Supporting the Wound Healing Process—Curcumin, Resveratrol and Baicalin in In Vitro Wound Healing Studies
Abstract
1. Introduction
2. Results and Discussion
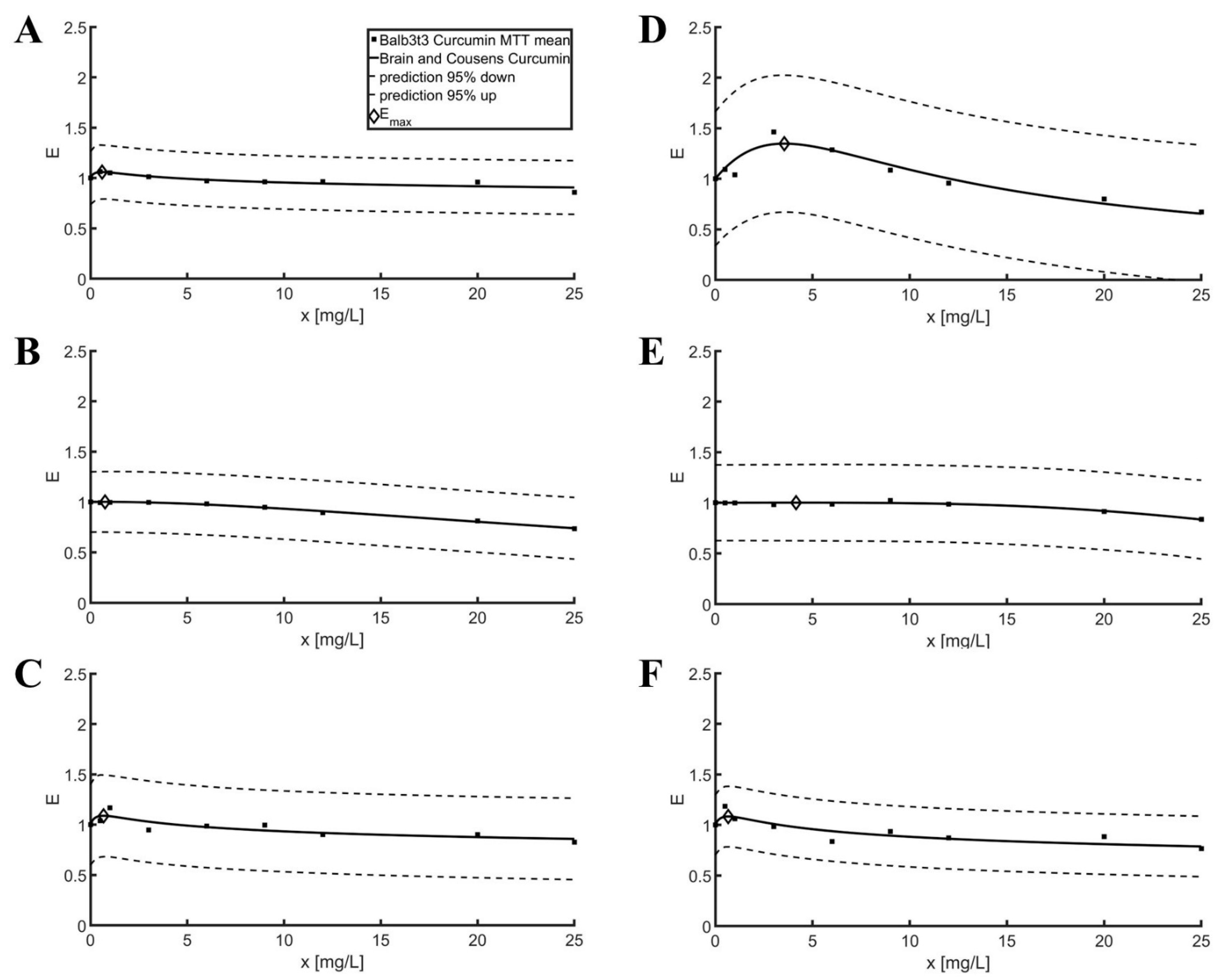
2.1. Model Fit and Statistical Analysis for MTT Test Results
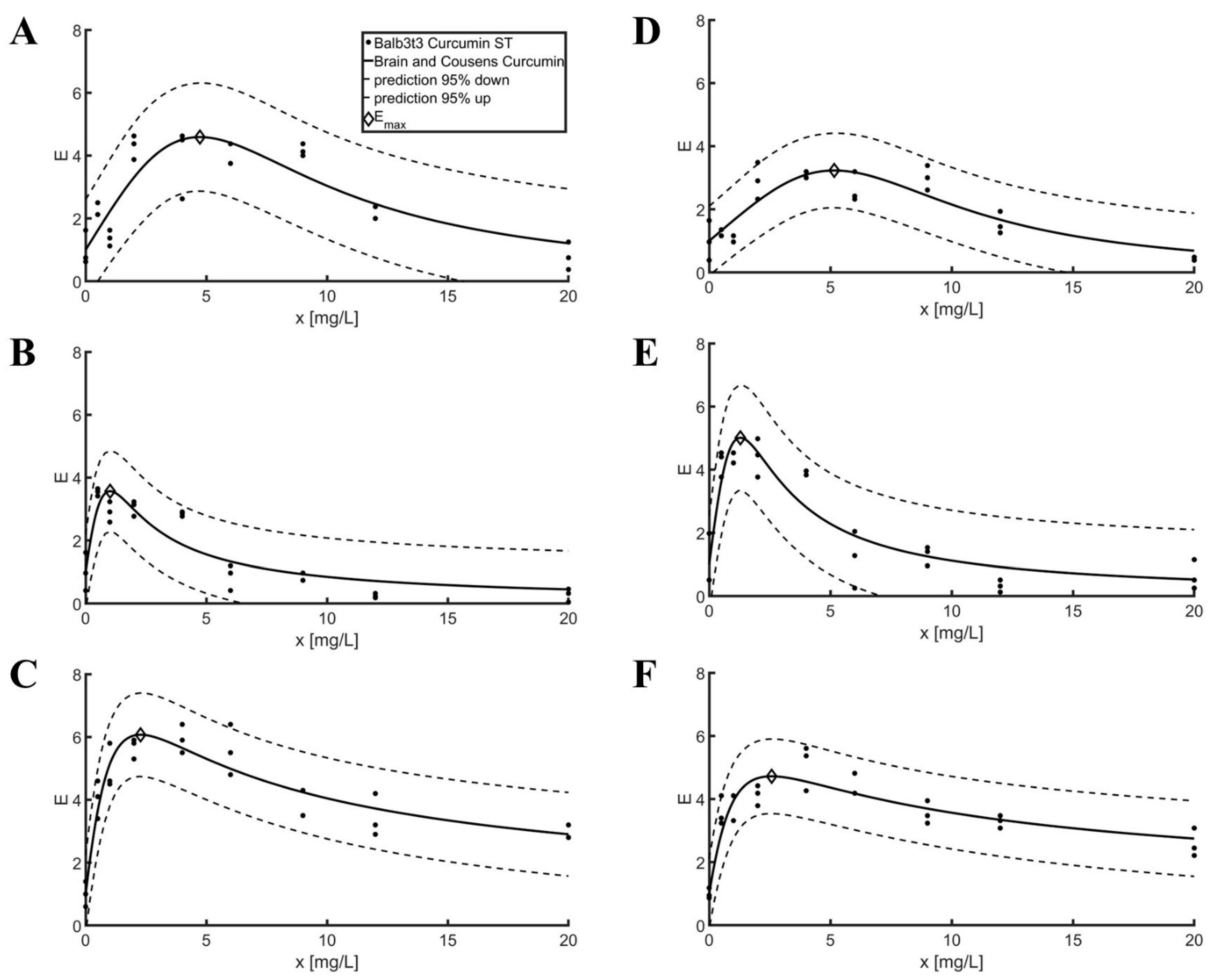
2.2. Model Fit for Scratch Assay Tests
3. Discussion
3.1. Model Fit and Statistical Analysis for MTT Test Results
3.2. Model Fit for Scratch Assay Tests
4. Materials and Methods
4.1. Bioflavonoids
4.2. Cell Lines
4.2.1. L929
4.2.2. Balb3t3
4.3. Cell Viability
4.4. Scratch Assay
4.5. Model Fit and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Beers, E.H. Palliative Wound Care: Less Is More. Surg. Clin. N. Am. 2019, 99, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes: Cellular Mechanisms of Wound Repair. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Kuhnke, J.L.; Keast, D.; Rosenthal, S.; Evans, R.J. Health Professionals’ Perspectives on Delivering Patient-Focused Wound Management: A Qualitative Study. J. Wound Care 2019, 28, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Atkin, L. Chronic Wounds: The Challenges of Appropriate Management. Br. J. Community Nurs. 2019, 24, S26–S32. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in Wound Healing: An Updated Review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and Its Topical Formulations for Wound Healing Applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and Anti-Inflammatory Potential of Curcumin Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin-Induced Angiogenesis Hastens Wound Healing in Diabetic Rats. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.K.; Irkorucu, O.; Ucan, B.H.; Tascilar, O.; Emre, A.U.; Karakaya, K.; Bahadir, B.; Acikgoz, S.; Pasaoglu, H.; Ankarali, H.; et al. The Effects of Resveratrol on the Healing of Left Colonic Anastomosis. J. Investig. Surg. 2009, 22, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Günal, M.Y.; Ayla, Ş.; Bedri, N.; Beker, M.Ç.; Çağlayan, A.B.; Aslan, İ.; Özdemir, E.M.; Yeşilada, E.; Kılıç, Ü. The Effects of Topical Liposomal Resveratrol on Incisional and Excisional Wound Healing Process. Turkderm. Turk. Arch. Dermatol. Venereol. 2019, 53, 128–134. [Google Scholar] [CrossRef]
- Brâkenhielm, E.; Cao, R.; Cao, Y. Suppression of Angiogenesis, Tumor Growth, and Wound Healing by Resveratrol, a Natural Compound in Red Wine and Grapes. FASEB J. 2001, 15, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y.; et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FoxO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharm. 2019, 10, 421. [Google Scholar] [CrossRef]
- Yurdagul, A.; Kleinedler, J.J.; McInnis, M.C.; Khandelwal, A.R.; Spence, A.L.; Orr, A.W.; Dugas, T.R. Resveratrol Promotes Endothelial Cell Wound Healing under Laminar Shear Stress through an Estrogen Receptor-α-Dependent Pathway. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, 797–806. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.P.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef]
- Pignet, A.L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A Randomized Exploratory Trial to Assess the Effects of Resveratrol on VEGF and TNF-α 2 Expression in Endometriosis Women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef] [PubMed]
- Moore, O.A. The Extraction, Anticancer Effect, Bioavailability, and Nanotechnology of Baicalin. J. Nutr. Med. Diet. Care 2016, 2, 1–12. [Google Scholar] [CrossRef]
- You, J.; Cheng, J.; Yu, B.; Duan, C.; Peng, J. Baicalin, a Chinese Herbal Medicine, Inhibits the Proliferation and Migration of Human Non-Small Cell Lung Carcinoma (NSCLC) Cells, A549 and H1299, by Activating the SIRT1/AMPK Signaling Pathway. Med. Sci. Monit. 2018, 24, 2126–2133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lu, J.; Mori, T.; Smith-Powell, L.; Synold, T.W.; Chen, S.; Wen, W. Baicalin Increases VEGF Expression and Angiogenesis by Activating the ERRα/PGC-1α Pathway. Cardiovasc. Res. 2011, 89, 426–435. [Google Scholar] [CrossRef]
- Alshehabat, M.; Hananeh, W.; Ismail, Z.B.; Rmilah, S.A.; Abeeleh, M.A. Wound Healing in Immunocompromised Dogs: A Comparison between the Healing Effects of Moist Exposed Burn Ointment and Honey. Vet. World 2020, 13, 2793–2797. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Li, Z.; Li, B.; Wang, H. Baicalin Regulates MRNA Expression of VEGF-c, Ang-1/Tie2, TGF-β and Smad2/3 to Inhibit Wound Healing in Streptozotocin-Induced Diabetic Foot Ulcer Rats. J. Biochem. Mol. Toxicol. 2021, 35, e22893. [Google Scholar] [CrossRef]
- ISO 10993:5; Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Alqahtani, M.S.; Alqahtani, A.; Kazi, M.; Ahmad, M.Z.; Alahmari, A.; Alsenaidy, M.A.; Syed, R. Wound-Healing Potential of Curcumin Loaded Lignin Nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102020. [Google Scholar] [CrossRef]
- Celen, C.; Keçeciler, C.; Yapar, E.A.; Gökçe, E.H.; Nalbantsoy, A. Evaluation of Resveratrol Organogels Prepared by Micro-Irradiation: Fibroblast Proliferation through in Vitro Wound Healing. Turk. J. Biochem. 2018, 43, 385–392. [Google Scholar] [CrossRef]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin Upregulates Transforming Growth Factor-Β1, Its Receptors, and Vascular Endothelial Growth Factor Expressions in an in Vitro Human Gingival Fibroblast Wound Healing Model. BMC Oral Health 2021, 21, 535. [Google Scholar] [CrossRef]
- Lantto, T.A.; Colucci, M.; Závadová, V.; Hiltunen, R.; Raasmaja, A. Cytotoxicity of Curcumin, Resveratrol and Plant Extracts from Basil, Juniper, Laurel and Parsley in SH-SY5Y and CV1-P Cells. Food Chem. 2009, 117, 405–411. [Google Scholar] [CrossRef]
- Eroğlu, I.; Gökçe, E.H.; Tsapis, N.; Tanriverdi, S.T.; Gökçe, G.; Fattal, E.; Özer, O. Evaluation of Characteristics and in Vitro Antioxidant Properties of RSV Loaded Hyaluronic Acid-DPPC Microparticles as a Wound Healing System. Colloids Surf. B Biointerfaces 2015, 126, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of Gellan-Cholesterol Nanohydrogels Embedding Baicalin and Evaluation of Their Wound Healing Activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, C.; Li, L.; Liu, J.; Gao, Y.; Mu, K.; Chen, D.; Lu, A.; Ren, Y.; Li, Z. Baicalin Alleviates Bleomycin-Induced Pulmonary Fibrosis and Fibroblast Proliferation in Rats via the PI3K/AKT Signaling Pathway. Mol. Med. Rep. 2020, 21, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kang, J.H.; Lee, S.A.; Park, I.H.; Lee, H.M. Baicalin Down-Regulates IL-1β-Stimulated Extracellular Matrix Production in Nasal Fibroblasts. PLoS ONE 2016, 11, e0168195. [Google Scholar] [CrossRef]
- Mirzahosseinipour, M.; Khorsandi, K.; Hosseinzadeh, R.; Ghazaeian, M.; Shahidi, F.K. Antimicrobial Photodynamic and Wound Healing Activity of Curcumin Encapsulated in Silica Nanoparticles. Photodiagnosis Photodyn. Ther. 2020, 29, 101639. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2019, 29, 1806883. [Google Scholar] [CrossRef]
- Qiu, W.; Han, H.; Li, M.; Li, N.; Wang, Q.; Qin, X.; Wang, X.; Yu, J.; Zhou, Y.; Li, Y.; et al. Nanofibers Reinforced Injectable Hydrogel with Self-Healing, Antibacterial, and Hemostatic Properties for Chronic Wound Healing. J. Colloid Interface Sci. 2021, 596, 312–323. [Google Scholar] [CrossRef]
- Dev, S.K.; Choudhury, P.K.; Srivastava, R.; Sharma, M. Antimicrobial, Anti-Inflammatory and Wound Healing Activity of Polyherbal Formulation. Biomed. Pharmacother. 2019, 111, 555–567. [Google Scholar] [CrossRef]
- Tewtrakul, S.; Tungcharoen, P.; Sudsai, T.; Karalai, C.; Ponglimanont, C.; Yodsaoue, O. Antiinflammatory and Wound Healing Effects of Caesalpinia sappan L. Phytother. Res. 2015, 29, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Lukiswanto, B.S.; Miranti, A.; Sudjarwo, S.A.; Primarizky, H.; Yuniarti, W.M. Evaluation of Wound Healing Potential of Pomegranate (Punica Granatum) Whole Fruit Extract on Skin Burn Wound in Rats (Rattus Norvegicus). J. Adv. Vet. Anim. Res. 2019, 6, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, Characterization and Pre-Clinical Trials of an Innovative Wound Healing Dressing Based on Propolis (EPP-AF®)-Containing Self-Microemulsifying Formulation Incorporated in Biocellulose Membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Ritz, C.; Streibig, J.C. Improved Empirical Models Describing Hormesis. Environ. Toxicol. Chem. 2005, 24, 3166–3172. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration in Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Brain, P.; Cousens, R. An Equation to Describe Dose Responses Where There Is Stimulation of Growth at Low Doses. Weed Res. 1989, 29, 93–96. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Stanek, E.J.; Nascarella, M.A. Evidence for Hormesis in Mutagenicity Dose-Response Relationships. Mutat. Res. 2011, 726, 91–97. [Google Scholar] [CrossRef]
| Parameters | Goodness of Fit | |||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | Substance | b | e | f | xop [mg/L] | Emax | R2 | RMSE |
| Balb3t | Curcumin | 1.06 | 0.61 | 1.85 | 0.59 | 1.06 | 0.12 | 0.13 |
| Resveratrol | 1.57 | 39.07 | 0.0042 | 0.76 | 1.00 | 0.25 | 0.15 | |
| Baicalin | 1.09 | 0.74 | 1.59 | 0.67 | 1.09 | 0.12 | 0.20 | |
| L929 | Curcumin | 1.66 | 6.24 | 0.25 | 3.55 | 1.35 | 0.27 | 0.34 |
| Resveratrol | 3.53 | 38.98 | 0.00032 | 4.15 | 1.00 | 0.06 | 0.19 | |
| Baicalin | 1.12 | 1.00 | 1.16 | 0.67 | 1.08 | 0.31 | 0.15 | |
| Cell Line | Substance | Dose [mg/L] | p-Value | Hypothesis |
|---|---|---|---|---|
| Balb3t3 | Curcumin | 0.5 | 0.073 | 0 |
| 1 | 0.102 | 0 | ||
| Resveratrol | 0.5 | 0.890 | 0 | |
| 1 | 0.921 | 0 | ||
| Baicalin | 0.5 | 0.356 | 0 | |
| 1 | 0.002 | 1 | ||
| L929 | Curcumin | 3 | Not tested | |
| 6 | 0.009 | 1 | ||
| Resveratrol | 3 | 0.708 | 0 | |
| 6 | 0.749 | 0 | ||
| Baicalin | 0.5 | Not tested | ||
| 1 | 0.117 | 0 | ||
| Parameters | Goodness of Fit | |||||||
|---|---|---|---|---|---|---|---|---|
| Cell Line | Substance | b | e | f | xop | Emax | R2 | RMSE |
| Balb3t3 | Curcumin | 2.57 | 6.21 | 1.24 | 4.73 | 4.59 | 0.74 | 0.78 |
| Resveratrol | 1.91 | 1.15 | 5.31 | 1.01 | 3.56 | 0.82 | 0.57 | |
| Baicalin | 1.53 | 1.69 | 6.44 | 2.28 | 6.07 | 0.87 | 0.60 | |
| L929 | Curcumin | 2.96 | 7.34 | 0.65 | 5.16 | 3.23 | 0.76 | 0.53 |
| Resveratrol | 2.09 | 1.49 | 5.98 | 1.29 | 5.01 | 0.84 | 0.74 | |
| Baicalin | 1.43 | 1.68 | 4.82 | 2.57 | 4.72 | 0.81 | 0.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagiełło, K.; Uchańska, O.; Matyja, K.; Jackowski, M.; Wiatrak, B.; Kubasiewicz-Ross, P.; Karuga-Kuźniewska, E. Supporting the Wound Healing Process—Curcumin, Resveratrol and Baicalin in In Vitro Wound Healing Studies. Pharmaceuticals 2023, 16, 82. https://doi.org/10.3390/ph16010082
Jagiełło K, Uchańska O, Matyja K, Jackowski M, Wiatrak B, Kubasiewicz-Ross P, Karuga-Kuźniewska E. Supporting the Wound Healing Process—Curcumin, Resveratrol and Baicalin in In Vitro Wound Healing Studies. Pharmaceuticals. 2023; 16(1):82. https://doi.org/10.3390/ph16010082
Chicago/Turabian StyleJagiełło, Kacper, Oliwia Uchańska, Konrad Matyja, Mateusz Jackowski, Benita Wiatrak, Paweł Kubasiewicz-Ross, and Ewa Karuga-Kuźniewska. 2023. "Supporting the Wound Healing Process—Curcumin, Resveratrol and Baicalin in In Vitro Wound Healing Studies" Pharmaceuticals 16, no. 1: 82. https://doi.org/10.3390/ph16010082
APA StyleJagiełło, K., Uchańska, O., Matyja, K., Jackowski, M., Wiatrak, B., Kubasiewicz-Ross, P., & Karuga-Kuźniewska, E. (2023). Supporting the Wound Healing Process—Curcumin, Resveratrol and Baicalin in In Vitro Wound Healing Studies. Pharmaceuticals, 16(1), 82. https://doi.org/10.3390/ph16010082










