Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection, Data Collection Process and Data Items
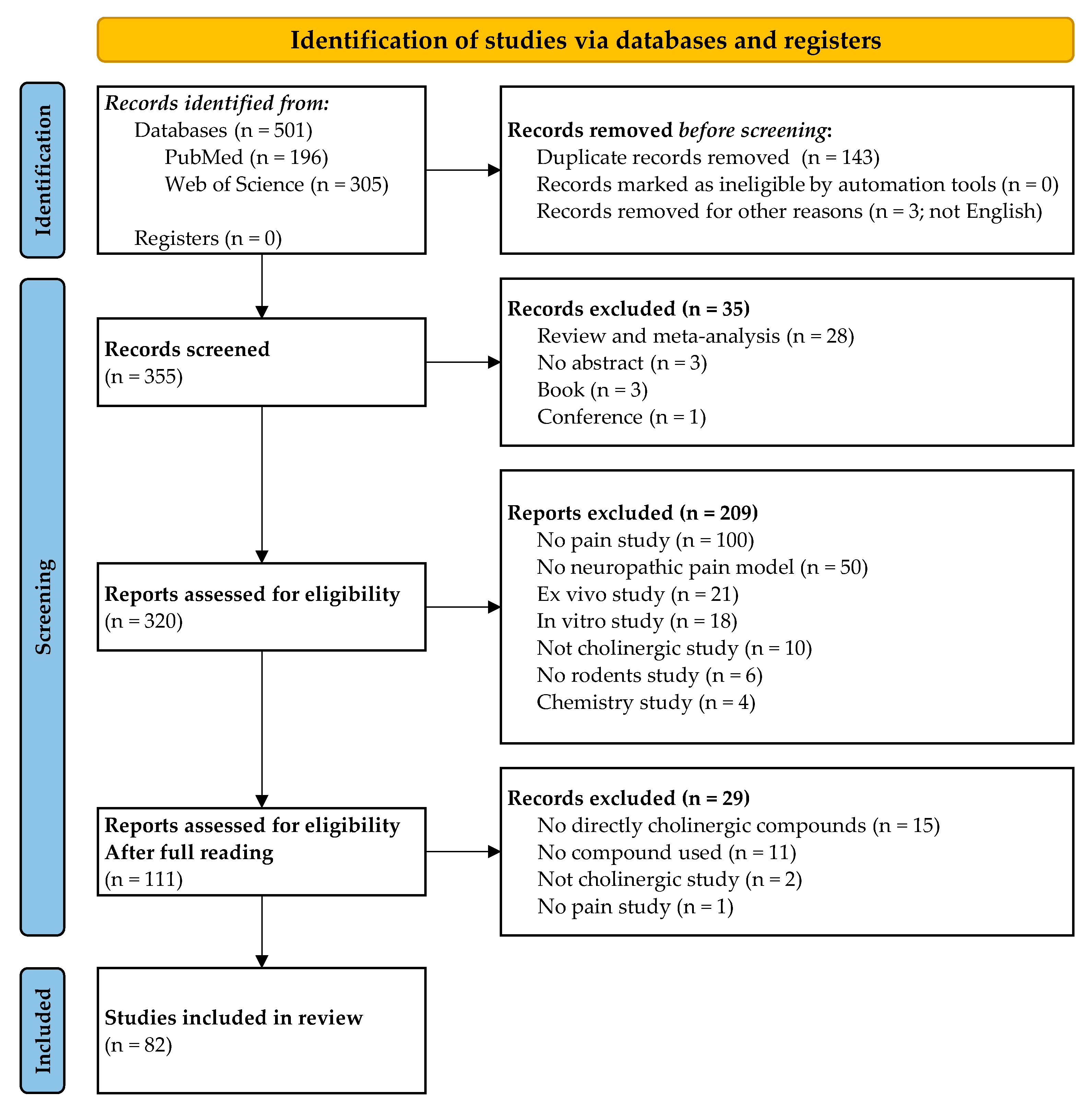
3. Results
3.1. Study Selection and Characteristics
- -
- Chronic constriction injury (CCI, n = 24);
- -
- Partial sciatic nerve ligation (PSL, n = 18);
- -
- Spinal nerve ligation (SNL, n = 13);
- -
- Spared nerve injury (SNI, n = 2);
- -
- Common peroneal nerve ligation (CPNL, n = 2);
- -
- Cuff model (CM, n = 1);
- -
- Sciatic nerve crush injury (SCNI, n = 1);
- -
- Sciatic nerve transection (SNT, n = 1);
- -
- Tibial nerve transection (TNT, n = 1).
3.2. Pharmacological Compounds Increasing the Acetylcholine Neurotransmission
| Compounds | Targets | Doses, Routes | Models | Species (Sex) | Behavioral Assays | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Ambenonium chloride | AChE inhibitors | 0.05 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs Conditioned place preference | ↘ M 0 spontaneous pain | [34] |
| Donepezil | 0.3–1 mg/kg, i.p. | Traumatic | Rat (♂) | Paw pressure | ↘ M | [20] | |
| 0.3–1.0 mg/kg, i.p. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M (0.6 and 1 mg/kg) | [31] | ||
| 5–10 mg/kg, p.o. | CIPN + traumatic | Rat (♂) | Electronic Von Frey Paw immersion 10 °C and 46 °C | ↘ M + T | [32] | ||
| 5 mg/kg, p.o. | CIPN | Rat (♂) | Electronic Von Frey Tail immersion 10 °C | ↘ M + T | [33] | ||
| Huperzine-A | 0.1–0.15 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs Conditioned place preference | ↘ M 0 spontaneous pain | [34] | |
| Neostigmine | 2 mg, i.t. | Traumatic | Rat (♂) | Von Frey hairs | ↘ M | [36] | |
| 0.3–3 ng, i.t. | Traumatic | Mouse (♂) | Plantar test Von Frey hairs | Dose-dependant ↘ M + T (3 ng) | [38] | ||
| 0.1–0.5 µg, i.t. | Diabetic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (0.5 µg) | [37] | ||
| Physostigmine | 15 nmol, i.t. | Traumatic | Mouse (♂) | Von Frey hairs | ↘ M | [35] | |
| BoNT/A | Acetylcholine exocytosis inhibitors | 15 pg, i.p. | Traumatic | Mouse (♂) | Electronic Von Frey | ↘ M | [39] |
| BoNT/A Dysport® BoNT/A Botox® | 20 U/kg, s.p. | CIPN | Rat (♂) | Paw pressure | ↘ M | [40] | |
| Citicoline | Acetylcholine precursor | 0.4–0.8 mL (100 µmol/L), p.n. | Traumatic | Rat (♂) | Von Frey hairs | ↘ M | [41] |
| 0.5–2 µmol, i.c.v. | Traumatic | Rat (♂) | Paw pressure | Dose- and time-dependent ↘ M (1 and 2 µmol) | [42] | ||
| 0.5–2 µmol, i.c.v. | CIPN | Rat (♂) | Paw pressure | Dose- and time-dependent ↘ M (1 and 2 µmol) | [43] |
3.3. Pharmacological Compounds Targeting Nicotinic Acetylcholine Receptors
3.3.1. Unspecific Targeting of Nicotinic Acetylcholine Receptors
| Compounds | Targets | Doses, Routes | Models | Species (Sex) | Behavioral Assays | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Epibatidine | nAChRs agonists | 0.03–0.3 nmol, i.t. | Traumatic | Mouse (♂) | Electronic Von Frey Plantar test | Dose-dependent ↘ M (all doses effective) | [18] |
| 0.3–10.0 µg/kg, s.c. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M (all doses effective) | [55] | ||
| 0.036–0.36 pmol, i.t. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M | [48] | ||
| Nicotine | 0.1–1.5 mg/kg, s.c. | Traumatic | Mouse (♂) | Von Frey hairs | Dose- and time-dependent↘ M (1 and 1.5 mg/kg) | [45] | |
| 0.1–10 nmol, i.t. | Traumatic | Rat (♂) | Electronic Von Frey | Dose- and time-dependent↘ M (1 nmol) | [46] | ||
| 3–30 nmol, i.t. | Traumatic | Mouse (♂) | Electronic Von Frey Plantar test | Dose-dependent ↘ M (10 and 30 nmol) | [18] | ||
| 2.2–6.5 nmol, i.t. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M | [48] | ||
| 0.25–1.75 mg/kg, i.p. | Traumatic | Mouse (♂ + ♀) | Von Frey hairs | Dose-dependent ↘ M | [49] | ||
| 0.25–25 µg, i.c.v. | Traumatic | Mouse (♂ + ♀) | Von Frey hairs | Dose-dependent ↘ M | [49] | ||
| 0.25–17.5 µg, i.t. | Traumatic | Mouse (♂ + ♀) | Von Frey hairs | Dose-dependent ↘ M | [49] | ||
| 25–100 µg, i.pl. | Traumatic | Mouse (♂ + ♀) | Von Frey hairs | Dose-dependent ↘ M | [49] | ||
| 4 or 10 mg/kg/day, s.c. | Traumatic | Rat (♂) | Paw pressure | ↗ M | [50] | ||
| 20 nmol/day/4 days, p.n. 1–20 nmol, p.n. | Traumatic | Mouse (♂) | Von Frey hairs Plantar test | ↘ M (preventive effect) Dose-dependent ↘ M + T (5 and 20 nmol) | [47] | ||
| 2 mg/kg, s.c. | Traumatic | Mouse (♂) | Von Frey hair | ↘ T | [51] | ||
| 1 mg/kg, i.v. | Traumatic | Mouse (♂) | Von Frey hairs Plantar test Tail-flick | ↘ M + T | [52] | ||
| 20 nmol, p.n. | Traumatic | Mouse (♂) | Von Frey hair Plantar test | ↘ M + T | [53] | ||
| 0.3–0.9 mg/kg, i.p. 24 mg/kg/day, s.c. | CIPN | Mouse (♂) | Von Frey hairs | Dose-dependent ↘ M (0.6 and 0.9 mg/kg) ↘ M (preventive effect) | [44] | ||
| S(-)-nornicotine | 5–20 mg/kg, i.p. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M (20 mg/kg) | [54] | |
| R(+)-nornicotine | 10–15 mg/kg, i.p. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M (15 mg/kg) | [54] |
3.3.2. Specific Targeting of α4β2 Nicotinic Acetylcholine Receptors
| Compounds | Targets | Doses, Routes | Models | Species (Sex) | Behavioral Assays | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| [123/125I]5IA | α4β2 nAChR agonists | 1–100 nmol, i.c.v. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (50 and 100 nmol) | [57] |
| 1–10 nmol, i.c.v. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (3 and 10 nmol) | [63] | ||
| A-366833 | 1.9–19 µmol/kg, i.p. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (all doses effective) | [64] | |
| 1–6 mg/kg, i.p. | Traumatic + Diabetic +CIPN | Rat (♂) | Paw pressure | ↘ M | [65] | ||
| A-85380 | 0.125–1 µmol/kg, i.p. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (0.75 and 1 µmol/kg | [66] | |
| <pmol, i.t. | Traumatic | Rat (♂) | Paw pressure | 0 M | [48] | ||
| NS9283 | 35 mmol/kg, i.p. | Traumatic | Rat (♂) | Von Frey hair | 0 M | [67] | |
| ABT-418 | 1–20 nmol, p.n. | Traumatic | Mouse (♂) | Von Frey hairs Plantar test | ↘ M + T | [53] | |
| ABT-594 | 3–100 µg/kg, s.c. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (10–100 µg/kg) | [55] | |
| 0.01–0.3 µmol/kg, i.p. | CIPN | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (0.1–0.3 µmol/kg) | [59] | ||
| Bee venom | 0.25 mg/kg, s.c. | CIPN | Rat (♂) | Tail immersion test | ↘ T | [61] | |
| C-9515 | 0.001–0.01 mg/kg, i.p. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (0.003 and 0.01 mg/kg) | [58] | |
| RJR-2403 | 1–100 nmol, i.t. | Traumatic | Rat (♂) | Electronic Von Frey | ↘ M | [46] | |
| TC-2559 | 2.28–22.8 µmol/kg, s.c. 20 nmol, p.n. | Traumatic | Mouse (♂) | Von Frey hairs | Dose-dependent ↘ M (22.8 µmol/kg) ↘ M | [56] | |
| 1–20 nmol, p.n. | Traumatic | Mouse (♂) | Von Frey hairs Plantar test | ↘ M + T | [53] | ||
| 0.3–3 mg/kg, i.p. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (1 and 3 mg/kg) | [62] | ||
| 20 nmol, p.n. 10 mg/kg, s.c. | Diabetic | Mouse (♂) | Von Frey hair | ↘ M | [60] | ||
| Sazetidine A | α4β2 nAChR partial agonist | 0.2–20 nmol, p.n. | Traumatic | Mouse (♂) | Von Frey hairs | Dose-dependent ↘ M (20 nmol) | [56] |
3.3.3. Specific Targeting of α7 Nicotinic Acetylcholine Receptors
| Compounds | Targets | Doses, Routes | Models | Species (Sex) | Behavioral Assays | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| (R)-ICH3 | α7 nAChR agonist | 30 mg/kg, p.o. | CIPN | Rat (♂) | Paw pressure Von Frey hairs Cold plate (4 °C) | ↘ M + T | [70] |
| Choline | 100 nmol, i.t. | Traumatic | Rat (♂) | Electronic Von Frey | ↘ M | [46] | |
| Cobratoxin | 0.56–4.5 µg/kg, i.t. | Traumatic | Rat (♂) | Paw pressure Tail-flick | Dose-dependent ↘ M + T (1.12 and 4.5 µg/kg) | [68] | |
| DM489 | 3–10 mg/kg, p.o. | Diabetic | Mouse (♂) | Cold plate 4 °C | ↘ T | [73] | |
| 10–30 mg/kg, p.o. | CIPN | Mouse (♂) | Cold plate 4 °C | ↘ T | [73] | ||
| GAT107 | 1–10 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hair Plantar test | Dose- and time-dependent ↘ M (3 and 10 mg/kg) ↘ T | [72] | |
| PAM-4 | 1–2 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs | Dose- and time-dependent ↘ M (2 mg/kg) | [74] | |
| PHA-543613 | 12 μg, i.t. | Traumatic | Rat (♂) | Electronic von frey Plantar test | ↘ M + T | [71] | |
| 1–6 mg/kg, s.c. | Traumatic | Mouse (♂) | Von Frey hairs | Dose-dependent ↘ M (6 mg/kg) | [69] | ||
| PNU-28298 | 1–30 mg kg, p.o. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M | [75] | |
| PNU-282987 | 30 mg/kg, p.o. | CIPN | Rat (♂) | Paw pressure | ↘ M | [70] | |
| 1 µg/kg, i.t. | Traumatic | Rat (♂) | Paw pressure Tail-flick | ↘ M + T | [68] | ||
| TC-7020 | 1–10 mg/kg/day, s.c. | Traumatic | Rat (♂) | Von Frey hairs | ↘ M | [76] | |
| NS6740 | α7 nAChR silent agonist | 1–9 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs | Dose-dependent ↘ M (9 mg/kg) | [77] |
| R-47 | 0–10 mg/kg, p.o. | CIPN | Mouse (♂) | Von Frey hairs | Dose- and time-dependent ↘ M (5 and 10 mg/kg) | [78] | |
| PAM-2 | α7 nAChR PAM | 3 mg/kg, p.o. | Diabetic + CIPN | Mouse (♂) | Cold plate 4 °C | ↘ T | [73] |
| 2–8 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs | Dose-dependent ↘ M (6 and 8 mg/kg) | [79] | ||
| NS1738 | 30 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs Plantar test | 0 M + T | [69] | |
| PNU-120596 | 1–8 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs Plantar test | ↘ M + T | [69] | |
| α-conopeptides Eu1.6 | α3β4/α7 nAChR antagonist | 0.5–24.9 μg/kg, i.m. | Traumatic | Rat (♂) | Paw pressure | ↘ M | [80] |
| DXL-A-24 | α7 nAChR/ m4AChR agonist | 0.25–1 mg/kg, p.o. daily | Traumatic | Rat (♂) | Von Frey hairs Hot plate 50 °C | Dose- and time-dependent ↘ M + T (0.5 and 1 mg/kg) | [81] |
3.3.4. Specific Targeting of α9/α10 Nicotinic Acetylcholine Receptors
| Compounds | Targets | Doses, Routes | Models | Species (Sex) | Behavioral Assays | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| (±)-18-MC | α9/α10 nAChR antagonist | 72 mg/kg, p.o. | CIPN | Mouse (♂) | Cold plate 4 °C | ↘ T | [90] |
| (+)-catharanthine | 36–72 mg/kg, p.o. | CIPN | Mouse (♂) | Cold plate 4 °C | ↘ T | [90] | |
| α-conotoxin AuIB | 0.02–2 nmol, i.t. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (2 nmol) | [82] | |
| 0.36–36 µg, i.m. | Traumatic | Rat (♂) | Von Frey hairs | ↘ M | [83] | ||
| α-conotoxin MII | 0.02–2 nmol, i.t. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (2 nmol) | [82] | |
| 0.36–36 µg, i.m. | Traumatic | Rat (♂) | Von Frey hairs | ↘ M | [83] | ||
| α-conotoxin Mr1.1 [S4Dap] | 0.5–25 µg/kg, nr | Traumatic | Rat (♂) | Electronic Von Frey | Dose-dependent ↘ M (25 µg/kg) | [91] | |
| α-conotoxin RgIA | 2–10 nmol, i.m. | Traumatic | Rat (♂) | Paw pressure Electronic Von Frey | ↘ M | [84] | |
| α-conotoxin RgIA4 | 0.02–0.2 nmol, i.m. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M (0.2 nmol) | [92] | |
| 100 mg/kg, i.m. | CIPN | Mouse (♂) | Hot plate 47 °C Electronic Von Frey Acetone test | ↘ T + M | [93] | ||
| 2–10 nmol, i.m. | CIPN | Rat (♂) | Cold plate 4 °C | ↘ T | [94] | ||
| 0.128–80 µg/kg, s.c. | CIPN | Mouse + Rat (♂) | Paw pressure Cold plate 4 °C | Dose-dependent ↘ M + T (all doses effective) | [95] | ||
| 40 µg/kg, s.c. | CIPN | Mouse (nr) | Cold plate (decrease of 10 °C/min) | ↘ T | [96] | ||
| 40 µg/kg, s.c. | CIPN | Mouse (nr) | Cold plate (decrease of 10 °C/min) | ↘ T | [97] | ||
| 16–80 µg/kg, s.c. | CIPN | Rat (♂) | Von Frey hairs Cold plate 5 °C Plantar test | Time-dependent ↘ M 0 T | [98] | ||
| α-conotoxin RgIA-5474 | 4–40 µg/kg, s.c. | CIPN | Mouse (nr) | Cold plate (decrease of 10 °C/min) | ↘ T | [99] | |
| α-conotoxin Vc1.1 | 0.02–2 nmol, i.t. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (2 nmol) | [82] | |
| 0.36–36 µg, i.m. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (all doses effective) | [83] | ||
| 0.36–3.6 µg, i.m. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M (3.6 µg) | [101] | ||
| 0.036–0.36 µg, i.m. | Traumatic | Rat (♂) | Paw pressure | Dose-dependent ↘ M | [92] | ||
| 60 µg, i.m. | Traumatic | Rat (♂) | Von Frey hairs | ↘ M | [85] | ||
| 27.2–54.2 μg/kg, i.m. | Traumatic | Rat (♂) | Paw pressure | ↘ M | [80] | ||
| [2,8]-alkyne Vc1.1 3 | 0.03–0.1 mg/kg, i.m. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (0.1 mg/kg) | [100] | |
| Vc1.1[N9R] | 0.3–15 nmol/kg, i.m. | Traumatic | Rat (♂) | Paw pressure | ↘ M | [86] | |
| [P6O]Vc.1.1 | 60 µg, i.m. | Traumatic | Rat (♂) | Von Frey hairs | 0 M | [85] | |
| GeXIVA[1,2] | 0.5–2 nmol, i.m. | Traumatic | Rat (♂) | Electronic Von Frey | Dose-dependent ↘ M (All doses effective) | [104] | |
| 0.3–1.2 nmol, i.m. | Traumatic | Rat (♂) | Electronic Von Frey Von Frey hairs | ↘ M | [87] | ||
| 32–128 nmol/kg, i.m. | CIPN | Rat (♂) | Von Frey hairs + acetone test + tail-flick | Dose-dependent ↘ (128 nmol/kg) 0 T | [102] | ||
| 0.45 mg/kg, i.m. | CIPN | Rat (♂) | Von Frey hairs Tail-flick | ↘ M + T | [103] | ||
| GeXIVA[1,4] | 0.5–2 nmol, i.m. | Traumatic | Rat (♂) | Electronic Von Frey | Dose-dependent ↘ M (1 and 2 nmol) | [104] | |
| Oligoarginine R8 | 20 mg/kg, i.m. | CIPN | Mouse (♂) | Hot plate 47 °C Electronic Von Frey Acetone test | ↘ M + T | [93] | |
| ZZ-204G | 3.6–3600 µg/kg, i.p. | Traumatic | Rat (♂) | Paw pressure Tail-flick | Dose-dependent ↘ M (360 and 3600 µg/kg) 0 T | [89] | |
| ZZ1-61c | 100 µg/kg/day, i.p. | CIPN | Rat (♂) | Paw pressure | ↘ M | [88] |
3.4. Pharmacological Compounds Targeting Muscarinic Acetylcholine Receptors
| Compounds | Targets | Doses, Routes | Models | Species (Sex) | Behavioral Assays | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Oxotremorine | mAChRs non-selective agonist | 5–10 μg, i.t. | Traumatic | Rat (♂) | Von Frey hairs Ethyl chloride spray Plantar test | ↘ M + T | [105] |
| PTAC | m2-4 AChRs agonist m1-3-5 AChRs antagonist | 0.05–0.1 mg/kg, i.p. | Traumatic | Mouse (♂) | Von Frey hairs | ↘ M | [106] |
| Scopolamine | mAChRs antagonist | 0.4 μg/μL, ACC | Traumatic | Rat (♂) | Daily autotomy scores | ↘ autotomy | [107] |
| DXL-A-24 | m4AChR + α7 nAChR agonist | 0.25–1 mg/kg, p.o. | Traumatic | Rat (♂) | Von Frey hairs | Dose-dependent ↘ M (0.5 and 1 mg/kg) | [81] |
| McNA-343 | m1/m4AChRs agonist | 18.9–1890 pmol, ACC | Traumatic | Rat (♂) | Electronic Von Frey | Dose-dependent ↘ M (189 and 1890 pmol) | [108] |
| 3–10 μg, i.t. | Traumatic | Mouse (♂) | Plantar test Von Frey hairs Paw pressure | ↘ M 0 T | [38] | ||
| PPBI | m1AChR agonist | 0.2–100 mmol/kg, p.o. | Traumatic + CIPN | Mouse + Rat (♂) | Tail immersion 13 °C Acetone test Plantar test | Dose-dependent ↘ M + T | [109] |
| Pirenzepine | m1AChR antagonist | 10 mg/kg/d, s.c. | Diabetic + CIPN | Mouse + Rat (♂ + ♀) | Von Frey hairs | ↘ M | [110] |
| VU0255035 | 10 mg/kg, i.p. | Diabetic | Mouse + Rat (♂ + ♀) | Von Frey hairs | ↘ M | [110] |
4. Discussion
Limitations of This Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; Lester, D.; Clay Smither, F.; Balakhanlou, E. Peripheral Neuropathic Pain. NeuroRehabilitation 2020, 47, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [CrossRef]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Cuménal, M.; Selvy, M.; Kerckhove, N.; Bertin, C.; Morez, M.; Courteix, C.; Busserolles, J.; Balayssac, D. The Safety of Medications Used to Treat Peripheral Neuropathic Pain, Part 2 (Opioids, Cannabinoids and Other Drugs): Review of Double-Blind, Placebo-Controlled, Randomized Clinical Trials. Expert Opin. Drug Saf. 2021, 20, 51–68. [Google Scholar] [CrossRef]
- Selvy, M.; Cuménal, M.; Kerckhove, N.; Courteix, C.; Busserolles, J.; Balayssac, D. The Safety of Medications Used to Treat Peripheral Neuropathic Pain, Part 1 (Antidepressants and Antiepileptics): Review of Double-Blind, Placebo-Controlled, Randomized Clinical Trials. Expert Opin. Drug Saf. 2020, 19, 707–733. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Torrance, N.; Ferguson, J.A.; Afolabi, E.; Bennett, M.I.; Serpell, M.G.; Dunn, K.M.; Smith, B.H. Neuropathic Pain in the Community: More under-Treated than Refractory? Pain 2013, 154, 690–699. [Google Scholar] [CrossRef]
- Naser, P.V.; Kuner, R. Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018, 387, 135–148. [Google Scholar] [CrossRef]
- Colangelo, C.; Shichkova, P.; Keller, D.; Markram, H.; Ramaswamy, S. Cellular, Synaptic and Network Effects of Acetylcholine in the Neocortex. Front. Neural Circuits 2019, 13, 24. [Google Scholar] [CrossRef]
- Elgoyhen, A.B. The A9α10 Acetylcholine Receptor: A Non-Neuronal Nicotinic Receptor. Pharmacol. Res. 2023, 190, 106735. [Google Scholar] [CrossRef]
- Hone, A.J.; McIntosh, J.M. Nicotinic Acetylcholine Receptors: Therapeutic Targets for Novel Ligands to Treat Pain and Inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.N.T.; Abraham, N.; Lewis, R.J. Structure-Function of Neuronal Nicotinic Acetylcholine Receptor Inhibitors Derived From Natural Toxins. Front. Neurosci. 2020, 14, 609005. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Gebhart, G.F. Tonic Cholinergic Inhibition of Spinal Mechanical Transmission. Pain 1991, 46, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Xie, W.; Inoue, M.; Ueda, H. Evidence for the Tonic Inhibition of Spinal Pain by Nicotinic Cholinergic Transmission through Primary Afferents. Mol. Pain 2007, 3. [Google Scholar] [CrossRef]
- Rashid, M.H.; Ueda, H. Neuropathy-Specific Analgesic Action of Intrathecal Nicotinic Agonists and Its Spinal GABA-Mediated Mechanism. Brain Res. 2002, 953, 53–62. [Google Scholar] [CrossRef]
- Fiorino, D.F.; Garcia-Guzman, M. Muscarinic Pain Pharmacology: Realizing the Promise of Novel Analgesics by Overcoming Old Challenges. In Muscarinic Receptors; Fryer, A.D., Christopoulos, A., Nathanson, N.M., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 208, pp. 191–221. ISBN 978-3-642-23273-2. [Google Scholar]
- Kimura, M.; Hayashida, K.; Eisenach, J.C.; Saito, S.; Obata, H. Relief of Hypersensitivity after Nerve Injury from Systemic Donepezil Involves Spinal Cholinergic and γ-Aminobutyric Acid Mechanisms. Anesthesiology 2013, 118, 173–180. [Google Scholar] [CrossRef]
- Yoon, S.; Kwon, Y.; Kim, H.; Roh, D.; Kang, S.; Kim, C.; Han, H.; Kim, K.; Yang, I.; Beitz, A. Intrathecal Neostigmine Reduces the Zymosan-Induced Inflammatory Response in a Mouse Air Pouch Model via Adrenomedullary Activity: Involvement of Spinal Muscarinic Type 2 Receptors. Neuropharmacology 2005, 49, 275–282. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, S.; Han, H.; Sood, A.K.; Lopez-Berestein, G.; Pan, H. Role of M2, M3, and M4 Muscarinic Receptor Subtypes in the Spinal Cholinergic Control of Nociception Revealed Using siRNA in Rats. J. Neurochem. 2009, 111, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Shelukhina, I.; Paddenberg, R.; Kummer, W.; Tsetlin, V. Functional Expression and Axonal Transport of A7 nAChRs by Peptidergic Nociceptors of Rat Dorsal Root Ganglion. Brain Struct. Funct. 2015, 220, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-G.; Choi, I.-S.; Cho, J.-H.; Jang, I.-S. Cholinergic Modulation of Primary Afferent Glutamatergic Transmission in Rat Medullary Dorsal Horn Neurons. Neuropharmacology 2013, 75, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Chen, S.-R.; Pan, H.-L. Regulation of Glutamate Release From Primary Afferents and Interneurons in the Spinal Cord by Muscarinic Receptor Subtypes. J. Neurophysiol. 2007, 97, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-R.; Chen, H.; Yuan, W.-X.; Wess, J.; Pan, H.-L. Differential Regulation of Primary Afferent Input to Spinal Cord by Muscarinic Receptor Subtypes Delineated Using Knockout Mice. J. Biol. Chem. 2014, 289, 14321–14330. [Google Scholar] [CrossRef]
- Iwamoto, E.T.; Marion, L. Adrenergic, Serotonergic and Cholinergic Components of Nicotinic Antinociception in Rats. J. Pharmacol. Exp. Ther. 1993, 265, 777–789. [Google Scholar]
- Jareczek, F.J.; White, S.R.; Hammond, D.L. Plasticity in Brainstem Mechanisms of Pain Modulation by Nicotinic Acetylcholine Receptors in the Rat. eNeuro 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Macon, C.J.; Nguyen, T.M.; Coates, M.B.; Guyenet, P.G. Cholinergic Neurons in the Mouse Rostral Ventrolateral Medulla Target Sensory Afferent Areas. Brain Struct. Funct. 2013, 218, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Kimura, M.; Saito, S.; Obata, H. Dexmedetomidine Decreases Hyperalgesia in Neuropathic Pain by Increasing Acetylcholine in the Spinal Cord. Neurosci. Lett. 2012, 529, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, J.; Bayet-Robert, M.; Dalmann, R.; El Guerrab, A.; Aissouni, Y.; Graveron-Demilly, D.; Chalus, M.; Pinguet, J.; Eschalier, A.; Richard, D.; et al. Cholinergic Neurotransmission in the Posterior Insular Cortex Is Altered in Preclinical Models of Neuropathic Pain: Key Role of Muscarinic M2 Receptors in Donepezil-Induced Antinociception. J. Neurosci. 2015, 35, 16418–16430. [Google Scholar] [CrossRef]
- Selvy, M.; Mattévi, C.; Dalbos, C.; Aissouni, Y.; Chapuy, E.; Martin, P.-Y.; Collin, A.; Richard, D.; Dumontet, C.; Busserolles, J.; et al. Analgesic and Preventive Effects of Donepezil in Animal Models of Chemotherapy-Induced Peripheral Neuropathy: Involvement of Spinal Muscarinic Acetylcholine M2 Receptors. Biomed. Pharmacother. 2022, 149, 112915. [Google Scholar] [CrossRef]
- Zuo, Z.-X.; Wang, Y.-J.; Liu, L.; Wang, Y.; Mei, S.-H.; Feng, Z.-H.; Wang, M.; Li, X.-Y. Huperzine A Alleviates Mechanical Allodynia but Not Spontaneous Pain via Muscarinic Acetylcholine Receptors in Mice. Neural Plast. 2015, 2015, 453170. [Google Scholar] [CrossRef] [PubMed]
- Dhanasobhon, D.; Medrano, M.-C.; Becker, L.J.; Moreno-Lopez, Y.; Kavraal, S.; Bichara, C.; Schlichter, R.; Inquimbert, P.; Yalcin, I.; Cordero-Erausquin, M. Enhanced Analgesic Cholinergic Tone in the Spinal Cord in a Mouse Model of Neuropathic Pain. Neurobiol. Dis. 2021, 155, 105363. [Google Scholar] [CrossRef] [PubMed]
- Paqueron, X.; Li, X.; Eisenach, J.C. P75-Expressing Elements Are Necessary for Anti-Allodynic Effects of Spinal Clonidine and Neostigmine. Neuroscience 2001, 102, 681–686. [Google Scholar] [CrossRef]
- Chen, S.R.; Khan, G.M.; Pan, H.L. Antiallodynic Effect of Intrathecal Neostigmine Is Mediated by Spinal Nitric Oxide in a Rat Model of Diabetic Neuropathic Pain. Anesthesiology 2001, 95, 1007–1012. [Google Scholar] [CrossRef]
- Takasu, K.; Honda, M.; Ono, H.; Tanabe, M. Spinal Alpha(2)-Adrenergic and Muscarinic Receptors and the NO Release Cascade Mediate Supraspinally Produced Effectiveness of Gabapentin at Decreasing Mechanical Hypersensitivity in Mice after Partial Nerve Injury. Br. J. Pharmacol. 2006, 148, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. The Analgesic Effect on Neuropathic Pain of Retrogradely Transported Botulinum Neurotoxin A Involves Schwann Cells and Astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef]
- Favre-Guilmard, C.; Auguet, M.; Chabrier, P.-E. Different Antinociceptive Effects of Botulinum Toxin Type A in Inflammatory and Peripheral Polyneuropathic Rat Models. Eur. J. Pharmacol. 2009, 617, 48–53. [Google Scholar] [CrossRef]
- Emril, D.R.; Wibowo, S.; Meliala, L.; Susilowati, R. Cytidine 5′-Diphosphocholine Administration Prevents Peripheral Neuropathic Pain after Sciatic Nerve Crush Injury in Rats. J. Pain Res. 2016, 9, 287–291. [Google Scholar] [CrossRef][Green Version]
- Bagdas, D.; Sonat, F.A.; Hamurtekin, E.; Sonal, S.; Gurun, M.S. The Antihyperalgesic Effect of Cytidine-5′-Diphosphate-Choline in Neuropathic and Inflammatory Pain Models. Behav. Pharmacol. 2011, 22, 589–598. [Google Scholar] [CrossRef]
- Kanat, O.; Bagdas, D.; Ozboluk, H.Y.; Gurun, M.S. Preclinical Evidence for the Antihyperalgesic Activity of CDP-Choline in Oxaliplatin-Induced Neuropathic Pain. J. BUON 2013, 18, 1012–1018. [Google Scholar]
- Kyte, S.L.; Toma, W.; Bagdas, D.; Meade, J.A.; Schurman, L.D.; Lichtman, A.H.; Chen, Z.-J.; Del Fabbro, E.; Fang, X.; Bigbee, J.W.; et al. Nicotine Prevents and Reverses Paclitaxel-Induced Mechanical Allodynia in a Mouse Model of CIPN. J. Pharmacol. Exp. Ther. 2018, 364, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Ergun, D.; Jackson, A.; Toma, W.; Schulte, M.K.; Damaj, M.I. Allosteric Modulation of A4β2* Nicotinic Acetylcholine Receptors: Desformylflustrabromine Potentiates Antiallodynic Response of Nicotine in a Mouse Model of Neuropathic Pain. Eur. J. Pain 2018, 22, 84–93. [Google Scholar] [CrossRef]
- Abdin, M.J.; Morioka, N.; Morita, K.; Kitayama, T.; Kitayama, S.; Nakashima, T.; Dohi, T. Analgesic Action of Nicotine on Tibial Nerve Transection (TNT)-Induced Mechanical Allodynia through Enhancement of the Glycinergic Inhibitory System in Spinal Cord. Life Sci. 2006, 80, 9–16. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Tominaga, S.; Nakamura, J.; Fukazawa, Y.; Ozaki, M.; Kishioka, S. Activation of Nicotinic Acetylcholine Receptors on Bone Marrow-Derived Cells Relieves Neuropathic Pain Accompanied by Peripheral Neuroinflammation. Neurochem. Int. 2012, 61, 1212–1219. [Google Scholar] [CrossRef]
- Young, T.; Wittenauer, S.; Parker, R.; Vincler, M. Peripheral Nerve Injury Alters Spinal Nicotinic Acetylcholine Receptor Pharmacology. Eur. J. Pharmacol. 2008, 590, 163–169. [Google Scholar] [CrossRef]
- Wieskopf, J.S.; Mathur, J.; Limapichat, W.; Post, M.R.; Al-Qazzaz, M.; Sorge, R.E.; Martin, L.J.; Zaykin, D.V.; Smith, S.B.; Freitas, K.; et al. The Nicotinic A6 Subunit Gene Determines Variability in Chronic Pain Sensitivity via Cross-Inhibition of P2X2/3 Receptors. Sci. Transl. Med. 2015, 7, 287ra72. [Google Scholar] [CrossRef] [PubMed]
- Josiah, D.T.; Vincler, M.A. Impact of Chronic Nicotine on the Development and Maintenance of Neuropathic Hypersensitivity in the Rat. Psychopharmacology 2006, 188, 152–161. [Google Scholar] [CrossRef]
- Xanthos, D.N.; Beiersdorf, J.W.; Thrun, A.; Ianosi, B.; Orr-Urtreger, A.; Huck, S.; Scholze, P. Role of A5-Containing Nicotinic Receptors in Neuropathic Pain and Response to Nicotine. Neuropharmacology 2015, 95, 37–49. [Google Scholar] [CrossRef]
- Brunori, G.; Schoch, J.; Mercatelli, D.; Ozawa, A.; Toll, L.; Cippitelli, A. Influence of Neuropathic Pain on Nicotinic Acetylcholine Receptor Plasticity and Behavioral Responses to Nicotine in Rats. Pain 2018, 159, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Peripheral Alpha4beta2 Nicotinic Acetylcholine Receptor Signalling Attenuates Tactile Allodynia and Thermal Hyperalgesia after Nerve Injury in Mice. Acta Physiol. (Oxf.) 2015, 213, 462–471. [Google Scholar] [CrossRef]
- Holtman, J.R.; Crooks, P.A.; Johnson-Hardy, J.K.; Wala, E.P. The Analgesic and Toxic Effects of Nornicotine Enantiomers Alone and in Interaction with Morphine in Rodent Models of Acute and Persistent Pain. Pharmacol. Biochem. Behav. 2010, 94, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Kesingland, A.C.; Gentry, C.T.; Panesar, M.S.; Bowes, M.A.; Vernier, J.M.; Cube, R.; Walker, K.; Urban, L. Analgesic Profile of the Nicotinic Acetylcholine Receptor Agonists, (+)-Epibatidine and ABT-594 in Models of Persistent Inflammatory and Neuropathic Pain. Pain 2000, 86, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Kobayashi, D.; Saika, F.; Matsuzaki, S.; Kishioka, S. Inhibition of Peripheral Macrophages by Nicotinic Acetylcholine Receptor Agonists Suppresses Spinal Microglial Activation and Neuropathic Pain in Mice with Peripheral Nerve Injury. J. Neuroinflamm. 2018, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Iida, Y.; Yoneyama, T.; Kawai, T.; Ogawa, M.; Magata, Y.; Saji, H. In Vivo Relationship between Thalamic Nicotinic Acetylcholine Receptor Occupancy Rates and Antiallodynic Effects in a Rat Model of Neuropathic Pain: Persistent Agonist Binding Inhibits the Expression of Antiallodynic Effects. Synapse 2011, 65, 77–83. [Google Scholar] [CrossRef]
- Li, W.; Cai, J.; Wang, B.H.; Huang, L.; Fan, J.; Wang, Y. Antinociceptive Effects of Novel Epibatidine Analogs through Activation of A4β2 Nicotinic Receptors. Sci. China Life Sci. 2018, 61, 688–695. [Google Scholar] [CrossRef]
- Lynch, J.J.; Wade, C.L.; Mikusa, J.P.; Decker, M.W.; Honore, P. ABT-594 (a Nicotinic Acetylcholine Agonist): Anti-Allodynia in a Rat Chemotherapy-Induced Pain Model. Eur. J. Pharmacol. 2005, 509, 43–48. [Google Scholar] [CrossRef]
- Saika, F.; Kiguchi, N.; Matsuzaki, S.; Kobayashi, D.; Kishioka, S. Inflammatory Macrophages in the Sciatic Nerves Facilitate Neuropathic Pain Associated with Type 2 Diabetes Mellitus. J. Pharmacol. Exp. Ther. 2019, 368, 535–544. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, M.J.; Yoon, I.; Li, D.X.; Bae, H.; Kim, S.K. Nicotinic Acetylcholine Receptors Mediate the Suppressive Effect of an Injection of Diluted Bee Venom into the GV3 Acupoint on Oxaliplatin-Induced Neuropathic Cold Allodynia in Rats. Biol. Pharm. Bull. 2015, 38, 710–714. [Google Scholar] [CrossRef]
- Cheng, L.-Z.; Han, L.; Fan, J.; Huang, L.-T.; Peng, L.-C.; Wang, Y. Enhanced Inhibitory Synaptic Transmission in the Spinal Dorsal Horn Mediates Antinociceptive Effects of TC-2559. Mol. Pain 2011, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Iida, Y.; Tominaga, A.; Yoneyama, T.; Ogawa, M.; Magata, Y.; Nishimura, H.; Kuge, Y.; Saji, H. Nicotinic Acetylcholine Receptors Expressed in the Ventralposterolateral Thalamic Nucleus Play an Important Role in Anti-Allodynic Effects. Br. J. Pharmacol. 2010, 159, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Bunnelle, W.H.; Anderson, D.J.; Faltynek, C.; Dyhring, T.; Ahring, P.K.; Rueter, L.E.; Curzon, P.; Buckley, M.J.; Marsh, K.C.; et al. A-366833: A Novel Nicotinonitrile-Substituted 3,6-Diazabicyclo[3.2.0]-Heptane Alpha4beta2 Nicotinic Acetylcholine Receptor Selective Agonist: Synthesis, Analgesic Efficacy and Tolerability Profile in Animal Models. Biochem. Pharmacol. 2007, 74, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Jabaris, S.L.; Jayarajan, P.; Abraham, R.; Shanmuganathan, D.; Rasheed, M.A.; Royapalley, P.K.; Goura, V. Antinociceptive Activity of A4β2* Neuronal Nicotinic Receptor Agonist A-366833 in Experimental Models of Neuropathic and Inflammatory Pain. Eur. J. Pharmacol. 2011, 668, 155–162. [Google Scholar] [CrossRef]
- Rueter, L.E.; Kohlhaas, K.L.; Curzon, P.; Surowy, C.S.; Meyer, M.D. Peripheral and Central Sites of Action for A-85380 in the Spinal Nerve Ligation Model of Neuropathic Pain. Pain 2003, 103, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Zhu, C.; Malysz, J.; Campbell, T.; Shaughnessy, T.; Honore, P.; Polakowski, J.; Gopalakrishnan, M. A4β2 Neuronal Nicotinic Receptor Positive Allosteric Modulation: An Approach for Improving the Therapeutic Index of A4β2 nAChR Agonists in Pain. Biochem. Pharmacol. 2011, 82, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Liang, Q.; Zhu, Q.; Ding, D.; Yin, Q.; Tao, J.; Jiang, X. Nicotinic Acetylcholine Receptor A7 Subunit Is Involved in the Cobratoxin-Induced Antinociception in an Animal Model of Neuropathic Pain. Toxicon 2015, 93, 31–36. [Google Scholar] [CrossRef]
- Freitas, K.; Ghosh, S.; Ivy Carroll, F.; Lichtman, A.H.; Imad Damaj, M. Effects of A7 Positive Allosteric Modulators in Murine Inflammatory and Chronic Neuropathic Pain Models. Neuropharmacology 2013, 65, 156–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Cesare Mannelli, L.; Pacini, A.; Matera, C.; Zanardelli, M.; Mello, T.; De Amici, M.; Dallanoce, C.; Ghelardini, C. Involvement of A7 nAChR Subtype in Rat Oxaliplatin-Induced Neuropathy: Effects of Selective Activation. Neuropharmacology 2014, 79, 37–48. [Google Scholar] [CrossRef]
- Ji, L.; Chen, Y.; Wei, H.; Feng, H.; Chang, R.; Yu, D.; Wang, X.; Gong, X.; Zhang, M. Activation of Alpha7 Acetylcholine Receptors Reduces Neuropathic Pain by Decreasing Dynorphin A Release from Microglia. Brain Res. 2019, 1715, 57–65. [Google Scholar] [CrossRef]
- Bagdas, D.; Wilkerson, J.L.; Kulkarni, A.; Toma, W.; AlSharari, S.; Gul, Z.; Lichtman, A.H.; Papke, R.L.; Thakur, G.A.; Damaj, M.I. The A7 Nicotinic Receptor Dual Allosteric Agonist and Positive Allosteric Modulator GAT107 Reverses Nociception in Mouse Models of Inflammatory and Neuropathic Pain. Br. J. Pharmacol. 2016, 173, 2506–2520. [Google Scholar] [CrossRef]
- Arias, H.R.; Ghelardini, C.; Lucarini, E.; Tae, H.-S.; Yousuf, A.; Marcovich, I.; Manetti, D.; Romanelli, M.N.; Elgoyhen, A.B.; Adams, D.J.; et al. (E)-3-Furan-2-Yl-N-p-Tolyl-Acrylamide and Its Derivative DM489 Decrease Neuropathic Pain in Mice Predominantly by A7 Nicotinic Acetylcholine Receptor Potentiation. ACS Chem. Neurosci. 2020, 11, 3603–3614. [Google Scholar] [CrossRef]
- Bagdas, D.; Sevdar, G.; Gul, Z.; Younis, R.; Cavun, S.; Tae, H.-S.; Ortells, M.O.; Arias, H.R.; Gurun, M.S. (E)-3-Furan-2-Yl-N-Phenylacrylamide (PAM-4) Decreases Nociception and Emotional Manifestations of Neuropathic Pain in Mice by A7 Nicotinic Acetylcholine Receptor Potentiation. Neurol. Res. 2021, 43, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Di Cesare Mannelli, L.; Bonaccini, L.; Ronzoni, S.; Bartolini, A.; Ghelardini, C. Protective Effect of Alpha7 nAChR: Behavioural and Morphological Features on Neuropathy. Pain 2010, 150, 542–549. [Google Scholar] [CrossRef]
- Loram, L.C.; Taylor, F.R.; Strand, K.A.; Maier, S.F.; Speake, J.D.; Jordan, K.G.; James, J.W.; Wene, S.P.; Pritchard, R.C.; Green, H.; et al. Systemic Administration of an Alpha-7 Nicotinic Acetylcholine Agonist Reverses Neuropathic Pain in Male Sprague Dawley Rats. J. Pain 2012, 13, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Bagdas, D.; Kulkarni, A.R.; Gould, T.; AlSharari, S.D.; Thakur, G.A.; Damaj, M.I. The Analgesic-like Properties of the Alpha7 nAChR Silent Agonist NS6740 Is Associated with Non-Conducting Conformations of the Receptor. Neuropharmacology 2015, 91, 34–42. [Google Scholar] [CrossRef]
- Toma, W.; Kyte, S.L.; Bagdas, D.; Jackson, A.; Meade, J.A.; Rahman, F.; Chen, Z.-J.; Del Fabbro, E.; Cantwell, L.; Kulkarni, A.; et al. The A7 Nicotinic Receptor Silent Agonist R-47 Prevents and Reverses Paclitaxel-Induced Peripheral Neuropathy in Mice without Tolerance or Altering Nicotine Reward and Withdrawal. Exp. Neurol. 2019, 320, 113010. [Google Scholar] [CrossRef]
- Bagdas, D.; Targowska-Duda, K.M.; López, J.J.; Perez, E.G.; Arias, H.R.; Damaj, M.I. The Antinociceptive and Antiinflammatory Properties of 3-Furan-2-Yl-N-p-Tolyl-Acrylamide, a Positive Allosteric Modulator of A7 Nicotinic Acetylcholine Receptors in Mice. Anesth. Analg. 2015, 121, 1369–1377. [Google Scholar] [CrossRef]
- Liu, Z.; Bartels, P.; Sadeghi, M.; Du, T.; Dai, Q.; Zhu, C.; Yu, S.; Wang, S.; Dong, M.; Sun, T.; et al. A Novel α-Conopeptide Eu1.6 Inhibits N-Type (CaV2.2) Calcium Channels and Exhibits Potent Analgesic Activity. Sci. Rep. 2018, 8, 1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, H.; Liang, Y.; Wang, X.; Du, X.; Li, R.; Jiang, Y.; Ye, J. Antinociceptive Effect of Spirocyclopiperazinium Salt Compound DXL-A-24 and the Underlying Mechanism. Neurochem. Res. 2019, 44, 2786–2795. [Google Scholar] [CrossRef] [PubMed]
- Napier, I.A.; Klimis, H.; Rycroft, B.K.; Jin, A.H.; Alewood, P.F.; Motin, L.; Adams, D.J.; Christie, M.J. Intrathecal α-Conotoxins Vc1.1, AuIB and MII Acting on Distinct Nicotinic Receptor Subtypes Reverse Signs of Neuropathic Pain. Neuropharmacology 2012, 62, 2202–2207. [Google Scholar] [CrossRef]
- Klimis, H.; Adams, D.J.; Callaghan, B.; Nevin, S.; Alewood, P.F.; Vaughan, C.W.; Mozar, C.A.; Christie, M.J. A Novel Mechanism of Inhibition of High-Voltage Activated Calcium Channels by α-Conotoxins Contributes to Relief of Nerve Injury-Induced Neuropathic Pain. Pain 2011, 152, 259–266. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Cinci, L.; Micheli, L.; Zanardelli, M.; Pacini, A.; McIntosh, J.M.; Ghelardini, C. α-Conotoxin RgIA Protects against the Development of Nerve Injury-Induced Chronic Pain and Prevents Both Neuronal and Glial Derangement. Pain 2014, 155, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Nevin, S.T.; Clark, R.J.; Klimis, H.; Christie, M.J.; Craik, D.J.; Adams, D.J. Are Alpha9alpha10 Nicotinic Acetylcholine Receptors a Pain Target for Alpha-Conotoxins? Mol. Pharmacol. 2007, 72, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Xu, N.; Liu, Z.; Ding, R.; Yu, S.; Dong, M.; Wang, S.; Shen, J.; Tae, H.-S.; Adams, D.J.; et al. Targeting of N-Type Calcium Channels via GABAB-Receptor Activation by α-Conotoxin Vc1.1 Variants Displaying Improved Analgesic Activity. J. Med. Chem. 2018, 61, 10198–10205. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhangsun, D.; Harvey, P.J.; Kaas, Q.; Wu, Y.; Zhu, X.; Hu, Y.; Li, X.; Tsetlin, V.I.; Christensen, S.; et al. Cloning, Synthesis, and Characterization of AO-Conotoxin GeXIVA, a Potent A9α10 Nicotinic Acetylcholine Receptor Antagonist. Proc. Natl. Acad. Sci. USA 2015, 112, E4026–E4035. [Google Scholar] [CrossRef]
- Wala, E.P.; Crooks, P.A.; McIntosh, J.M.; Holtman, J.R. Novel Small Molecule A9α10 Nicotinic Receptor Antagonist Prevents and Reverses Chemotherapy-Evoked Neuropathic Pain in Rats. Anesth. Analg. 2012, 115, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Holtman, J.R.; Dwoskin, L.P.; Dowell, C.; Wala, E.P.; Zhang, Z.; Crooks, P.A.; McIntosh, J.M. The Novel Small Molecule A9α10 Nicotinic Acetylcholine Receptor Antagonist ZZ-204G Is Analgesic. Eur. J. Pharmacol. 2011, 670, 500–508. [Google Scholar] [CrossRef]
- Arias, H.R.; Tae, H.-S.; Micheli, L.; Yousuf, A.; Ghelardini, C.; Adams, D.J.; Di Cesare Mannelli, L. Coronaridine Congeners Decrease Neuropathic Pain in Mice and Inhibit A9α10 Nicotinic Acetylcholine Receptors and CaV2.2 Channels. Neuropharmacology 2020, 175, 108194. [Google Scholar] [CrossRef]
- Liang, J.; Tae, H.-S.; Zhao, Z.; Li, X.; Zhang, J.; Chen, S.; Jiang, T.; Adams, D.J.; Yu, R. Mechanism of Action and Structure-Activity Relationship of α-Conotoxin Mr1.1 at the Human A9α10 Nicotinic Acetylcholine Receptor. J. Med. Chem. 2022, 65, 16204–16217. [Google Scholar] [CrossRef] [PubMed]
- Vincler, M.; Wittenauer, S.; Parker, R.; Ellison, M.; Olivera, B.M.; McIntosh, J.M. Molecular Mechanism for Analgesia Involving Specific Antagonism of Alpha9alpha10 Nicotinic Acetylcholine Receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 17880–17884. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, I.A.; Palikova, Y.A.; Palikov, V.A.; Korolkova, Y.V.; Kazakov, V.A.; Egorova, N.S.; Garifulina, A.I.; Utkin, Y.N.; Tsetlin, V.I.; Kryukova, E.V. α-Conotoxin RgIA and Oligoarginine R8 in the Mice Model Alleviate Long-Term Oxaliplatin Induced Neuropathy. Biochimie 2022, 194, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Micheli, L.; Maresca, M.; Branca, J.J.V.; McIntosh, J.M.; Ghelardini, C.; Di Cesare Mannelli, L. The A9α10 Nicotinic Receptor Antagonist α-Conotoxin RgIA Prevents Neuropathic Pain Induced by Oxaliplatin Treatment. Exp. Neurol. 2016, 282, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Romero, H.K.; Christensen, S.B.; Di Cesare Mannelli, L.; Gajewiak, J.; Ramachandra, R.; Elmslie, K.S.; Vetter, D.E.; Ghelardini, C.; Iadonato, S.P.; Mercado, J.L.; et al. Inhibition of A9α10 Nicotinic Acetylcholine Receptors Prevents Chemotherapy-Induced Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2017, 114, E1825–E1832. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Hone, A.J.; Roux, I.; Kniazeff, J.; Pin, J.-P.; Upert, G.; Servent, D.; Glowatzki, E.; McIntosh, J.M. RgIA4 Potently Blocks Mouse A9α10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front. Cell. Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.N.; Christensen, S.B.; McIntosh, J.M. RgIA4 Prevention of Acute Oxaliplatin-Induced Cold Allodynia Requires A9-Containing Nicotinic Acetylcholine Receptors and CD3+ T-Cells. Cells 2022, 11, 3561. [Google Scholar] [CrossRef]
- Huynh, P.N.; Giuvelis, D.; Christensen, S.; Tucker, K.L.; McIntosh, J.M. RgIA4 Accelerates Recovery from Paclitaxel-Induced Neuropathic Pain in Rats. Mar. Drugs 2019, 18, 12. [Google Scholar] [CrossRef]
- Gajewiak, J.; Christensen, S.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Olivera, B.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide That Acts Through a Non-Opioid Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef]
- Belgi, A.; Burnley, J.V.; MacRaild, C.A.; Chhabra, S.; Elnahriry, K.A.; Robinson, S.D.; Gooding, S.G.; Tae, H.-S.; Bartels, P.; Sadeghi, M.; et al. Alkyne-Bridged α-Conotoxin Vc1.1 Potently Reverses Mechanical Allodynia in Neuropathic Pain Models. J. Med. Chem. 2021, 64, 3222–3233. [Google Scholar] [CrossRef]
- Satkunanathan, N.; Livett, B.; Gayler, K.; Sandall, D.; Down, J.; Khalil, Z. Alpha-Conotoxin Vc1.1 Alleviates Neuropathic Pain and Accelerates Functional Recovery of Injured Neurones. Brain Res. 2005, 1059, 149–158. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The A9α10 Nicotinic Acetylcholine Receptor Antagonist AO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.; Hong, X.; Li, X.; Gao, J.; Zhang, H.; Zheng, A. Lyophilization Serves as an Effective Strategy for Drug Development of the A9α10 Nicotinic Acetylcholine Receptor Antagonist α-Conotoxin GeXIVA[1,2]. Mar. Drugs 2021, 19, 121. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Wu, Y.; Huang, Y.; Yu, S.; Ding, Q.; Zhangsun, D.; Luo, S. Anti-Hypersensitive Effect of Intramuscular Administration of AO-Conotoxin GeXIVA[1,2] and GeXIVA[1,4] in Rats of Neuropathic Pain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 66, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Meyerson, B.A.; Linderoth, B. Muscarinic Receptor Activation Potentiates the Effect of Spinal Cord Stimulation on Pain-Related Behavior in Rats with Mononeuropathy. Neurosci. Lett. 2008, 436, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Zuo, Z.-X.; Zhang, M.; Feng, Z.-H.; Yan, M.; Li, X.-Y. The Analgesic Effects of (5R,6R)6-(3-Propylthio-1,2,5-Thiadiazol-4-Yl)-1-Azabicyclo[3.2.1] Octane on a Mouse Model of Neuropathic Pain. Anesth. Analg. 2017, 124, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Legaspi, J.M.; López-Avila, A.; Coffeen, U.; del Angel, R.; Pellicer, F. Scopolamine into the Anterior Cingulate Cortex Diminishes Nociception in a Neuropathic Pain Model in the Rat: An Interruption of “Nociception-Related Memory Acquisition”? Eur. J. Pain 2003, 7, 425–429. [Google Scholar] [CrossRef]
- Koga, K.; Matsuzaki, Y.; Migita, K.; Shimoyama, S.; Eto, F.; Nakagawa, T.; Matsumoto, T.; Terada, K.; Mishima, K.; Furue, H.; et al. Stimulating Muscarinic M1 Receptors in the Anterior Cingulate Cortex Reduces Mechanical Hypersensitivity via GABAergic Transmission in Nerve Injury Rats. Brain Res. 2019, 1704, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.W.; Martino, G.; Coupal, M.; Lindberg, M.; Schroeder, P.; Santhakumar, V.; Valiquette, M.; Sandin, J.; Widzowski, D.; Laird, J. Broad Analgesic Activity of a Novel, Selective M1 Agonist. Neuropharmacology 2017, 123, 233–241. [Google Scholar] [CrossRef]
- Calcutt, N.A.; Smith, D.R.; Frizzi, K.; Sabbir, M.G.; Chowdhury, S.K.R.; Mixcoatl-Zecuatl, T.; Saleh, A.; Muttalib, N.; Van der Ploeg, R.; Ochoa, J.; et al. Selective Antagonism of Muscarinic Receptors Is Neuroprotective in Peripheral Neuropathy. J. Clin. Investig. 2017, 127, 608–622. [Google Scholar] [CrossRef]
- Ghazisaeidi, S.; Muley, M.M.; Salter, M.W. Neuropathic Pain: Mechanisms, Sex Differences, and Potential Therapies for a Global Problem. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 565–583. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Sex Differences in Pain: A Tale of Two Immune Cells. Pain 2016, 157, S2–S6. [Google Scholar] [CrossRef]
- Salehi, B.; Sestito, S.; Rapposelli, S.; Peron, G.; Calina, D.; Sharifi-Rad, M.; Sharopov, F.; Martins, N.; Sharifi-Rad, J. Epibatidine: A Promising Natural Alkaloid in Health. Biomolecules 2018, 9, 6. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Belgi, A.; Husselbee, B.W.; Spanswick, D.; Norton, R.S.; Robinson, A.J. α-Conotoxin Peptidomimetics: Probing the Minimal Binding Motif for Effective Analgesia. Toxins 2020, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.L.; Deba, F.; Crowley, M.L.; Hamouda, A.K.; McMahon, L.R. Advances in the In Vitro and In Vivo Pharmacology of Alpha4beta2 Nicotinic Receptor Positive Allosteric Modulators. Neuropharmacology 2020, 168, 108008. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gurun, M.S.; Flood, P.; Papke, R.L.; Damaj, M.I. New Insights on Neuronal Nicotinic Acetylcholine Receptors as Targets for Pain and Inflammation: A Focus on A7 nAChRs. Curr. Neuropharmacol. 2018, 16, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Shiers, S.; Klein, R.M.; Price, T.J. Quantitative Differences in Neuronal Subpopulations between Mouse and Human Dorsal Root Ganglia Demonstrated with RNAscope in Situ Hybridization. Pain 2020, 161, 2410–2424. [Google Scholar] [CrossRef] [PubMed]
- Umana, I.C.; Daniele, C.A.; McGehee, D.S. Neuronal Nicotinic Receptors as Analgesic Targets: It’s a Winding Road. Biochem. Pharmacol. 2013, 86, 1208–1214. [Google Scholar] [CrossRef]
- Gotti, C.; Clementi, F. Neuronal Nicotinic Receptors: From Structure to Pathology. Prog. Neurobiol. 2004, 74, 363–396. [Google Scholar] [CrossRef]
- Del Bufalo, A.; Cesario, A.; Salinaro, G.; Fini, M.; Russo, P. Alpha9 Alpha10 Nicotinic Acetylcholine Receptors as Target for the Treatment of Chronic Pain. Curr. Pharm. Des. 2014, 20, 6042–6047. [Google Scholar] [CrossRef]
- Ditre, J.W.; Heckman, B.W.; Zale, E.L.; Kosiba, J.D.; Maisto, S.A. Acute Analgesic Effects of Nicotine and Tobacco in Humans: A Meta-Analysis. Pain 2016, 157, 1373–1381. [Google Scholar] [CrossRef]
- Hahn, E.J.; Rayens, M.K.; Kirsh, K.L.; Passik, S.D. Brief Report: Pain and Readiness to Quit Smoking Cigarettes. Nicotine Tob. Res. 2006, 8, 473–480. [Google Scholar] [CrossRef]
- Pandya, A.A.; Yakel, J.L. Effects of Neuronal Nicotinic Acetylcholine Receptor Allosteric Modulators in Animal Behavior Studies. Biochem. Pharmacol. 2013, 86, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-C.; Yu, J.-T.; Wang, H.-F.; Tan, M.-S.; Meng, X.-F.; Wang, C.; Jiang, T.; Zhu, X.-C.; Tan, L. Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2014, 41, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Chepkin, S.C.; Ye, W.; Bullen, C.; Lancaster, T. Nicotine Replacement Therapy versus Control for Smoking Cessation. Cochrane Database Syst. Rev. 2018, 2018, CD000146. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Arons, C.; Rollema, H.; Berlin, I.; Hajek, P.; Fagerström, K.; Els, C.; McRae, T.; Russ, C. Varenicline: Mode of Action, Efficacy, Safety and Accumulated Experience Salient for Clinical Populations. Curr. Med. Res. Opin. 2020, 36, 713–730. [Google Scholar] [CrossRef]
- Nair, A. Publication Bias—Importance of Studies with Negative Results! Indian J. Anaesth. 2019, 63, 505. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montigné, E.; Balayssac, D. Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies. Pharmaceuticals 2023, 16, 1363. https://doi.org/10.3390/ph16101363
Montigné E, Balayssac D. Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies. Pharmaceuticals. 2023; 16(10):1363. https://doi.org/10.3390/ph16101363
Chicago/Turabian StyleMontigné, Edouard, and David Balayssac. 2023. "Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies" Pharmaceuticals 16, no. 10: 1363. https://doi.org/10.3390/ph16101363
APA StyleMontigné, E., & Balayssac, D. (2023). Exploring Cholinergic Compounds for Peripheral Neuropathic Pain Management: A Comprehensive Scoping Review of Rodent Model Studies. Pharmaceuticals, 16(10), 1363. https://doi.org/10.3390/ph16101363







