Abstract
Natural products are an important source of drug lead compounds, and natural products with significant biological activity are constantly being discovered and used in clinical practice. At present, natural products play an important role in the targeted therapy of cancer, cardiovascular and cerebrovascular diseases, nervous system diseases, and autoimmune diseases. Meanwhile, in recent years, the rise of protein-targeted degradation technologies, such as proteolysis-targeting chimeras (PROTACs) and molecular glues, has provided a new solution for drug resistance caused by clinical molecular-targeting drugs. It is noteworthy that natural products and their derivatives, as important components of PROTACs and molecular glues, play an important role in the development of protein-targeting drugs. Hence, this review summarized the protein-targeted degradation agents based on natural products, such as PROTACs and molecular glues. More natural products with the potential to be used in the development of PROTACs and molecular glues as targeted protein degradation agents are still being investigated.
1. Introduction
As important sources of drug lead compounds, natural products play pivotal roles in drug discovery and development. It has been reported that, in the past 40 years, about 50% of the new drugs on the market came directly or indirectly from natural products. Natural medicines such as morphine, penicillin, aspirin, and paclitaxel are considered some of the most important active molecules affecting the course of human history. In recent years, drugs from natural sources have been found to have obvious advantages in antitumor, anti-infection, and neurological disease treatment. Moreover, the high probability of natural product discovery and structural diversity along with various properties provides more opportunities and possibilities for drug research and development [1,2]. To date, drugs obtained from natural products have been widely used in the clinical treatment of various diseases, such as paclitaxel for its anticancer ability; butylphthalide for its anti-cerebral ischemia ability; puerarin for its unique cardiovascular protection; the drug arsenic trioxide for the treatment of acute promyelocytic leukemia; and artemisinin, which saves millions of patients with malaria every year. Since the concept of tumor-targeted therapy was proposed, small-molecule-targeted drugs have been a research hotspot. With the continuous progress of isolation and identification technology, natural products with novel structures and significant activities have been discovered. Meanwhile, some new targets and mechanisms of the active compounds have been gradually clarified. Natural products and their derivatives, such as romidepsin and rapamycin, have become an important source of molecular-targeted drugs [3,4]. Compared with traditional chemotherapy drugs, molecular-targeted drugs have the advantages of low systemic side effects and good efficacy in disease treatment [5,6]. However, due to the cross-linking of signaling pathways resulting in drug resistance, the therapeutic effect of these molecular-targeted drugs has been limited to patients with the target gene aberration and is short-lived [7]. Because of this, in some cases, molecular-targeted drugs are not effective, and some proteins have poor targeting and druggability. Some proteins are resistant to molecular-targeted drugs, and others lack good targeting and druggability, such as disease-related transcription factors, skeleton proteins, and other proteins without enzymatic activity. In recent years, proteolysis-targeting chimeras (PROTACs) and molecular glue have evolved into two of the most important technologies for targeting protein degradation.
The concept of PROTACs was first proposed by the team of Professor Craig M. Crews of Yale University and Professor Raymond J. Deshaies of California Institute of Technology. PROTAC technology is a targeted inducible protein degradation chimera formed by linking target protein ligands and E3 ubiquitin-ligase enzyme (E3) ligands through suitable linking chains. A PROTAC can simultaneously recruit the target protein and a specific E3 in vivo to achieve target protein ubiquitination degradation (Figure 1), which is a promising disease treatment strategy for target protein degradation via the ubiquitin-proteasome pathway [8,9]. Compared with traditional inhibitors, it has the advantages of high selectivity and potent activity and is expected to degrade non-generic targets and overcome drug resistance. Since the first polypeptide-based PROTAC molecule degrading MetAp-2 was reported in 2001, the technology has become an emerging strategy for target protein degradation using the ubiquitin-proteasome system. The earliest PROTAC was designed based on peptides, but its poor cell permeability and chemical stability meant that it did not have the potential to be developed into drugs. Researchers gradually shifted their focus to the development of small-molecule PROTACs, and they have been well-developed. To date, PROTACs have been successfully used in the degradation of different types of target proteins associated with a variety of diseases. In the PROTAC strategy, the following have been reported as targets: nuclear proteins, such as estrogen receptorα (ERα); cyclin-dependent kinases (CDKs); the breakpoint cluster region-c-abl (BCR-ABL) fusion protein; transmembrane proteins, such as human epidermal growth factor receptor-2 (HER2); c-mesenchymal-epithelial transition factor (c-Met); and cytoplasmic proteins, such as Brutons tyrosine kinase (BTK) and mouse double minute 2 (MDM2) [10].
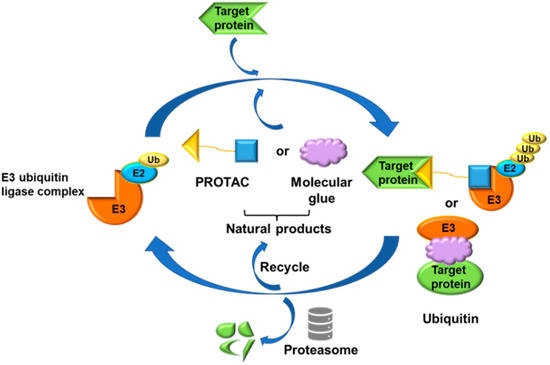
Figure 1.
Mechanism of action of PROTAC and molecular glue technology.
Molecular glues are another class of small-molecule protein degraders acting via E3 ubiquitin ligases, the concept of which was proposed in the early 1990s. Although both molecular glue and PROTACs are protein degraders, they have different mechanisms of action and molecular structural characteristics. Molecular glue has high affinity and selectivity, so it can directly bind to proteins in order to form ternary complexes, and it can promote the dimerization or co-localization of two proteins [11]. By narrowing the distance between the target protein and the E3 ligase, molecular glue can induce the ubiquitination and degradation of the target protein, which is more in line with Lipinski’s five rules of drug-like (Figure 1). Molecular glue has a small molecular weight and good druggability, but it is difficult to design. According to recent research, some PROTACs designed target proteins play an anticancer role through the formation of molecular glue that can degrade new substrates. Some experts believe that the best PROTAC is made of molecular glue [12]. Therefore, in the design and verification of PROTACs, the two mechanisms should be considered comprehensively.
In recent years, natural products and their derivatives have played important roles in the design of PROTACs and molecular glues. The activities of natural products may occur due to interactions with multiple targets, but their affinities with one target are not very strong. The complexity of these mechanisms of action poses a huge challenge in identifying their targets. PROTACs and molecular glues can degrade target proteins effectively and specifically, and they bind to target proteins at lower dosages, which could be an efficient strategy for confirming the indetectable targets of natural products and for increasing their activities dramatically. Therefore, PROTACs and molecular glues that are designed based on natural products have great potential for protein degradation. This review summarized the progress of protein-targeting degradation agents based on natural products, such as PROTACs and molecular glues. The classes of these natural products include hormones, flavonoids, alkaloids, terpenoids, vitamins, microorganisms, and peptides. More natural products applicable for developing PROTACs and molecular glues are waiting to be explored. The toolbox of protein-targeted degradation agents will be significantly expanded.
2. Natural Products and Their Derivatives in PROTAC Design
2.1. Derived from Hormones
According to reports, not only is a malignant tumor one of the diseases with the highest morbidity in the world, but it also has a high mortality rate among major diseases, causing serious harm to human health. It has been difficult to achieve satisfactory results using traditional cancer treatment methods, such as surgery, chemotherapy, and radiotherapy, due to serious damage to the body and poor drug selectivity. At present, tumor-targeted therapy is the main direction of tumor therapy due to its specificity and targeting, and emerging protein degradation technologies, such as PROTACs, are also constantly opening up new fields of tumor-targeted therapy. Natural products contain endogenous compounds from plants and animals that play a positive role in regulating biological activities. At present, using natural products to fight cancer is the dominant direction of cancer drug development.
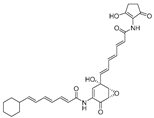
Breast cancer, as one of the most common malignant tumors, has seriously harmed the health of women all over the world. ERα is a member of the steroid hormone nuclear receptor family, and more than half of patients with breast cancer are ERα-positive [13]. Estrogen is a class of steroid compounds with a wide range of biological activities. Free estrogen enters target cells through passive diffusion or specific active transport, and it binds with the nuclear estrogen receptor (ER) to exert biological effects. Currently, antagonizing the interaction between estrogen and ERα is an important method for the treatment of breast cancer [14]. In addition, due to the existence of drug resistance, the use of selective estrogen receptor degraders (SERDs) to target the degradation of ERα is also an important direction in the development of breast cancer therapy. Estradiol (E2) is the most biologically active natural estrogen. Previous studies have shown that 17β-estradiol (Table 1 can be used as a ligand to recruit ER in the PROTAC design, inducing the specific degradation of ER through the ubiquitin-proteasome system [15]. Data from a previous study have shown that ligands linked to von Hippel-Lindau (VHL) E3 ligase via the C7α site of estradiol have higher affinity for estrogen receptors and more efficient ER degradation than C16α-based PROTACs, superior even to tamoxifen (Table 2). PROTACs, as an emerging strategy for protein degradation, have successfully achieved the ubiquitination of ERα [16]. Designing a new type of selective estrogen receptor degrader based on the PROTAC model is conducive to further expanding the PROTAC toolbox [17]. In addition, breast cancer growth and metastasis are dependent on angiogenesis, and ER is a potential anti-angiogenic target. PROTACs utilizing the E3 ubiquitin ligase pVHL ligand HIF-1α octapeptide combined with E2-Octa-[Ala] have endothelial-cell-targeting activity at low doses, and the use of ER-targeted PROTACs as probes for angiogenesis can be used to study the mechanisms of angiogenesis in animal models of diseases [18].
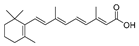
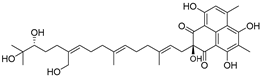
Similarly, androgen receptor (AR) inhibitors are the main targeted drugs used for prostate cancer, and they are effective in metastatic castration-resistant prostate cancer (mCRPC). In the latest report in Nature on the sales of prostate cancer drugs, AR-targeted therapies account for 58% [19]. In recent years, a series of highly selective AR degradation PROTACs have been developed. Not only do these compounds effectively reduce AR protein levels in prostate cancer cell lines, but they also effectively inhibit cell growth in AR-positive breast cancer cell lines [20,21]. Dihydrotestosterone (Table 1) is a natural androgen that has made an important contribution to the pioneering work in the design and synthesis of PROTAC molecules for the degradation of AR. Preliminary experimental results have shown that AR degradation depends on the recruitment of E3 ligands and testosterone to bind to their respective targets, and the use of the green fluorescence protein (GFP) fusion protein technique provides a convenient method to monitor PROTAC-induced degradation (Table 2) [22,23]. The specific synthesis processes of the PROTACs discussed in this section are shown in Scheme 1, Scheme 2, Scheme 3 and Scheme 4.

Scheme 1.
Synthesis of C16-derivatized estradiol–based PROTAC.
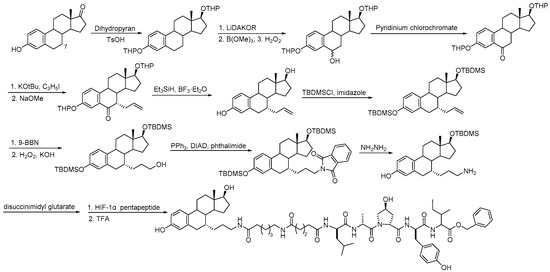
Scheme 2.
Synthesis of C7-derivatized estradiol–based PROTAC.
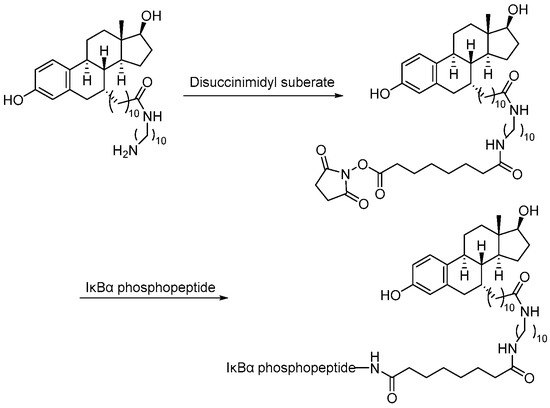
Scheme 3.
Synthesis of estradiol–based PROTAC.
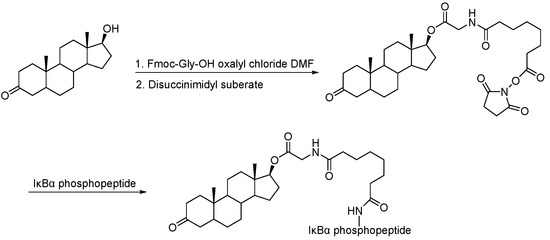
Scheme 4.
Synthesis of dihydroxytestosterone–based PROTAC.

Table 1.
Natural products 1–2.
Table 1.
Natural products 1–2.
| No. | Natural Products | Structure | Structure Classification | Source of Compounds | Reference |
|---|---|---|---|---|---|
| 1 | 17β-estradiol | 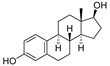 | Sterides | Estrogen | [15,22] |
| 2 | Dihydroxytestosterone | 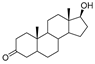 | Sterides | Androgen | [22] |

Table 2.
Natural product targets 1–3.
Table 2.
Natural product targets 1–3.
| No. | Target | E3 | Cell Type | Reference |
|---|---|---|---|---|
| 1 | ERα | pVHL and SCFβ-TRCP | MCF-7 cells | [15] |
| 2 | ERα | pVHL and SCFβ-TRCP | 293T cells | [22] |
| 3 | AR | SCFβ-TRCP | 293T cells | [22] |
2.2. Derived from Flavonoids, Alkaloids, and Terpenoids
Apigenin (Table 3), a flavonoid natural product with anticancer activity, can directly interact with the ligand-activated transcription factor aryl hydrocarbon receptor (AhR) [24,25]. Using apigenin derivatives as ligands for recruiting AhR, the degradation of AhR was efficiently induced by the E3 ubiquitin ligase pVHL (Table 4) [26]. In addition, activated AhR also had E3 ubiquitin ligase activity [27]. Wogonin (Table 3) is a natural flavonoid extracted from the Chinese herbal medicine Scutellaria baicalensis, which has various biological activities, such as anti-inflammatory and antitumor activities, and it can improve diabetes [28,29]. Wogonin can directly bind to cyclin-dependent kinase 9 (CDK9) and selectively inhibit the activity of CDK9. A series of PROTACs targeting CDK9 can be designed based on wogonin. Previous results showed that the PROTACs obtained by linking wogonin and CRBN with triazole groups can selectively degrade CDK9 (Table 4) [30].
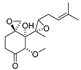
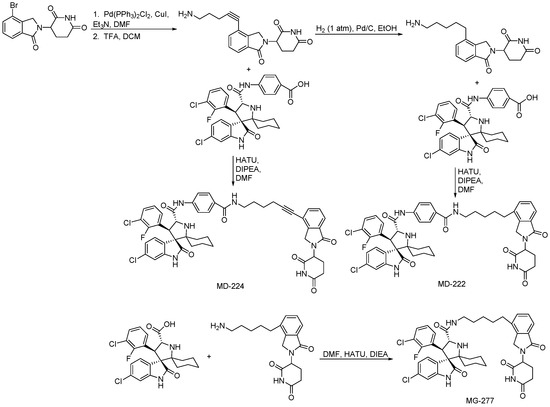
As the core skeleton of various natural products, oxindole spiro compounds (Table 3) have antimalarial, anticancer, antihypertensive, and other drug activities [31,32]. The E3 ubiquitin ligase MDM2 binds to p53 in order to ubiquitinate and degrade p53, and it is a major cellular endogenous inhibitor of p53. In human cancers, the overexpression of MDM2 leads to the inactivation of the tumor suppressor gene p53. Therefore, MDM2 can be used as a target of p53 activation, and the targeted degradation of MDM2-p53 can overcome the accumulation and potential adverse effects of MDM2 due to the activation of p53 [33,34]. The compounds MD-222 and MD-224, which utilize the previously reported oxindole spiro derivative MI-1061 as a ligand for recruiting MDM2 and a ligand for the ubiquitin ligase CRBN, are effective in mice and degrade MDM2 in vitro, and MD-224 efficiently induces the degradation of MDM2 in human leukemia cells at a concentration of 1 nM (Table 4) [35]. Researchers soon found that MG-277, obtained by removing the benzoic acid portion of the MDM2 inhibitor segment of MD-222, achieved significant cell growth inhibitory activity by inducing G1 to S phase transition 1 (GSPT1) protein degradation. This suggests that the designed phthalimide-coupled degradation agent can act as either a true PROTAC of the target protein or as a molecular glue (Table 4) [36]. Ursolic acid (UA) is a natural triterpenoid widely distributed in many plants (Table 3). Researchers connected UA and the CRBN ligand thalidomide through different linking chains and found that compound 1B with 3-Polyoxyether (POE-3) as the linking chain could inhibit the proliferation of A549, Huh7, HepG2, and other cancer cell lines and that it has significant in vitro antitumor activity (with an IC value of 0.23 to 0.39 μM) (Table 4) [37]. At the same time, the researchers confirmed that UA has the targeted binding properties of MDM2 and that the UA-PROTAC also promoted the expressions of P21 and PUMA proteins downstream of MDM2, thereby inhibiting the proliferation of A549 cells and promoting apoptosis.
Indirubin (Table 3), isolated from the traditional Chinese medicine Indigo Naturalis, is a new type of bisindole alkaloid antitumor drug that has a good curative effect on leukemia. By linking the natural product indirubin, a selective inhibitor of HDAC6, with the E3 ubiquitin ligase CRBN ligand pomalidomide, PROTAC compounds that can selectively degrade HDAC6 can be designed (Table 4) [38]. Targeting the STAT3 pathway has been shown to eliminate resistance to EGFR inhibitors in head and neck squamous cell carcinomas. Researchers designed and synthesized a PROTAC that specifically degrades STAT3 using the triterpenoid toosendanin compound (Table 3). This PROTAC has significant inhibitory activity in head and neck cancer and colorectal cancer tumor models (Table 4) [39]. Recently, based on the previously discovered molecular mechanism of broad-spectrum pentacyclic triterpenes inhibiting virus entry into host cells, Zhou and Xiao’s team linked oleanolic acid (OA) binding to influenza virus hemagglutinin protein (HA) to the CRBN and VHL ligands of E3 ligase by using PROTAC targeted protein degradation technology (Table 3) [40]. In this study, the PROTAC molecule was constructed to degrade hemagglutinin at both the molecular and cellular levels, and compound V3 effectively degraded the A/WSN/33 (H1N1) virus protein (DC50 = 1.44 μM) during viral replication (Table 4).
The efficacy of traditional Chinese medicine components and natural products includes realized through their interactions with multiple targets, and their affinities with target proteins are not very strong. The complexity of these mechanisms of action brings great challenges to the identification of their targets. In view of the fact that PROTAC molecules can effectively and specifically degrade target proteins without strong affinity, recent studies have demonstrated the potential of PROTAC technology in identifying the targets of traditional Chinese medicine components and natural products. In an article in 2020, PROTAC technology was combined with a quantitative proteomic analysis to identify the unknown target of the multikinase inhibitor sorafenib [41]. Recently, the research team at Shenyang Pharmaceutical University creatively introduced PROTAC technology into the field of traditional Chinese medicine research [42]. The combination of the PROTAC technique with quantitative proteomics and the molecular interaction detection technique represented by a microscale thermophoresis (MST) assay were proposed. It is proved that V-maf musculoaponeurotic fibrosarcoma oncogene homolog F (MAFF) is the target of a series of lathyrane diterpenoids obtained from the traditional Chinese medicine Euphorbia lathyris, and it is clarified that the anti-inflammatory effect of the lathyrane diterpenoid ZCY020 is based on the Nrf2/HO-1 signaling pathway. The core skeleton of the ZCY-001 compound lathyrol (Table 3) is linked to thalidomide (an E3 ligase CRBN ligand) by a PEG linker, and the resulting PROTAC molecule ZCY-PROTAC strongly degrades MAFF in a dose-dependent manner (Table 4). The specific synthesis processes of the PROTACs discussed in this section are shown in Scheme 5, Scheme 6, Scheme 7, Scheme 8, Scheme 9, Scheme 10, Scheme 11 and Scheme 12.
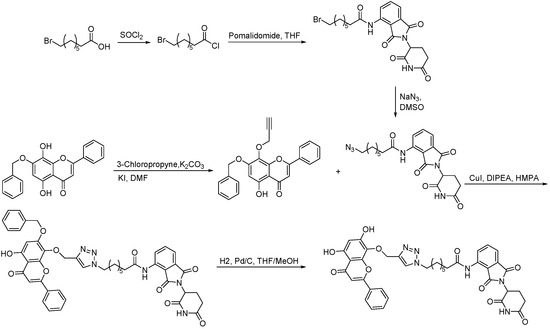
Scheme 5.
Synthesis of wogonin–based PROTAC.
Scheme 6.
Synthesis of apigenin–based PROTAC.

Scheme 7.
Synthesis of indirubin–based PROTAC.
Scheme 8.
Synthesis of MI-1061– and MI-2103–based PROTAC.

Scheme 9.
Synthesis of ursolic-acid–based PROTAC.

Scheme 10.
Synthesis of toosendanin–based PROTAC.

Scheme 11.
Synthesis of oleanolic-acid–based PROTAC.

Scheme 12.
Synthesis of the lathyrol-derivatized–based PROTAC.

Table 3.
Natural products 3–11.
Table 3.
Natural products 3–11.
| No. | Natural Products | Structure | Structure Classification | Source of Compounds | Reference |
|---|---|---|---|---|---|
| 3 | Apigenin | 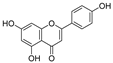 | Flavonoid | Apple, celery, tea, fragrant plant, honey, etc | [26] |
| 4 | Wogonin | 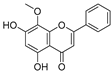 | Flavonoid | Scutellaria baicalensis | [30] |
| 5 | MI-1061 | 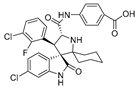 | Alkaloid | Spirooxindole derivative | [35] |
| 6 | MI-2103 | 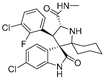 | Alkaloid | Spirooxindole derivative | [36] |
| 7 | Ursolic acid | 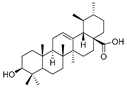 | Triterpenoid | Ligustrum lucidum Ait | [37] |
| 8 | Indirubin | 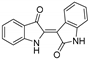 | Bisindole alkaloid | Indigo naturalis | [38] |
| 9 | Toosendanin | 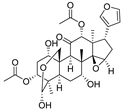 | Triterpenoid | Melia azedarach L. and Melia Toosendanin Sieb. et Zucc | [39] |
| 10 | Oleanolic acid | 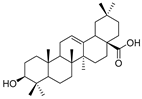 | Triterpenoid | Olea europaea l | [40] |
| 11 | Lathyrol | 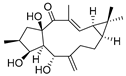 | Lathyrane diterpenoids | Euphorbia lathyris | [42] |

Table 4.
Natural product targets 4–12.
Table 4.
Natural product targets 4–12.
| No. | Target | E3 | Cell Type | Reference |
|---|---|---|---|---|
| 4 | AhR | pVHL | Immortalized mouse hepatocyte cells and CV-1 cells (monkey kidney cell line) | [26] |
| 5 | CDK9 | CRBN | MCF-7 and L02 cells | [30] |
| 6 | MDM2 | CRBN | RS4;11 cells | [35] |
| 7 | MDM2 | CRBN | RS4;11, MOLM-13, MDA-MB-468, MV-4-11, HL-60, and MDA-MB-231 cells | [36] |
| 8 | MDM2 | CRBN | A549, Huh7, and HepG2 cells | [37] |
| 9 | HDAC6 | CRBN | K562, HeLa, and THP-1 cells | [38] |
| 10 | STAT3 | CRBN and VHL | CAL33 and HCT116 cells | [39] |
| 11 | Hemagglutinin protein | CRBN and VHL | Human embryonic kidney 293T cells | [40] |
| 12 | MAFF | CRBN | Mouse RAW264.7 macrophage and human embryonic kidney 293T (HEK293T) cells | [42] |
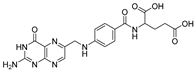
2.3. Derived from Vitamins
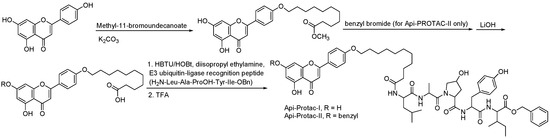
Retinoic acid (RA) is a metabolic intermediate of vitamin A (retinol) in the body, and cellular retinoic-acid-binding proteins are important regulators of RA activity (Table 5) [43]. Studies have shown that an increased level of cellular retinoic-acid-binding protein 1 (CRABP1) in tumor cytoplasm is associated with RA resistance and that an increased level of cellular retinoic-acid-binding protein 2 (CRABP2) in the nucleus is associated with RA sensitivity, so RA is a potential tumor therapeutic target [44]. Studies have shown that the use of the Cullin4B-Ring (CRL4B) E3 ligase complex component AhR to recruit degradants and a small-molecule chimera composed of all-trans retinoic acid and AhR ligands connected by a suitable linker chain can effectively degrade CRABPs (Table 6) [45]. In addition, natural products can also be used as tools for the targeted delivery of PROTACs in vivo to achieve the selective degradation of target proteins in cancer cells. Folic acid, a water-soluble B vitamin, was first extracted and isolated from spinach leaves. Folic acid (Table 5) deficiency may induce cancer [46]. Folate receptor alpha (FOLR1) is highly expressed in a variety of cancers, including ovarian, lung, and breast cancers, but it has low or no expression in normal tissues and cells. FOLR1 is the most well-defined target for drug delivery to cancer cells, and other receptors, such as FOLR2 and FOLR3, have less affinity for folic acid than FOLR1 [47]. Based on PROTACs, a FOLR1-targeted drug delivery strategy is provided. A folic acid group is installed on the E3 ubiquitin ligase ligand. After entering cancer cells, folic acid-PROTAC is preferentially transported to cancer cells with high FOLR1 expressions. Intracellular hydrolase-catalyzed release and subsequently released PROTACs designed for specific target proteins recruit endogenous VHL E3 ubiquitin ligases to efficiently ubiquitinate and degrade cancer-cell-associated BRD, MEK, and ALK proteins (Table 6) [48]. The specific synthesis processes of the PROTACs discussed in this section are shown in Scheme 13 and Scheme 14.

Table 5.
Natural products 12–13.

Table 6.
Natural product targets 13–16.

Scheme 13.
Synthesis of retinoic-acid–based PROTAC.

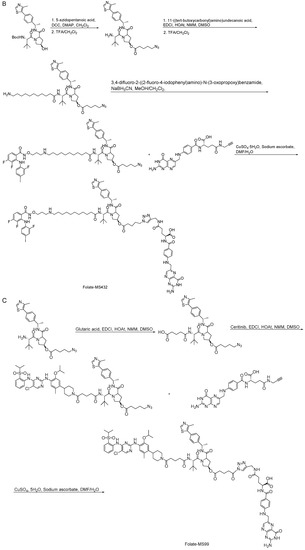
Scheme 14.
Synthesis of folate–based PROTACs. (A) Synthesis of folate-ARV-771. (B) Synthesis of folate-MS432. (C) Synthesis of folate-MS99.
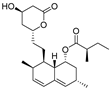
2.4. Derived from Microorganisms
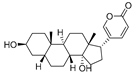
Ovalicin (Table 7) is one of the earliest microbial-derived immunosuppressants produced by fungi, and it has a strong inhibitory effect on lymphocyte proliferation and the DNA synthesis of lymphoma cells [49]. Ovalicin can covalently inhibit MetAP2, which plays a catalytic role in the cleavage of the N-terminal methionine of newly synthesized proteins in cells, thus regulating the growth cycle of endothelial cells and inhibiting tumor angiogenesis and growth [50]. Ovalicin was selected as the ligand of the MetAP2 protein, and the first PROTAC for the ubiquitination labeling and degradation of MetAP2 was designed and synthesized by properly binding to the phospopeptide binding to SKp1-Cullin-Fbox (SCF) of the ubiquitin ligase E3 system (Table 8) [51]. Because of the reduced efficacy of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase, HMGCR) accumulation after statin treatment, researchers found that, by inducing the degradation of HMGCR, the accumulation of HMGCR can be eliminated and cholesterol can be reduced [52]. Inspired by PROTACs, researchers designed and synthesized the PROTAC molecule P22A based on the E3 ligase CRBN. P22A can promote the expression of endogenous HMGCR proteins in Insig-1- and Insig-2-deficient Chinese hamster ovary (CHO) cells (SRD15 cells) [53]. The HMGCR inhibitor lovastatin (Table 7), which is also derived from microorganisms, is a drug used for the treatment of hypercholesterolemia. It can prevent the development of atherosclerosis, can reduce the incidence of myocardial infarction, and has a good effect on cardiovascular diseases [54]. Using lovastatin acid and VHL ligand conjugation, a powerful HMGCR-targeting PROTAC (21c) was identified that efficiently degrades HMGCR in Insig-silenced HepG2 cells (DC50 = 120 nmol/L). Furthermore, the corresponding lactone prodrug 21b has been shown to afford high plasma exposures referring to the active ingredient 21c, leading to efficient HMGCR degradation and promising cholesterol-lowering potency in vivo (Table 8) [55]. Microbial metabolites can be important sources for discovering natural PROTAC molecules. Recently, the first PROTAC molecule derived from a microbial natural product was reported [56]. The microbial natural product APL-16-5 (Table 7), based on the PROTAC mechanism, simultaneously binds to the E3 ubiquitin ligase TRIM25 and influenza virus PA subunit in order to induce TRIM25-dependent PA ubiquitination and degradation (Table 8). The TRIM25/APL-16-5/PA complex blocks viral RNA replication and has anti-influenza A virus activity in vivo. Apl-16-5 and its derivatives can also be used as TRIM25 ligands to further expand their applications. In addition, researchers suggest that the phenalenone fragment mainly contributes to interactions with PA and that the diterpene fragment determines the binding to TRIM25. The specific synthesis processes of the PROTACs discussed in this section are shown in Scheme 15 and Scheme 16.

Table 7.
Natural products 14–16.

Table 8.
Natural product targets 17–19.
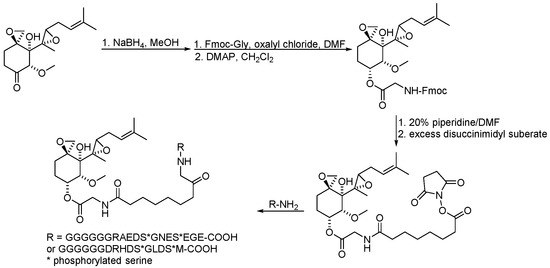
Scheme 15.
Synthesis of ovalicin–based PROTAC.

Scheme 16.
Synthesis of lovastatin–based PROTAC.
2.5. Derived from Peptides
PROTACs designed using peptides to recruit target proteins have certain limitations in applications. However, PROTACs based on peptides are not only easy to modify but also have large protein-protein interaction surfaces, which have certain therapeutic potential for oncoproteins with unknown interactions [57,58]. β-catenin is an extremely attractive cancer target that plays an important role in the Wnt/β-catenin signaling pathway. As a β-catenin-targeted stapled peptide, xStAx (sequence: Ac-RRWPRSILDSHVRRVWR-NH2), derived from the tumor suppressor gene Axin, has been found for the first time to target and degrade β-catenin [59]. The xStAx-VHLL obtained by coupling xStAx with the VHL ligand not only promoted the continuous degradation of β-catenin in the tumor cells of various mouse tumor models but also strongly inhibited Wnt signaling. In addition, xStAx-VHLL also has a significant inhibitory effect on the survival of tumor organoids from patients with colorectal cancer. Researchers designed a cell-permeable peptide-based PROTAC. It was based on the homodimerized leucine-zipper-like motif in the C-terminus domain of the cell-cycle-related and expression-elevated proteins in tumors (CREPT, also named RPRD1B) to induce their degradation in vivo. In pancreatic cancer cells, PROTACs can induce CREPT degradation in a proteasome-dependent manner. Therefore, the use of protein-based dimeric structure interaction motifs may be a new approach to designing PROTACs [60].
In addition, natural products and their derivatives can also be used as ligands to recruit E3 ubiquitin ligases in PROTACs. Bestatin is a natural product derived from streptomyces olivaceus (Table 9). Studies have shown that small hybrid molecules composed of amide derivatives of bestatin and polymeric probes induce Huntingtin protein (Htt) aggregates in Huntington’s disease (HD) to form complexes with the E3 ubiquitin ligase cIAP1, thereby leading to the proteasomal degradation of mHtt, and this approach can also be used to target other aggregation-prone proteins responsible for neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and other polyQ (Table 10) [61]. Nimbolide, a triterpenoid derived from the leaves and flowers of Azadirachta indica L., can inhibit tumor genesis and metastasis (Table 9). Researchers found that the natural product nimbolide is a covalent ligand of the E3 ligase RNF114 via an activity-based proteome profiling (ABPP) platform, and they further confirmed that the recruitment of RNF114 by nimbolide can be used to target protein degradation (Table 10). The PROTACs formed by nimbolide coupled with BCR-ABL fused with the oncogene inhibitor dasatinib BT1 selectively degrade BCR-ABL but not c-ABL in leukemia cells, compared with previously reported CRBN- and VHL-recruited BCR-ABL inhibitors [62,63]. The specific synthesis processes of the PROTACs discussed in this section are shown in Scheme 17 and Scheme 18.

Table 9.
Natural products 17–18.

Table 10.
Natural product targets 20–21.
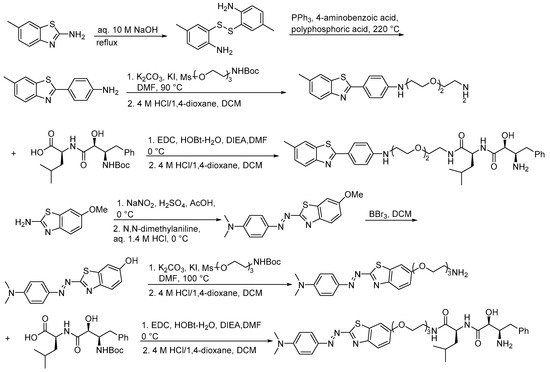
Scheme 17.
Synthesis of bestatin–based PROTAC.

Scheme 18.
Synthesis of nimbolide–based PROTAC.
3. Molecular Glue Degradation Agents Derived from Natural Products
Protein-protein interactions induced by molecular glues also have great application potential in the field of protein degradation. Currently, molecular glues are being examined in clinical studies, but most of them were accidentally discovered, and there is no good drug development strategy at present, so the discovery of new molecular glues is very challenging. Ubiquitin ligases regulate a variety of plant hormone signaling pathways. Studies have shown that, when an F-box protein transport inhibitor response 1 (TIR1) protein binds to auxin (Table 11), it will initiate SCF ubiquitin ligase to degrade downstream auxin/indole-3-acetic acid (Aux/IAA) transcription factors. These proteins are assembled into SCFTIR1 protein complexes in plants. Studies have shown that auxin can regulate the degradation of Aux/IAA proteins by directly binding to TIR1 (Table 12). The regulatory mechanism of TIR1 by auxin suggests that there may be a small molecule in ubiquitin ligases that facilitates protein-protein interactions [64]. Researchers found that the leucine-rich repeat domain of TIR1 contains a phytate cofactor present in plants and eukaryotes (InsP6/phytate), which recognizes auxin and Aux/IAA polypeptide substrates through a single surface pocket [65].

Table 11.
Natural products 19–21.

Table 12.
Natural product targets 22–24.
Nomura’s team found that a manumycin polyketide, asukamycin (Table 11), has multiple electrophilic sites, targets Cys374 of the E3 ligase UBR7 in breast cancer cells, and participates in the molecular glue interaction of the new substrate tumor suppressor TP53 in order to form a UBR7-asukamycin-TP53 complex to exert anticancer effects (Table 12). Combined with chemical proteomic studies, it was found that asukamycin can also serve as a probe for the rational identification of molecular glues from electrophilic natural products [66]. Recently, a natural-product-derived molecular glue has been identified using a human proteome chip (Table 11). The study identified a natural-derived small-molecule bufalin that promotes the rapid degradation of E2F transcription factor 2 (E2F2) and inhibits hepatocellular carcinoma (Table 12). In addition, the pyrone structure of bufalin has been suggested as a potential structural group for targeting the atypical E3 ligase Zinc finger protein 91 (ZFP91). Its carbonyl-conjugated structure may possess electrophilic addition capability in order to covalently bind to the cysteine in ZFP91 [67].
4. Perspectives and Conclusions
This review summarized the progress of protein-targeting degradation agents based on natural products, such as PROTACs and molecular glues (Figure 2). The classes of these natural products include hormones, flavonoids, alkaloids, terpenoids, vitamins, microorganisms, and peptides. In conclusion, PROTACs and molecular glues based on natural products and their derivatives have good application prospects in the targeted degradation of disease-related proteins. In addition, PROTACs mainly degrade intracellular proteins, but 40% of gene products are extracellular and membrane-associated proteins, such as growth factors, cytokines, and chemokines, which cause abnormalities in various diseases, such as pain and inflammation signaling. Lysosome-targeted chimera (LYTAC) is a degradation technology targeting extracellular proteins, and it was reported in Nature by the research team of Professor Bertozzi of Stanford University. The study confirmed that LYTAC successfully degraded the epidermal growth factor receptor (EGFR), programmed death-ligand 1 (PD-L1), and apolipoprotein E4 [68]. Stanford researchers have also shown that LYTACs can target and degrade important proteins in Alzheimer’s disease and cancer in cells. The structure of an LYTAC is similar to that of a PROTAC. One side of an LYTAC is an oligopeptide group that can bind to the transmembrane receptor CI-M6PR on the cell surface, the other side is an antibody or small molecule that can bind to the target protein, and the two sides are connected by a linker. For example, LYTAC AB-2 is a conjugation of the anti-EGFR monogram lowering antibody cetuximab and the CI-M6PR ligand M6Pn sugar polypeptide. The complex can be engulfed by the cell membrane to form transport vesicles and then transferred to the lysosome for degradation under the action of CI-M6PR. The receptor CI-M6PR can be recycled and returned to the cell membrane in order to degrade EGFR. In addition, there are other emerging targeting protein technologies, such as light-controlled target protein degradation (photo-PROTAC), autophagy-mediated target protein degradation (AUTAC), and autophagosome-tethering compounds (ATTECs). These techniques not only expand the pathway of protein degradation but also improve the accuracy of protein degradation.

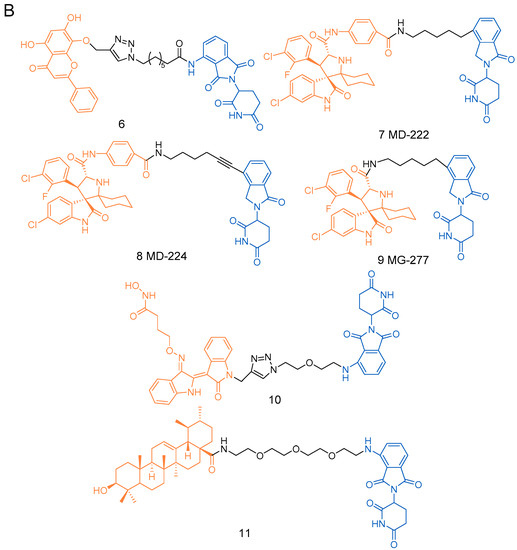

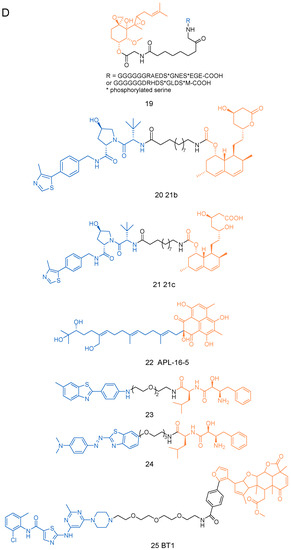
Figure 2.
The structures of PROTACs presented in this review. (A) The structures of PROTACs 1–5f. (B) The structures of PROTACs 6–11f. (C) The structures of PROTACs 12-18f. (D) The structures of PROTACs 19–25f.
In addition, signal transduction associated with disease is an extremely complex and multifactorial regulatory process. Therefore, single-target therapy is often insufficient to curb disease progression, and new multi-target drugs or drug combinations need to be designed to block related signal transduction for different pathways and mechanisms to achieve the effect of treating the disease and reduce the drug resistance [69,70]. Recently, novel dual PROTACs with gefitinib/olaparib and CRBN/VHL E3 ligands as substrates have been reported to degrade EGFR and PARP in cancer cells simultaneously [71]. As the first successful case of dual PROTACs, this technology will greatly broaden the application of PROTAC methods and open up a new field for drug discovery. In addition, recent studies have shown that natural products have structural diversity, high activity, and low toxicity and that their therapeutic effects are generally achieved through multi-target therapy [72]. Therefore, the use of natural products is one of the important ways to obtain multi-targeted drugs, and they are also a hot direction of multi-targeted drug research. Meanwhile, there has been increasing interest in the use of PROTAC and molecular glue strategies to recognize natural product target proteins. With the help of proteomics and other technologies, protein-targeted degradation agents based on natural products will have great application potential in the field of protein-targeted degradation.
Author Contributions
Conceptualization, Y.T. and H.S.; methodology, Y.L.; software, Y.L.; validation, Y.L., Y.T. and H.S.; formal analysis, Y.L.; investigation, Y.J.; resources, Y.J. and X.W.; data curation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, Y.T. and H.S.; visualization, Y.T.; supervision, Y.T.; project administration, Y.T.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the financial support from the National Natural Sciences Foundation of China (Grant No. 82173710), the CAMS Innovation Fund for Medical Science (CIFMS) (Grant No. 2021-I2M-1-028, 2022-I2M-2-002), and the Beijing Natural Science Foundation (Grant No. 7192129).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful for the National Natural Sciences Foundation of China, the CAMS Innovation Fund for Medical Science (CIFMS), and the Beijing Natural Science Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2013, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Vandermolen, K.M.; Mcculloch, W.; Pearce, C.J.; Oberlies, N.H. Romidepsin (Istodax, NSC 630176, FR901228, FK228, depsipeptide): A natural product recently approved for cutaneous T-cell lymphoma. J. Antibiot. Vol. 2011, 64, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Cottens, S.; Kallen, J.; Schuler, W.; Sedrani, R. Derivation of rapamycin: Adventures in natural product chemistry. CHIMIA Int. J. Chem. 2019, 73, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Varma, P.V.C. Molecular targeted therapy: Cancer therapy of the future. WebmedCentral Med. Educ. 2012, 3, WMC003496. [Google Scholar] [CrossRef]
- Kanat, O.; O’Neil, B.; Shahda, S. Targeted therapy for advanced gastric cancer: A review of current status and future prospects. World J. Gastrointest. Oncol. 2015, 7, 401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spaans, J.N.; Goss, G.D. Drug resistance to molecular targeted therapy and its consequences for treatment decisions in non-small-cell lung cancer. Front. Oncol. 2014, 4, 190. [Google Scholar] [CrossRef]
- Nalawansha, D.A.; Crews, C.M. PROTACs: An emerging therapeutic modality in precision medicine. Cell Chem. Biol. 2020, 27, 998–1014. [Google Scholar] [CrossRef]
- Hu, B.C.; Zhou, Y.R.; Sun, D.J.; Yang, Y.Y.; Liu, Y.; Li, X.Z.; Li, H.; Chen, L. PROTACs: New method to degrade transcription regulating proteins. Eur. J. Med. Chem. 2020, 207, 112698. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Q.; Chang, Q.; Hu, W.W.; Hu, G.Y.; Li, Q.B. Application of small molecule PROTAC in different target studies. J. Pharm. Sci. 2020, 7, 446–452. [Google Scholar] [CrossRef]
- Gerry, C.J.; Schreiber, S.L. Unifying principles of bifunctional, proximity-inducing small molecules. Nat. Chem. Biol. 2020, 16, 369–378. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Tandon, I.; Wu, S.; Tang, W. Development of MDM2 degraders based on ligands derived from ugi reactions: Lessons and discoveries. Eur. J. Med. Chem. 2021, 219, 113425. [Google Scholar] [CrossRef]
- Eric, A.; Ariazi, J.L.A.; Fernando Cordera, V. Craig Jordan. Estrogen receptors as therapeutic targets in breast cancer. Curr. Top. Med. Chem. 2006, 6, 181–202. [Google Scholar] [CrossRef]
- Covaleda, A.M.; van den Berg, H.; Vervoort, J.; van der Saag, P.; Strom, A.; Gustafsson, J.A.; Rietjens, I.; Murk, A.J. Influence of cellular ERa/ERb ratio on the ERa-agonist induced proliferation of human T47D breast cancer cells. Toxicol. Sci. 2008, 105, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Cyrus, K.; Wehenkel, M.; Choi, E.Y.; Lee, H.; Kim, K.B. Jostling for position: Optimizing linker location in the design of estrogen receptor-targeting PROTACs. ChemMedChem 2010, 5, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xiang, H.; Luo, G. Targeting estrogen receptor α for degradation with PROTACs: A promising approach to overcome endocrine resistance. Eur. J. Med. Chem. 2020, 206, 112689. [Google Scholar] [CrossRef]
- Wang, L.; Guillen, V.S.; Sharma, N.; Flessa, K.; Sharma, A. New class of selective estrogen receptor degraders (SERDs): Expanding the toolbox of PROTAC degrons. ACS Med. Chem. Lett. 2018, 9, 803–808. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Baek, S.H.; Lee, H.; Kim, K.; Mohan, R. Use of PROTACS as molecular probes of angiogenesis. Bioorg. Med. Chem. Lett. 2005, 15, 2724–2727. [Google Scholar] [CrossRef]
- do Pazo, C.; Webster, R.M. The prostate cancer drug market. Nature reviews. Drug Discov. 2021, 20, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, L.; Xiang, W.; Qin, C.; Wang, S. Discovery of highly potent and efficient PROTAC degraders of androgen receptor (AR) by employing weak binding affinity VHL E3 ligase ligands. J. Med. Chem. 2019, 62, 11218–11231. [Google Scholar] [CrossRef] [PubMed]
- Lzab, E.; Xin, H.; Jlb, E.; Meb, E.; Swbcd, E. A highly potent PROTAC androgen receptor (AR) degrader ARD-61 effectively inhibits AR-positive breast cancer cell growth in vitro and tumor growth in vivo. Neoplasia 2020, 22, 522–532. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Verma, R.; Ransick, A.; Stein, B.; Crews, C.M.; Deshaies, R.J. Development of protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol. Cell. Proteom. Mcp 2003, 2, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Schneekloth, J.S.; Fonseca, F.N.; Koldobskiy, M.; Mandal, A.; Crews, C.M. Chemical genetic control of protein levels: Selective in vivo targeted degradation. J. Am. Chem. Soc. 2004, 126, 3748–3754. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Lin, I.C.; Kuo, P.L.; Lin, C.C. Anti-proliferative effect of apigenin and its apoptotic induction in human Hep G2 cells. Cancer Lett. 2006, 237, 207–214. [Google Scholar] [CrossRef]
- Puppala, D.; Gairola, C.G.; Swanson, H.I. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis 2007, 28, 639–647. [Google Scholar] [CrossRef]
- Lee, H.; Puppala, D.; Choi, E.Y.; Swanson, H.; Kim, K.B. Targeted degradation of the aryl hydrocarbon receptor by the PROTAC approach: A useful chemical genetic tool. ChemBioChem 2007, 8, 2058–2062. [Google Scholar] [CrossRef] [PubMed]
- Berg, P. Differential usage of nuclear export sequences regulates intracellular localization of the dioxin (aryl hydrocarbon) receptor. J. Biol. Chem. 2001, 276, 43231–43238. [Google Scholar] [CrossRef]
- Zheng, Z.C.; Zhu, W.; Lei, L.; Liu, X.Q.; Wu, Y.G. Wogonin ameliorates renal inflammation and fibrosis by inhibiting NF-κB and TGF-β1/Smad3 signaling pathways in diabetic nephropathy. Drug Des. Dev. Ther. 2020, 14, 4135–4148. [Google Scholar] [CrossRef]
- Wang, J.; Ge, R.; Qiu, X.; Xu, Q.; Wei, L.; Li, Z. Discovery and synthesis of novel wogonin derivatives with potent antitumor activity in vitro. Proc. Eur. J. Med. Chem. 2018, 140, 421–434. [Google Scholar] [CrossRef]
- Bian, J.; Jie, R.; Li, Y.; Wang, J.; Xi, X.; Feng, Y.; Hui, T.; Wang, Y.; Li, Z. Discovery of wogonin-based PROTACs against CDK9 and capable of achieving antitumor activity. Bioorganic Chem. 2018, 81, 373–381. [Google Scholar] [CrossRef]
- Aguilar, A.; Sun, W.; Liu, L.; Lu, J.F.; McEachern, D.; Bernard, D.; Deschamps, J.R.; Wang, S. Design of Chemically Stable, Potent, and Efficacious MDM2 Inhibitors That Exploit the Retro-Mannich Ring-Opening-Cyclization Reaction Mechanism in Spiro-oxindoles. J. Med. Chem. 2014, 57, 10486–10498. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Hines, J.; Lartigue, S.; Dong, H.Q.; Qian, Y.M.; Crews, C.M. MDM2-recruiting PROTAC offers superior, synergistic anti-proliferative activity via simultaneous degradation of BRD4 and stabilization of p53. Cancer Res. 2018, 79, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wurz, R.P.; Cee, V.J. Targeted degradation of MDM2 as a new approach to improve the efficacy of MDM2-p53 inhibitors. J. Med. Chem. 2018, 62, 445–447. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Aguilar, A.; McEachern, D.; Przybranowski, S.; Liu, L.; Yang, C.Y.; Wang, M.; Han, X.; Wang, S. Discovery of MD-224 as a first-in-Class, highly potent, and efficacious proteolysis targeting chimera murine double minute 2 degrader capable of achieving complete and durable tumor regression. J. Med. Chem. 2019, 62, 448–466. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Aguilar, A.; Liu, Z.; Wang, S. Simple structural modifications converting a bona fide MDM2 PROTAC degrader into a molecular glue molecule: A cautionary tale in the design of PROTAC degraders. J. Med. Chem. 2019, 62, 9471–9487. [Google Scholar] [CrossRef]
- Qi, Z.; Yang, G.; Deng, T.; Wang, J.; Zhou, H.; Popov, S.; Shults, E.; Wang, C. Design and linkage optimization of ursane-thalidomide-based PROTACs and identification of their targeted-degradation properties to MDM2 protein. Bioorganic Chem. 2021, 111, 104901. [Google Scholar] [CrossRef]
- Cao, Z.; Gu, Z.; Lin, S.; Chen, D.; Wang, J.; Zhao, Y.; Li, Y.; Liu, T.; Li, Y.; Wang, Y. Attenuation of NLRP3 inflammasome activation by indirubin-derived PROTAC targeting HDAC6. ACS Chem. Biol. 2021, 16, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Luan, X.; Jin, J.M.; Chen, H.Z.; Wu, Y.; Zhou, Q. A kind of PROTAC compound based on toosendanin with STAT3 degrading activity and its preparation method and application. CN 202111198270.4 [P], 31 December 2021. [Google Scholar]
- Li, H.W.; Wang, S.X.; Ma, W.X.; Cheng, B.Y.; Yi, Y.L.; Ma, X.Y.; Xiao, S.; Zhang, L.; Zhou, D. Discovery of pentacyclic triterpenoid PROTACs as a class of effective hemagglutinin protein degraders. J. Med. Chem. 2022, 65, 7154–7169. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.S.; Wang, P.; Liu, X.L.; Fu, Q.Y.; Xie, Y.T.; Zhou, Y.; Qi, X.B.; Huang, N. Identification of PDE6D as a Potential Target of Sorafenib via PROTAC Technology. Available online: https://www.biorxiv.org/content/10.1101/2020.05.06.079947v2 (accessed on 30 September 2020).
- Wu, Y.L.; Yang, Y.Y.; Wang, W.; Sun, D.J.; Liang, J.; Zhu, M.; Li, H.; Chen, L.X. PROTAC technology as a novel tool to identify the target of lathyrane diterpenoids. Acta Pharm. Sin. B 2022, 12, 4262–4265. [Google Scholar] [CrossRef]
- Favorskaya, I.; Kainov, Y.; Chemeris, G.; Komelkov, A.; Zborovskaya, I.; Tchevkina, E. Expression and clinical significance of CRABP1 and CRABP2 in non-small cell lung cancer. Tumour. Biol. 2014, 35, 10295–10300. [Google Scholar] [CrossRef]
- Liu, R.Z.; Garcia, E.; Glubrecht, D.D.; Poon, H.Y.; Mackey, J.R.; Godbout, R. CRABP1 is associated with a poor prognosis in breast cancer: Adding to the complexity of breast cancer cell response to retinoic acid. Mol. Cancer 2015, 14, 129. [Google Scholar] [CrossRef]
- Ohoka, N.; Tsuji, G.; Shoda, T.; Fujisato, T.; Naito, M. Development of small molecule chimeras that recruit AhR E3 ligase to target proteins. ACS Chem. Biol. 2019, 14, 2822–2832. [Google Scholar] [CrossRef]
- Sahar, S.; Stepan, M.; Pogribny, I.P.; Wang, W.; Francois, H.T.; Deng, L.; Jacquetta, T.; Jill, J.S.; Rima, R. Studies of methionine cycle intermediates (SAM, SAH), DNA methylation and the impact of folate deficiency on tumor numbers in Min mice. Carcinogenesis 2002, 23, 61–65. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Liu, Y.; Shen, Y.; Wei, W. Cancer selective target degradation by folate-caged PROTACs. J. Am. Chem. Soc. 2021, 143, 7380–7387. [Google Scholar] [CrossRef] [PubMed]
- Lazáry, S.; Sthelin, H. Immunosuppressive and specific antimitotic effects of ovalicin. Experientia 1968, 24, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E.C.; Su, Z.; Niwayama, S.; Ramsay, C.A. Molecular recognition of angiogenesis inhibitors fumagillin and ovalicin by methionine aminopeptidase 2. Proc. Natl. Acad. Sci. USA 1998, 95, 15183–15188. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Y.; Li, H.; Tang, J.J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.K.; Shi, X.J.; Cui, H.W.; Tang, J.; et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat. Commun. 2018, 9, 5138. [Google Scholar] [CrossRef]
- Li, M.X.; Yang, Y.Q.; Zhao, Q.Y.; Wu, Y.; Song, L.; Yang, H.Y.; He, M.; Gao, H.; Song, B.L.; Luo, J.; et al. Degradation versus inhibition: Development of proteolysis-targeting chimeras for overcoming statin-induced compensatory upregulation of 3hydroxy-3-methylglutaryl coenzyme a reductase. J. Med. Chem. 2020, 63, 4908–4928. [Google Scholar] [CrossRef]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.S.; Li, Z.B.; Lin, X.; Li, X.Y.; Chen, Y.; Xi, K.; Xiao, M.; Wei, H.; Zhu, L.; Xiang, H. Discovery of an orally active VHL-recruiting PROTAC that achieves robust HMGCR degradation and potent hypolipidemic activity invivo. Acta Pharm. Sin. B 2020, 11, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Y.; Wang, J.; Pang, X.; Liu, Z.L.; Li, Q.J.; Yi, D.R.; Zhang, Y.; Fang, X.; Zhang, T.; Zhou, R.; et al. An anti-influenza A virus microbial metabolite acts by degrading viral endonuclease PA. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Deng, Q.W.; Zhao, H.; Xie, M.S.; Chen, L.J.; Yin, F.; Qin, X.; Zheng, W.; Zhao, Y.J.; Li, Z. Development of stabilized peptide-based PROTACs against estrogen receptor α. ACS Chem. Biol. 2017, 13, 628–635. [Google Scholar] [CrossRef]
- Ivanov, A.; Khuri, F.R.; Fu, H. Targeting protein-protein interactions as an anticancer strategy. Trends Pharmacol. Sci. 2013, 34, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, X.; Zhao, L.; Wang, Y.; Chen, Y.G. A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discov. 2020, 6, 35. [Google Scholar] [CrossRef]
- Ma, D.; Zou, Y.; Chu, Y.; Liu, Z.; Chang, Z. A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer. Theranostics 2020, 10, 3708–3721. [Google Scholar] [CrossRef]
- Tomoshige, S.; Nomura, S.; Ohgane, K.; Hashimoto, Y.; Ishikawa, M. Discovery of Small Molecules that Induce the Degradation of Huntingtin. Angew. Chem. 2017, 56, 11530–11533. [Google Scholar] [CrossRef]
- Tong, B.; Spradlin, J.N.; Novaes, L.; Zhang, E.; Nomura, D.K. A nimbolide-based kinase degrader preferentially degrades oncogenic BCR-ABL. ACS Chem. Biol. 2020, 15, 1788–1794. [Google Scholar] [CrossRef]
- Burslem, G.M.; Schultz, A.R.; Bondeson, D.P.; Eide, C.A.; Crews, C.M. Targeting BCR-ABL1 in chronic myeloid leukemia by PROTAC-mediated targeted protein degradation. Cancer Res. 2019, 79, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef]
- Tan, X.; Calderon-Villalobos, L.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Okumura, M.; Mcgregor, L.M.; Brittain, S.M.; Nomura, D.K. Manumycin polyketides act as molecular glues between UBR7 and P53. Nat. Chem. Biol. 2020, 16, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Heng, Y.; Zhuo, F.F.; Yang, Z.; Zhao, M.M.; Guo, Q.; Liu, Y.; Liu, D.; Zeng, K.W.; Tu, P.F. Atypical E3 ligase ZFP91 promotes small-molecule-induced E2F2 transcription factor degradation for cancer therapy. EBioMedicine 2022, 86, 104353. [Google Scholar] [CrossRef]
- Banik, S.M.; Pedram, K.; Wisnovsky, S.; Ahn, G.; Bertozzi, C.R. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584, 1–7. [Google Scholar] [CrossRef]
- Upadhyay, H.C. Coumarin-1,2,3-triazole hybrid molecules: An emerging scaffold for combating drug resistance. Curr. Top. Med. Chem. 2021, 21, 737–752. [Google Scholar] [CrossRef]
- Xu, J.; Regan-Fendt, K.; Deng, S.; Carson, W.E.; Payne, P.R.O.; Li, F. Diffusion mapping of drug targets on disease signaling network elements reveals drug combination strategies. Pac. Symp. Biocomput. 2018, 23, 92–103. [Google Scholar] [CrossRef]
- Zheng, M.; Huo, J.; Gu, X.; Wu, C.; Li, H. Rational design and synthesis of novel dual protacs for simultaneous degradation of EGFR and PARP. J. Med. Chem. 2021, 64, 7839–7852. [Google Scholar] [CrossRef]
- Newman, D.J.; Giddings, L.A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014, 13, 123–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).