, M.; Choudhary, M.I. Antileishmanial activity of diterpene lactones from Suregada multiflora and their semisynthetic derivatives. Curr. Bioact. Compd. 2020, 16, 53–57. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Das, G.; Talapatra, B. Stereostructures and molecular conformations of six diterpene lactones from Gelonium multiflorum. Phytochemistry 1989, 28, 1181–1185. [Google Scholar] [CrossRef]
- Talapatra, B.; Das, G.; Das, A.K.; Biswas, K.; Talapatra, S.K. Stereostructures and conformations of four diterpene lactones from Gelonium multiflorum. Phytochemistry 1998, 49, 1353–1359. [Google Scholar] [CrossRef]
- Mangisa, M.; Tembu, V.J.; Fouche, G.; Nthambeleni, R.; Peter, X.; Langat, M.K. Ent-abietane diterpenoids from Suregada zanziberiensis Baill. (Euphorbiaceae), their cytotoxic and anticancer properties. Nat. Prod. Res. 2018, 33, 3240–3247. [Google Scholar] [CrossRef]
- He, S.L.; Yan, R.Y.; Zuo, L.; Chen, R.Y. Ent-Abietane and ent-Kaurane diterpenoids from the Roots of Suregada glomerulata. Planta Med. 2009, 75, 641–646. [Google Scholar] [CrossRef]
- Yan, R.Y.; Tan, Y.X.; Cui, X.Q.; Chen, R.; Yu, D.Q. Diterpenoids from the roots of Suregada glomerulata. J. Nat. Prod. 2008, 71, 195–198. [Google Scholar] [CrossRef]
- Yan, R.Y.; Wang, H.Q.; Lui, C.; Chen, R.Y.; Yu, D.Q. Three new water-soluble alkaloids from the leaves of Suregada glomerulata (Blume) Baill. Fitoterapia 2011, 82, 247–250. [Google Scholar] [CrossRef]
- Sengupta, P.; Khastgir, H.N. Terpenoids, and related compounds-III: Bauerenol and multiflorenol from gelonium multiflorum A. Juss. The structure of multiflorenol. Tetrahedron 1963, 19, 123–132. [Google Scholar] [CrossRef]
- Kite, G.C.; Hoffmann, P.; Lees, D.C.; Wurdack, K.J.; Gillespie, L.J. α-Homonojirimycin and other polyhydroxyalkaloids in Suregada Roxb. ex Rottl. (Euphorbiaceae). Biochem. Syst. Ecol. 2005, 33, 1183–1186. [Google Scholar] [CrossRef]
- Gondal, H.Y. Isolation and Biotransformation of Some Bioactive Compounds from Medicinal Plants. Ph.D. Thesis, H.E.J. Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Karachi, Pakistan, 2004; pp. 1–8. [Google Scholar]
- Parveen, N.; Khan, N.U. Luteolin 7, 4′-dimethyl ether 3′-glucoside from Gelonium multiflorum. Phytochemistry 1987, 26, 2130–2131. [Google Scholar] [CrossRef]
- Das, B.; Chakravarthy, A.K. Three flavone glycosides from gelonium multiflorum. Phytochemistry 1993, 33, 493–496. [Google Scholar] [CrossRef]
- Yan, R.Y.; Wang, H.Q.; Kang, J.; Chen, R.Y. Pyrrolidine-type iminosugars from leaves of Suregada glomerulata. Carbohydr. Res. 2014, 384, 9–12. [Google Scholar] [CrossRef]
- Khuntong, S.; Sudprasert, W. Extraction and basic testing for antibacterial activity of the chemical constituents in Suregada multiflorum. Kasetsart J. Nat. Sci. 2008, 42, 429–434. [Google Scholar]
- Islam, M.S.; Zahan, R.; Alam, M.B.; Nazn, M.; Sarkar, G.C.; Mosaddik, M.A.; Haque, M.E. Studies on antibacterial and insecticidal activities of Suregada multiflora. LARCJI 2011, 2, 62–67. [Google Scholar]
- Valente, C.; Pedro, M.; Duarte, A.; Nascimento, M.S.J.; Abreu, P.M.; Ferreira, M.J. Bioactive diterpenoids, a new jatrophane and two ent-abietanes, and other constituents from Euphorbia pubescens. J. Nat. Prod. 2004, 67, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V. Crystal structure and antibacterial evaluation of epifriedelinol isolated from Vitex peduncularis Wall. ex Schauer. Arab. J. Chem. 2019, 12, 2289–2292. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Guan, S.H.; Li, X.N.; Chen, G.T.; Zhang, J.Q.; Huang, H.L.; Liu, X.; Guo, D.A. Cytotoxic diterpenes from Euphorbia helioscopia. J. Nat. Prod. 2008, 71, 873–876. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Wang, L.; Zhang, F.; Zhang, Y. Research Progress on Chemical Constituents and Anticancer Pharmacological Activities of Euphorbia lunulata Bunge. BioMed Res. Int. 2020, 2020, 3618941. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhou, Y.J.; Bai, X.; He, P. Jolkinolide B from Euphorbia fischeriana Steud induces apoptosis in human leukemic U937 cells through PI3K/Akt and XIAP pathways. Mol. Cell. 2011, 32, 451–457. [Google Scholar] [CrossRef]
- Gao, C.; Yan, X.; Wang, B. Jolkinolide B induces apoptosis and inhibits tumor growth in mouse melanoma B16F10 cells by altering glycolysis. Sci. Rep. 2016, 6, 36114. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Cui, H.; Yang, H.; Du, X.; Yue, L.; Liu, J.; Lin, Y. Anti-metastatic effect of jolkinolide B and the mechanism of activity in breast cancer MDA-MB-231 cells. Oncol. Lett. 2015, 10, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, J.; Cao, S. Bauerenol inhibits proliferation, migration, and invasion of retinoblastoma cells via induction of apoptosis, autophagy, and cell cycle arrest. Trop. J. Pharm. Res. 2022, 21, 1377–1382. [Google Scholar] [CrossRef]
- Luo, H.; Wang, A. Induction of apoptosis in K562 cells by jolkinolide B. Can. J. Physiol. Pharmacol. 2006, 84, 959–965. [Google Scholar] [CrossRef]
- Aljamali, N.M.; Al-zubaidy, Z.H.; Enad, A.H. Bacterial infection and common bacterial diseases: A Review. Pharm. Nanotechnol. 2021, 3, 13–23. [Google Scholar]
- Kuroshima, K.N.; Campos-Buzzi, F.; Yunes, R.A.; Monache, F.D.; Filho, V.C. Chemical composition and antinociceptive properties of Hyeronima alchorneoides. Leaves. Pharm. Biol. 2005, 43, 573–578. [Google Scholar] [CrossRef]
- Bourinbaiar, A.S.; Lee-Huang, S. The activity of plant-derived antiretroviral proteins MAP30 and GAP31 against herpes simplex virus infection in vitro. Biochem. Biophys. Res. Commun. 1996, 219, 923–929. [Google Scholar] [CrossRef]
- Kigondua, E.V.M.; Rukunga, G.M.; Keriko, J.M.; Tonui, W.K.; Gathirwa, J.W.; Kirira, P.G.; Irungu, B.; Ingonga, J.M.; Ndiege, I.O. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J. Ethnopharmacol. 2009, 123, 504–50963. [Google Scholar] [CrossRef]
- Kumar, S.P.; Viswanathan, M.B.G.; Venkatesan, M.; Balakrishna, K. Bauerenol, a triterpenoid from Indian Suregada angustifolia: Induces reactive oxygen species-mediated P38MAPK activation and apoptosis in human hepatocellular carcinoma (HepG2) cells. Tumor Biol. 2017, 39, 1010428317698387. [Google Scholar]
- Muhammad, I.; Midiwo, J.; Tekwani, B.L.; Samoylenko, V.; Sahu, R.; Machumi, F.; Rahman, A.A.; Walker, L.A.; Hester, J.P. Antileishmanial Activity of Kenyan Medicinal Plants; University of Nairobi: Nairobi, Kenya, 2008; pp. 1–2. [Google Scholar]
- Omulokoli, E.; Khan, B.; Chhabra, S.C. Antiplasmodial activity of four Kenyan medicinal plants. J. Ethnopharmacol. 1997, 56, 133–137. [Google Scholar] [CrossRef]
- Mohana, D.C.; Raveesha, K.A. Antibacterial activity of Caesalpinia coriaria (Jacq.) Willd. against plant pathogenic Xanthomonas pathovars: An eco-friendly approach. JAST 2006, 2, 317–327. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangisa, M.; Kemboi, D.; Fouche, G.; Nthambeleni, R.; Langat, M.K.; Tarirai, C.; Cheek, M.; Gonyela, O.; Tembu, V.J. Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of Suregada Genus: A Review. Pharmaceuticals 2023, 16, 1390. https://doi.org/10.3390/ph16101390
Mangisa M, Kemboi D, Fouche G, Nthambeleni R, Langat MK, Tarirai C, Cheek M, Gonyela O, Tembu VJ. Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of Suregada Genus: A Review. Pharmaceuticals. 2023; 16(10):1390. https://doi.org/10.3390/ph16101390
Chicago/Turabian StyleMangisa, Mandisa, Douglas Kemboi, Gerda Fouche, Rudzani Nthambeleni, Moses Kiprotich Langat, Clemence Tarirai, Martin Cheek, Odwa Gonyela, and Vuyelwa Jacqueline Tembu. 2023. "Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of Suregada Genus: A Review" Pharmaceuticals 16, no. 10: 1390. https://doi.org/10.3390/ph16101390
APA StyleMangisa, M., Kemboi, D., Fouche, G., Nthambeleni, R., Langat, M. K., Tarirai, C., Cheek, M., Gonyela, O., & Tembu, V. J. (2023). Ethnomedicinal Uses, Phytochemistry and Pharmacological Properties of Suregada Genus: A Review. Pharmaceuticals, 16(10), 1390. https://doi.org/10.3390/ph16101390