Serpentine Enhances Insulin Regulation of Blood Glucose through Insulin Receptor Signaling Pathway
Abstract
:1. Introduction
2. Results
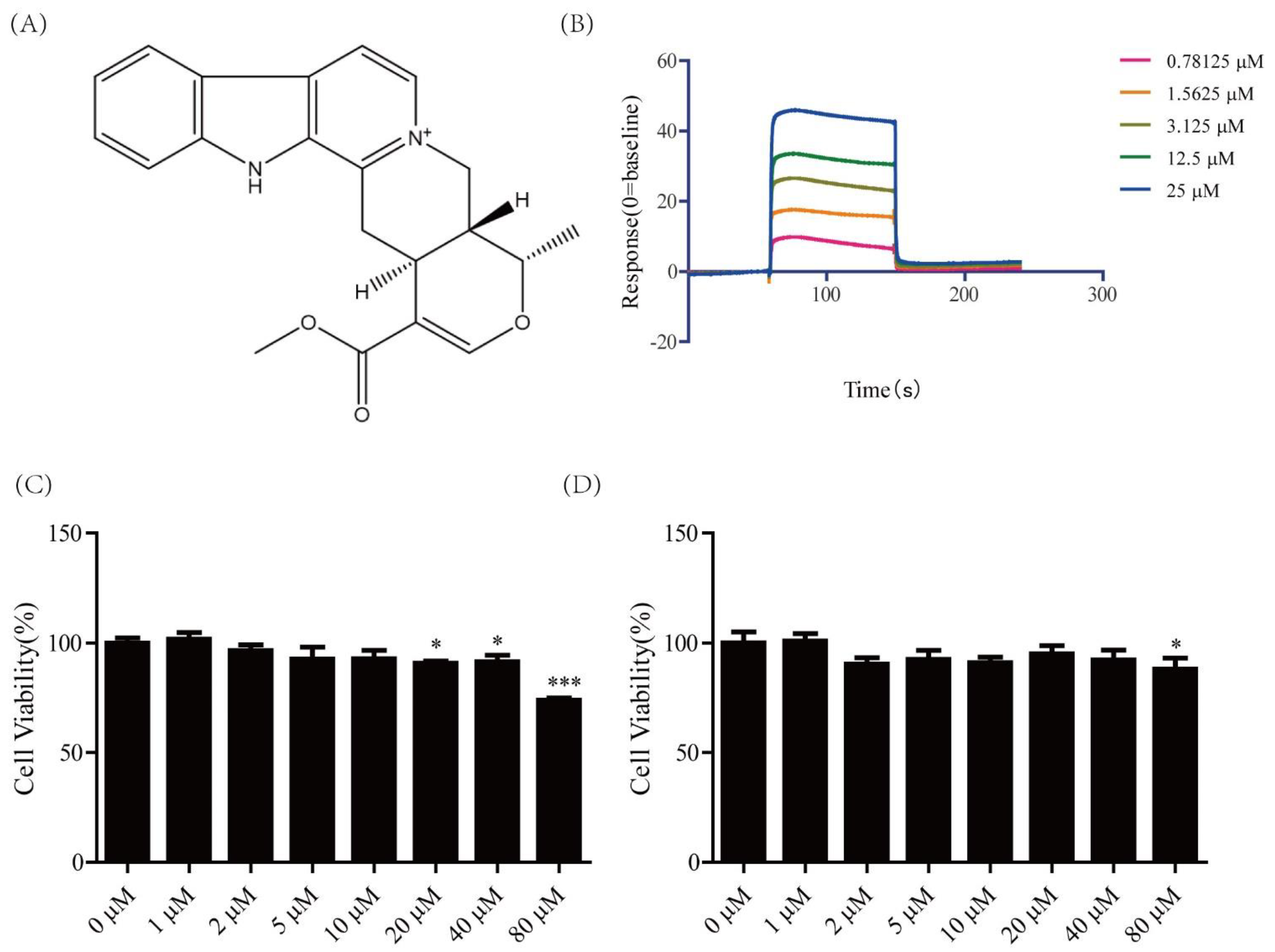
2.1. Cytotoxicity of Serpentine and Affinity of Serpentine for Insulin Receptor
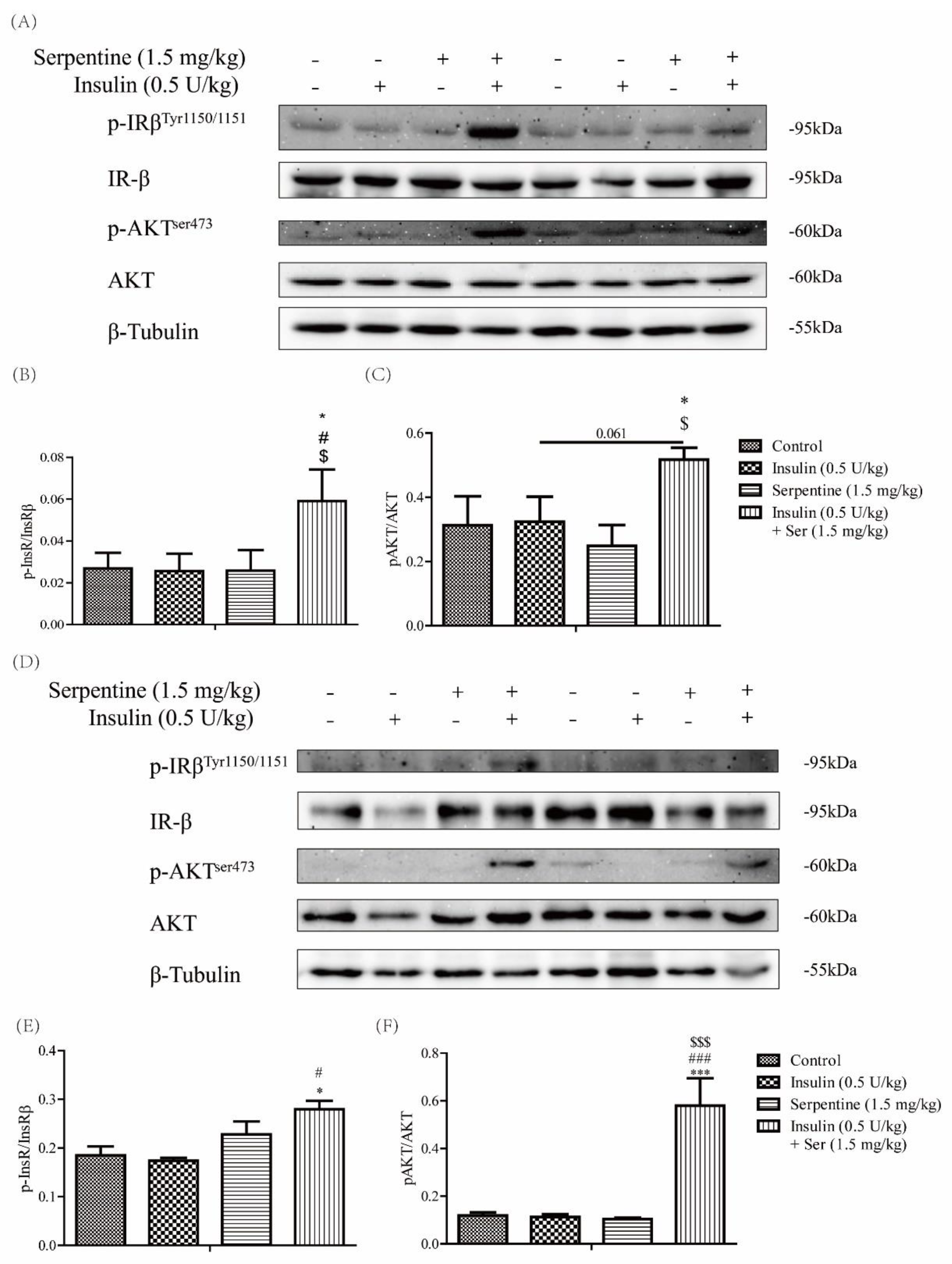
2.2. Effects of Serpentine on the Insulin Signaling Pathway Activated by Low-Dose Insulin
2.3. Effects of Serpentine on Blood Glucose and Hormone Levels in Mice with STZ/HFD-Induced Type 2 Diabetes Treated with Insulin
2.4. Effects of Serpentine and Insulin Treatment on the Insulin Signaling Pathway in Target Organs
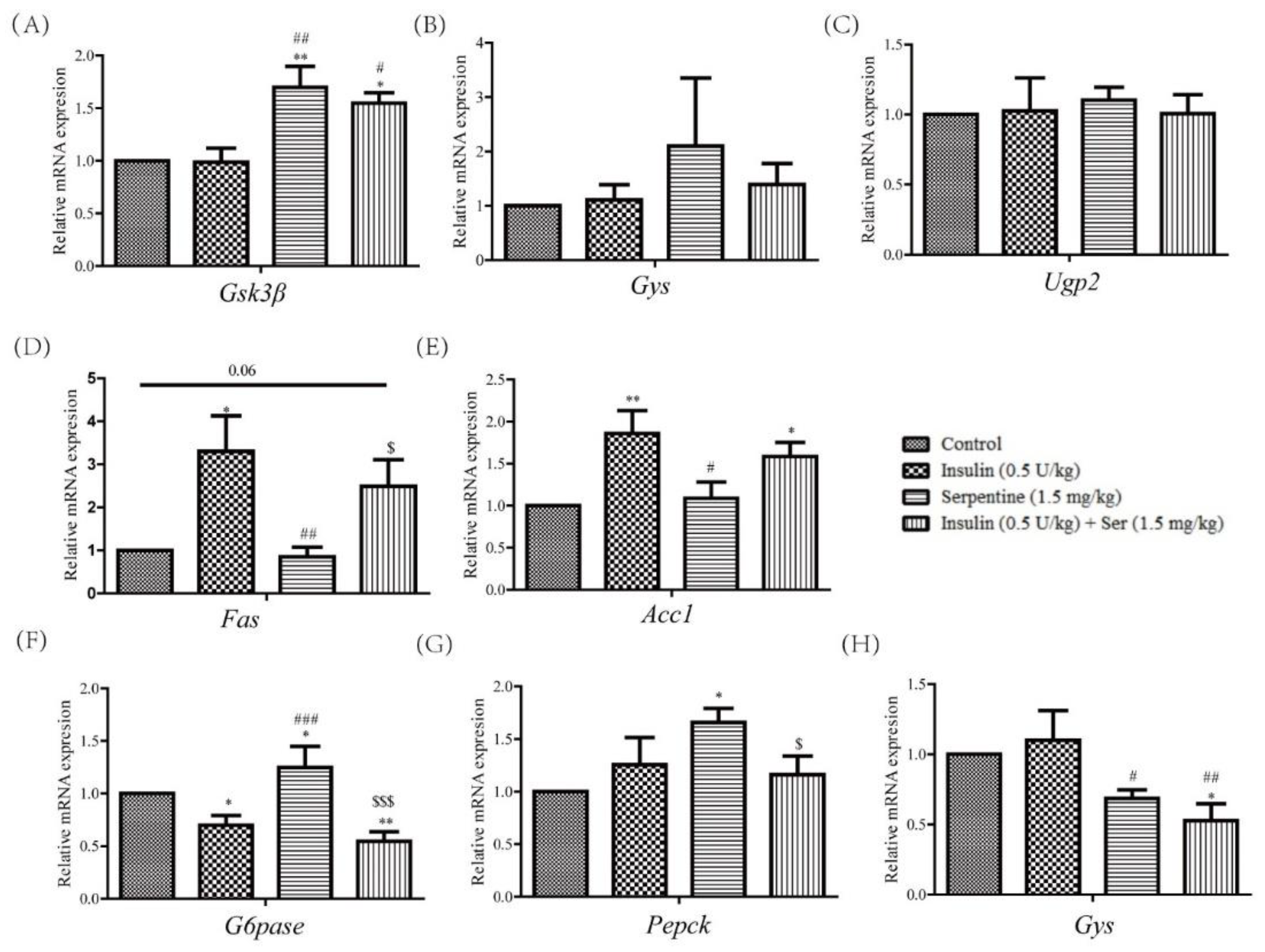
2.5. Effects of Serpentine and Insulin Treatment on Genes Related to Gluconeogenesis, Glycogen Synthesis, and Fat Synthesis
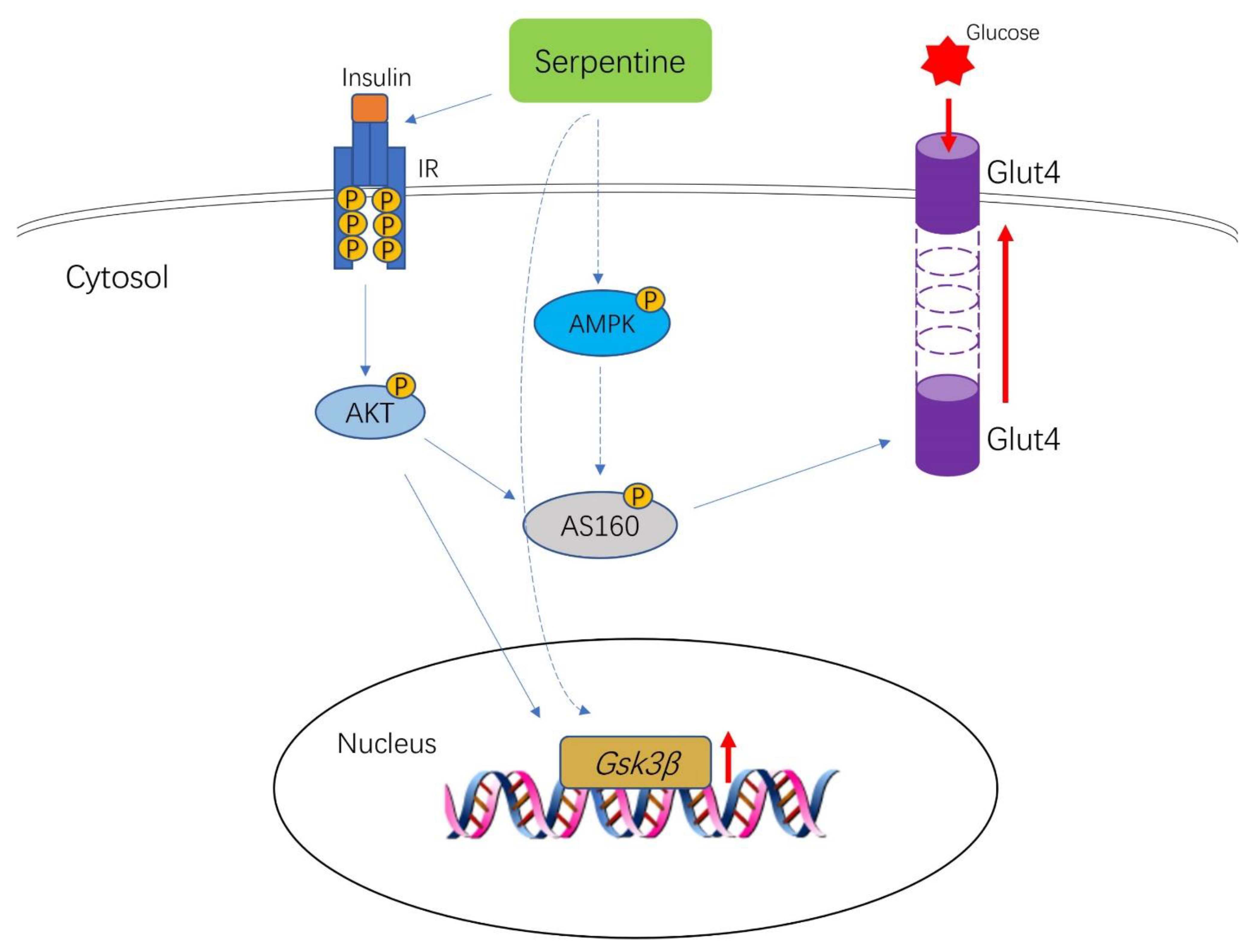
2.6. Serpentine Promotes Glucose Uptake through the AMPK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Animals
4.3. Cell Viability Assay
4.4. Western Blot Analysis
4.5. Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.6. Glucose Uptake Assay
4.7. SPR Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pilch, P.F. The insulin receptor: Structure, function, and signaling. Am. J. Physiol. 1994, 266, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Pagadala, J.; Miller, D.; Steinle, J.J. Reduced insulin receptor signaling in retinal Müller cells cultured in high glucose. Mol. Vis. 2013, 19, 804–811. [Google Scholar]
- Haruta, T.; Morris, A.J.; Rose, D.W.; Nelson, J.G.; Mueckler, M.; Olefsky, J.M. Insulin-stimulated GLUT4 Translocation Is Mediated by a Divergent Intracellular Signaling Pathway. J. Biol. Chem. 1995, 270, 27991–27994. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [Green Version]
- Hedrington, M.S.; Pulliam, L.; Davis, S.N. Basal insulin treatment in type 2 diabetes. Diabetes Technol. Ther. 2011, 13 (Suppl. 1), S33–S42. [Google Scholar] [CrossRef] [Green Version]
- Chantelau, E.; Spraul, M.; Mühlhauser, I.; Gause, R.; Berger, M. Long-term safety, efficacy and side-effects of continuous subcutaneous insulin infusion treatment for Type 1 (insulin-dependent) diabetes mellitus: A one centre experience. Diabetologia 1989, 32, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, S.A.; Ding, V.; Li, Z.; Szalkowski, D.; Zhang, B.B. Activation of Insulin Signal Transduction Pathway and Anti-diabetic Activity of Small Molecule Insulin Receptor Activators. J. Biol. Chem. 2000, 275, 36590–36595. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.H.; Seol, H.J.; Jeon, S.J.; Son, K.H.; Lee, J.R. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza Bunge. Phytomedicine 2009, 16, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-G.; Chen, F.; Li, P.; Quan, L.; Chen, J.; Yu, L.; Ding, H.; Li, C.; Chen, L.; Gao, Z.; et al. Natural product vindoline stimulates insulin secretion and efficiently ameliorates glucose homeostasis in diabetic murine models. J. Ethnopharmacol. 2013, 150, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Nammi, S.; Boini, M.K.; Lodagala, S.D.; Behara, R.B. The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Complement. Altern. Med. 2003, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, R.R. A comparative evaluation of some blood sugar lowering agents of plant origin. J. Ethnopharmacol. 1999, 67, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, R.R.; Sarkar, S.K.; Ganguly, S.; Banerjee, R.N.; Basu, T.K. Hypoglycemic and antihyperglycemic effect of leaves of Vinca rosea Linn. Indian J. Physiol. Pharmacol. 1991, 35, 145–151. [Google Scholar]
- Islam, M.A.; Akhtar, M.A.; Islam, M.R.; Hossain, M.S.; Ahmed, M. Antidiabetic and hypolipidemic effects of different fractions of Catharanthus roseus (L.) on normal and streptozotocin induced diabetic rats. J. Sci. Res. 2009, 1, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, B.; Singh, R. Catharanthus roseus (L.) G. Don: A review of its ethnobotany, phytochemistry, ethnopharmacology and toxicities. J. Ethnopharmacol. 2022, 284, 114647. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, S.S.; Shanker, K.; Kalra, A. Endophytes enhance the production of root alkaloids ajmalicine and serpentine by modulating the terpenoid indole alkaloid pathway in Catharanthus roseus roots. J. Appl. Microbiol. 2020, 128, 1128–1142. [Google Scholar] [CrossRef]
- Uniyal, G.C.; Bala, S.; Mathur, A.K.; Kulkarni, R.N. Symmetry C18 column: A better choice for the analysis of indole alkaloids of Catharanthus roseus. Phytochem. Anal. 2001, 12, 206–210. [Google Scholar] [CrossRef]
- Das, A.; Sarkar, S.; Bhattacharyya, S.; Gantait, S. Biotechnological advancements in Catharanthus roseus (L.) G. Don. Appl. Microbiol. Biotechnol. 2020, 104, 4811–4835. [Google Scholar] [CrossRef]
- Yoshida, T.; Sato, M.; Ozawa, T.; Umezawa, Y. An SPR-based screening method for agonist selectivity for insulin signaling pathways based on the binding of phosphotyrosine to its specific binding protein. Anal. Chem. 2000, 72, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Youngren, J.F.; Manchem, V.P.; Kozlowski, M.; Zhang, B.B.; Maddux, B.A.; Goldfine, I.D. Small molecule insulin receptor activators potentiate insulin action in insulin-resistant cells. Diabetes 2001, 50, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Nason, S.; Antipenko, J.; Finan, B.; Shalev, A.; DiMarchi, R.; Habegger, K.M. Hepatic mTORC2 Signaling Facilitates Acute Glucagon Receptor Enhancement of Insulin-Stimulated Glucose Homeostasis in Mice. Diabetes 2022, 71, 2123–2135. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [Green Version]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]
- Cohen, P.; Nimmo, H.G.; Proud, C.G. How does insulin stimulate glycogen synthesis? Biochem. Soc. Symp. 1978, 43, 69–95. [Google Scholar]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S52–S59. [Google Scholar] [CrossRef]
- Kramer, H.F.; Witczak, C.A.; Fujii, N.; Jessen, N.; Taylor, E.B.; Arnolds, D.E.; Sakamoto, K.; Hirshman, M.F.; Goodyear, L.J. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 2006, 55, 2067–2076. [Google Scholar] [CrossRef] [Green Version]
- Salt, I.P.; Hardie, D.G. AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System. Circ. Res. 2017, 120, 1825–1841. [Google Scholar] [CrossRef] [Green Version]
- Watson, R.T.; Kanzaki, M.; Pessin, J.E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004, 25, 177–204. [Google Scholar] [CrossRef]
- Manchem, V.P.; Goldfine, I.D.; Kohanski, R.A.; Cristobal, C.P.; Lum, R.T.; Schow, S.R.; Shi, S.; Spevak, W.R.; Laborde, E.; Toavs, D.K.; et al. A novel small molecule that directly sensitizes the insulin receptor in vitro and in vivo. Diabetes 2001, 50, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shaqha, W.M.; Khan, M.; Salam, N.; Azzi, A.; Chaudhary, A.A. Anti-diabetic potential of Catharanthus roseus Linn. and its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Complement. Altern. Med. 2015, 15, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkreathy, H.M.; Ahmad, A. Catharanthus roseus Combined with Ursolic Acid Attenuates Streptozotocin-Induced Diabetes through Insulin Secretion and Glycogen Storage. Oxid. Med. Cell Longev. 2020, 2020, 8565760. [Google Scholar] [CrossRef] [PubMed]
- Tiong, S.H.; Looi, C.Y.; Arya, A.; Wong, W.F.; Hazni, H.; Mustafa, M.R.; Awang, K. Vindogentianine, a hypoglycemic alkaloid from Catharanthus roseus (L.) G. Don (Apocynaceae). Fitoterapia 2015, 102, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.C.; Mustafa, M.R.; Awang, K. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770–9784. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095489. [Google Scholar] [CrossRef]
- Da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607. [Google Scholar] [CrossRef]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta 2006, 1760, 1505–1512. [Google Scholar] [CrossRef]
- Rout, D.; Chandra Dash, U.; Kanhar, S.; Swain, S.K.; Sahoo, A.K. The modulatory role of prime identified compounds in the bioactive fraction of Homalium zeylanicum in high-fat diet fed-streptozotocin-induced type 2 diabetic rats. J. Ethnopharmacol. 2020, 260, 113099. [Google Scholar] [CrossRef]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [Green Version]
- Ratzmann, K.P.; Schulz, B. Further support for inhibition of endogenous insulin secretion by exogenous insulin. Exp. Clin. Endocrinol. 1985, 85, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, P.; Wang, Q. Insulin as a physiological modulator of glucagon secretion. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E751–E761. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.R.; Son, Y.J.; Lee, Y.; Lee, H.J.; Yeo, J.; Lee, M.J.; Lee, M.K. Korean naked waxy barley (saechalssal) extract reduces blood glucose in diabetic mice by modulating the PI3K-Akt-GSK3β pathway. Biomed. Pharmacother. 2022, 150, 112976. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wen, X.; Zhang, Z.; Xie, M.; Zhou, J. Phillyrin ameliorates diabetic nephropathy through the PI3K/Akt/GSK-3β signalling pathway in streptozotocin-induced diabetic mice. Hum. Exp. Toxicol. 2021, 40, S487–S496. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S.; Huerta-Bahena, J.; Pelton, J.T.; Hruby, V.J.; Trivedi, D.; García-Sáinz, J.A. Metabolic effects and cyclic AMP levels produced by glucagon, (1-N alpha-Trinitrophenylhistidine,12-homoarginine)glucagon and forskolin in isolated rat hepatocytes. Biochim. Biophys. Acta 1984, 804, 434–441. [Google Scholar] [CrossRef]
- Park, D.R.; Park, K.H.; Kim, B.J.; Yoon, C.S.; Kim, U.H. Exercise ameliorates insulin resistance via Ca2+ signals distinct from those of insulin for GLUT4 translocation in skeletal muscles. Diabetes 2015, 64, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Ferdowsi, P.V.; Ahuja, K.D.K.; Beckett, J.M.; Myers, S. Capsaicin and Zinc Promote Glucose Uptake in C2C12 Skeletal Muscle Cells through a Common Calcium Signalling Pathway. Int. J. Mol. Sci. 2022, 23, 2207. [Google Scholar] [CrossRef]
- Baus, D.; Heermeier, K.; De Hoop, M.; Metz-Weidmann, C.; Gassenhuber, J.; Dittrich, W.; Welte, S.; Tennagels, N. Identification of a novel AS160 splice variant that regulates GLUT4 translocation and glucose-uptake in rat muscle cells. Cell Signal. 2008, 20, 2237–2246. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.; Chen, D.; Foldes, R.; Chan, T.H.; Dou, Q.P. Novel epigallocatechin gallate analogs as potential anticancer agents: A patent review (2009–present). Expert Opin. Ther. Pat. 2013, 23, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lii, C.K.; Lo, C.W.; Lin, Y.H.; Yang, Y.C.; Huang, C.S.; Chen, H.W. 14-Deoxy-11,12-Didehydroandrographolide Ameliorates Glucose Intolerance Enhancing the LKB1/AMPK[Formula: See text]/TBC1D1/GLUT4 Signaling Pathway and Inducing GLUT4 Expression in Myotubes and Skeletal Muscle of Obese Mice. Am. J. Chin. Med. 2021, 49, 1473–1491. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Alsalmi, F.A. Influence of olive leaves extract on hepatorenal injury in streptozotocin diabetic rats. Saudi J. Biol. Sci. 2019, 26, 1865–1874. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-Actin | 5′-GAGACCTTCAACACCCCAGC-3′ | 5′-ATGTCACGCACGATTTCCC-3′ |
| Gys | 5′-ATCTTCTTCGTCTTCCGCATC-3′ | 5′-GACACTGAGCAGGGCTTTTCC-3′ |
| G6pase | 5′-AAAAAGCCAACGTATGGATTCCG-3′ | 5′-CAGCAAGGTAGATCCGGGA-3′ |
| Pepck | 5′-TTTGATGCCCAAGGCAACTT-3′ | 5′-ATCGATGCCTTCCCAGTAAA-3′ |
| Fas | 5′-CTGGCATTCGTGATGGAGTC-3′ | 5′-TGTTTCCCCTGAGCCATGTA-3′ |
| Acc1 | 5′-CGCTCGTCAGGTTCTTATTG-3′ | 5′-TTTCTGCAGGTTCTCAATGC-3′ |
| Ugp2 | 5′-TAACCAAGGGCACTGTAGGGA-3′ | 5′-GGAGCTGCAATTAAAAGTTTCG-3′ |
| Gsk3β | 5′-TCCATTCCTTTGGAATCTGC-3′ | 5′-CAATTCAGCCAACACACAGC-3′ |
| α-Tubulin | 5′-CCACAAGTTTGATCTGTTGCAT-3′ | 5′-GCAGCAACTAGTATCCCTGTCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, G.; Liu, X.; Chen, M.; Zeng, Y.; Li, Y.; Wu, X.; Wang, X.; Sheng, J. Serpentine Enhances Insulin Regulation of Blood Glucose through Insulin Receptor Signaling Pathway. Pharmaceuticals 2023, 16, 16. https://doi.org/10.3390/ph16010016
Wang Y, Liu G, Liu X, Chen M, Zeng Y, Li Y, Wu X, Wang X, Sheng J. Serpentine Enhances Insulin Regulation of Blood Glucose through Insulin Receptor Signaling Pathway. Pharmaceuticals. 2023; 16(1):16. https://doi.org/10.3390/ph16010016
Chicago/Turabian StyleWang, Yinghao, Guanfu Liu, Xutao Liu, Minhua Chen, Yuping Zeng, Yuyan Li, Xiaoyun Wu, Xuanjun Wang, and Jun Sheng. 2023. "Serpentine Enhances Insulin Regulation of Blood Glucose through Insulin Receptor Signaling Pathway" Pharmaceuticals 16, no. 1: 16. https://doi.org/10.3390/ph16010016
APA StyleWang, Y., Liu, G., Liu, X., Chen, M., Zeng, Y., Li, Y., Wu, X., Wang, X., & Sheng, J. (2023). Serpentine Enhances Insulin Regulation of Blood Glucose through Insulin Receptor Signaling Pathway. Pharmaceuticals, 16(1), 16. https://doi.org/10.3390/ph16010016







