Formation and Characterisation of Posaconazole Hydrate Form
Abstract
1. Introduction
2. Results
2.1. Investigation of Posaconazole Form I Transformation
2.2. Investigation of the Mixing Method for the Production of Pure Posaconazole Form-S
2.3. Characterisation of Posaconazole Form-S
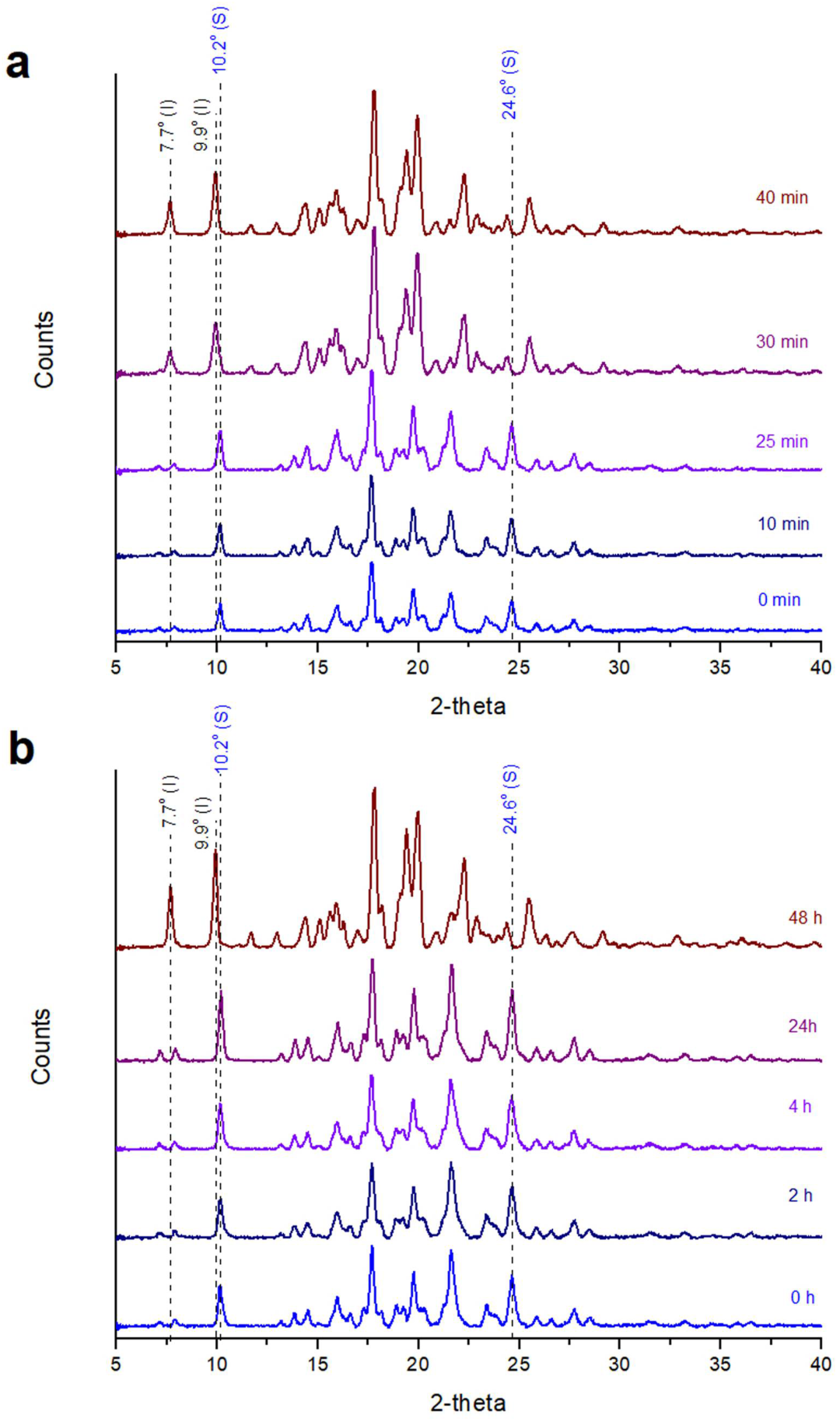
2.3.1. X-ray Powder Diffraction (XRPD)
Determination of the Crystallographic Data of Posaconazole Form-S through XRPD
Stability of Posaconazole Form-S through XRPD
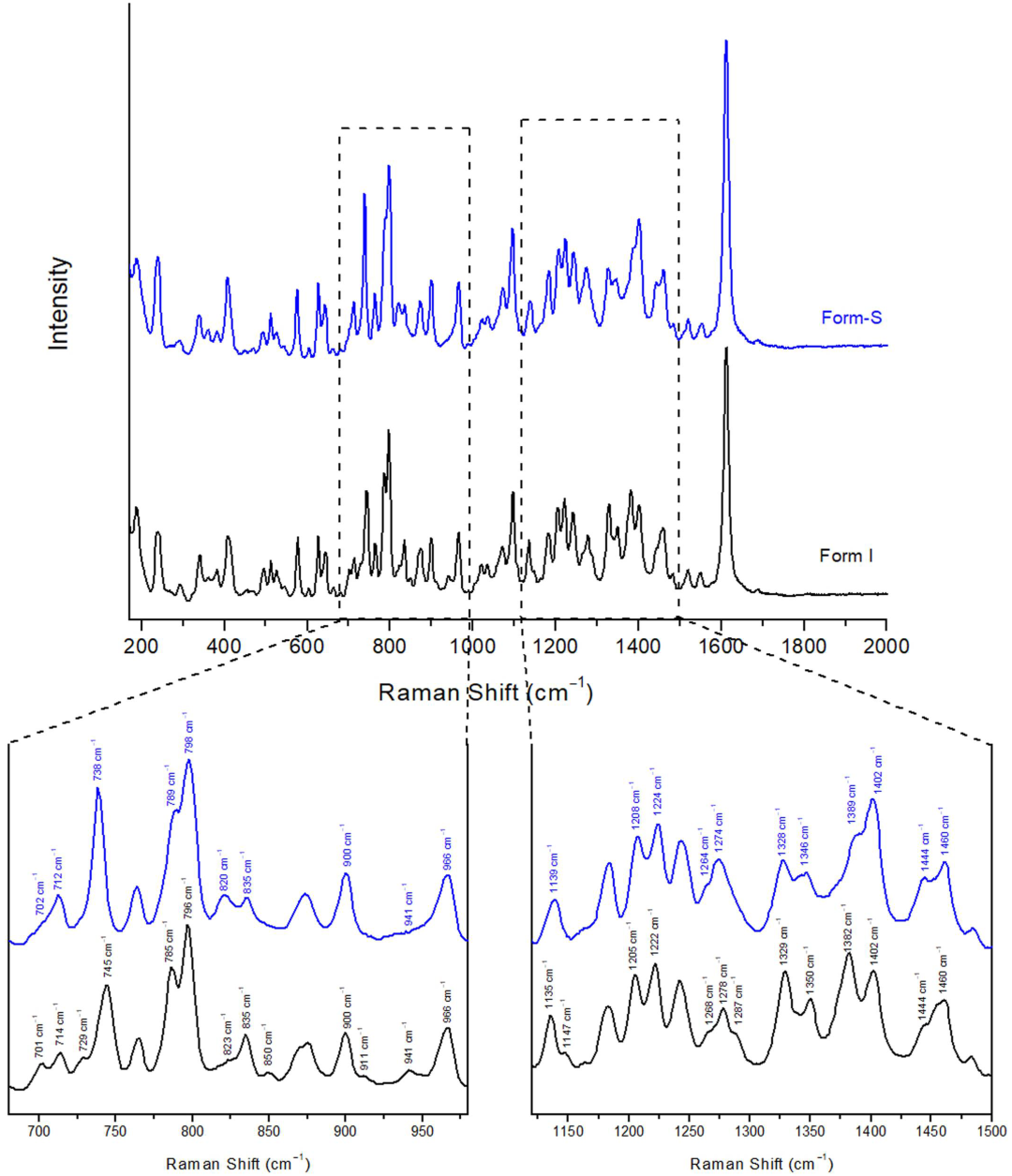
2.3.2. Raman Spectroscopy
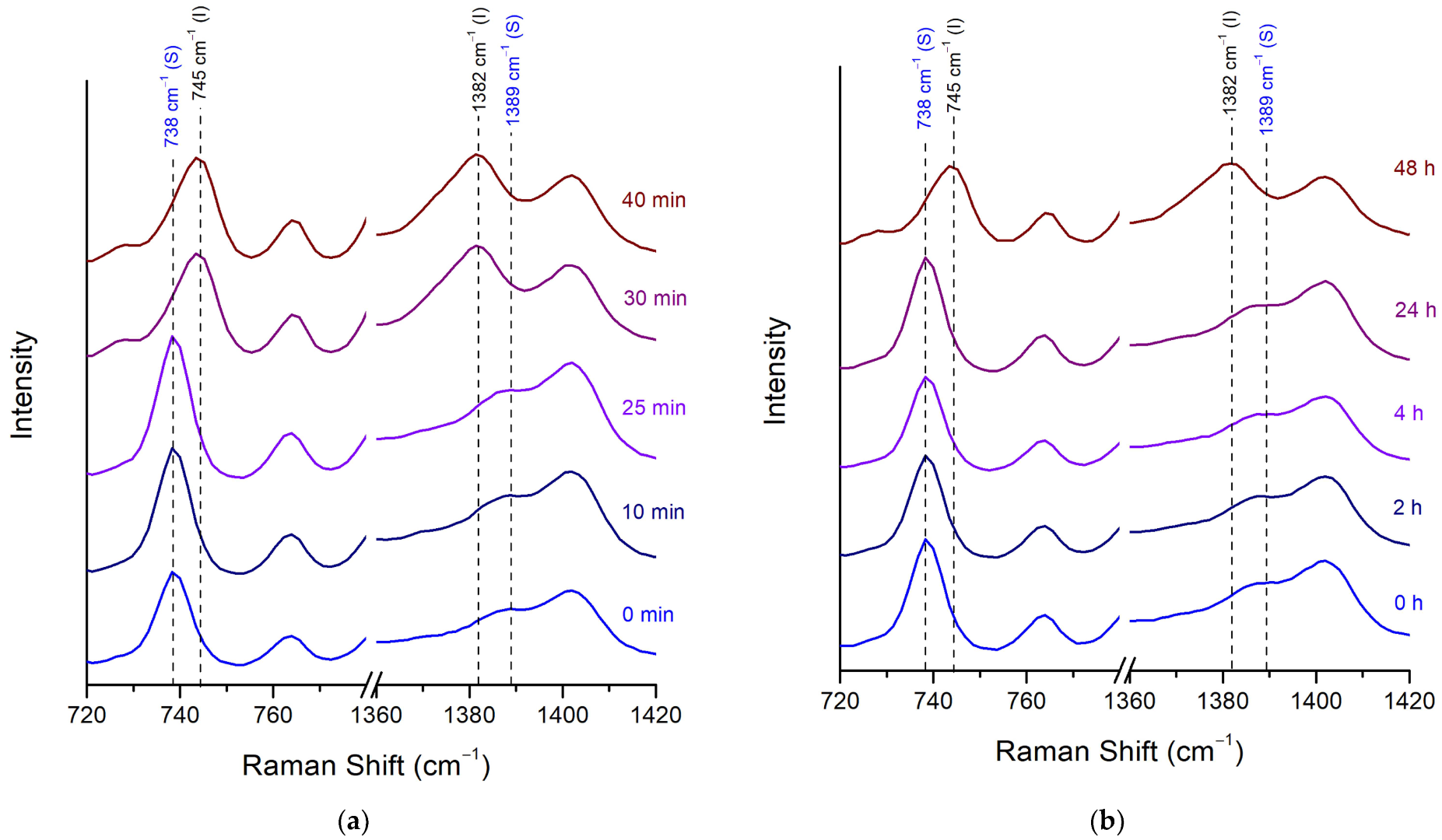
Stability of Posaconazole Form-S through Raman Spectroscopy
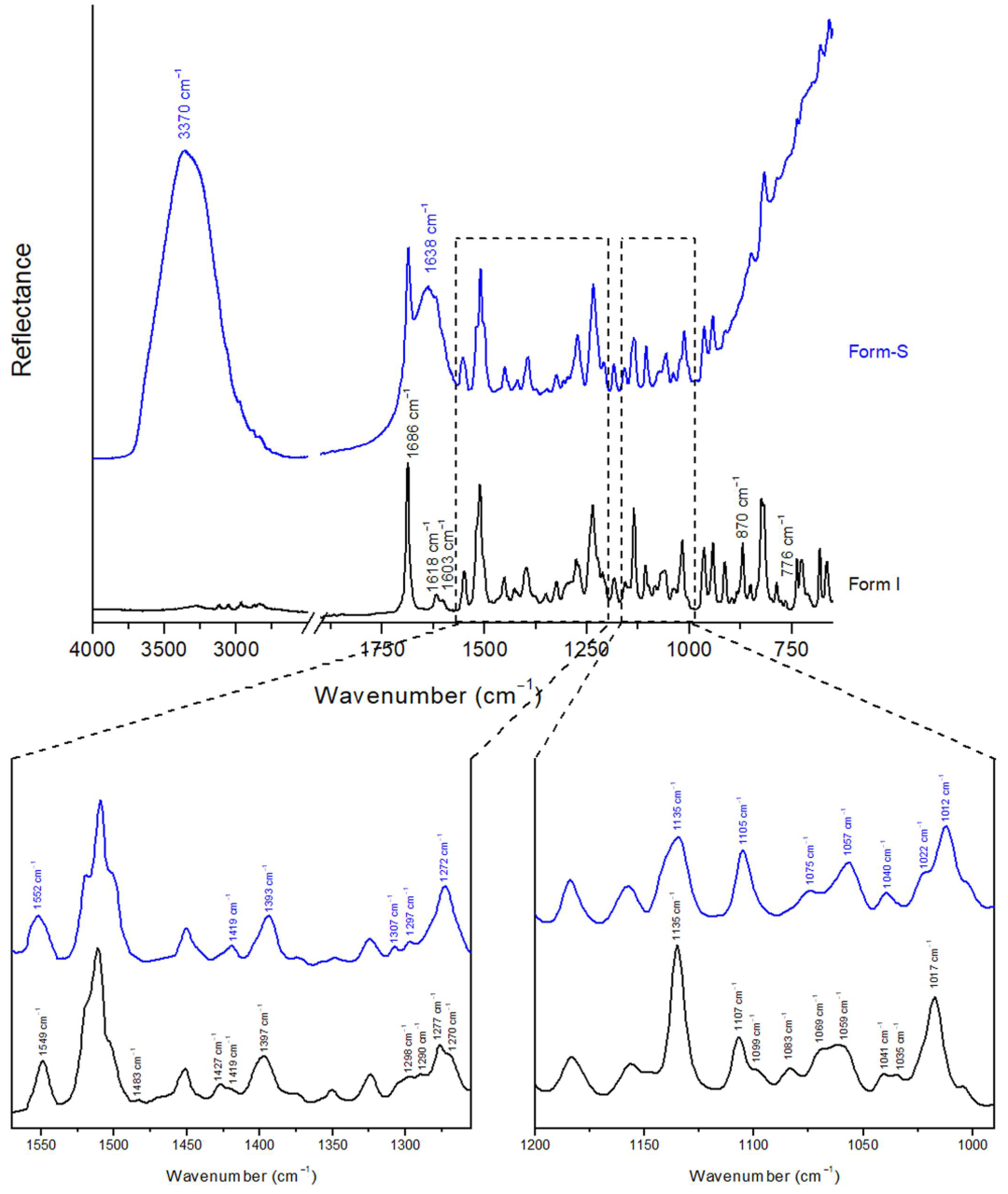
2.3.3. Attenuated Total Reflection (ATR) Spectroscopy
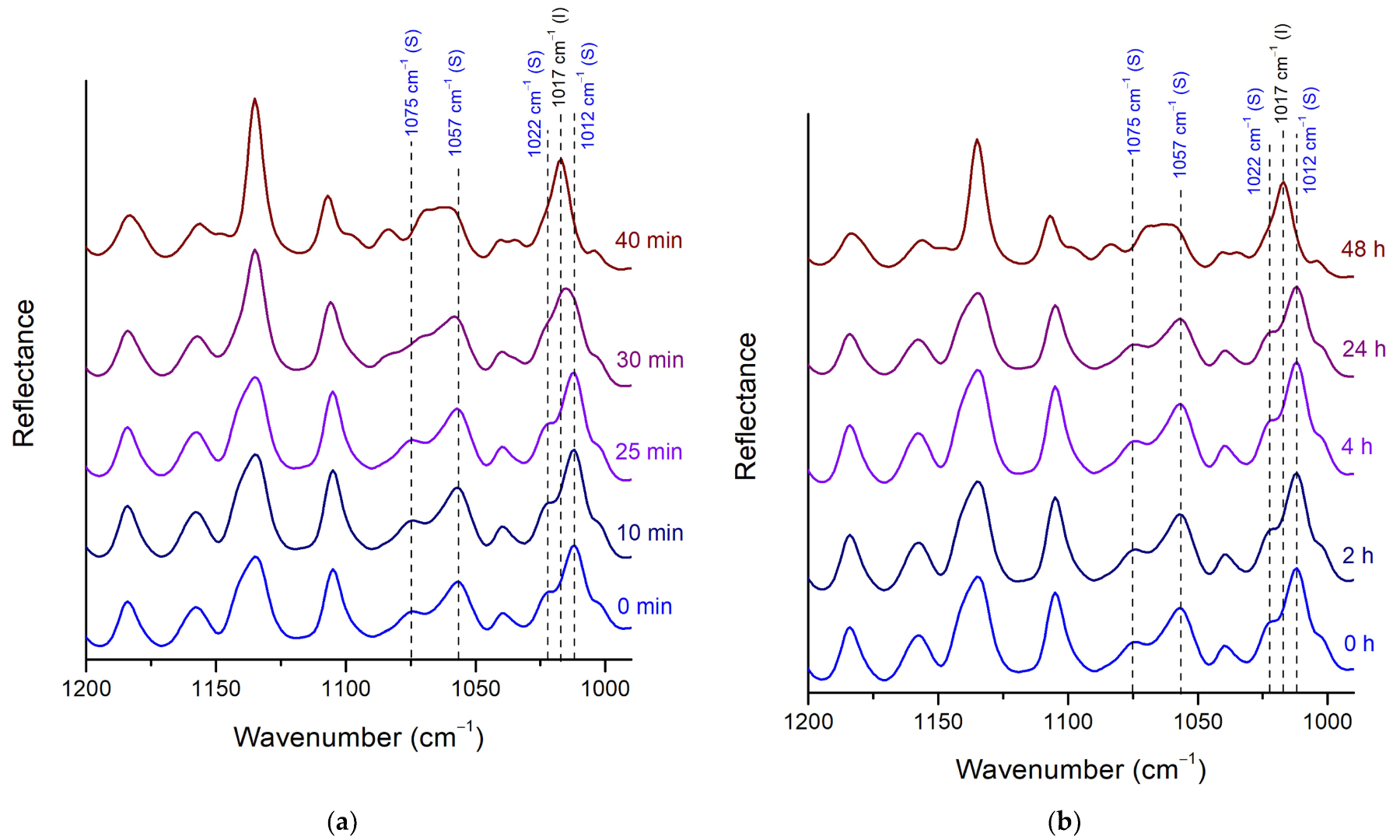
Stability of Posaconazole Form-S through ATR Spectroscopy
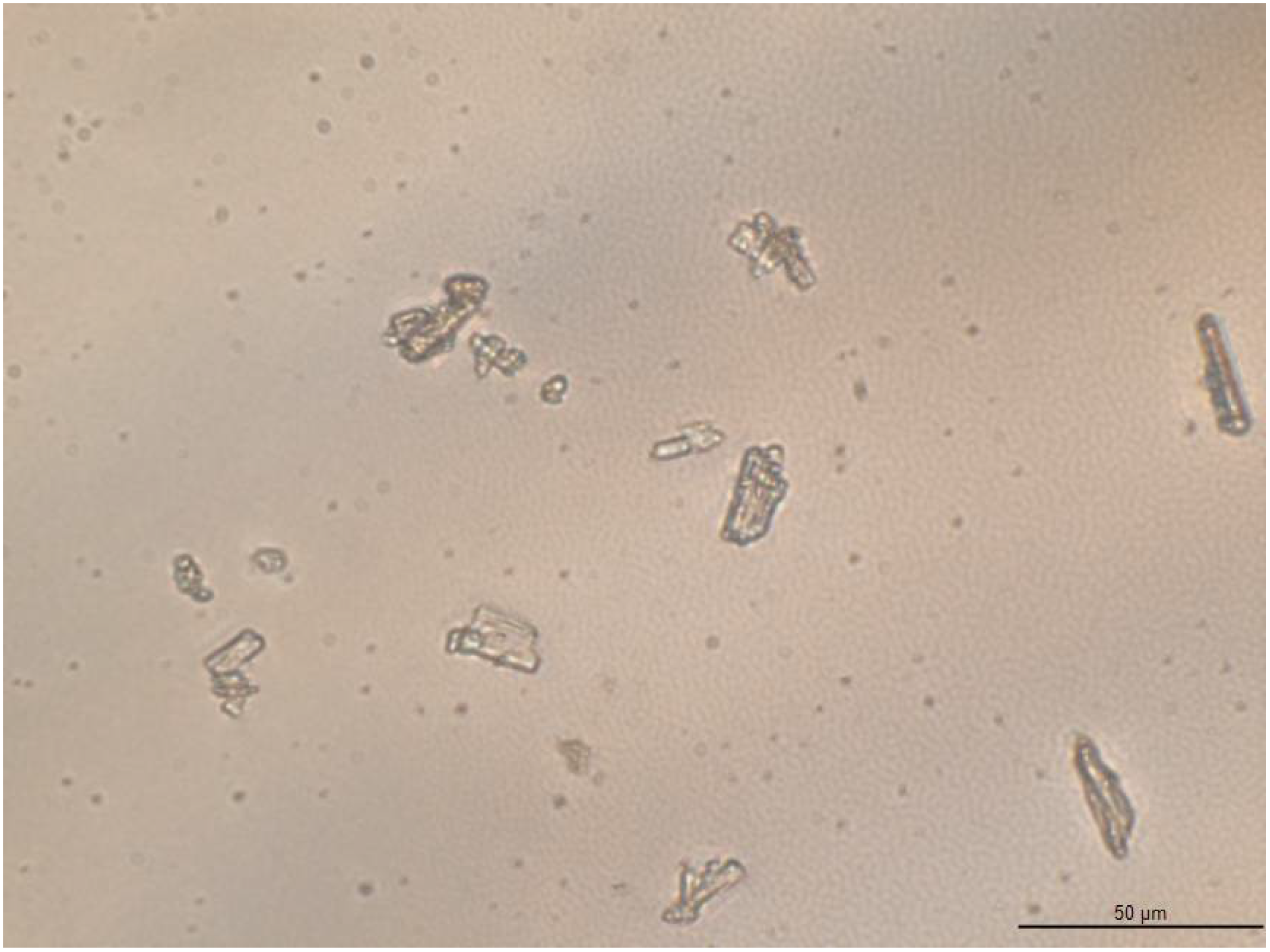
2.3.4. Optical Microscopy
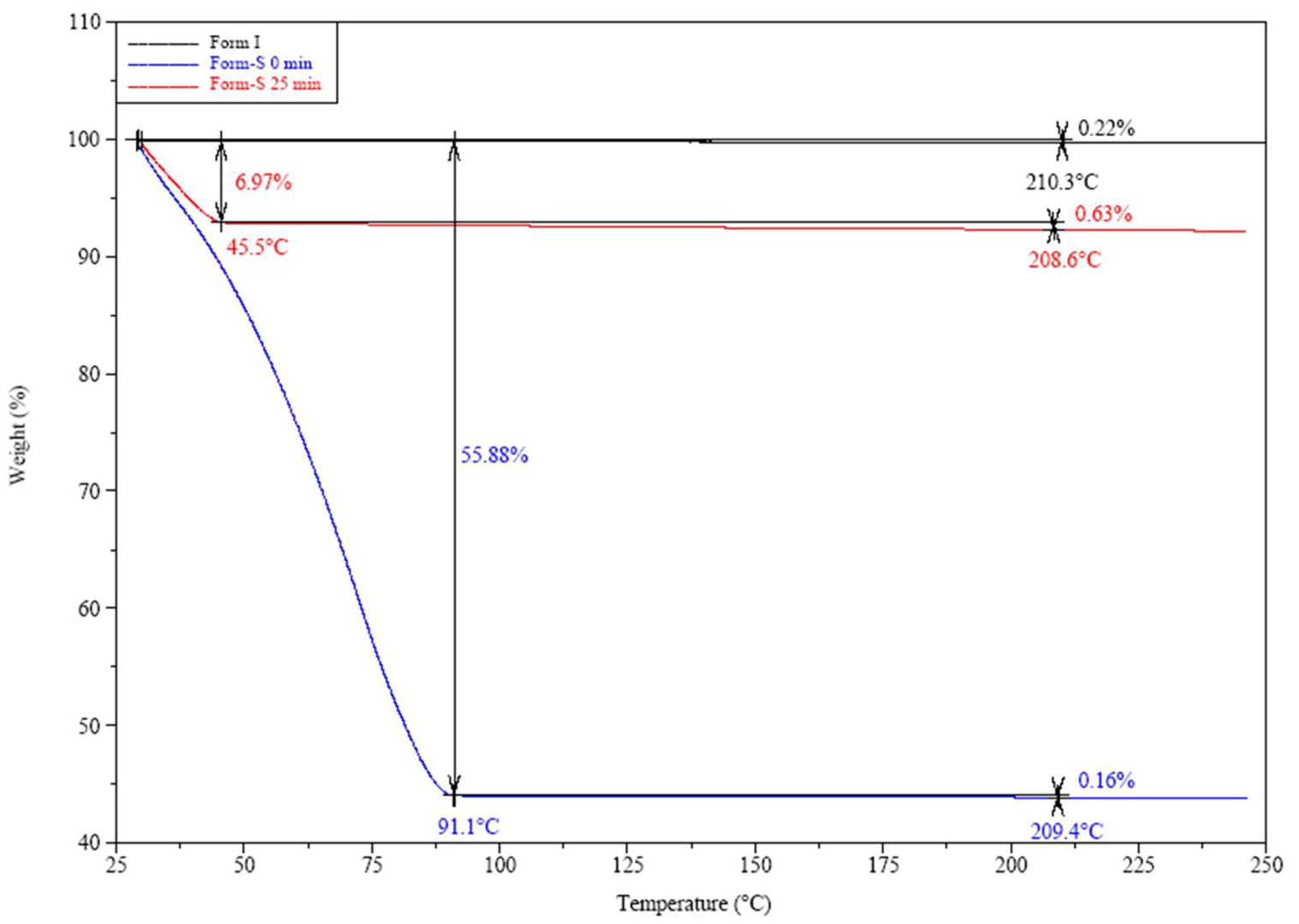
2.3.5. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3. Discussion
4. Materials and Methods
4.1. Materials and Samples
4.2. The Isolation of Pure Form-S
4.3. X-ray Powder Diffraction (XRPD)
4.4. Micro-Raman Spectroscopy
4.5. Attenuated Total Reflection (ATR) Spectroscopy
4.6. Optical Microscopy
4.7. Thermogravimetric Analysis (TGA)
4.8. Conatct Angle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nutan, M.T.H.; Reddy, I.K. Chapter 2: General Principles of Suspensions. In Pharmaceutical Suspensions: From Formulation Development to Manufacturing; Kulshreshtha, A.K., Singh, O.N., Wall, G.M., Eds.; Springer Science+Business Media: New York, NY, USA, 2010; pp. 39–65. [Google Scholar]
- Nash, R.A. Chapter 1: Pharmaceutical Suspensions. In Pharmaceutical Dosage Forms: Disperse Systems, 2nd ed.; Lieberman, H.A., Rieger, M.M., Banker, G.S., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; Volume 2. [Google Scholar]
- Ofner, C.M.; Schnaare, R.I. Section 9: Suspensions. Dupont: Nutrition & Biosciences. Available online: https://www.pharma.dupont.com/content/dam/dupont/amer/us/en/nutrition-health/general/pharmaceuticals/documents/problem-solver-documents/Suspensions.pdf (accessed on 19 March 2022).
- Wiacek, A.E.; Przykaza, K. Wettability and Stability of Naproxen, Ibuprofen and/or Cyclosporine A/Silica Delivery Systems. Colloids Interfaces 2022, 6, 11. [Google Scholar] [CrossRef]
- Stokes, G.G. On the effect of internal friction of fluids on the motion of pendulums. Trans. Camb. Philos. Soc. 1856, 9 Pt 2, 8–106. [Google Scholar]
- Yang, J.; Heinichen, N.; Velankar, S.S. The effect of particle wettability on the of rheology particulate suspensions with capillary force. Colloids Surf. A 2018, 558, 164–170. [Google Scholar]
- Frederick, K.J. Performance and Problems of Pharmaceutical Suspensions. J. Pharm. Sci. 1961, 50, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Brog, J.P.; Chanez, C.L.; Crochet, A.; Fromm, K.M. Polymorphism, what it is and how to identify it: A systematic review. RSC Adv. 2013, 3, 16905–16931. [Google Scholar] [CrossRef]
- Purohit, R.; Venugopalan, P. Polymorphism: An Overview. Resonance 2009, 14, 882–893. [Google Scholar] [CrossRef]
- Nogueira, B.A.; Castiglioni, C.; Fausto, R. Color polymorphism in organic crystals. Commun. Chem. 2020, 3, 34. [Google Scholar] [CrossRef]
- Ainurofiq, A.; Dinda, K.E.; Pangestika, M.W.; Himawati, U.; Wardhani, W.D.; Sipahutar, Y.T. The effect of polymorphism on active pharmaceutical ingredients: A review. Int. J. Res. Pharm. Sci. 2020, 11, 1621–1630. [Google Scholar] [CrossRef]
- Groll, A.H.; Walsh, T. Posaconazole: Clinical pharmacology and potential for management of fungal infections. Expert Rev. Anti-Infect. Ther. 2005, 3, 467–487. [Google Scholar] [CrossRef]
- Chen, L.; Krekels, E.H.J.; Verweij, P.E.; Buil, J.B.; Knibbe, C.A.J.; Brüggemann, R.J.M. Pharmacokinetics and Pharmacodynamics of posaconazole. Drugs 2020, 80, 671–695. [Google Scholar] [CrossRef]
- European Medicines Agency. Noxafil: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/noxafil-epar-product-information_en.pdf (accessed on 18 March 2022).
- European Medicines Agency. Noxafil, INN-Posaconazole. Available online: https://www.ema.europa.eu/en/documents/overview/noxafil-epar-summary-public_en.pdf (accessed on 18 March 2022).
- Kwon, D.S.; Mylonakis, E. Posaconazole: A new broad-spectrum antifungal agent. Expert Opin. Pharmacother. 2007, 8, 1167–1178. [Google Scholar] [CrossRef]
- Moore, J.N.; Healy, J.R.; Kraft, W.K. Pharmacologic and clinical evaluation of posaconazole. Expert Rev. Clin. Pharmacol. 2015, 8, 321–334. [Google Scholar] [CrossRef]
- Hof, H. A new, broad-spectrum azole antifungal: Posaconazole—mechanisms of action and resistance, spectrum of activity. Mycoses 2006, 49 (Suppl. S1), 2–6. [Google Scholar] [CrossRef]
- Keating, G.M. posaconazole: Adis Drug Profile. Drugs 2005, 65, 1553–1567. [Google Scholar] [CrossRef]
- Andrews, D.; Leong, W.; Sudhakar, A. Crystalline Antifungal Polymorph. U.S. Patent 6,958,337 B2, 25 October 2005. [Google Scholar]
- Wieser, J.; Pichler, A.; Hotter, A.; Griesser, U.; Langes, C. A Crystalline Form of Posaconazole. U.S. Patent 2011/0065722 A1, 17 March 2011. [Google Scholar]
- Gharpure, M.; Krishna, V.; Sanikommu, S.R.; Chaudhari, G.; Verdia, J.; Khan, M.A. Process for Preparation of Posaconazole and Crystalline Polymorphic Form V of Posaconazole. International Patent 2011/158248 A2, 22 December 2011. [Google Scholar]
- Wieser, J.; Pichler, A.; Hotter, A.; Griesser, U.; Langes, C. Crystalline Form of Posaconazole. U.S. Patent 2012/0101277 Al, 26 April 2012. [Google Scholar]
- Wieser, J.; Pichler, A.; Hotter, A.; Griesser, U.; Langes, C.; Laschober, C. Pharmaceutical Compositions Containing a Crystalline Form of Posaconazole. U.S. Patent 2011/10105525 Al, 5 May 2011. [Google Scholar]
- Badone, D.; Negri, C.; Repetti, A. A Crystalline Form of Posaconazole. International Patent 2015/092595 A1, 25 June 2015. [Google Scholar]
- Reddy, M.S.; Rajan, S.T.; Eswaraiah, S.; Vishnuvardhan, S. Process for the Preparation of Triazole Antifungal Drug, its Intermediates and Polymorphs Thereof. International Patent 2013/042138 A2, 28 March 2013. [Google Scholar]
- McQuiston, D.K.; Mucalo, M.R.; Saunders, G.C. The structure of posaconazole and its solvates with methanol, and dioxane and water: Difluorophenyl as a hydrogen bond donor. J. Mol. Struct. 2019, 1179, 477–486. [Google Scholar] [CrossRef]
- Charyulu, P.V.R.; Gowda, D.J.C.; Rajmahendra, S.; Raman, M. Crystalline Forms of Posaconazole Intermediate and Process for the Preparation of Amorphous Posaconazole. U.S. Patent 2020/0087289 A1, 19 March 2020. [Google Scholar]
- European Medicines Agency. Posaconazole AHCL—INN-Posaconazole. Available online: https://www.ema.europa.eu/en/documents/assessment-report/posaconazole-ahcl-epar-public-assessment-report_en.pdf (accessed on 20 March 2022).
- Lykouras, M.; Fertaki, S.; Orkoula, M.; Kontoyannis, C. Sample Preparation of posaconazole Oral Suspensions for Identification of the Crystal Form of the Active Pharmaceutical Ingredient. Molecules 2020, 25, 6032. [Google Scholar] [CrossRef]
- Chieng, N.; Rades, T.; Aaltonen, J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J. Pharm. Biomed. Anal. 2011, 55, 618–644. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Brittain, H.G.; Suryanarayanan, R. Chapter 9: Thermoanalytical and Crystallographic Methods. In Polymorphism in Pharmaceutical Solids, 2nd ed.; Brittain, H.G., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; pp. 318–346. [Google Scholar]
- Brittain, H.G. Chapter 10: Vibrational Spectroscopy. In Polymorphism in Pharmaceutical Solids, 2nd ed.; Brittain, H.G., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; pp. 347–380. [Google Scholar]
- Kumar, A.; Singh, P.; Nanda, A. Hot stage microscopy and its applications in pharmaceutical characterization. Appl. Microsc. 2020, 50, 12. [Google Scholar] [CrossRef]
- Lu, J.; Rohani, S. Preparation and Characterization of Theophylline−Nicotinamide Cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- European Pharmacopoeia 11.1.2.9.45. Wettability of Porous Solids Including Powders. Available online: https://pheur.edqm.eu/ (accessed on 26 October 2022).
- Boultif, A.; Louër, D. Powder pattern indexing with the dichotomy method. J. Appl. Cryst. 2004, 37, 724–731. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). PreDICT. Available online: https://www.icdd.com/predict/ (accessed on 10 May 2022).
- Blanton, J.R.; Papoular, R.J.; Louër, D. PreDICT: A graphical user interface to the DICVOL14 indexing software program for powder diffraction data. Powder Diffr. 2019, 34, 233–241. [Google Scholar] [CrossRef]
- de Wolff, P.M. A Simplified Criterion for the Reliability of a Powder Pattern Indexing. J. Appl. Crystallogr. 1968, 1, 108–113. [Google Scholar] [CrossRef]
- Smith, G.S.; Snyder, R.L. FN: A Criterion for Rating Powder Diffraction Patterns and Evaluating the Reliability of Powder-Pattern Indexing. J. Appl. Crystallogr. 1979, 12, 60–65. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.Y.; Yu, C.C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef] [PubMed]
- Coker, D.F.; Reimers, J.R.; Watts, R.O. The Infrared Absorption Spectrum of Water. Aust. J. Phys. 1982, 35, 623–638. [Google Scholar] [CrossRef]
- Papaspyridakou, P.; Lykouras, M.; Kontoyannis, C.; Orkoula, M. Comparative Study of Sample Carriers for the Identification of Volatile Compounds in Biological Fluids Using Raman Spectroscopy. Molecules 2022, 27, 3279. [Google Scholar] [CrossRef]



| Constituents | Role | Concentration (mg/mL) |
|---|---|---|
| Posaconazole Form I | API | 40.0 |
| Polysorbate 80 | Non-ionic surfactant | 15.0 |
| Simethicone | Anti-foaming agent | 3.0 |
| Sodium Benzoate | Preservative | 2.0 |
| Xanthan Gum | Suspending agent | 3.0 |
| Glycerol | Co-solvent Sweetening agent | 100.0 |
| Liquid Glucose | Sweetening agent | 300.0 |
| Titanium Dioxide | Opacifier agent | 4.0 |
| Artificial Cherry Flavour | Flavouring agent | 5.0 |
| Citric Acid Monohydrate | Buffering agent | 1.5 |
| Sodium Citrate Dihydrate | Buffering agent | 0.6 |
| Purified Water | Solvent | q.s. 1.0 mL |
| Sample Preparation Method | Posaconazole Polymorphism |
|---|---|
| Manual Shaking 1 min | Form-S with traces of Form I |
| Manual Shaking 5 min | Form-S with traces of Form I |
| Vortex 1 min | Form-S with traces of Form I |
| Vortex 2 min | Form-S (lack of repeatability) |
| Vortex 5 min | Form-S with traces of Form I |
| Magnetic Stir 250 rpm 30 min | Form-S with traces of Form I |
| Magnetic Stir 500 rpm 30 min | Form-S with traces of Form I |
| Magnetic Stir 750 rpm 30 min | Form-S with traces of Form I |
| Magnetic Stir 1000 rpm 30 min | Form-S (lack of repeatability) |
| Magnetic Stir 1000 rpm 5 min | Form-S and Form I |
| Magnetic Stir 1000 rpm 15 min | Form-S with traces of Form I |
| Magnetic Stir 1000 rpm 60 min | Form-S with traces of Form I |
| Sonication 1 min | Form-S and Form I |
| Sonication 2 min | Form-S with traces of Form I |
| Sonication 5 min | Form-S and Form I |
| Sonication 10 min | Form-S |
| Sonication 15 min | Form-S |
| Crystallographic Data | Form I | Form-S |
|---|---|---|
| Crystal System | Monoclinic | Monoclinic |
| Bravais Crystal Lattice | Simple Monoclinic | Simple Monoclinic |
| M20 (de Wolff) | 207.4 | 24.3 |
| F20 (Smith-Snyder) | 505.5 (0.0009, 43) | 74.00 (0.0079, 34) |
| Edge a | (12.536 ± 0.001) Å | (12.380 ± 0.005) Å |
| Edge b | (6.348 ± 0.0001) Å | (6.305 ± 0.003) Å |
| Edge c | (22.780 ± 0.001) Å | (23.126 ± 0.016) Å |
| Angle β | 96.387° ± 0.002° | 93.140° ± 0.034° |
| Unit Cell Volume (V) | (1801.48 ± 0.10) Å3 | (1802.47 ± 1.68) Å3 |
| Raman Shift (cm−1) | Posaconazole Form I | Posaconazole Form-S |
|---|---|---|
| 701–714 cm−1 | Medium double peak at 701 cm−1 and 714 cm−1 | Shoulder at 702 cm−1 and medium peak at 712 cm−1 |
| 729–745 cm−1 | Shoulder at 729 cm−1 and strong peak at 745 cm−1 | Single strong peak at 738 cm−1 |
| 785–798 cm−1 | Strong double peak at 785 cm−1 and 796 cm−1 | Shoulder at 789 cm−1 and strong peak at 798 cm−1 |
| 820–835 cm−1 | Broad shoulder at 823 cm−1 followed by a medium peak at 835 cm−1 | Medium double peak at 820 cm−1 and 835 cm−1 |
| 850 cm−1 | Weak single peak at 850 cm−1 | End of a shoulder due to the peak at 835 cm−1 |
| 911 cm−1 | Shoulder at 911 cm−1 at the descent of the single peak at 900 cm−1 | No shoulder and no peak at 911 cm−1 |
| 941–966 cm−1 | Weak peak at 941 cm−1 followed by a strong peak at 966 cm−1 | Broad shoulder at 941 cm−1 at the ascent of the strong peak at 966 cm−1 |
| 1135–1147 cm−1 | Medium single peak at 1135 cm−1 followed by a shoulder at 1147 cm−1 | Medium single peak at 1139 cm−1 without shoulder at 1147 cm−1 |
| 1205–1224 cm−1 | Strong double peak at 1205 cm−1 and 1222 cm−1 | Strong double peak at 1208 cm−1 and 1224 cm−1 |
| 1264–1287 cm−1 | Strong peak at 1278 cm−1 with two shoulders at 1268 cm−1 and 1287 cm−1 | Strong peak at 1274 cm−1 with only one shoulder at 1264 cm−1 |
| 1328–1350 cm−1 | Strong double peak at 1329 cm−1 and 1350 cm−1 | Strong double peak at 1328 cm−1 and 1346 cm−1 |
| 1382–1402 cm−1 | Strong double peak at 1382 cm−1 and 1402 cm−1 The peak at 1382 cm−1 is of higher intensity | Shoulder at 1389 cm−1 and strong single peak at 1402 cm−1 The peak at 1402 cm−1 is of higher intensity |
| 1444–1460 cm−1 | Shoulder at 1444 cm−1 and medium peak at 1460 cm−1 | Medium double peak at 1444 cm−1 and 1460 cm−1 |
| Wavenumber (cm−1) | Posaconazole Form I | Posaconazole Form-S |
|---|---|---|
| 3800–2700 cm−1 | Multiple weak peaks | Very strong broad peak with centre at 3370 cm−1 corresponding to water |
| 1770–1570 cm−1 | Strong single peak at 1686 cm−1 and weak double peak at 1618 cm−1 and 1603 cm−1 | Strong broad peak with centre at 1638 cm−1, corresponding to water and overlapping the peaks at 1686 cm−1, 1618 cm−1 and 1603 cm−1 |
| 1552–1549 cm−1 | Medium single peak at 1549 cm−1 | Medium single peak at 1552 cm−1 |
| 1483 cm−1 | Very weak single peak at 1483 cm−1 | No peak |
| 1427–1419 cm−1 | Weak peak at 1427 cm−1 with shoulder at 1419 cm−1 | Weak single peak at 1419 cm−1 |
| 1397–1393 cm−1 | Medium single peak at 1397 cm−1 | Medium single peak at 1393 cm−1 |
| 1307–1290 cm−1 | Ascent at 1307 cm−1 of the weak double peak at 1298 cm−1 and 1290 cm−1 | Weak double peak at 1307 cm−1 and 1297 cm−1 |
| 1277–1270 cm−1 | Medium peak at 1277 cm−1 with shoulder at 1270 cm−1 | Medium single peak at 1272 cm−1 |
| 1135 cm−1 | Strong and sharp single peak at 1135 cm−1 | Medium broad single peak at 1135 cm−1 |
| 1107–1099 cm−1 | Medium peak at 1107 cm−1 with shoulder at 1099 cm−1 | Medium single peak at 1105 cm−1 |
| 1083 cm−1 | Weak single peak at 1083 cm−1 | No peak |
| 1075–1057 cm−1 | Medium broad double-like peak at 1069–1059 cm−1 | Medium double peak at 1075 cm−1 and 1057 cm−1, stronger at 1057 cm−1 |
| 1041–1035 cm−1 | Weak double peak at 1041 cm−1 and 1035 cm−1 | Weak single peak at 1040 cm−1 |
| 1022–1012 cm−1 | Medium single peak at 1017 cm−1 | Medium peak at 1012 cm−1 with shoulder at 1022 cm−1 |
| 900–650 cm−1 | Multiple strong, medium and weak peaks Medium single peak at 870 cm−1 Weak single peak at 776 cm−1 | Ascent of a very broad peak, corresponding to water, with weak overlapped peaks of Form-S No peaks were detected at 870 cm−1 and 776 cm−1 even after 20 min drying |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lykouras, M.; Orkoula, M.; Kontoyannis, C. Formation and Characterisation of Posaconazole Hydrate Form. Pharmaceuticals 2023, 16, 65. https://doi.org/10.3390/ph16010065
Lykouras M, Orkoula M, Kontoyannis C. Formation and Characterisation of Posaconazole Hydrate Form. Pharmaceuticals. 2023; 16(1):65. https://doi.org/10.3390/ph16010065
Chicago/Turabian StyleLykouras, Michail, Malvina Orkoula, and Christos Kontoyannis. 2023. "Formation and Characterisation of Posaconazole Hydrate Form" Pharmaceuticals 16, no. 1: 65. https://doi.org/10.3390/ph16010065
APA StyleLykouras, M., Orkoula, M., & Kontoyannis, C. (2023). Formation and Characterisation of Posaconazole Hydrate Form. Pharmaceuticals, 16(1), 65. https://doi.org/10.3390/ph16010065







