Favipiravir—Tautomeric and Complexation Properties in Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Tautomerism of Favipiravir
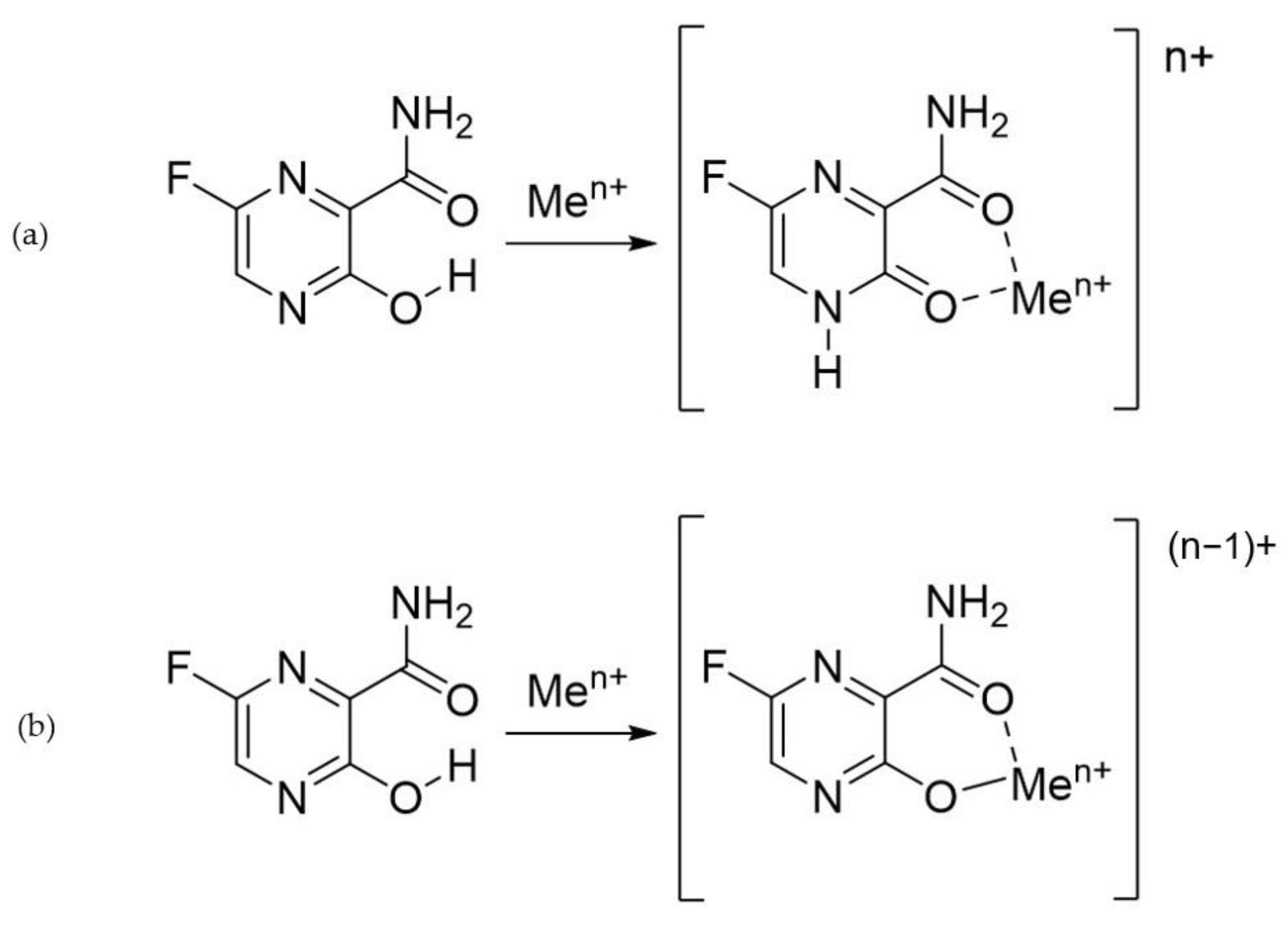
2.2. Complex Formation
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pospisil, P.; Ballmer, P.; Scapozza, L.; Folkers, G. Tautomerism in Computer-Aided Drug Design. J. Recept. Signal Transduct. 2003, 23, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Hall, C.D.; El-Gendy, B.E.-D.M.; Draghici, B. Tautomerism in Drug Discovery. J. Comput. Aided Mol. Des. 2010, 24, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. Let’s Not Forget Tautomers. J. Comput. Aided Mol. Des. 2009, 23, 693. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.I. Theoretical Consideration of In-Solution Tautomeric Equilibria in Relation to Drug Design. In Tautomerism: Concepts and Applications in Science and Technology; Antonov, L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 113–146. ISBN 978-3-527-69571-3. [Google Scholar]
- Sayle, R.A. So You Think You Understand Tautomerism? J. Comput. Aided Mol. Des. 2010, 24, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. Experimental and PKa Prediction Aspects of Tautomerism of Drug-like Molecules. Drug Discov. Today Technol. 2018, 27, 59–64. [Google Scholar] [CrossRef]
- Göller, A.H. Reliable Gas-Phase Tautomer Equilibria of Drug-like Molecule Scaffolds and the Issue of Continuum Solvation. J. Comput. Aided Mol. Des. 2022, 36, 805–824. [Google Scholar] [CrossRef]
- Borbulevych, O.Y.; Martin, R.I.; Westerhoff, L.M. The Critical Role of QM/MM X-Ray Refinement and Accurate Tautomer/Protomer Determination in Structure-Based Drug Design. J. Comput. Aided Mol. Des. 2021, 35, 433–451. [Google Scholar] [CrossRef]
- Milletti, F.; Storchi, L.; Sforna, G.; Cross, S.; Cruciani, G. Tautomer Enumeration and Stability Prediction for Virtual Screening on Large Chemical Databases. J. Chem. Inf. Model. 2009, 49, 68–75. [Google Scholar] [CrossRef]
- Seidel, T.; Wolber, G. Ligand-Side Tautomer Enumeration and Scoring for Structure-Based Drug-Design. J. Cheminform. 2010, 2, P32. [Google Scholar] [CrossRef]
- Wahl, O.; Sander, T. Tautobase: An Open Tautomer Database. J. Chem. Inf. Model. 2020, 60, 1085–1089. [Google Scholar] [CrossRef]
- Guasch, L.; Yapamudiyansel, W.; Peach, M.L.; Kelley, J.A.; Barchi, J.J.; Nicklaus, M.C. Experimental and Chemoinformatics Study of Tautomerism in a Database of Commercially Available Screening Samples. J. Chem. Inf. Model. 2016, 56, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Kuno-Maekawa, M.; Sangawa, H.; Uehara, S.; Kozaki, K.; Nomura, N.; Egawa, H.; Shiraki, K. Mechanism of Action of T-705 against Influenza Virus. AAC 2005, 49, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (Favipiravir) and Related Compounds: Novel Broad-Spectrum Inhibitors of RNA Viral Infections. Antivir. Res. 2009, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a Broad Spectrum Inhibitor of Viral RNA Polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an Anti-Influenza Drug against Life-Threatening RNA Virus Infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Konstantinova, I.D.; Andronova, V.L.; Fateev, I.V.; Esipov, R.S. Favipiravir and Its Structural Analogs: Antiviral Activity and Synthesis Methods. Acta Nat. 2022, 14, 16–38. [Google Scholar] [CrossRef]
- Smyk, J.M.; Majewska, A. Favipiravir in the Battle with Respiratory Viruses. MRMC 2022, 22, 2224–2236. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A New and Emerging Antiviral Option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, S.; Gao, J. Discovering Drugs to Treat Coronavirus Disease 2019 (COVID-19). DD&T 2020, 14, 58–60. [Google Scholar] [CrossRef]
- Odnovorov, A.I.; Grebennikova, T.V.; Pleteneva, T.V. Specific Influenza Therapy: Current State and Prospects (Review). Razrab. Regist. Lek. Sredstv 2020, 9, 83–91. [Google Scholar] [CrossRef]
- Rattanaumpawan, P.; Jirajariyavej, S.; Lerdlamyong, K.; Palavutitotai, N.; Saiyarin, J. Real-World Effectiveness and Optimal Dosage of Favipiravir for Treatment of COVID-19: Results from a Multicenter Observational Study in Thailand. Antibiotics 2022, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Vora, A.; Venugopal, K.; Dadhich, P.; Daxini, A.; Bhagat, S.; Patil, S.; Barkate, H. Real-World Experience with Favipiravir for the Treatment of Mild-to-Moderate COVID-19 in India. POR 2022, 13, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kulzhanova, S.A.; Konkayeva, M.E.; Smagulova, Z.K.; Beisenbieva, N.N.; Tuleshova, G.T.; Utegenova, A.M.; Turebaeva, G.O.; Nurakhmetova, G.A. A Retrospective Analysis of Treatment of Covid-19 with Favipiravir. J. Biomed. Clin. Res. 2022, 15, 74–78. [Google Scholar] [CrossRef]
- Balykova, L.A.; Zaslavskaya, K.Y.; Pavelkina, V.F.; Pyataev, N.A.; Selezneva, N.M.; Kirichenko, N.V.; Ivanova, A.Y.; Rodoman, G.V.; Kolontarev, K.B.; Skrupsky, K.S.; et al. Effectiveness and safety of favipiravir infusion in patients hospitalized with covid-19. Farm. Farmakol. 2022, 10, 113–126. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clin. Pharmacol. Ther. 2020, 108, 242–247. [Google Scholar] [CrossRef]
- Hayden, F.G.; Lenk, R.P.; Stonis, L.; Oldham-Creamer, C.; Kang, L.L.; Epstein, C. Favipiravir Treatment of Uncomplicated Influenza in Adults: Results of Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Trials. J. Infect. Dis. 2022, 226, 1790–1799. [Google Scholar] [CrossRef]
- Ruzhentsova, T.A.; Oseshnyuk, R.A.; Soluyanova, T.N.; Dmitrikova, E.P.; Mustafaev, D.M.; Pokrovskiy, K.A.; Markova, T.N.; Rusanova, M.G.; Kostina, N.E.; Agafina, A.S.; et al. Phase 3 Trial of Coronavir (Favipiravir) in Patients with Mild to Moderate COVID-19. Am. J. Transl. Res. 2021, 13, 12575–12587. [Google Scholar] [CrossRef]
- Golan, Y.; Campos, J.A.S.; Woolson, R.; Cilla, D.; Hanabergh, R.; Gonzales-Rojas, Y.; Lopez, R.; Finberg, R.; Balboni, A. Favipiravir in Patients With Early Mild-to-Moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Trial. Clin. Infect. Dis. 2022, ciac712. [Google Scholar] [CrossRef]
- Doi, Y.; Ishihara, T.; Banno, S.; Ando, M.; Kondo, M. Favipiravir for Symptomatic COVID-19: A Nationwide Observational Cohort Study. J. Infect. Chemother. 2022, S1341321X22002914. [Google Scholar] [CrossRef]
- Chen, C.; Huang, J.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; Luo, Y.; Zhang, J.; et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Salam, A.; Horby, P.; Hayden, F.G.; Chen, C.; Pan, J.; Zheng, J.; Lu, B.; Guo, L.; et al. Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection. J. Infect. Dis. 2020, 221, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Alshamsi, R.K.; Thomas, D.; Hussein, S. COVID-19 Clearance with Favipiravir—A Retrospective Study. J. Commun. Dis. 2022, 258–262. [Google Scholar] [CrossRef]
- Wattana, K.; Uitrakul, S.; Leesakulpisut, N.; Khunkit, P. Potential Drug Interaction Between Favipiravir and Warfarin in Patients With COVID-19: A Real-World Observational Study. J. Clin. Pharma 2022. ahead of print. [Google Scholar] [CrossRef]
- Lowe, D.M.; Brown, L.-A.K.; Chowdhury, K.; Davey, S.; Yee, P.; Ikeji, F.; Ndoutoumou, A.; Shah, D.; Lennon, A.; Rai, A.; et al. Favipiravir, Lopinavir-Ritonavir, or Combination Therapy (FLARE): A Randomised, Double-Blind, 2 × 2 Factorial Placebo-Controlled Trial of Early Antiviral Therapy in COVID-19. PLoS Med. 2022, 19, e1004120. [Google Scholar] [CrossRef] [PubMed]
- Go, N.; Mellow, P.A.; Dewi, B.D.N. COVID-19 Therapy: Comparison effectivity between remdesivir and favipiravir. JWMJ 2022, 4, 268–273. [Google Scholar] [CrossRef]
- Beak, P.; Fry, F.S. Equilibrium between 2-Hydroxypyridine and 2-Pyridone in the Gas Phase. J. Am. Chem. Soc. 1973, 95, 1700–1702. [Google Scholar] [CrossRef]
- Beak, P. Energies and Alkylations of Tautomeric Heterocyclic Compounds: Old Problems—New Answers. Acc. Chem. Res. 1977, 10, 186–192. [Google Scholar] [CrossRef]
- Beak, P.; Covington, J.B.; Smith, S.G.; White, J.M.; Zeigler, J.M. Displacement of Protomeric Equilibriums by Self-Association: Hydroxypyridine-Pyridone and Mercaptopyridine-Thiopyridone Isomer Pairs. J. Org. Chem. 1980, 45, 1354–1362. [Google Scholar] [CrossRef]
- Elguero, J. (Ed.) The Tautomerism of Heterocycles; Advances in Heterocyclic Chemistry: Supplement; Academic Press: New York, NY, USA, 1976; ISBN 978-0-12-020651-3. [Google Scholar]
- Tobias, S.; Günther, H. Tautomerism in OH-, SH-, and NH2-Substituted Pyrazines—A Carbon-13 and Nitrogen-15 NMR Study. Tetrahedron Lett. 1982, 23, 4785–4788. [Google Scholar] [CrossRef]
- Elguero, J.; Katritzky, A.R.; Denisko, O.V. Prototropic Tautomerism of Heterocycles: Heteroaromatic Tautomerism—General Overview and Methodology. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 76, pp. 1–84. ISBN 978-0-12-020776-3. [Google Scholar]
- Elguero, J. Tautomerism: A Historical Perspective. In Tautomerism; Antonov, L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–10. ISBN 978-3-527-69571-3. [Google Scholar]
- Schulman, S.G.; Underberg, W.J.M. Excitation wavelength dependence of prototropic dissociation and tautomerism of salicylamide in the lowest excited singlet state. Photochem. Photobiol. 1979, 29, 937–941. [Google Scholar] [CrossRef]
- Woolfe, G.J.; Thistlethwaite, P.J. Excited-State Prototropic Reactivity in Salicylamide and Salicylanilide. J. Am. Chem. Soc. 1980, 102, 6917–6923. [Google Scholar] [CrossRef]
- Nishiya, T.; Yamauchi, S.; Hirota, N.; Baba, M.; Hanazaki, I. Fluorescence Studies of Intramolecularly Hydrogen-Bonded o-Hydroxyacetophenone, Salicylamide, and Related Molecules. J. Phys. Chem. 1986, 90, 5730–5735. [Google Scholar] [CrossRef]
- Kramer, H.E.A. Tautomerism by Hydrogen Transfer in Salicylates, Triazoles and Oxazoles. In Photochromism; Elsevier: Amsterdam, The Netherlands, 2003; pp. 654–684. ISBN 978-0-444-51322-9. [Google Scholar]
- Sobczyk, L.; Chudoba, D.; Tolstoy, P.; Filarowski, A. Some Brief Notes on Theoretical and Experimental Investigations of Intramolecular Hydrogen Bonding. Molecules 2016, 21, 1657. [Google Scholar] [CrossRef] [PubMed]
- Antonov, L. Favipiravir Tautomerism: A Theoretical Insight. Theor. Chem. Acc. 2020, 139, 145. [Google Scholar] [CrossRef]
- Assis, L.C.; de Castro, A.A.; de Jesus, J.P.A.; da Cunha, E.F.F.; Nepovimova, E.; Krejcar, O.; Kuca, K.; Ramalho, T.C.; de Almeida La Porta, F. Theoretical Insights into the Effect of Halogenated Substituent on the Electronic Structure and Spectroscopic Properties of the Favipiravir Tautomeric Forms and Its Implications for the Treatment of COVID-19. RSC Adv. 2021, 11, 35228–35244. [Google Scholar] [CrossRef] [PubMed]
- Harismah, K.; Shahrtash, S.A.; Arabi, A.R.; Khadivi, R.; Mirzaei, M.; Akhavan-Sigari, R. Favipiravir Attachment to a Conical Nanocarbon: DFT Assessments of the Drug Delivery Approach. Comput. Theor. Chem. 2022, 1216, 113866. [Google Scholar] [CrossRef]
- Sedmidubská, B.; Luxford, T.F.M.; Kočišek, J. Electron Attachment to Isolated and Microhydrated Favipiravir. Phys. Chem. Chem. Phys. 2021, 23, 21501–21511. [Google Scholar] [CrossRef]
- Umar, Y. Theoretical Studies of the Rotational and Tautomeric States, Electronic and Spectroscopic Properties of Favipiravir and Its Structural Analogues: A Potential Drug for the Treatment of COVID-19. J. Taibah Univ. Sci. 2020, 14, 1613–1625. [Google Scholar] [CrossRef]
- Alver, Ö.; Parlak, C.; Umar, Y.; Ramasami, P. DFT/QTAIM Analysis of Favipiravir Adsorption on Pristine and Silicon Doped C20 Fullerenes. Main Group Met. Chem. 2019, 42, 143–149. [Google Scholar] [CrossRef]
- Negi, P.; Prakash, S.; Patil, V.M. Structure Based Drug Design Approach to Identify Potential SARS-CoV-2 Polymerase Inhibitors. COVID 2021, 2, 507–515. [Google Scholar] [CrossRef]
- Raczyńska, E.D.; Kosińska, W.; Ośmiałowski, B.; Gawinecki, R. Tautomeric Equilibria in Relation to Pi-Electron Delocalization. Chem. Rev. 2005, 105, 3561–3612. [Google Scholar] [CrossRef]
- Antonov, L.; Nedeltcheva, D. Resolution of Overlapping UV–Vis Absorption Bands and Quantitative Analysis. Chem. Soc. Rev. 2000, 29, 217–227. [Google Scholar] [CrossRef]
- Antonov, L.; Petrov, V. Quantitative Analysis of Undefined Mixtures—“Fishing Net” Algorithm. Anal. Bioanal. Chem. 2002, 374, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.C.; Antonov, L. Excited-State Intramolecular Proton Transfer: A Short Introductory Review. Molecules 2021, 26, 1475. [Google Scholar] [CrossRef] [PubMed]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.; Weissberger, A. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; Techniques of Chemistry; Wiley: New York, NY, USA, 1986; ISBN 978-0-471-08467-9. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06 Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 119, 525. [Google Scholar] [CrossRef]
- Antonov, L. Tautomerism in Azo and Azomethyne Dyes: When and If Theory Meets Experiment. Molecules 2019, 24, 2252. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, S.; Antonov, L.; Okuno, Y. Prediction of the Color of Dyes by Using Time-Dependent Density Functional Theory. Bulg. Chem. Commun. 2014, 46, 228–237. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
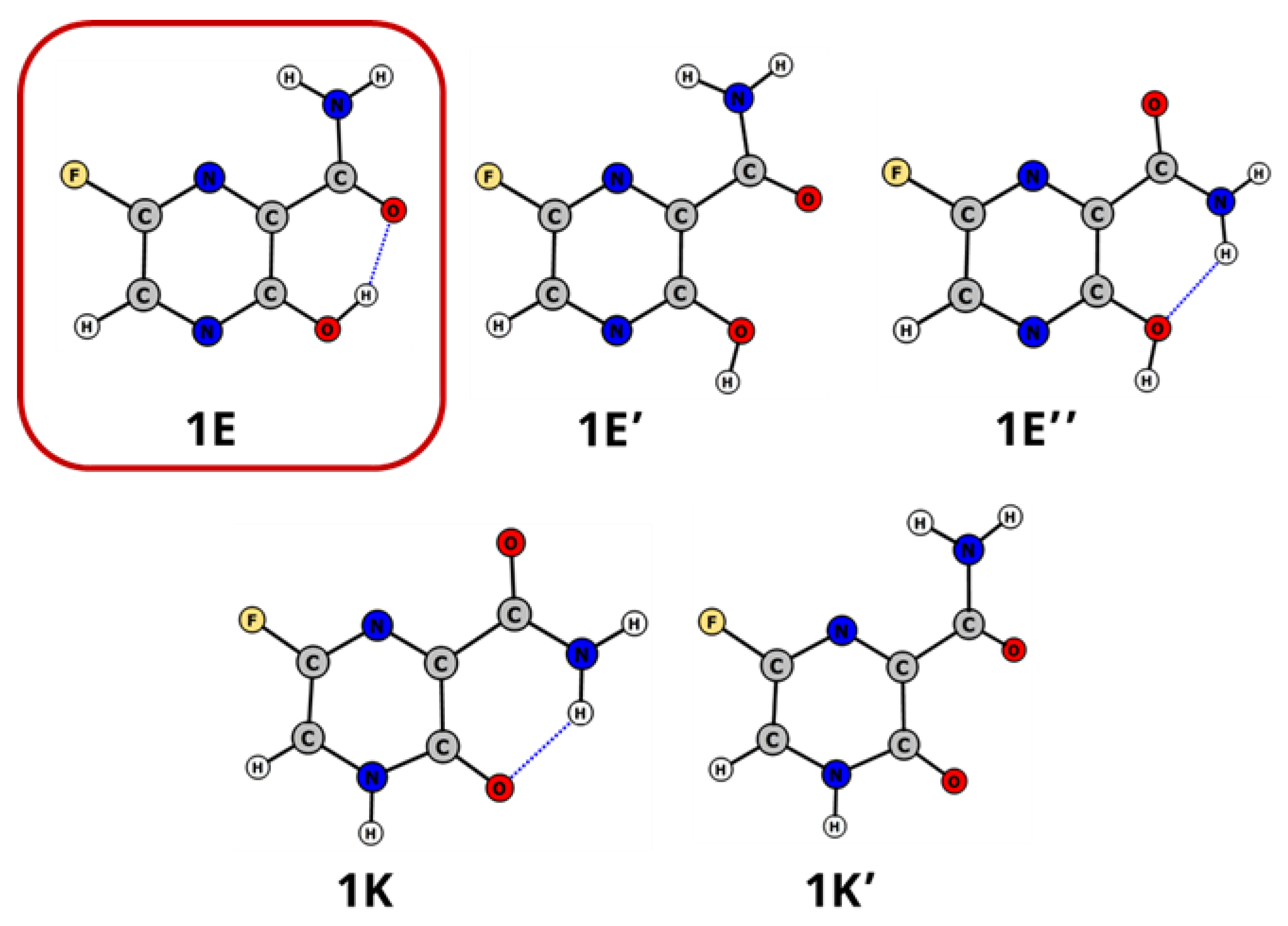
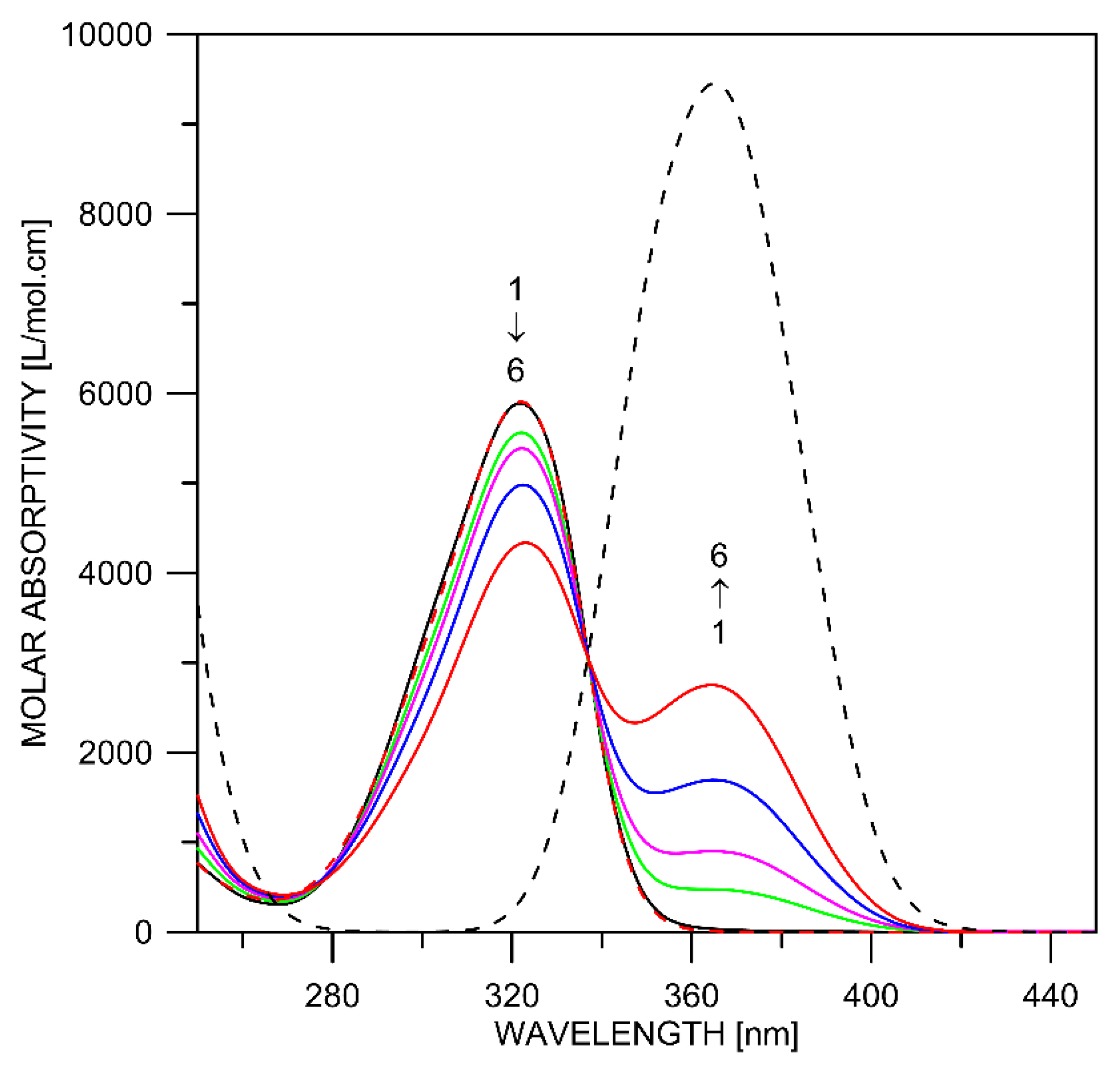

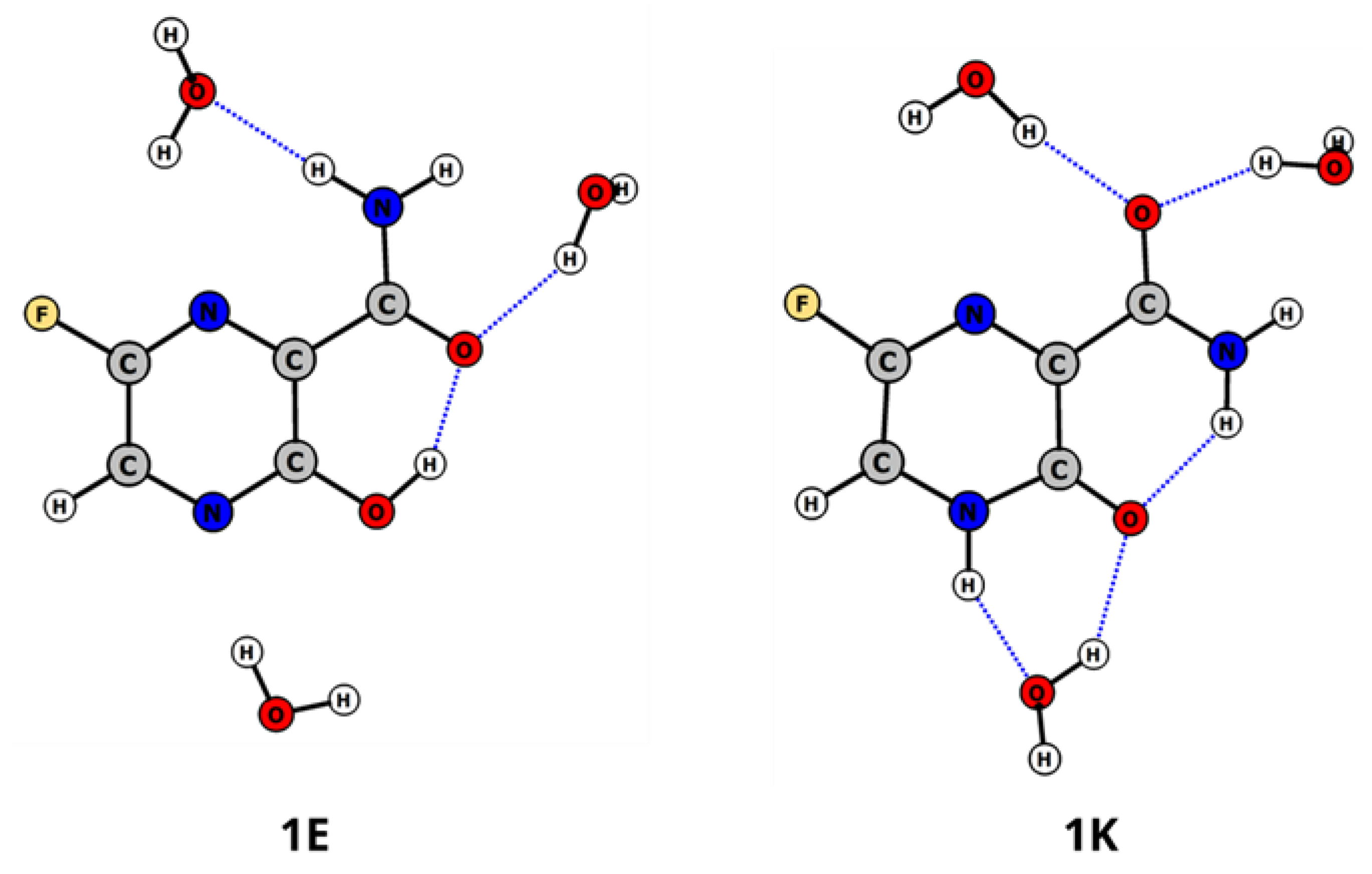
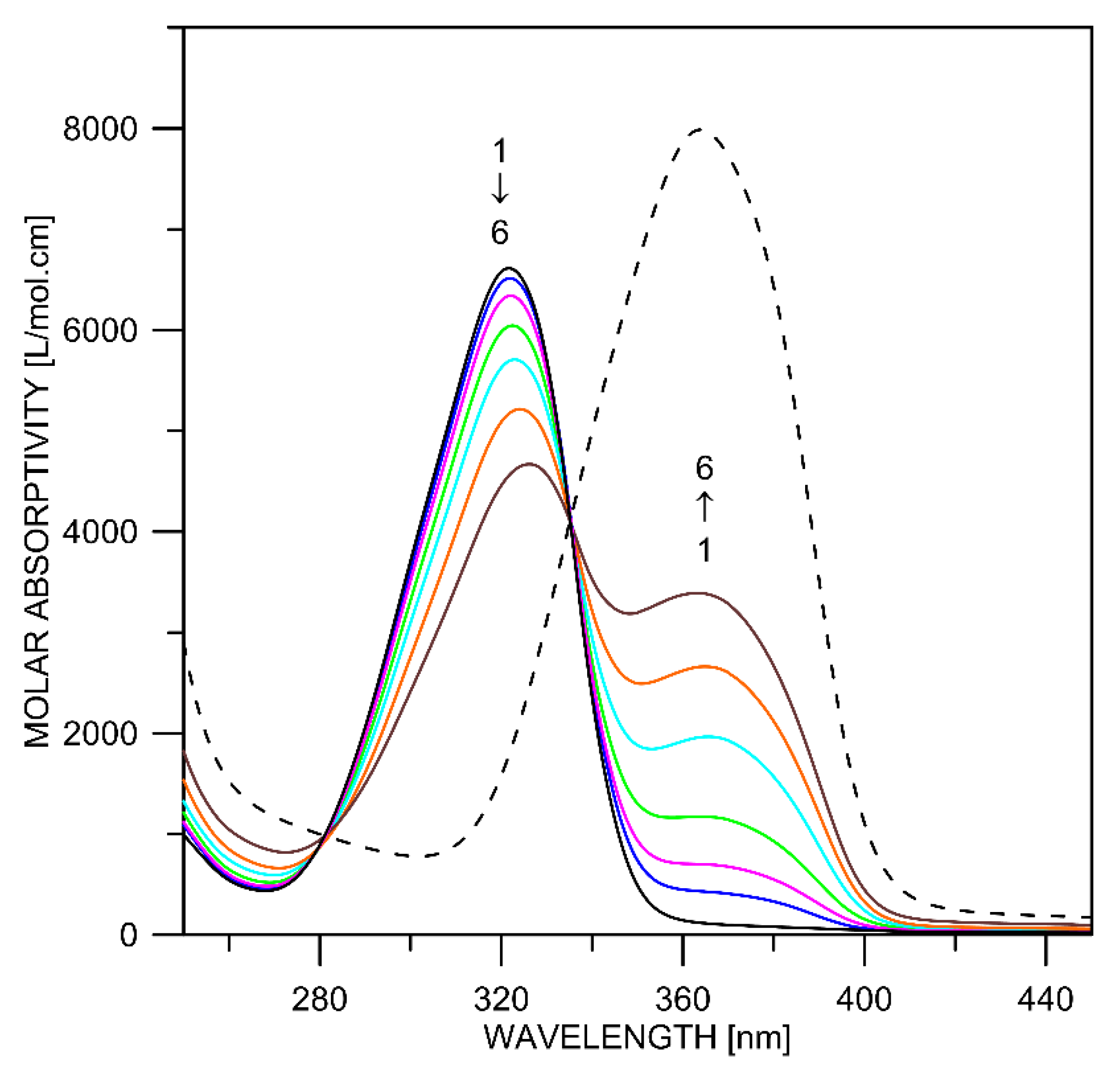
| Tautomer | Solvent | ΔE(298 K) [kcal/mol] | ΔE(0 K) [kcal/mol] | ΔG(298 K) [kcal/mol] | λmax *,** [nm] | f * | 1H NMR NH/OH [ppm] |
|---|---|---|---|---|---|---|---|
| 1E | Toluene | 0.00 | 0.00 | 0.00 | 305 | 0.21 | 13.28 |
| Acetonitrile | 0.00 | 0.00 | 0.00 | 301 | 0.20 | 13.61 | |
| Water | 0.00 | 0.00 | 0.00 | 301 | 0.20 | 13.63 | |
| 1K | Toluene | 10.70 | 10.84 | 10.45 | 340 | 0.18 | 9.09 |
| Acetonitrile | 6.87 | 7.13 | 6.81 | 340 | 0.19 | 9.70 | |
| Water | 6.66 | 6.93 | 6.61 | 340 | 0.19 | 9.73 |
| Water [vol. %] | 1E [%] | 1K [%] | KT * | ΔG298K [kcal/mol] |
|---|---|---|---|---|
| 0 | 100 | 0 | 0.00 | - |
| 20 | 95 | 5 | 0.05 | −1.74 |
| 40 | 90 | 10 | 0.11 | −1.30 |
| 60 | 82 | 18 | 0.22 | −0.90 |
| 80 | 71 | 29 | 0.41 | −0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deneva, V.; Slavova, S.; Kumanova, A.; Vassilev, N.; Nedeltcheva-Antonova, D.; Antonov, L. Favipiravir—Tautomeric and Complexation Properties in Solution. Pharmaceuticals 2023, 16, 45. https://doi.org/10.3390/ph16010045
Deneva V, Slavova S, Kumanova A, Vassilev N, Nedeltcheva-Antonova D, Antonov L. Favipiravir—Tautomeric and Complexation Properties in Solution. Pharmaceuticals. 2023; 16(1):45. https://doi.org/10.3390/ph16010045
Chicago/Turabian StyleDeneva, Vera, Sofia Slavova, Alina Kumanova, Nikolay Vassilev, Daniela Nedeltcheva-Antonova, and Luidmil Antonov. 2023. "Favipiravir—Tautomeric and Complexation Properties in Solution" Pharmaceuticals 16, no. 1: 45. https://doi.org/10.3390/ph16010045
APA StyleDeneva, V., Slavova, S., Kumanova, A., Vassilev, N., Nedeltcheva-Antonova, D., & Antonov, L. (2023). Favipiravir—Tautomeric and Complexation Properties in Solution. Pharmaceuticals, 16(1), 45. https://doi.org/10.3390/ph16010045







