Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs
Abstract
1. Introduction
2. Results
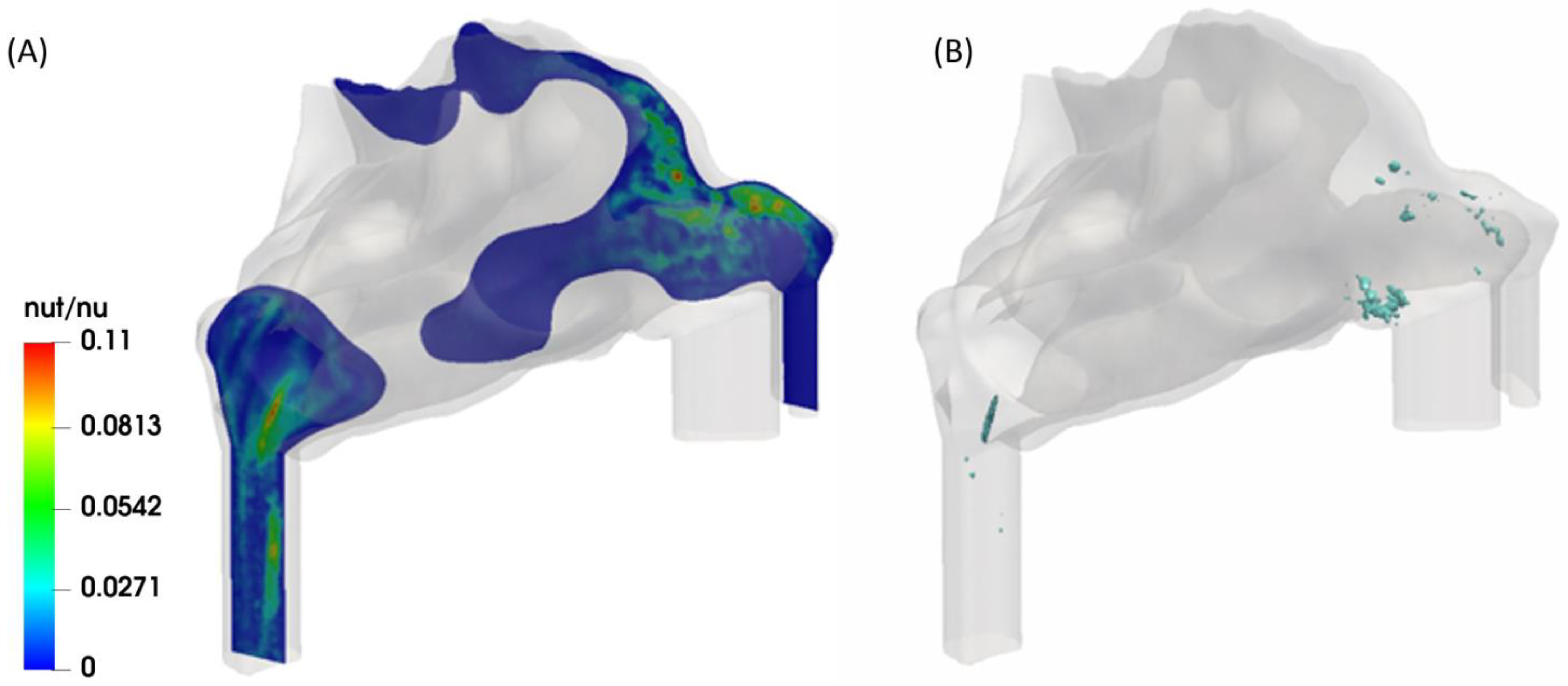
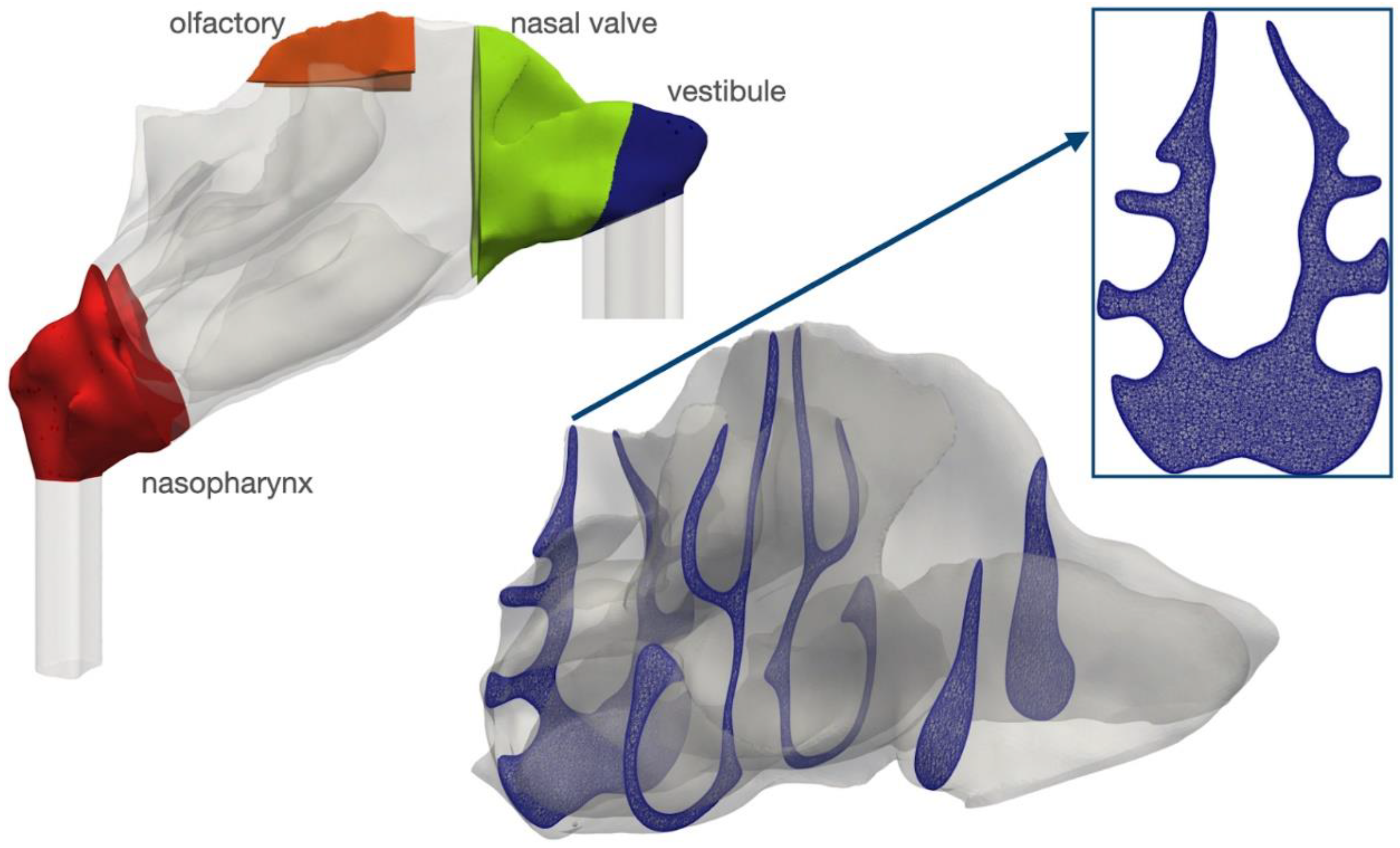
2.1. Mesh Quality
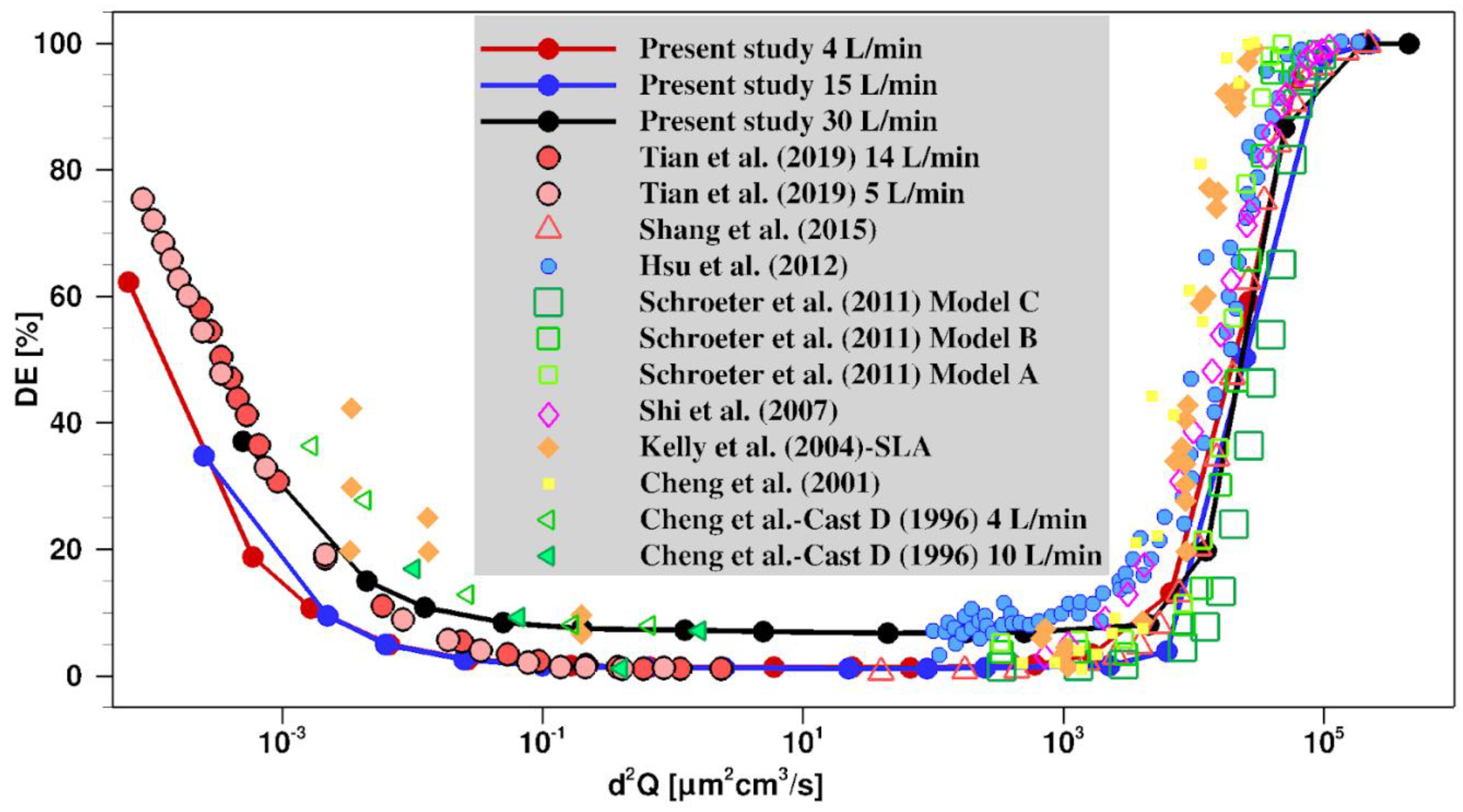
2.2. Validation of the Model
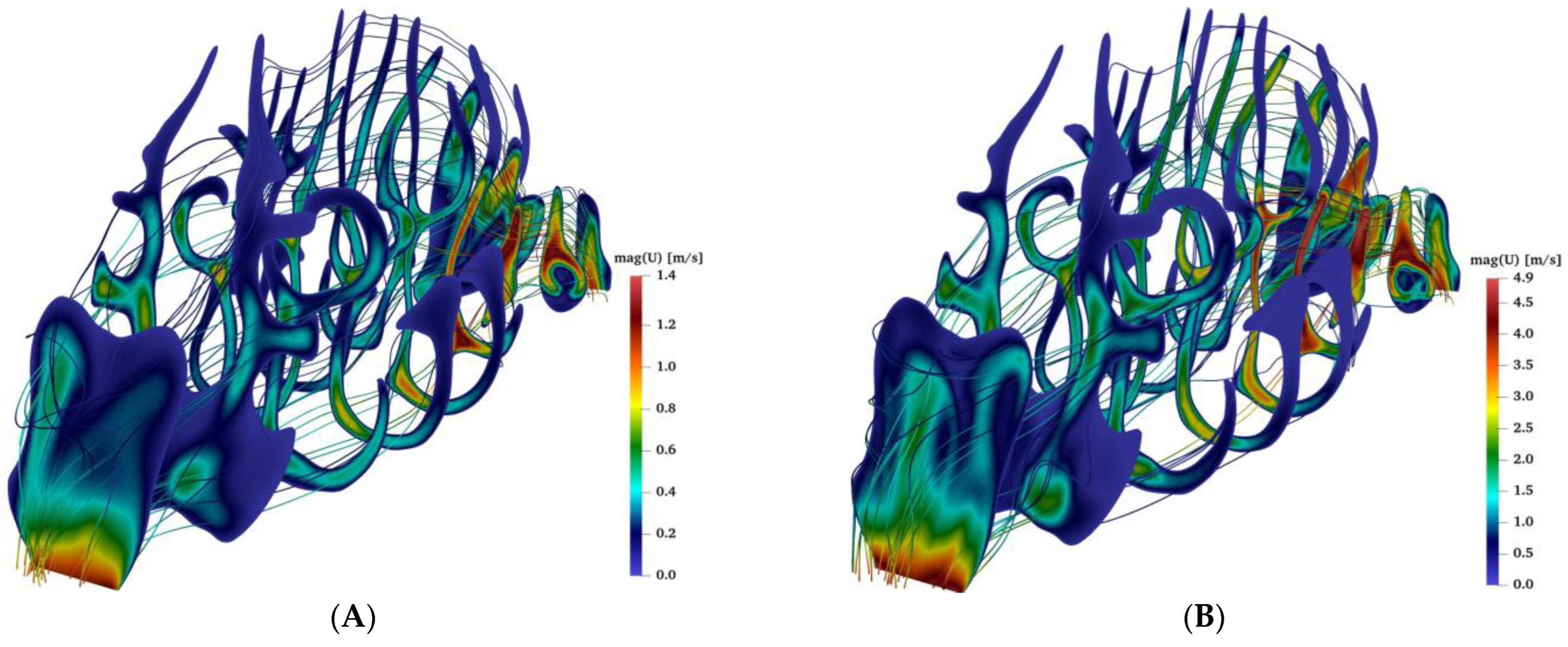
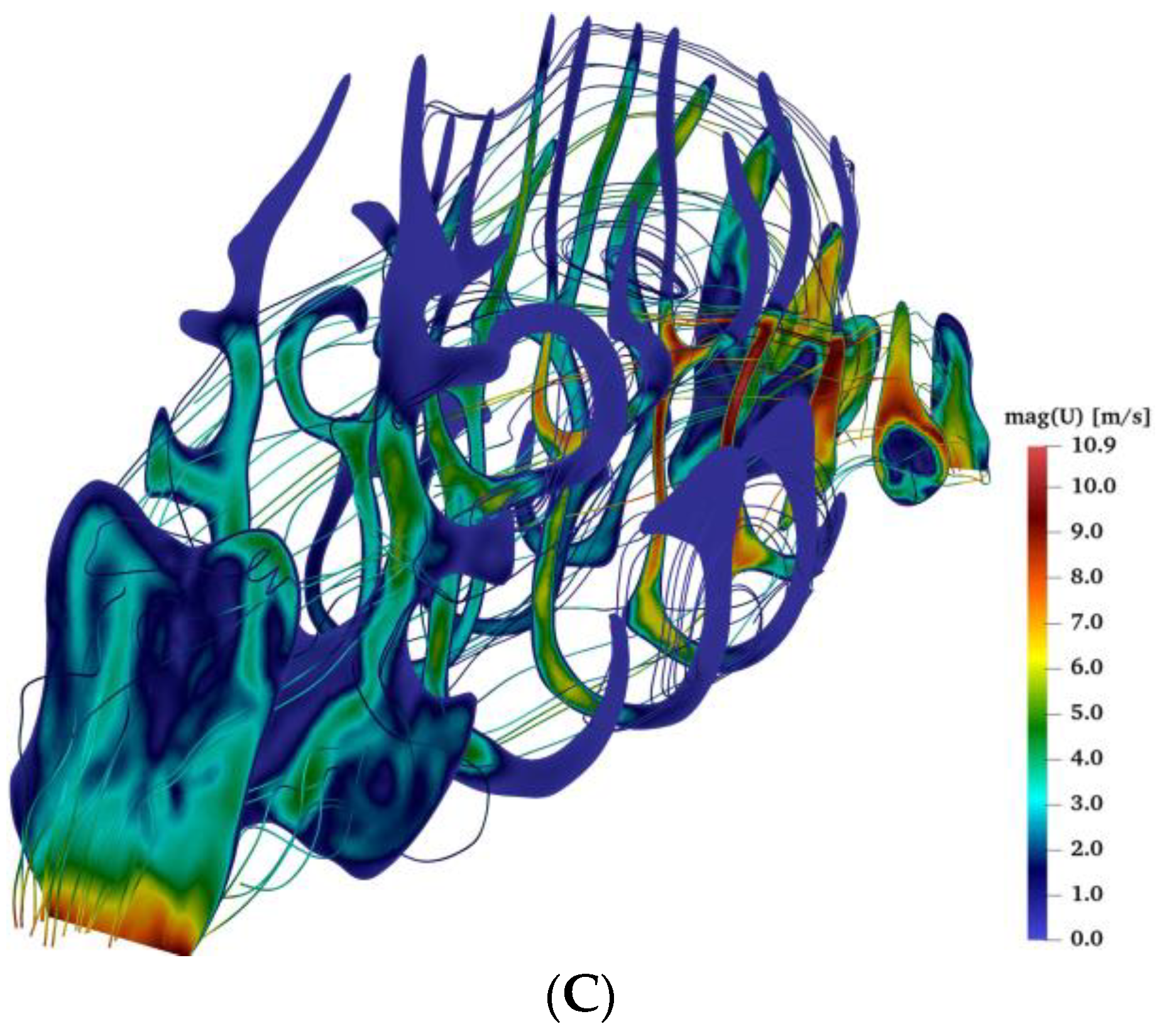
2.3. Airflow during Bilateral Aerosol Delivery
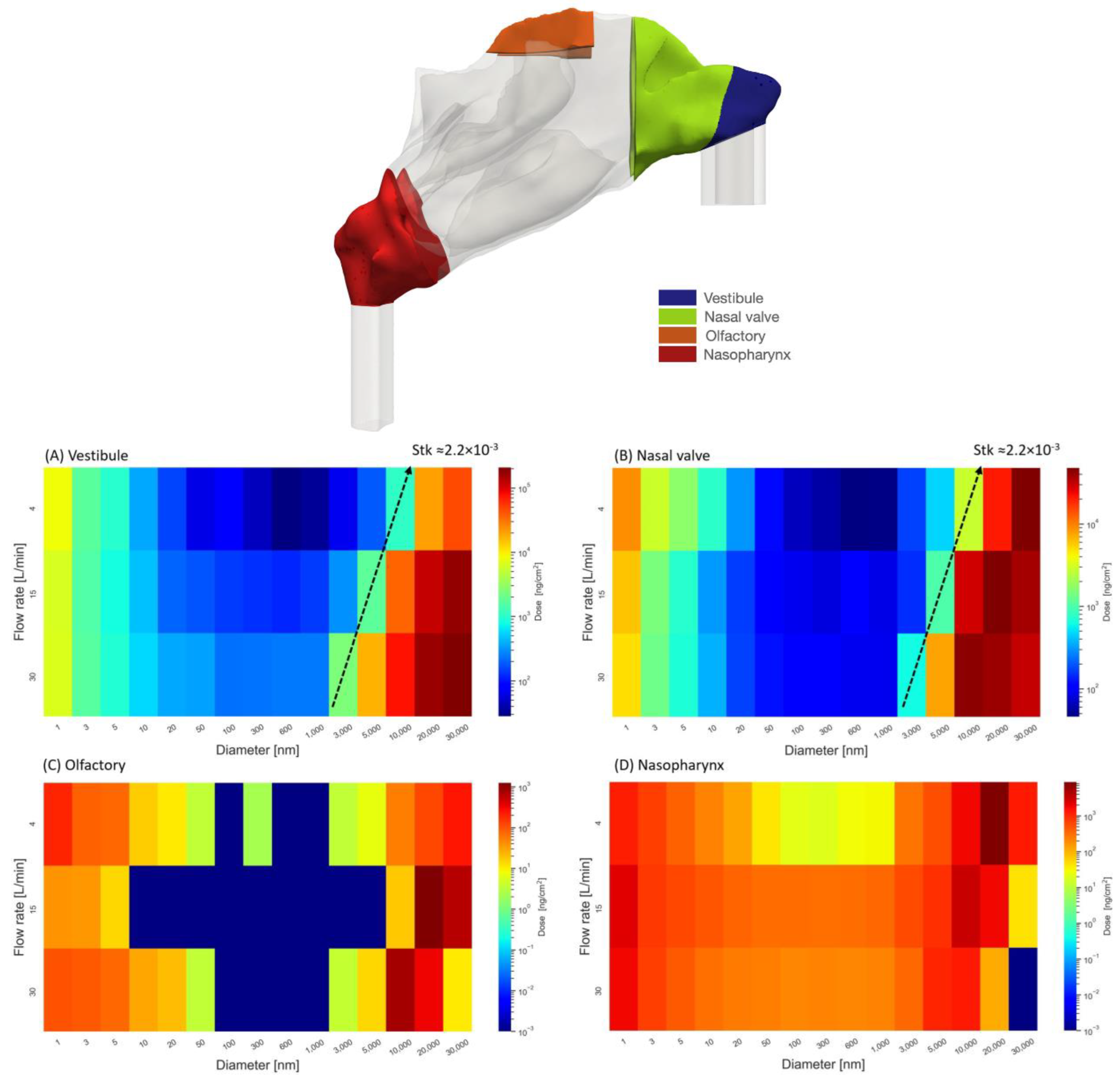
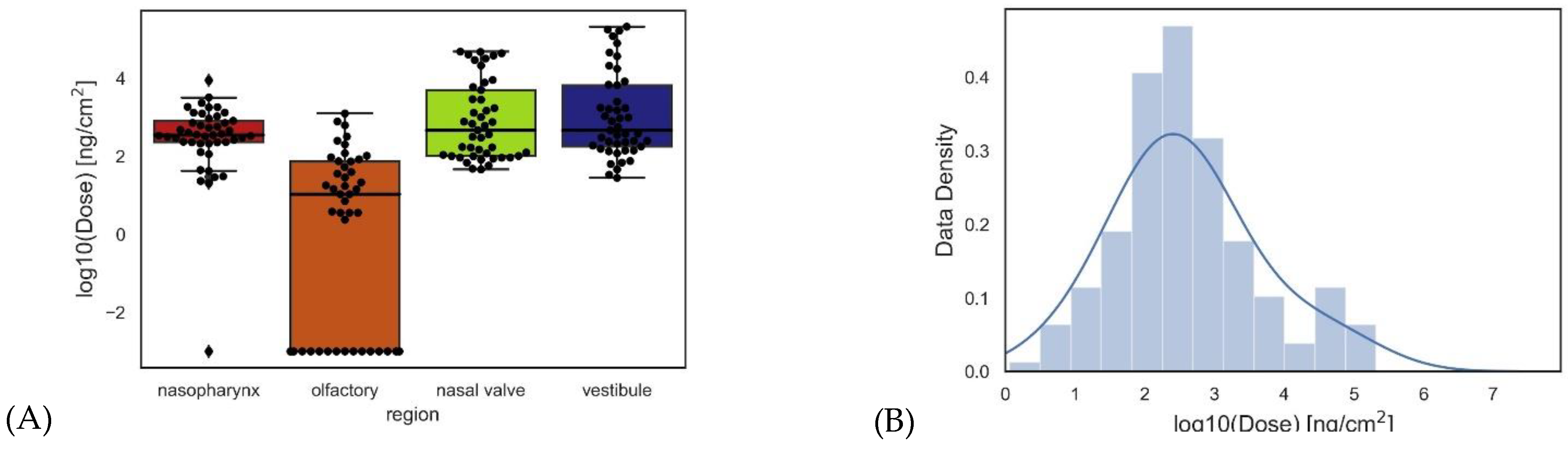
2.4. Regional Delivered Dose
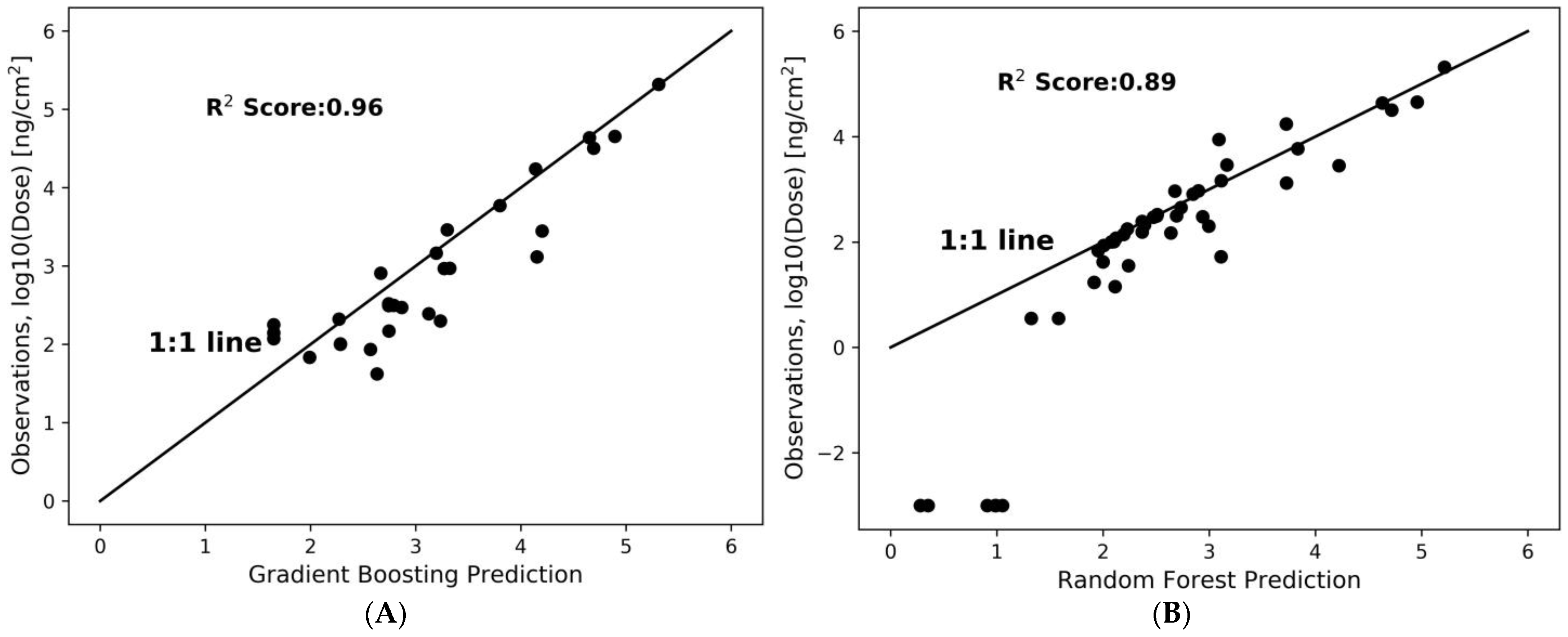
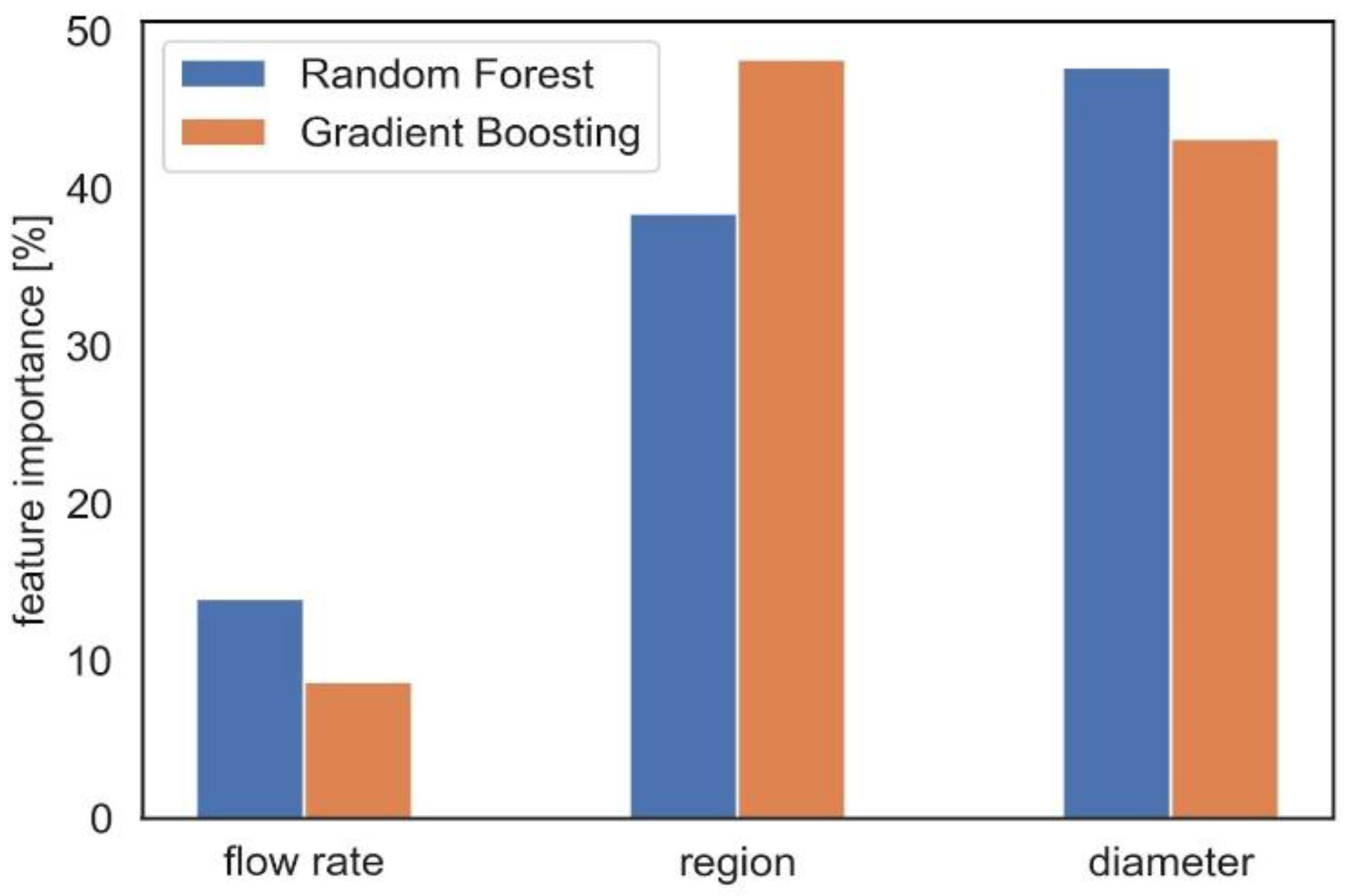
2.5. Dose Prediction Using Machine Learning
3. Discussion
4. Materials and Methods
4.1. Geometry
4.2. Numerical Modeling
5. Conclusions
6. Code Availability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suman, J.D. Current understanding of nasal morphology and physiology as a drug delivery target. Drug Deliv. Transl. Res. 2013, 3, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 12, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-S.; Yamada, Y.; Yeh, H.-C.; Swift, D.L. Diffusional deposition of ultrafine aerosols in a human nasal cast. J. Aerosol Sci. 1988, 19, 741–751. [Google Scholar] [CrossRef]
- Kelly, J.T.; Asgharian, B.; Kimbell, J.S.; Wong, B.A. Particle deposition in human nasal airway replicas manufactured by different methods. Part I: Inertial regime particles. Aerosol Sci. Technol. 2004, 38, 1063–1071. [Google Scholar] [CrossRef]
- Kelly, J.T.; Asgharian, B.; Kimbell, J.S.; Wong, B.A. Particle deposition in human nasal airway replicas manufactured by different methods. Part II: Ultrafine particles. Aerosol Sci. Technol. 2004, 38, 1072–1079. [Google Scholar] [CrossRef]
- Liu, Y.; Matida, E.A.; Johnson, M.R. Experimental measurements and computational modeling of aerosol deposition in the Carleton-Civic standardized human nasal cavity. J. Aerosol Sci. 2010, 41, 569–586. [Google Scholar] [CrossRef]
- Schroeter, J.D.; Tewksbury, E.W.; Wong, B.A.; Kimbell, J.S. Experimental measurements and computational predictions of regional particle deposition in a sectional nasal model. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 20–29. [Google Scholar] [CrossRef]
- Basu, S.; Holbrook, L.T.; Kudlaty, K.; Fasanmade, O.; Wu, J.; Burke, A.; Langworthy, B.W.; Farzal, Z.; Mamdani, M.; Bennett, W.D.; et al. Numerical evaluation of spray position for improved nasal drug delivery. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Pourmehran, O.; Arjomandi, M.; Cazzolato, B.; Tian, Z.; Vreugde, S.; Javadiyan, S.; Psaltis, A.J.; Wormald, P.-J. Acoustic drug delivery to the maxillary sinus. Int. J. Pharm. 2021, 606, 120927. [Google Scholar] [CrossRef]
- Pourmehran, O.; Cazzolato, B.; Tian, Z.; Arjomandi, M. Acoustically-driven drug delivery to maxillary sinuses: Aero-acoustic analysis. Eur. J. Pharm. Sci. 2020, 151, 105398. [Google Scholar] [CrossRef]
- Farnoud, A.; Baumann, I.; Rashidi, M.M.; Schmid, O.; Gutheil, E. Simulation of patient-specific bi-directional pulsating nasal aerosol dispersion and deposition with clockwise 45° and 90° nosepieces. Comput. Biol. Med. 2020, 123, 103816. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.; Tofighian, H.; Baumann, I.; Garcia, G.J.M.; Schmid, O.; Gutheil, E.; Rashidi, M.M. Large eddy simulations of airflow and particle deposition in pulsating bi-directional nasal drug delivery. Phys. Fluids 2020, 32, 101905. [Google Scholar] [CrossRef]
- Taheri, M.H.; Pourmehran, O.; Sarafraz, M.M.; Ahookhosh, K.; Farnoud, A.; Cui, X. Effect of swirling flow and particle-release pattern on drug delivery to human tracheobronchial airways. Biomech. Model. Mechanobiol. 2021, 20, 2451–2469. [Google Scholar] [CrossRef]
- Tian, L.; Shang, Y.; Chen, R.; Bai, R.; Chen, C.; Inthavong, K.; Tu, J. Correlation of regional deposition dosage for inhaled nanoparticles in human and rat olfactory. Part. Fibre Toxicol. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Farnoud, A.; Tofighian, H.; Baumann, I.; Martin, A.R.; Rashidi, M.M.; Menden, M.P.; Schmid, O. Pulsatile bi-directional aerosol flow affects aerosol delivery to the intranasal olfactory region: A patient-specific computational study. Front. Pharmacol. 2021, 12, 746420. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Z.; Si, X.A.; Zhou, Y. Nasal dilation effects on olfactory deposition in unilateral and bi-directional deliveries: In vitro tests and numerical modeling. Eur. J. Pharm. Sci. 2018, 118, 113–123. [Google Scholar] [CrossRef]
- Biglarian, M.; MomeniLarimi, M.; Firoozabadi, B.; Inthavong, K.; Farnoud, A. Targeted drug delivery with polydisperse particle transport and deposition in patient-specific upper airway during inhalation and exhalation. Respir. Physiol. Neurobiol. 2023, 308, 103986. [Google Scholar] [CrossRef]
- Farnoud, A.; Cui, X.; Baumann, I.; Gutheil, E. Numerical simulation of the dispersion and deposition of a spray carried by a pulsating airflow in a patient-specific human nasal cavity. At. Sprays 2017, 27, 913–928. [Google Scholar] [CrossRef]
- Leclerc, L.; Merhie, A.E.; Navarro, L.; Prévôt, N.; Durand, M.; Pourchez, J. Impact of acoustic airflow on intrasinus drug deposition: New insights into the vibrating mode and the optimal acoustic frequency to enhance the delivery of nebulized antibiotic. Int. J. Pharm. 2015, 494, 227–234. [Google Scholar] [CrossRef]
- Shi, H.; Kleinstreuer, C.; Zhang, Z. Dilute suspension flow with nanoparticle deposition in a representative nasal airway model. Phys. Fluids 2008, 20, 013301. [Google Scholar] [CrossRef]
- Inthavong, K.; Zhang, K.; Tu, J. Numerical modelling of nanoparticle deposition in the nasal cavity and the tracheobronchial airway. Comput. Methods Biomech. Biomed. Engin. 2011, 14, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Dong, J. From the Cover: Comparative Numerical Modeling of Inhaled Nanoparticle Deposition in Human and Rat Nasal Cavities. Toxicol. Sci. 2016, 152, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, A.; Ahookhosh, K.; Abdollahi, H.; Taheri, M.H.; Cui, X.; Nabaei, M.; Farnoud, A. Effect of laryngeal jet on dry powder inhaler aerosol deposition: A numerical simulation. Comput. Methods Biomech. Biomed. Engin. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ahookhosh, K.; Saidi, M.; Mohammadpourfard, M.; Aminfar, H.; Hamishehkar, H.; Farnoud, A.; Schmid, O. Flow Structure and Particle Deposition Analyses for Optimization of a Pressurized Metered Dose Inhaler (pMDI) in a Model of Tracheobronchial Airway. Eur. J. Pharm. Sci. 2021, 164, 105911. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, H.; Babamiri, A.; Ahookhosh, K.; Farnoud, A.; Nabaei, M. Effects of inhalation flow rate on particle deposition and flow structure in a model of tracheobronchial airway. In Proceedings of the 2021 28th National and 6th International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 25–26 November 2021; pp. 101–106. [Google Scholar]
- Garcia, G.J.M.; Schroeter, J.D.; Kimbell, J.S. Olfactory deposition of inhaled nanoparticles in humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- Inthavong, K.; Ma, J.; Shang, Y.; Dong, J.; Chetty, A.S.; Tu, J.; Frank-Ito, D. Geometry and airflow dynamics analysis in the nasal cavity during inhalation. Clin. Biomech. 2019, 66, 97–106. [Google Scholar] [CrossRef]
- Kiaee, M.; Wachtel, H.; Noga, M.L.; Martin, A.R.; Finlay, W.H. Regional deposition of nasal sprays in adults: A wide ranging computational study. Int. J. Numer. Method. Biomed. Eng. 2018, 34, e2968. [Google Scholar] [CrossRef]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef]
- Cosnier, F.; Seidel, C.; Valentino, S.; Schmid, O.; Bau, S.; Vogel, U.; Devoy, J.; Gaté, L. Retained particle surface area dose drives inflammation in rat lungs following acute, subacute, and subchronic inhalation of nanomaterials. Part. Fibre Toxicol. 2021, 18, 29. [Google Scholar] [CrossRef]
- Germano, M.; Piomelli, U.; Moin, P.; Cabot, W.H. A dynamic subgrid-scale eddy viscosity model. Phys. Fluids A Fluid Dyn. 1991, 3, 1760–1765. [Google Scholar] [CrossRef]
- Pelmard, J.; Norris, S.; Friedrich, H. LES grid resolution requirements for the modelling of gravity currents. Comput. Fluids 2018, 174, 256–270. [Google Scholar] [CrossRef]
- Shang, Y.D.; Inthavong, K.; Tu, J.Y. Detailed micro-particle deposition patterns in the human nasal cavity influenced by the breathing zone. Comput. Fluids 2015, 114, 141–150. [Google Scholar] [CrossRef]
- Hsu, D.-J.; Chuang, M.-H. In-vivo measurements of micrometer-sized particle deposition in the nasal cavities of Taiwanese adults. Aerosol Sci. Technol. 2012, 46, 631–638. [Google Scholar] [CrossRef]
- Schroeter, J.D.; Garcia, G.J.M.; Kimbell, J.S. Effects of surface smoothness on inertial particle deposition in human nasal models. J. Aerosol Sci. 2011, 42, 52–63. [Google Scholar] [CrossRef]
- Shi, H.; Kleinstreuer, C.; Zhang, Z. Modeling of inertial particle transport and deposition in human nasal cavities with wall roughness. J. Aerosol Sci. 2007, 38, 398–419. [Google Scholar] [CrossRef]
- Cheng, Y.; Holmes, T.; Gao, J.; Guilmette, R.; Li, S.; Surakitbanharn, Y.; Rowlings, C. Characterization of nasal spray pumps and deposition pattern in a replica of the human nasal airway. J. Aerosol Med. 2001, 14, 267–280. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yeh, H.C.; Guilmette, R.A.; Simpson, S.Q.; Cheng, K.H.; Swift, D.L. Nasal Deposition of Ultrafine Particles in Human Volunteers and Its Relationship to Airway Geometry. Aerosol Sci. Technol. 1996, 25, 274–291. [Google Scholar] [CrossRef]
- Lenz, A.-G.; Stoeger, T.; Cei, D.; Schmidmeir, M.; Semren, N.; Burgstaller, G.; Lentner, B.; Eickelberg, O.; Meiners, S.; Schmid, O. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air-liquid interface conditions. Am. J. Respir. Cell Mol. Biol. 2014, 51, 526–535. [Google Scholar] [CrossRef]
- Schmid, O.; Bolle, I.; Harder, V.; Karg, E.; Takenaka, S.; Schulz, H.; Ferron, G.A. Model for the deposition of aerosol particles in the respiratory tract of the rat. I. Nonhygroscopic particle deposition. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 291–307. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Capecelatro, J.; Longest, W.; Boerman, C.; Sulaiman, M.; Sundaresan, S. Recent developments in the computational simulation of dry powder inhalers. Adv. Drug Deliv. Rev. 2022, 188, 114461. [Google Scholar] [CrossRef] [PubMed]
- Moukalled, F.; Mangani, L.; Darwish, M. The Finite Volume Method in Computational Fluid Dynamics: An Advanced Introduction with OpenFOAM® and Matlab; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Sweby, P.K. High resolution schemes using flux limiters for hyperbolic conservation laws. SIAM J. Numer. Anal. 1984, 21, 995–1011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farnoud, A.; Tofighian, H.; Baumann, I.; Ahookhosh, K.; Pourmehran, O.; Cui, X.; Heuveline, V.; Song, C.; Vreugde, S.; Wormald, P.-J.; et al. Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs. Pharmaceuticals 2023, 16, 81. https://doi.org/10.3390/ph16010081
Farnoud A, Tofighian H, Baumann I, Ahookhosh K, Pourmehran O, Cui X, Heuveline V, Song C, Vreugde S, Wormald P-J, et al. Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs. Pharmaceuticals. 2023; 16(1):81. https://doi.org/10.3390/ph16010081
Chicago/Turabian StyleFarnoud, Ali, Hesam Tofighian, Ingo Baumann, Kaveh Ahookhosh, Oveis Pourmehran, Xinguang Cui, Vincent Heuveline, Chen Song, Sarah Vreugde, Peter-John Wormald, and et al. 2023. "Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs" Pharmaceuticals 16, no. 1: 81. https://doi.org/10.3390/ph16010081
APA StyleFarnoud, A., Tofighian, H., Baumann, I., Ahookhosh, K., Pourmehran, O., Cui, X., Heuveline, V., Song, C., Vreugde, S., Wormald, P.-J., Menden, M. P., & Schmid, O. (2023). Numerical and Machine Learning Analysis of the Parameters Affecting the Regionally Delivered Nasal Dose of Nano- and Micro-Sized Aerosolized Drugs. Pharmaceuticals, 16(1), 81. https://doi.org/10.3390/ph16010081






