Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain
Abstract
:1. Introduction
2. Results
2.1. Daily Cannabigerol Treatment Does Not Affect Weight or Induce Adverse Events in Neuropathic Mice
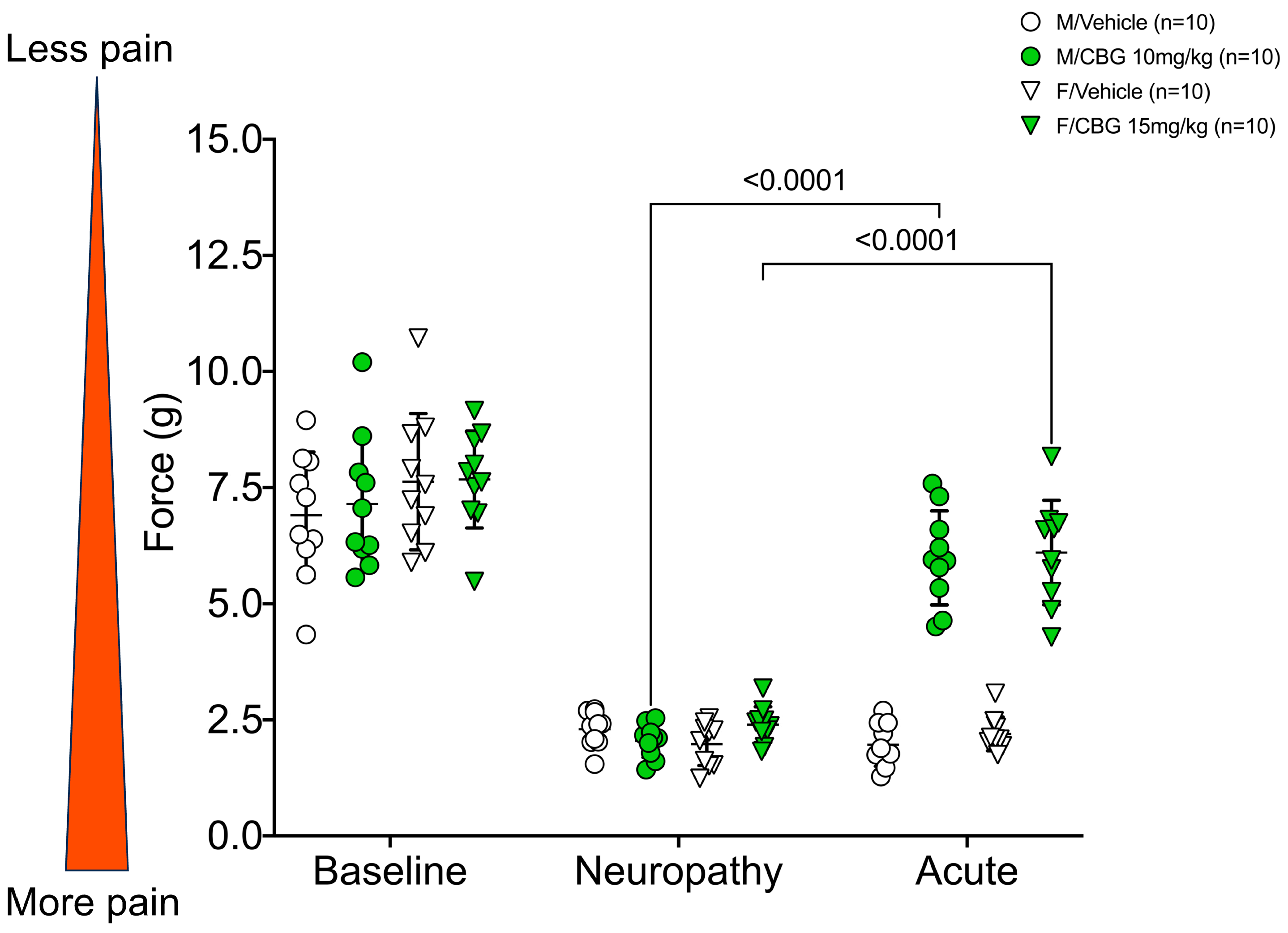
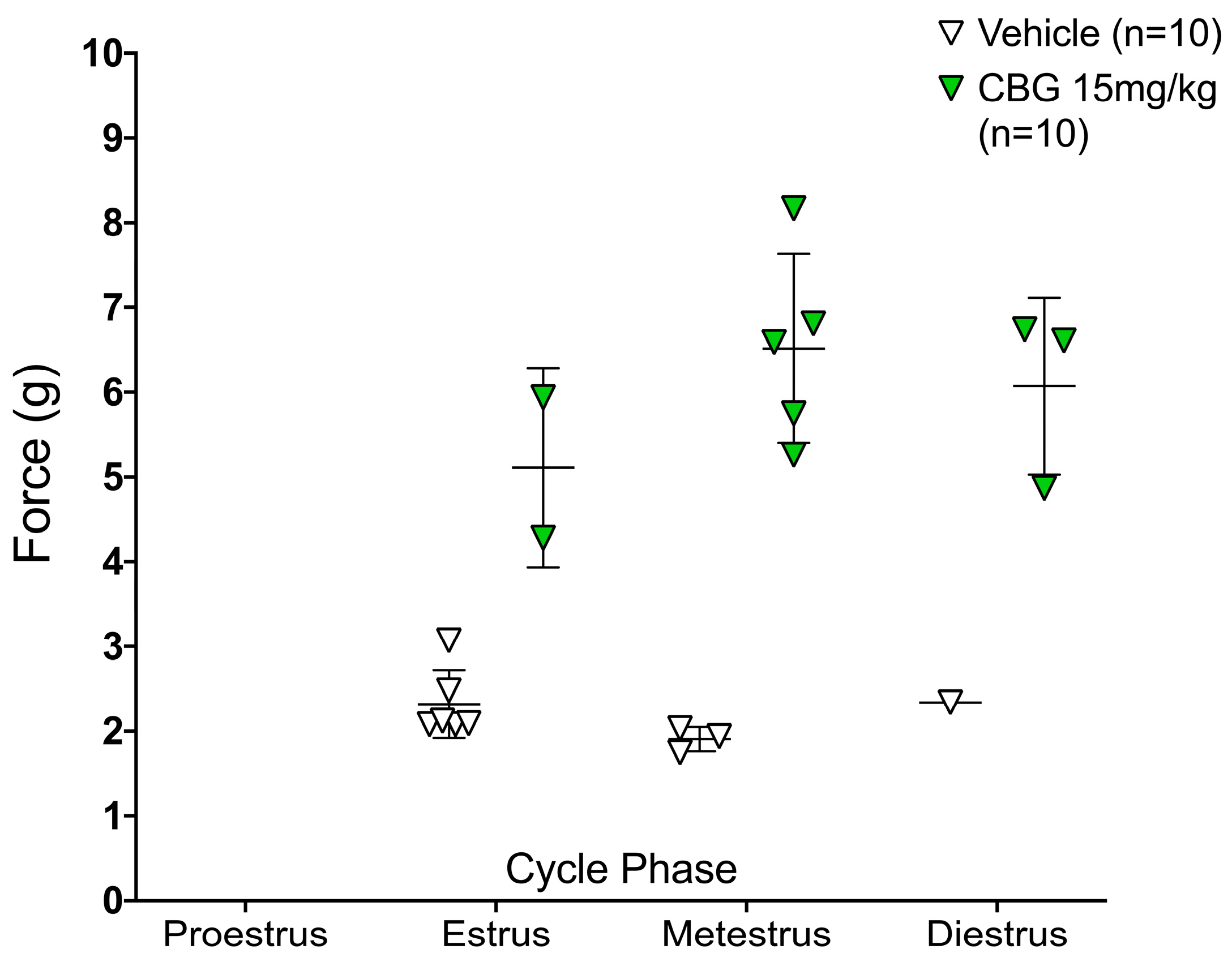
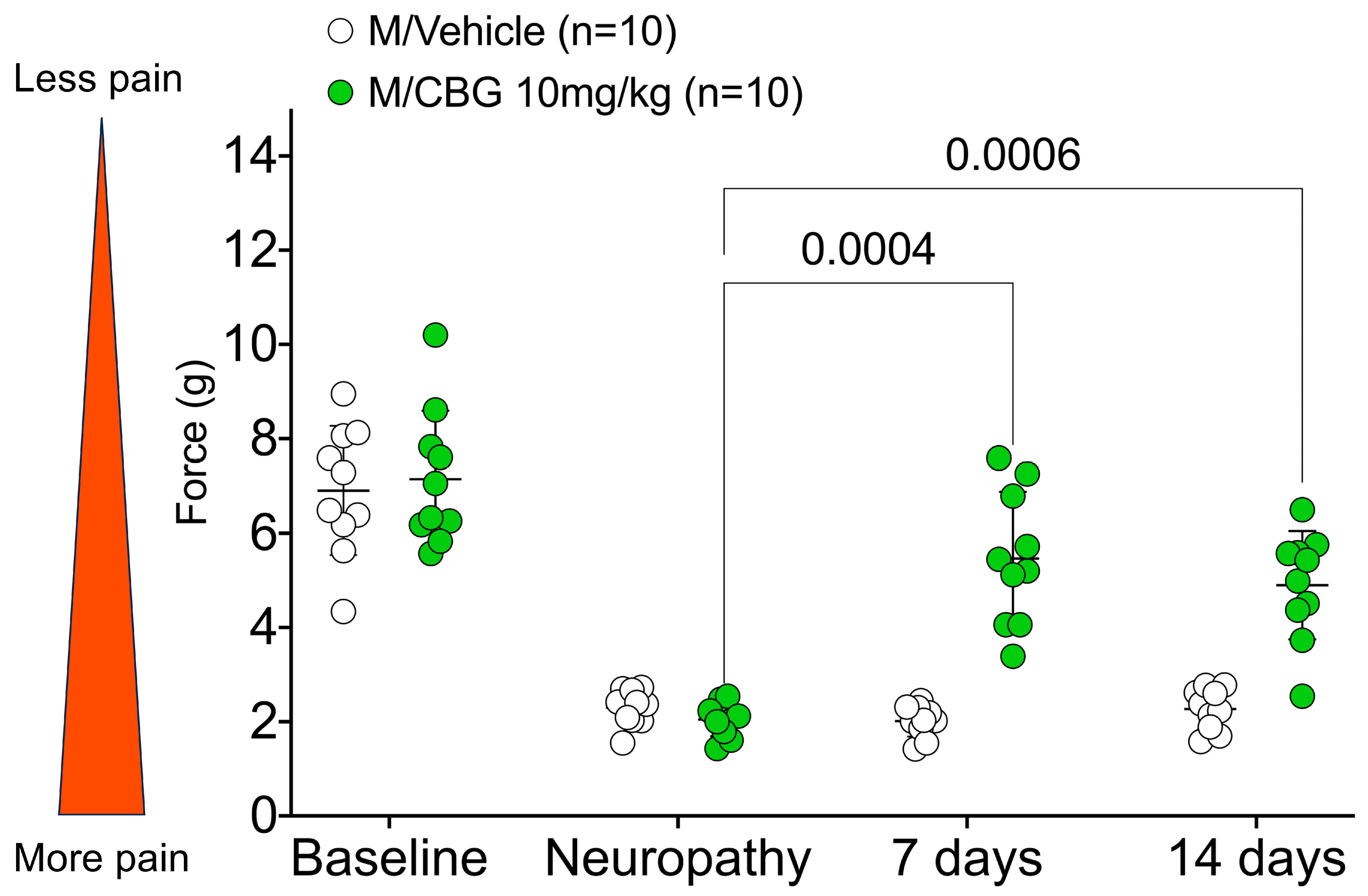
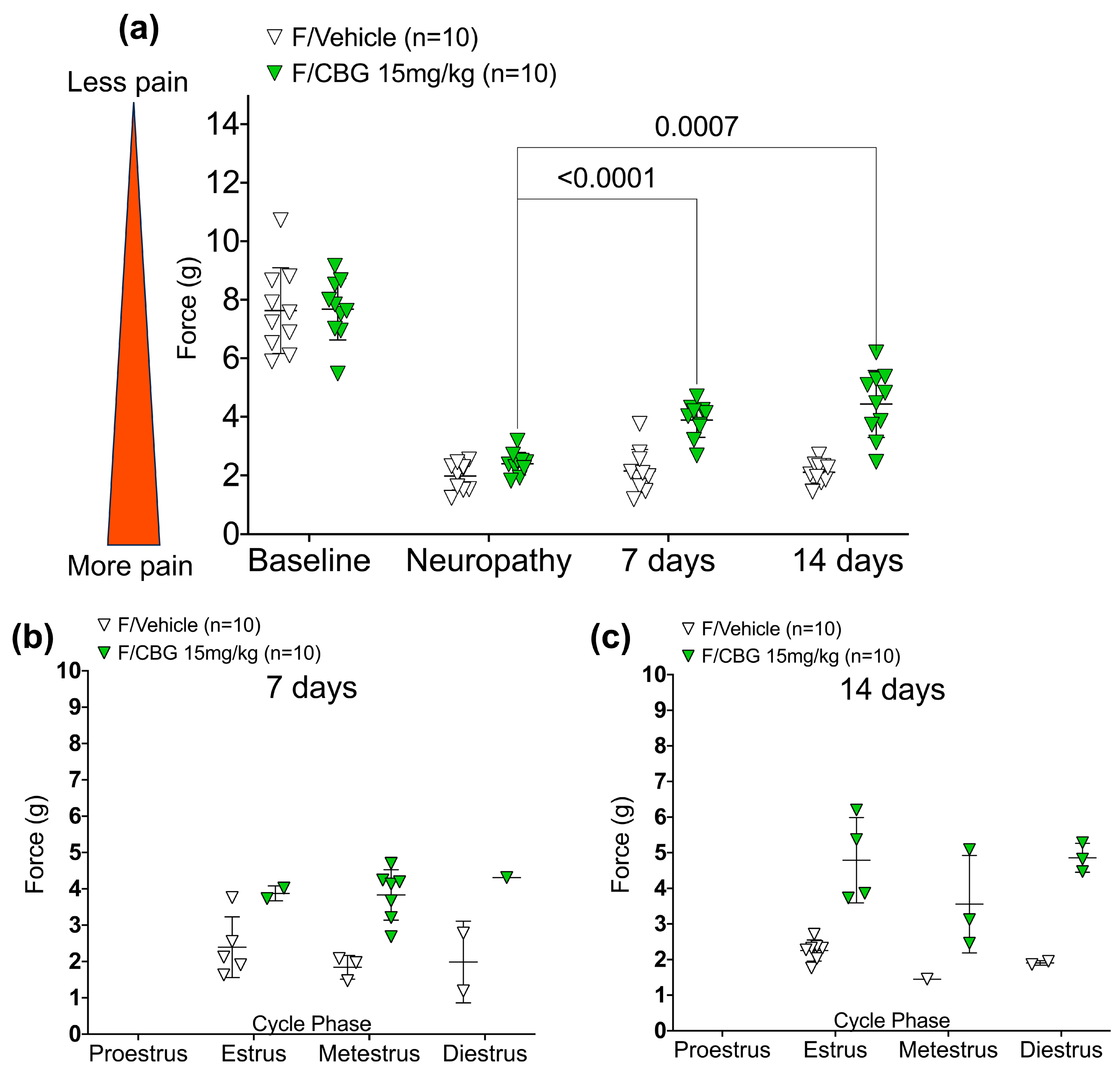
2.2. CBG Relieves CIPN Mechanical Hypersensitivity and Does Not Vary Based on Estrous Cycle Phase
2.3. Gene Expression Changes from Daily Administration of Cannabigerol in a Selected Panel of Cannabinoid and Pain-Related Targets
3. Discussion
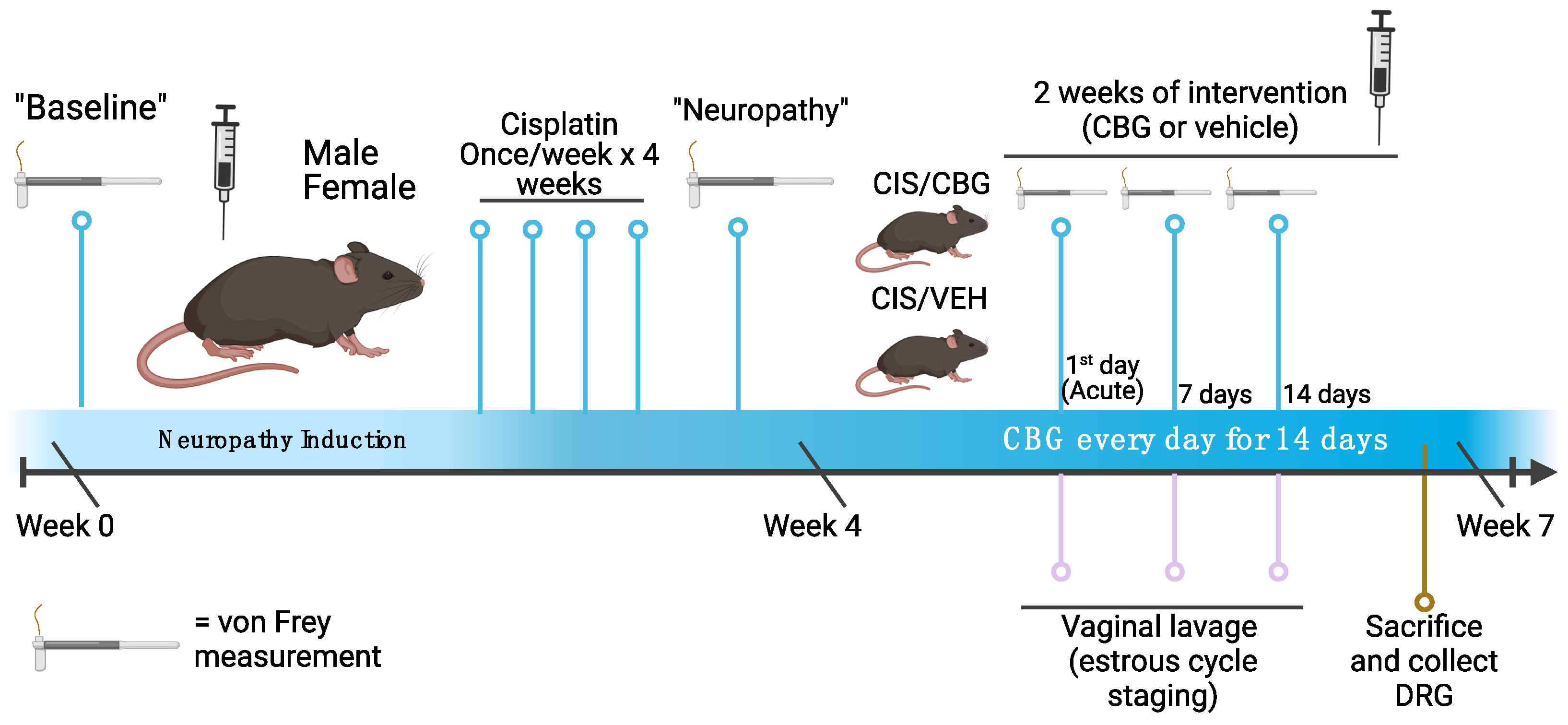
4. Materials and Methods
4.1. Animals
4.2. Cisplatin-Induced Neuropathy
4.3. Measurement of Mechanical Hypersensitivity—Von Frey
4.4. Drug Treatment Schedule and Analgesic Testing
4.5. Estrous Cycle Staging and Cytology
4.6. Dorsal Root Ganglia Extraction
4.7. RNA Extraction and RT-PCR
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.L.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of Duloxetine on Pain, Function, and Quality of Life Among Patients With Chemotherapy-Induced Painful Peripheral Neuropathy: A Randomized Clinical Trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.; Dockter, T.; Wolfe, E.G.; Pachman, D.R.; Wagner-Johnston, N.D.; Lazzara, K.M.; Sego, L.M.; Edwards, S.I.; Snow, C.I.; Hanna, N.; et al. Clinical course of patients with cisplatin (CDDP)-associated neuropathy compared to other neurotoxic chemotherapy. J. Clin. Oncol. 2019, 37, e23078. [Google Scholar] [CrossRef]
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B.; et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; LaFrance, E.M.; Craft, R.M. A Large-Scale Naturalistic Examination of the Acute Effects of Cannabis on Pain. Cannabis Cannabinoid Res. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mendiguren, A.; Aostri, E.; Rodilla, I.; Pujana, I.; Noskova, E.; Pineda, J. Cannabigerol modulates α(2)-adrenoceptor and 5-HT(1A) receptor-mediated electrophysiological effects on dorsal raphe nucleus and locus coeruleus neurons and anxiety behavior in rat. Front. Pharmacol. 2023, 14, 1183019. [Google Scholar] [CrossRef]
- Russo, E.B.; Cuttler, C.; Cooper, Z.D.; Stueber, A.; Whiteley, V.L.; Sexton, M. Survey of Patients Employing Cannabigerol-Predominant Cannabis Preparations: Perceived Medical Effects, Adverse Events, and Withdrawal Symptoms. Cannabis Cannabinoid Res. 2022, 7, 706–716. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Friedman, M.; Steinberg, D. The Antibacterial Effect of Cannabigerol toward Streptococcus mutans Is Influenced by the Autoinducers 21-CSP and AI-2. Biomedicines 2023, 11, 668. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Cannabigerol (CBG) attenuates mechanical hypersensitivity elicited by chemotherapy-induced peripheral neuropathy. Eur. J. Pain 2022, 26, 1950–1966. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Chung, J.; Abdeen, A.; Thompson, A.; Bouboukas, A.; Pinamont, W.J.; Yoshioka, N.K.; Sepulveda, D.E.; Raup-Konsavage, W.M.; Graziane, N.M.; et al. Distinctive Therapeutic Effects of Non-Euphorigenic Cannabis Extracts in Osteoarthritis. In Cannabis and Cannabinoid Research; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2022. [Google Scholar] [CrossRef]
- Salha, M.; Adenusi, H.; Dupuis, J.H.; Bodo, E.; Botta, B.; McKenzie, I.; Yada, R.Y.; Farrar, D.H.; Magolan, J.; Tian, K.V.; et al. Bioactivity of the cannabigerol cannabinoid and its analogues—The role of 3-dimensional conformation. Org. Biomol. Chem. 2023, 21, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Mueller, A.; Deuis, J.R.; Carter, D.A.; Vetter, I. Inflammatory and Neuropathic Gene Expression Signatures of Chemotherapy-Induced Neuropathy Induced by Vincristine, Cisplatin, and Oxaliplatin in C57BL/6J Mice. J. Pain 2020, 21, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, D.E.; Vrana, K.E.; Graziane, N.M.; Raup-Konsavage, W.M. Combinations of Cannabidiol and Δ(9)-Tetrahydrocannabinol in Reducing Chemotherapeutic Induced Neuropathic Pain. Biomedicines 2022, 10, 2548. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Limbird, L.E. Localization and trafficking of α2-adrenergic receptor subtypes in cells and tissues. Pharmacol. Ther. 1999, 84, 193–205. [Google Scholar] [CrossRef]
- Pereira, A.F.; Lisboa, M.R.P.; De Freitas Alves, B.W.; Da Silva, C.M.P.; Dias, D.B.S.; De Menezes, K.L.S.; Cesário, F.R.A.S.; De França, J.C.; De Oliveira, A.R.; Hallak, J.E.C.; et al. Endocannabinoid System Attenuates Oxaliplatin-Induced Peripheral Sensory Neuropathy Through the Activation of CB1 Receptors. Neurotox. Res. 2021, 39, 1782–1799. [Google Scholar] [CrossRef]
- La Porta, C.; Bura, S.A.; Aracil-Fernández, A.; Manzanares, J.; Maldonado, R. Role of CB1 and CB2 cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate. Pain 2013, 154, 160–174. [Google Scholar] [CrossRef]
- Armin, S.; Muenster, S.; Abood, M.; Benamar, K. GPR55 in the brain and chronic neuropathic pain. Behav. Brain Res. 2021, 406, 113248. [Google Scholar] [CrossRef]
- Deng, L.; Guindon, J.; Cornett, B.L.; Makriyannis, A.; Mackie, K.; Hohmann, A.G. Chronic Cannabinoid Receptor 2 Activation Reverses Paclitaxel Neuropathy Without Tolerance or Cannabinoid Receptor 1–Dependent Withdrawal. Biol. Psychiatry 2015, 77, 475–487. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behavi. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Vernail, V.L.; Bingaman, S.S.; Silberman, Y.; Raup-Konsavage, W.M.; Vrana, K.E.; Arnold, A.C. Acute Cannabigerol Administration Lowers Blood Pressure in Mice. Front. Physiol. 2022, 13, 871962. [Google Scholar] [CrossRef]
- Henderson-Redmond, A.N.; Crawford, L.C.; Sepulveda, D.E.; Hale, D.E.; Lesperance, J.J.; Morgan, D.J. Sex Differences in Tolerance to Delta-9-Tetrahydrocannabinol in Mice With Cisplatin-Evoked Chronic Neuropathic Pain. Front. Mol. Biosci. 2021, 8, 684115. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, J.D.; Craft, R.M.; LeResche, L.; Arendt-Nielsen, L.; Berkley, K.J.; Fillingim, R.B.; Gold, M.S.; Holdcroft, A.; Lautenbacher, S.; Mayer, E.A.; et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain 2007, 132 (Suppl. S1), S26–S45. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.; Ibuki, F.; Gonçalves, A.S.; Teixeira, M.J.; De Siqueira, S.R.D.T. Influence of Sexual Hormones on Neural Orofacial Perception. Pain Med. 2016, 18, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Klatzkin, R.R.; Mechlin, B.; Girdler, S.S. Menstrual cycle phase does not influence gender differences in experimental pain sensitivity. Eur. J. Pain 2010, 14, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wakley, A.A.; Craft, R.M. Antinociception and sedation following intracerebroventricular administration of Δ9-tetrahydrocannabinol in female vs. male rats. Behav. Brain Res. 2011, 216, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lunn, S.; Diaz, P.; O’Hearn, S.; Cahill, S.P.; Blake, A.; Narine, K.; Dyck, J.R.B. Human Pharmacokinetic Parameters of Orally Administered Δ9-Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis Cannabinoid Res. 2019, 4, 255–264. [Google Scholar] [CrossRef]
- Skosnik, P.D.; Krishnan, G.P.; Vohs, J.L.; O’Donnell, B.F. The effect of cannabis use and gender on the visual steady state evoked potential. Clin. Neurophysiol 2006, 117, 144–156. [Google Scholar] [CrossRef]
- Schnakenberg Martin, A.M.; D’Souza, D.C.; Newman, S.D.; Hetrick, W.P.; O’Donnell, B.F. Differential Cognitive Performance in Females and Males with Regular Cannabis Use. J. Int. Neuropsychol. Soc. 2021, 27, 570–580. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Haney, M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014, 136, 85–91. [Google Scholar] [CrossRef]
- Xu, Z.; Lee, M.-C.; Sheehan, K.; Fujii, K.; Rabl, K.; Rader, G.; Varney, S.; Sharma, M.; Eilers, H.; Kober, K.; et al. Chemotherapy for pain: Reversing inflammatory and neuropathic pain with the anticancer agent mithramycin A. Pain 2022, 10, 1097. [Google Scholar] [CrossRef]
- Meng, J.; Qiu, S.; Zhang, L.; You, M.; Xing, H.; Zhu, J. Berberine Alleviate Cisplatin-Induced Peripheral Neuropathy by Modulating Inflammation Signal via TRPV1. Front. Pharmacol. 2022, 12, 774795. [Google Scholar] [CrossRef] [PubMed]
- Seijffers, R.; Mills, C.D.; Woolf, C.J. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J. Neurosci. 2007, 27, 7911–7920. [Google Scholar] [CrossRef] [PubMed]
- Brumovsky, P.R. Dorsal root ganglion neurons and tyrosine hydroxylase--an intriguing association with implications for sensation and pain. Pain 2016, 157, 314–320. [Google Scholar] [CrossRef] [PubMed]
- McWhinney-Glass, S.; Winham, S.J.; Hertz, D.L.; Yen Revollo, J.; Paul, J.; He, Y.; Brown, R.; Motsinger-Reif, A.A.; McLeod, H.L. Cumulative genetic risk predicts platinum/taxane-induced neurotoxicity. Clin. Cancer Res. 2013, 19, 5769–5776. [Google Scholar] [CrossRef]
- Grassi, G.; Turri, C.; Seravalle, G.; Bertinieri, G.; Pierini, A.; Mancia, G. Effects of Chronic Clonidine Administration on Sympathetic Nerve Traffic and Baroreflex Function in Heart Failure. Hypertension 2001, 38, 286–291. [Google Scholar] [CrossRef]
- Guindon, J.; Deng, L.; Fan, B.; Wager-Miller, J.; Hohmann, A.G. Optimization of a cisplatin model of chemotherapy-induced peripheral neuropathy in mice: Use of vitamin C and sodium bicarbonate pretreatments to reduce nephrotoxicity and improve animal health status. Mol. Pain 2014, 10, 56. [Google Scholar] [CrossRef]
- Raup-Konsavage, W.M.; Sepulveda, D.E.; Morris, D.P.; Amin, S.; Vrana, K.E.; Graziane, N.M.; Desai, D. Efficient Synthesis for Altering Side Chain Length on Cannabinoid Molecules and Their Effects in Chemotherapy and Chemotherapeutic Induced Neuropathic Pain. Biomolecules 2022, 12, 1869. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse:Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Richner, M.; Jager, S.B.; Siupka, P.; Vaegter, C.B. Hydraulic Extrusion of the Spinal Cord and Isolation of Dorsal Root Ganglia in Rodents. JoVE 2017, 119, e55226. [Google Scholar] [CrossRef]
- Sleigh, J.N.; Weir, G.A.; Schiavo, G. A simple, step-by-step dissection protocol for the rapid isolation of mouse dorsal root ganglia. BMC Res. Notes 2016, 9, 82. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.M.; Walker, S.J.; Vrana, K.E. Quantitative RT-PCR: Pitfalls and potential. Biotechniques 1999, 26, 112–122+124–115. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| CBG (n = 10) | Vehicle (n = 10) | CBG (n = 10) | Vehicle (n = 10) | |||||
| Mean (g) | SD (g) | Mean (g) | SD (g) | Mean (g) | SD (g) | Mean (g) | SD (g) | |
| Start | 27.1 | 0.985 | 27.8 | 0.969 | 19.8 | 0.909 | 20.3 | 1.38 |
| 7 days | 27.0 | 1.17 | 27.6 | 0.7 | 20.2 | 1.01 | 20.5 | 1.59 |
| 14 days | 28.0 | 1.24 | 28.5 | 0.863 | 21.1 | 1.16 | 21.4 | 1.19 |
| Males 1 | Females 1 | ||||
|---|---|---|---|---|---|
| Gene | Name | % Change in Expression (CBG/Vehicle) | p Value | % Change in Expression (CBG/Vehicle) | p Value |
| Cnr1 | Cannabinoid Receptor 1 | 5% | 0.684 | −5% | 0.631 |
| Cnr2 | Cannabinoid Receptor 2 | −38% | 0.46 | 3% | 0.631 |
| Gpr55 | G-protein coupled receptor 55 | −10% | 0.661 | 18% | 0.579 |
| Faah | Fatty Acid Amide Hydrolase | 10% | 0.28 | −1% | 0.481 |
| Mgll | Monoglyceride Lipase | 15% | 0.105 | 2% | 0.999 |
| Atf3 | Activating Transcription Factor 3 | −17% | 0.043 * | −4% | 0.971 |
| Trpv1 | Transient Receptor Potential Cation Channel Subfamily V Member 1 | −1% | 0.853 | −6% | 0.28 |
| Adra2a | Adrenergic Receptor 2A | −7% | 0.356 | −10% | 0.796 |
| Adra2b | Adrenergic Receptor 2B | −4% | 0.661 | −31% | 0.796 |
| Adra2c | Adrenergic Receptor 2C | 3% | 0.912 | 7% | 0.912 |
| Drd2 | Dopamine Receptor D2 | 5% | 0.661 | −19% | 0.029 * |
| Gfap | Glial Fibrillary Acidic Protein | −5% | 0.999 | −59% | 0.684 |
| Oprm1 | Mu Opioid Receptor 1 | 7% | 0.166 | −9.5% | 0.007 * |
| Pparg | Peroxisome Proliferator Activated Receptor Gamma | 68% | 0.321 | 48% | 0.258 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachnani, R.; Sepulveda, D.E.; Booth, J.L.; Zhou, S.; Graziane, N.M.; Raup-Konsavage, W.M.; Vrana, K.E. Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain. Pharmaceuticals 2023, 16, 1442. https://doi.org/10.3390/ph16101442
Nachnani R, Sepulveda DE, Booth JL, Zhou S, Graziane NM, Raup-Konsavage WM, Vrana KE. Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain. Pharmaceuticals. 2023; 16(10):1442. https://doi.org/10.3390/ph16101442
Chicago/Turabian StyleNachnani, Rahul, Diana E. Sepulveda, Jennifer L. Booth, Shouhao Zhou, Nicholas M. Graziane, Wesley M. Raup-Konsavage, and Kent E. Vrana. 2023. "Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain" Pharmaceuticals 16, no. 10: 1442. https://doi.org/10.3390/ph16101442
APA StyleNachnani, R., Sepulveda, D. E., Booth, J. L., Zhou, S., Graziane, N. M., Raup-Konsavage, W. M., & Vrana, K. E. (2023). Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain. Pharmaceuticals, 16(10), 1442. https://doi.org/10.3390/ph16101442







