Tramadol and Tapentadol Induce Conditioned Place Preference with a Differential Impact on Rewarding Memory and Incubation of Craving
Abstract
1. Introduction
2. Results
2.1. Repeated Exposure to Tramadol and Tapentadol Leads to Changes in Place Preference
2.2. Tramadol, but Not Tapentadol, Causes CPP Memory for at Least 14 Days upon Conditioning
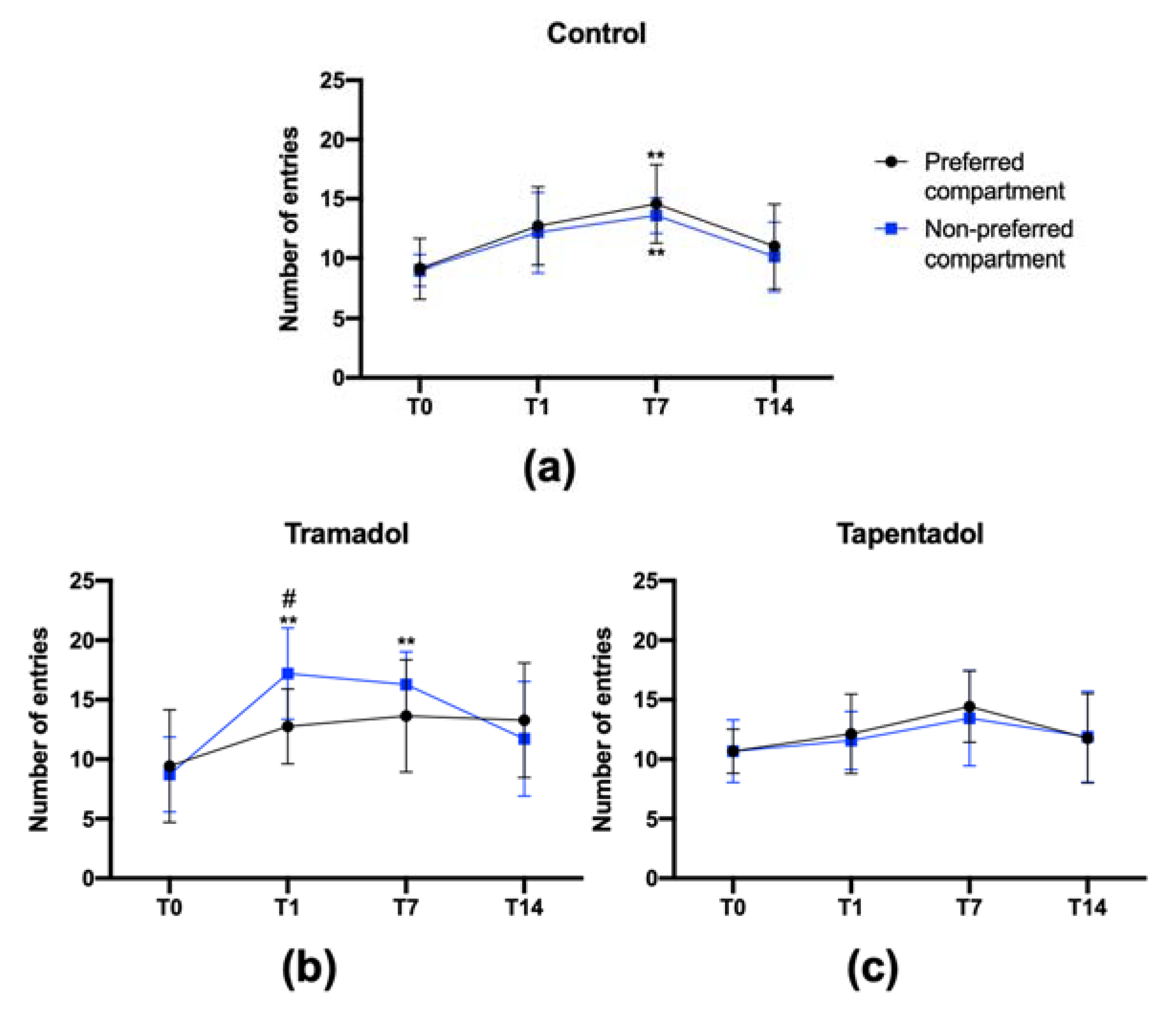
2.3. Tramadol, but Not Tapentadol, Shows Craving Properties
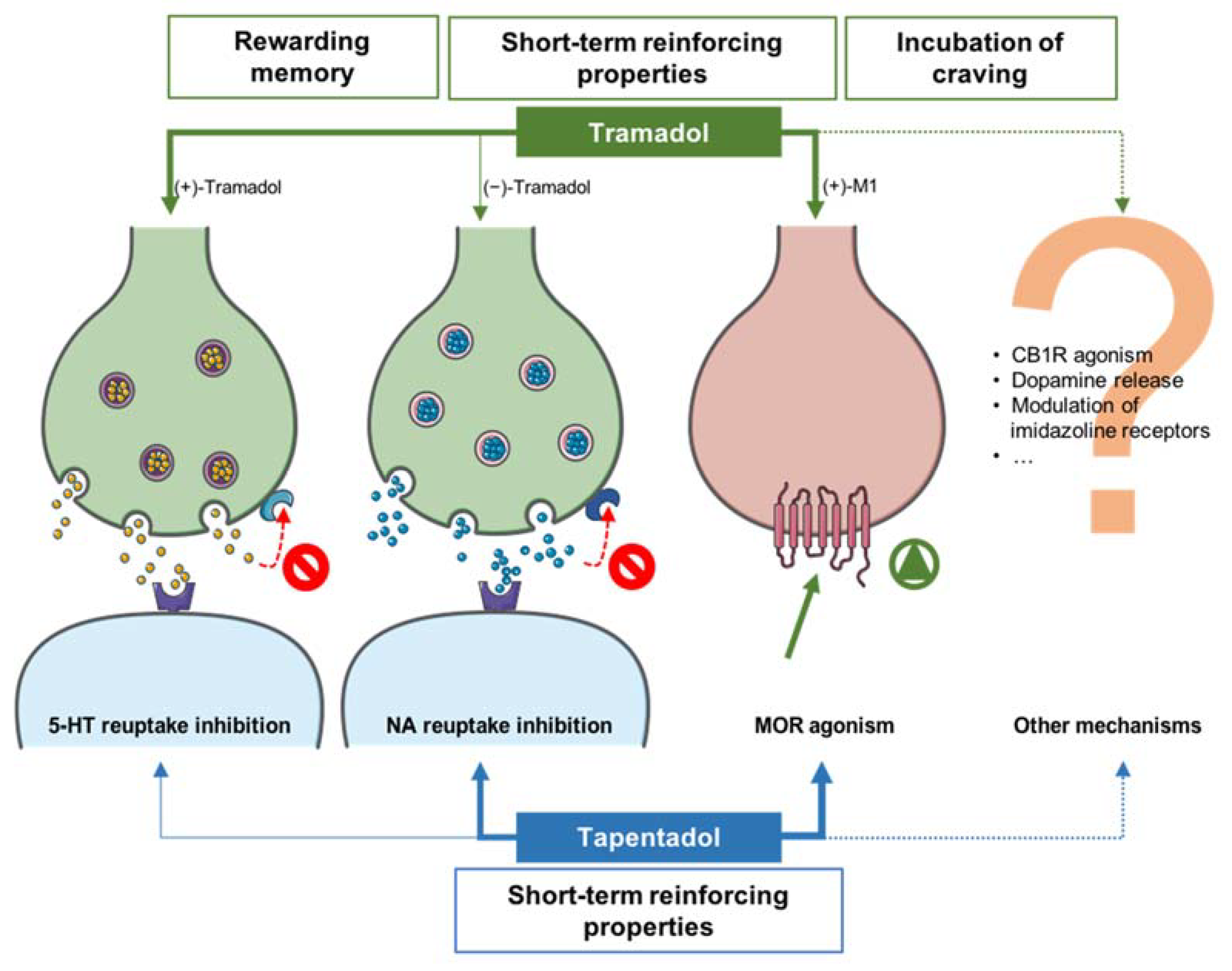
3. Discussion
4. Materials and Methods
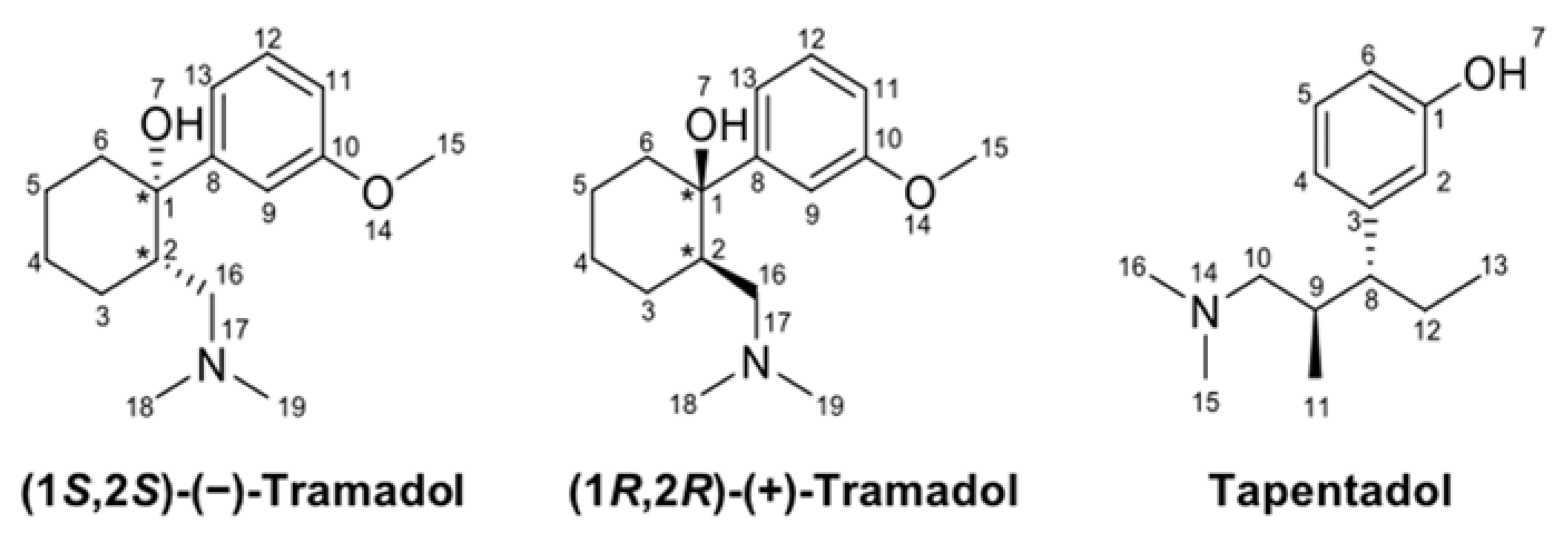
4.1. Chemicals
4.2. Experimental Models, Animal Housing and Handling
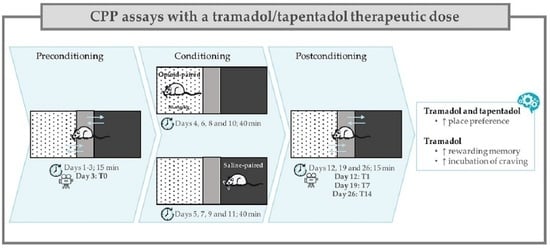
4.3. Conditioned Place Preference Assays
4.3.1. Apparatus and Environmental Conditions
4.3.2. Preconditioning
4.3.3. Conditioning
4.3.4. Postconditioning
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jayawardana, S.; Forman, R.; Johnston-Webber, C.; Campbell, A.; Berterame, S.; de Joncheere, C.; Aitken, M.; Mossialos, E. Global consumption of prescription opioid analgesics between 2009–2019: A country-level observational study. EClinicalMedicine 2021, 42, 101198. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, M.M.; Guo, J.J. Utilization, Spending, and Price of Opioid Medications in the US Medicaid Programs Between 1991 and 2019. Am. Health Drug Benefits 2022, 15, 31–37. [Google Scholar]
- Barbosa, J.; Faria, J.; Queiros, O.; Moreira, R.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative metabolism of tramadol and tapentadol: A toxicological perspective. Drug Metab. Rev. 2016, 48, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Barbosa, J.; Moreira, R.; Queiros, O.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur. J. Pain. 2018, 22, 827–844. [Google Scholar] [CrossRef]
- Wang, X.; Narayan, S.W.; Penm, J.; Patanwala, A.E. Efficacy and Safety of Tapentadol Immediate Release for Acute Pain: A Systematic Review and Meta-Analysis. Clin. J. Pain. 2020, 36, 399–409. [Google Scholar] [CrossRef]
- Raffa, R.B.; Buschmann, H.; Christoph, T.; Eichenbaum, G.; Englberger, W.; Flores, C.M.; Hertrampf, T.; Kogel, B.; Schiene, K.; Strassburger, W.; et al. Mechanistic and functional differentiation of tapentadol and tramadol. Expert. Opin. Pharm. 2012, 13, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.; Magnusson, P.; Coluzzi, F.; Breve, F.; LeQuang, J.A.K.; Varrassi, G. Multimechanistic Single-Entity Combinations for Chronic Pain Control: A Narrative Review. Cureus 2022, 14, e26000. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Kataoka, T.; Tasaki, Y.; Kondo, Y.; Sato, N.; Naiki, T.; Sakamoto, N.; Akechi, T.; Kimura, K. Efficacy of tapentadol for first-line opioid-resistant neuropathic pain in Japan. Jpn. J. Clin. Oncol. 2018, 48, 362–366. [Google Scholar] [CrossRef]
- Sommer, C.; Klose, P.; Welsch, P.; Petzke, F.; Hauser, W. Opioids for chronic non-cancer neuropathic pain. An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Eur. J. Pain. 2020, 24, 3–18. [Google Scholar] [CrossRef]
- Caraci, F.; Merlo, S.; Drago, F.; Caruso, G.; Parenti, C.; Sortino, M.A. Rescue of Noradrenergic System as a Novel Pharmacological Strategy in the Treatment of Chronic Pain: Focus on Microglia Activation. Front. Pharm. 2019, 10, 1024. [Google Scholar] [CrossRef]
- Bortolotto, V.; Grilli, M. Opiate Analgesics as Negative Modulators of Adult Hippocampal Neurogenesis: Potential Implications in Clinical Practice. Front. Pharm. 2017, 8, 254. [Google Scholar] [CrossRef]
- Burgess, G.; Williams, D. The discovery and development of analgesics: New mechanisms, new modalities. J. Clin. Investig. 2010, 120, 3753–3759. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.M.; Knaggs, R.; Farquhar-Smith, P.; Dickenson, A.H. Is tapentadol different from classical opioids? A review of the evidence. Br. J. Pain. 2016, 10, 217–221. [Google Scholar] [CrossRef]
- Meneghini, V.; Cuccurazzu, B.; Bortolotto, V.; Ramazzotti, V.; Ubezio, F.; Tzschentke, T.M.; Canonico, P.L.; Grilli, M. The noradrenergic component in tapentadol action counteracts mu-opioid receptor-mediated adverse effects on adult neurogenesis. Mol. Pharm. 2014, 85, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Cicero, T.J.; Adams, E.H.; Geller, A.; Inciardi, J.A.; Munoz, A.; Schnoll, S.H.; Senay, E.C.; Woody, G.E. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999, 57, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Zacny, J.P. Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005, 80, 273–278. [Google Scholar] [CrossRef]
- Epstein, D.H.; Preston, K.L.; Jasinski, D.R. Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: Lessons from tramadol. Biol. Psychol. 2006, 73, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Sprague, J.E.; Leifheit, M.; Selken, J.; Milks, M.M.; Kinder, D.H.; Nichols, D.E. In vivo microdialysis and conditioned place preference studies in rats are consistent with abuse potential of tramadol. Synapse 2002, 43, 118–121. [Google Scholar] [CrossRef]
- Randall, C.; Crane, J. Tramadol deaths in Northern Ireland: A review of cases from 1996 to 2012. J. Forensic. Leg. Med. 2014, 23, 32–36. [Google Scholar] [CrossRef]
- Faria, J.; Barbosa, J.; Leal, S.; Afonso, L.P.; Lobo, J.; Moreira, R.; Queiros, O.; Carvalho, F.; Dinis-Oliveira, R.J. Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar rats. Toxicology 2017, 385, 38–47. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Leal, S.; Afonso, L.P.; Lobo, J.; Queiros, O.; Moreira, R.; Carvalho, F.; Dinis-Oliveira, R.J. Acute administration of tramadol and tapentadol at effective analgesic and maximum tolerated doses causes hepato- and nephrotoxic effects in Wistar rats. Toxicology 2017, 389, 118–129. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Garcez, F.; Leal, S.; Afonso, L.P.; Nascimento, A.V.; Moreira, R.; Queiros, O.; Carvalho, F.; Dinis-Oliveira, R.J. Repeated Administration of Clinical Doses of Tramadol and Tapentadol Causes Hepato- and Nephrotoxic Effects in Wistar Rats. Pharmaceuticals 2020, 13, 149. [Google Scholar] [CrossRef]
- Barbosa, J.; Faria, J.; Garcez, F.; Leal, S.; Afonso, L.P.; Nascimento, A.V.; Moreira, R.; Pereira, F.C.; Queiros, O.; Carvalho, F.; et al. Repeated Administration of Clinically Relevant Doses of the Prescription Opioids Tramadol and Tapentadol Causes Lung, Cardiac, and Brain Toxicity in Wistar Rats. Pharmaceuticals 2021, 14, 97. [Google Scholar] [CrossRef]
- Bassiony, M.M.; Salah El-Deen, G.M.; Yousef, U.; Raya, Y.; Abdel-Ghani, M.M.; El-Gohari, H.; Atwa, S.A. Adolescent tramadol use and abuse in Egypt. Am. J. Drug. Alcohol. Abus. 2015, 41, 206–211. [Google Scholar] [CrossRef]
- Kitamura, A.; Higuchi, K.; Okura, T.; Deguchi, Y. Transport characteristics of tramadol in the blood-brain barrier. J. Pharm. Sci. 2014, 103, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Mahadevan, J.; Ithal, D.; Selvaraj, S.; Chand, P.K.; Murthy, P. Is tapentadol a potential Trojan horse in the postdextropropoxyphene era in India? Indian J. Pharmacol. 2018, 50, 44–46. [Google Scholar] [CrossRef]
- Kathiresan, P.; Pakhre, A.; Kattula, D.; Sarkar, S. Tapentadol Dependence: A Case Series. Prim. Care. Companion CNS Disord. 2019, 21. [Google Scholar] [CrossRef]
- Guay, D.R. Is tapentadol an advance on tramadol? Consult. Pharm 2009, 24, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Stoops, W.W.; Glaser, P.E.; Rush, C.R. Miotic and subject-rated effects of therapeutic doses of tapentadol, tramadol, and hydromorphone in occasional opioid users. Psychopharmacology 2013, 228, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Vosburg, S.K.; Dailey-Govoni, T.; Beaumont, J.; Butler, S.F.; Green, J.L. Characterizing the Experience of Tapentadol Nonmedical Use: Mixed Methods Study. JMIR Form. Res. 2022, 6, e16996. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, R.; Nabil, M.; Abdel-Aal, M.; Barakat, W. Nalbuphine could decrease the rewarding effect induced by tramadol in mice while enhancing its antinociceptive activity. Eur. J. Pharmacol. 2015, 758, 11–15. [Google Scholar] [CrossRef]
- Cha, H.J.; Song, M.J.; Lee, K.W.; Kim, E.J.; Kim, Y.H.; Lee, Y.; Seong, W.K.; Hong, S.I.; Jang, C.G.; Yoo, H.S.; et al. Dependence potential of tramadol: Behavioral pharmacology in rodents. Biomol. Ther. 2014, 22, 558–562. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.C.; Mead, A.N. Tramadol acts as a weak reinforcer in the rat self-administration model, consistent with its low abuse liability in humans. Pharm. Biochem. Behav 2010, 96, 279–286. [Google Scholar] [CrossRef]
- Tzschentke, T.M.; Bruckmann, W.; Friderichs, E. Lack of sensitization during place conditioning in rats is consistent with the low abuse potential of tramadol. Neurosci. Lett. 2002, 329, 25–28. [Google Scholar] [CrossRef]
- Sorodoc, V.; Rusu-Zota, G.; Nechita, P.; Moraru, C.; Manole, O.M. Effects of imidazoline agents in a rat conditioned place preference model of addiction. Naunyn Schmiedebergs Arch Pharm. 2022, 395, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jing, L.; Liu, Q.; Wen, R.T.; Li, J.X.; Li, Y.L.; Gong, Q.; Liang, J.H. Tramadol induces conditioned place preference in rats: Interactions with morphine and buprenorphine. Neurosci. Lett. 2012, 520, 87–91. [Google Scholar] [CrossRef]
- Nakamura, A.; Narita, M.; Miyoshi, K.; Shindo, K.; Okutsu, D.; Suzuki, M.; Higashiyama, K.; Suzuki, T. Changes in the rewarding effects induced by tramadol and its active metabolite M1 after sciatic nerve injury in mice. Psychopharmacology 2008, 200, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Adl, M.; Sadat-Shirazi, M.S.; Shahini, F.; Akbarabadi, A.; Khalifeh, S.; Borzabadi, S.; Nasehi, M.; Zarrindast, M.R. The role of cannabinoid 1 receptor in the nucleus accumbens on tramadol induced conditioning and reinstatement. Life Sci. 2020, 260, 118430. [Google Scholar] [CrossRef]
- Tzschentke, T.M. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007, 12, 227–462. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, G.; Zhou, K.; Zhu, Y. A Conditioned Place Preference Protocol for Measuring Incubation of Craving in Rats. J. Vis. Exp. 2018, 141, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Pan, Z.; Ma, Y. Increased entrances to side compartments indicate incubation of craving in morphine-induced rat and tree shrew CPP models. Pharm. Biochem. Behav. 2017, 159, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharm. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Chang, E.J.; Choi, E.J.; Kim, K.H. Tapentadol: Can It Kill Two Birds with One Stone without Breaking Windows? Korean J. Pain. 2016, 29, 153–157. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Tverdohleb, T.; Knezevic, I.; Candido, K.D. Unique pharmacology of tapentadol for treating acute and chronic pain. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 1475–1492. [Google Scholar] [CrossRef]
- Vadivelu, N.; Chang, D.; Helander, E.M.; Bordelon, G.J.; Kai, A.; Kaye, A.D.; Hsu, D.; Bang, D.; Julka, I. Ketorolac, Oxymorphone, Tapentadol, and Tramadol: A Comprehensive Review. Anesthesiol. Clin. 2017, 35, e1–e20. [Google Scholar] [CrossRef]
- Rutten, K.; De Vry, J.; Robens, A.; Tzschentke, T.M.; van der Kam, E.L. Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. Eur. J. Pain. 2011, 15, 299–305. [Google Scholar] [CrossRef]
- Miranda, H.F.; Pinardi, G. Antinociception, tolerance, and physical dependence comparison between morphine and tramadol. Pharm. Biochem. Behav 1998, 61, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Ide, S.; Minami, M.; Ishihara, K.; Uhl, G.R.; Sora, I.; Ikeda, K. Mu opioid receptor-dependent and independent components in effects of tramadol. Neuropharmacology 2006, 51, 651–658. [Google Scholar] [CrossRef]
- Butler, S.F.; McNaughton, E.C.; Black, R.A. Tapentadol abuse potential: A postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med. 2015, 16, 119–130. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Breve, F.; Taylor, R., Jr.; Raffa, R.B.; Strasburger, S.E.; LeQuang, J.A. Considering tapentadol as a first-line analgesic: 14 questions. Pain Manag. 2017, 7, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Fife, D.; Kihm, M.A.; Mastrogiovanni, G.; Yuan, Y. Comparison of the risks of shopping behavior and opioid abuse between tapentadol and oxycodone and association of shopping behavior and opioid abuse. Clin. J. Pain. 2014, 30, 1051–1056. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Fife, D.; Ma, Q.; Ryan, P.B. Comparison of the risks of opioid abuse or dependence between tapentadol and oxycodone: Results from a cohort study. J. Pain. 2013, 14, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Tjaderborn, M.; Jonsson, A.K.; Hagg, S.; Ahlner, J. Fatal unintentional intoxications with tramadol during 1995-2005. Forensic. Sci. Int. 2007, 173, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Barber, J. Examining the use of tramadol hydrochloride as an antidepressant. Exp. Clin. Psychopharmacol. 2011, 19, 123–130. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, J.; Leal, S.; Pereira, F.C.; Dinis-Oliveira, R.J.; Faria, J. Tramadol and Tapentadol Induce Conditioned Place Preference with a Differential Impact on Rewarding Memory and Incubation of Craving. Pharmaceuticals 2023, 16, 86. https://doi.org/10.3390/ph16010086
Barbosa J, Leal S, Pereira FC, Dinis-Oliveira RJ, Faria J. Tramadol and Tapentadol Induce Conditioned Place Preference with a Differential Impact on Rewarding Memory and Incubation of Craving. Pharmaceuticals. 2023; 16(1):86. https://doi.org/10.3390/ph16010086
Chicago/Turabian StyleBarbosa, Joana, Sandra Leal, Frederico C. Pereira, Ricardo Jorge Dinis-Oliveira, and Juliana Faria. 2023. "Tramadol and Tapentadol Induce Conditioned Place Preference with a Differential Impact on Rewarding Memory and Incubation of Craving" Pharmaceuticals 16, no. 1: 86. https://doi.org/10.3390/ph16010086
APA StyleBarbosa, J., Leal, S., Pereira, F. C., Dinis-Oliveira, R. J., & Faria, J. (2023). Tramadol and Tapentadol Induce Conditioned Place Preference with a Differential Impact on Rewarding Memory and Incubation of Craving. Pharmaceuticals, 16(1), 86. https://doi.org/10.3390/ph16010086