Brain Targeting of Quetiapine Fumarate via Intranasal Delivery of Loaded Lipospheres: Fabrication, In-Vitro Evaluation, Optimization, and In-Vivo Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Trial Formulations
2.2. Formulation’s Design
2.2.1. Entrapment Efficiency
2.2.2. Particle Size
2.2.3. Zeta Potential
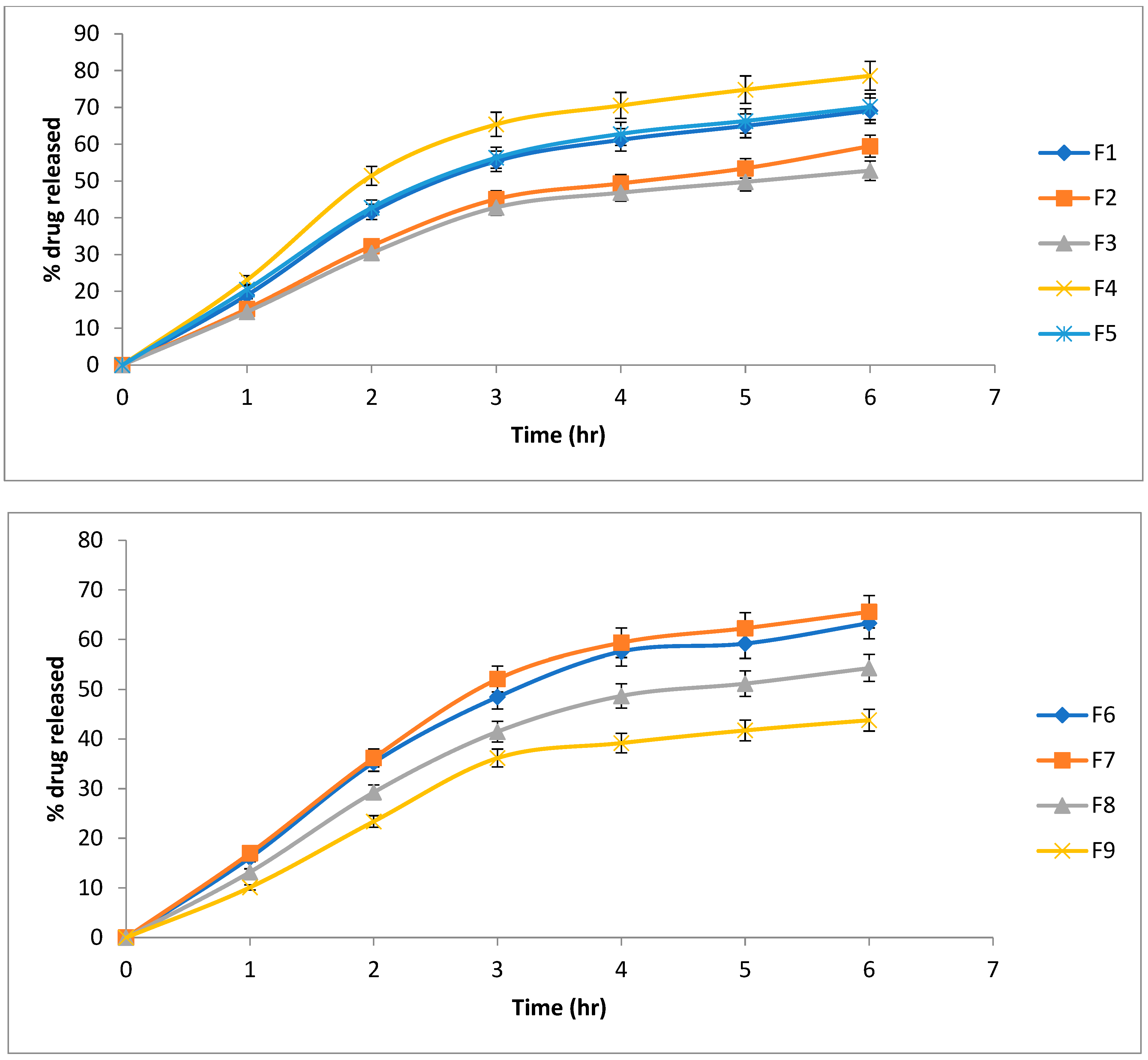
2.2.4. Drug Release Profiles and Kinetics
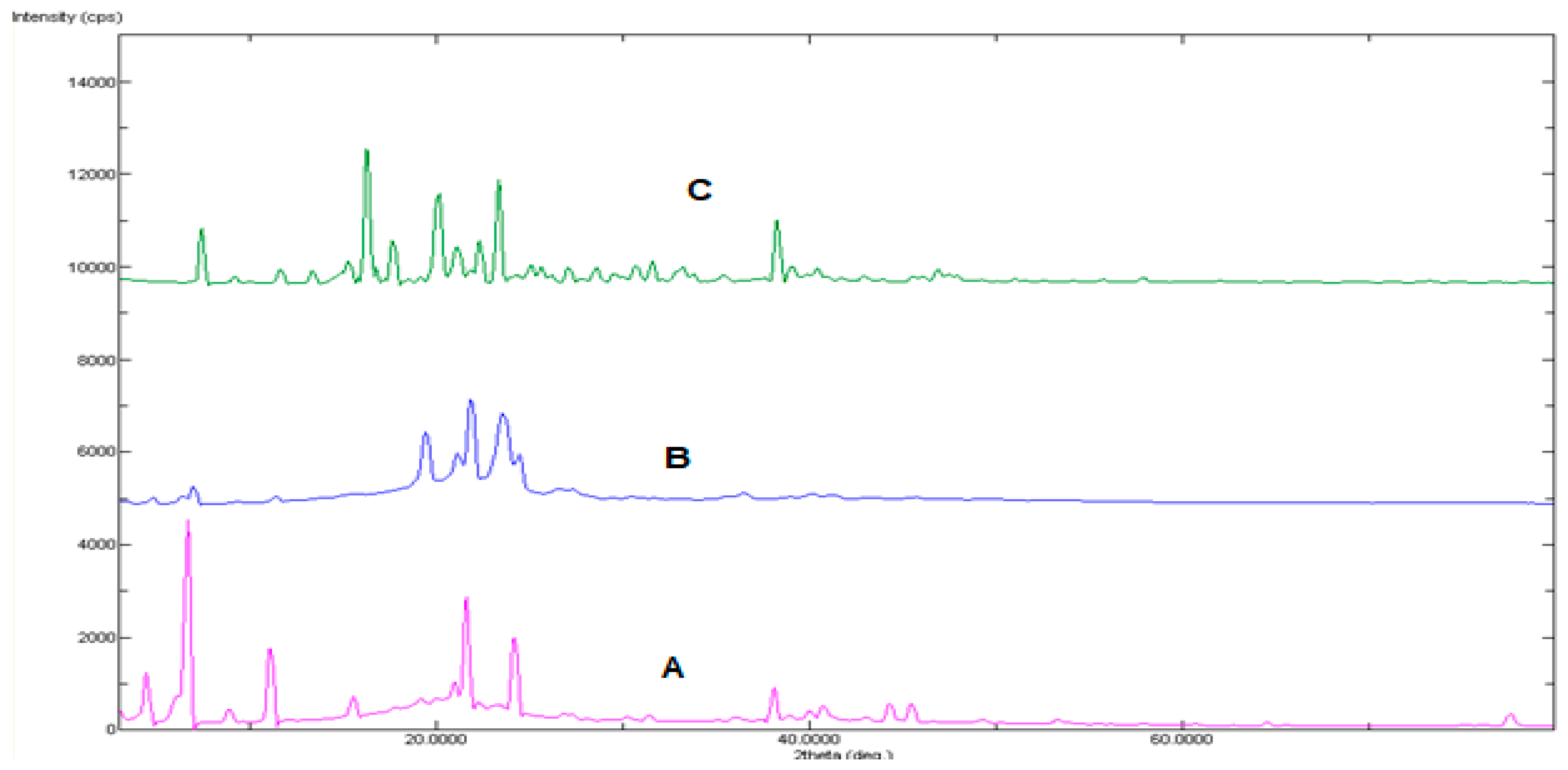
2.3. Powder X-ray Diffraction Examination
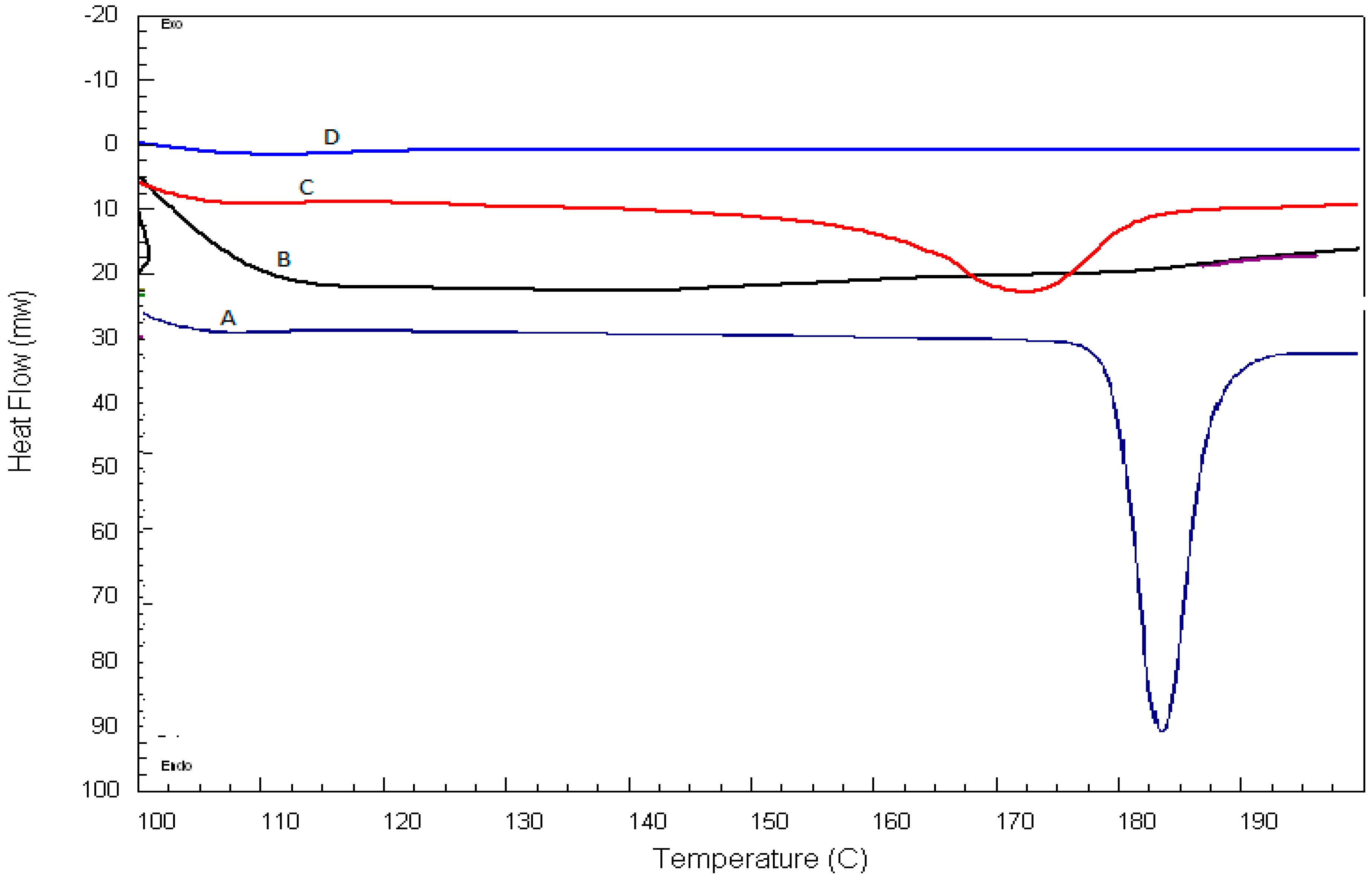
2.4. Differential Scanning Calorimetry
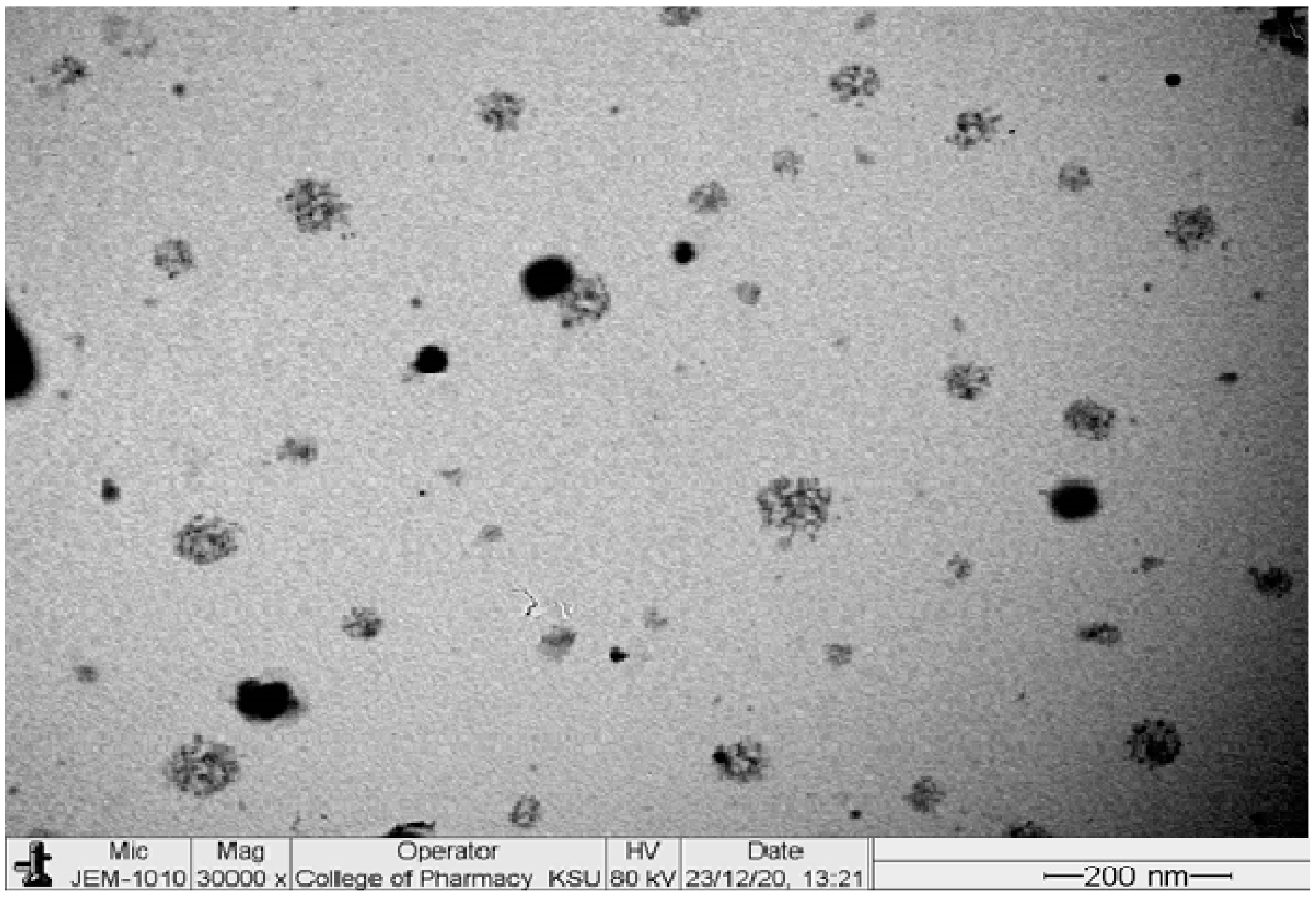
2.5. Transmission Electron Microscopy
2.6. Stability Study
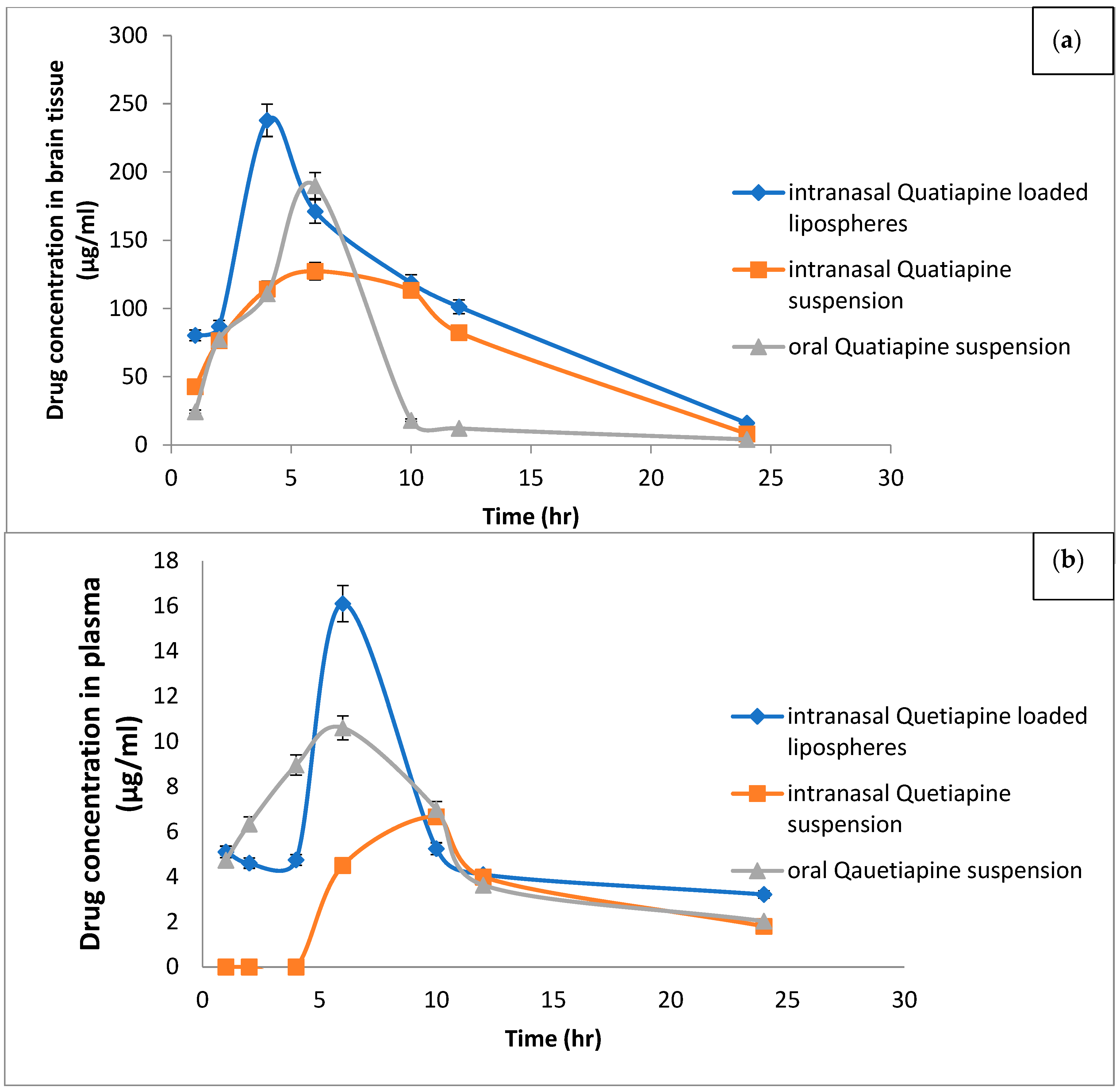
2.7. In Vivo Study
3. Material and Methods
3.1. Material
3.2. Animals
3.3. Study Design
3.4. Preparation of QT Loaded Lipospheres
3.5. Separation of Unentrapped QTF from the Prepared Lipospheres
3.6. Determination of Entrapment Efficiency.
3.7. Characterization of QTF Lipospheres
3.7.1. Morphological Description
3.7.2. Particle Size Analysis
3.7.3. Differential Scanning Calorimetry (DSC)
3.7.4. In Vitro Release of QTF from Lipospheres
3.7.5. Stability Study
3.8. In-Vivo Study
3.8.1. Separation of Plasma
3.8.2. Dissection and Preparation of Brain Sample
3.8.3. HPLC Conditions
3.8.4. Pharmacokinetic Parameters Analysis
3.9. Statistical Analysis of Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCutcheon, R.A.; Marques, T.R.; Howes, O.D. Schizophrenia—An overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Connell, C.; Worthington, M.; Elrafei, H.; Mulvaney, C.A.; Kaewchaluay, C. Quetiapine dose for people with schizophrenia. Cochrane Database Syst. Rev. 2019, 2019, CD013372. [Google Scholar] [CrossRef]
- Murasaki, M.; Koyama, T.; Kanba, S.; Takeuchi, M.; Shimizu, Y.; Arita, E.; Kuroishi, K.; Kamei, S. Multi-center, randomized, double-blind, placebo-controlled study of quetiapine extended-release formulation in Japanese patients with bipolar depression. Psychopharmacology 2018, 235, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-C.; Chen, K.-P.; Tsai, C.-J.; Wang, L.-J.; Chen, C.-K.; Lin, S.-K. Rapid initiation of quetiapine well tolerated as compared with the conventional initiation regimen in patients with schizophrenia or schizoaffective disorders. Kaohsiung J. Med Sci. 2011, 27, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chavan, B.S.; Sidana, A.; Das, S. Efficacy and Tolerability of Clozapine versus Quetiapine in Treatment-resistant Schizophrenia. Indian J. Psychol. Med. 2017, 39, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T. Quetiapine–Efficacy in different domains. Eur. Neuropsychopharmacol. 2001, 11, S385–S390. [Google Scholar] [CrossRef]
- Rothenberg, K.G.; Rajaram, R. Advances in Management of Psychosis in Neurodegenerative Diseases. Curr. Treat. Options Neurol. 2019, 21, 3. [Google Scholar] [CrossRef]
- Pae, C.-U.; Kim, T.-S.; Kim, J.-J.; Lee, S.-J.; Lee, C.-U.; Lee, C.; Paik, I.-H. Long-term treatment of adjunctive quetiapine for bipolar mania. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 763–766. [Google Scholar] [CrossRef]
- Zarezadeh, F.; Arbabi, M.; Shamabadi, A.; Naderi, S.; Hasanzadeh, A.; Ostadpour, M.; Samsami, F.S.; Akhondzadeh, S. Levetiracetam adjunct to quetiapine for the acute manic phase of bipolar disorder: A randomized, double-blind and placebo-controlled clinical trial of efficacy, safety and tolerability. Int. Clin. Psychopharmacol. 2021, 37, 46–53. [Google Scholar] [CrossRef]
- Patel, N.; Baldaniya, M.; Raval, M.; Sheth, N. Formulation and Development of In Situ Nasal Gelling Systems for Quetiapine Fumarate-Loaded Mucoadhesive Microemulsion. J. Pharm. Innov. 2015, 10, 357–373. [Google Scholar] [CrossRef]
- Krause, M.; Huhn, M.; Schneider-Thoma, J.; Rothe, P.; Smith, R.C.; Leucht, S. Antipsychotic drugs for elderly patients with schizophrenia: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2018, 28, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Thyrum, P.T.; Wong, Y.J.; Yeh, C. Single-dose pharmacokinetics of quetiapine in subjects with renal or hepatic impairment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2000, 24, 521–533. [Google Scholar] [CrossRef]
- Le Daré, B.; Ferron, P.-J.; Allard, P.-M.; Clément, B.; Morel, I.; Gicquel, T. New insights into quetiapine metabolism using molecular networking. Sci. Rep. 2020, 10, 19921. [Google Scholar] [CrossRef]
- Galeano, C.; Qiu, Z.; Mishra, A.; Farnsworth, S.L.; Hemmi, J.J.; Moreira, A.; Edenhoffer, P.; Hornsby, P.J. The Route by Which Intranasally Delivered Stem Cells Enter the Central Nervous System. Cell Transplant. 2018, 27, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef]
- Verekar, R.R.; Gurav, S.S.; Bolmal, U. Thermosensitive mucoadhesive in situ gel for intranasal delivery of Almotriptan malate: Formulation, characterization, and evaluation. J. Drug Deliv. Sci. Technol. 2020, 58, 101778. [Google Scholar] [CrossRef]
- Noorulla, K.; Yasir, M.; Muzaffar, F.; Roshan, S.; Ghoneim, M.M.; Almurshedi, A.S.; Tura, A.J.; Alshehri, S.; Gebissa, T.; Mekit, S.; et al. Intranasal delivery of chitosan decorated nanostructured lipid carriers of Buspirone for brain targeting: Formulation development, optimization and In-Vivo preclinical evaluation. J. Drug Deliv. Sci. Technol. 2022, 67, 102939. [Google Scholar] [CrossRef]
- Vyas, T.K.; Babbar, A.; Sharma, R.; Singh, S.; Misra, A. Intranasal Mucoadhesive Microemulsions of Clonazepam: Preliminary Studies on Brain Targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2001, 53, 3–22. [Google Scholar]
- Abdou, E.M.; Kandil, S.M.; El Miniawy, H.M. Brain targeting efficiency of antimigrain drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Aldawsari, H. Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin. J. Drug Deliv. Sci. Technol. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Hosny, K.M.; Hassan, A.H. Intranasal in situ gel loaded with saquinavir mesylate nanosized microemulsion: Preparation, characterization, and in vivo evaluation. Int. J. Pharm. 2014, 475, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, R.A.; Aboud, H.M.; Sayed, O.M. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J. Liposome Res. 2020, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Drechsler, M.; Mariani, P.; Carducci, F.; Servadio, M.; Melancia, F.; Ratano, P.; Campolongo, P.; Trezza, V.; Cortesi, R.; et al. Lipid nanoparticles for administration of poorly water soluble neuroactive drugs. Biomed. Microdevices 2017, 19, 44. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Kim, B.-D.; Na, K.; Choi, H.-K. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur. J. Pharm. Sci. 2005, 24, 199–205. [Google Scholar] [CrossRef]
- Waghule, T.; Rapalli, V.K.; Gorantla, S.; Saha, R.N.; Dubey, S.K.; Puri, A.; Singhvi, G. Nanostructured Lipid Carriers as Potential Drug Delivery Systems for Skin Disorders. Curr. Pharm. Des. 2020, 26, 4569–4579. [Google Scholar] [CrossRef]
- Nasr, M.; Mansour, S.; Mortada, N.; El Shamy, A.A. Lipospheres as Carriers for Topical Delivery of Aceclofenac: Preparation, Characterization and In Vivo Evaluation. AAPS PharmSciTech 2008, 9, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Toongsuwan, S.; Li, L.-C.; Erickson, B.K.; Chang, H.-C. Formulation and characterization of bupivacaine lipospheres. Int. J. Pharm. 2004, 280, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mohyeldin, S.M.; Samy, W.M.; Ragab, D.; Abdelmonsif, D.A.; Aly, R.G.; Elgindy, N.A. Precisely Fabricated Sulpiride-Loaded Nanolipospheres with Ameliorated Oral Bioavailability and Antidepressant Activity. Int. J. Nanomed. 2021, 16, 2013–2044. [Google Scholar] [CrossRef] [PubMed]
- Maniar, M.; Hannibal, D.; Amselem, S.; Xie, X.; Burch, R.; Domb, A. Characterization of lipospheres: Effect of carrier and phospholipid on the loading of drug into the lipospheres. Pharm. Res. 1991, 8, 175–185. [Google Scholar]
- Hanif, M.; Khan, H.U.; Maheen, S.; Shafqat, S.S.; Shah, S.; Masood, S.A.; Abbas, G.; Rizwan, M.; Rasheed, T.; Bilal, M. Formulation, characterization, and pharmacokinetic evaluation of Ivabradine-Nebivolol co-encapsulated lipospheres. J. Mol. Liq. 2021, 344, 117704. [Google Scholar] [CrossRef]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef]
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef]
- Tursilli, R.; Casolari, A.; Iannuccelli, V.; Scalia, S. Enhancement of melatonin photostability by encapsulation in lipospheres. J. Pharm. Biomed. Anal. 2006, 40, 910–914. [Google Scholar] [CrossRef]
- Leonardi, A.; Bucolo, C.; Drago, F.; Salomone, S.; Pignatello, R. Cationic solid lipid nanoparticles enhance ocular hypotensive effect of melatonin in rabbit. Int. J. Pharm. 2015, 478, 180–186. [Google Scholar] [CrossRef]
- Tan, Z.M.; Lai, G.P.; Pandey, M.; Srichana, T.; Pichika, M.R.; Gorain, B.; Bhattamishra, S.K.; Choudhury, H. Novel Approaches for the Treatment of Pulmonary Tuberculosis. Pharmaceutics 2020, 12, 1196. [Google Scholar] [CrossRef]
- Salama, H.; Mahmoud, A.; Kamel, A.O.; Hady, M.A.; Awad, G.A. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Pailla, S.R.; Talluri, S.; Rangaraj, N.; Ramavath, R.; Challa, V.S.; Doijad, N.; Sampathi, S. Intranasal Zotepine Nanosuspension: Intended for improved brain distribution in rats. DARU J. Pharm. Sci. 2019, 27, 541–556. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Tadros, M.I. Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers: In vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int. J. Pharm. 2013, 452, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Masters, D.B.; Domb, A.J. Liposphere Local Anesthetic Timed-Release for Perineural Site Application. Pharm. Res. 1998, 15, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Iannuccelli, V.; Sala, N.; Tursilli, R.; Coppi, G.; Scalia, S. Influence of liposphere preparation on butyl-methoxydibenzoylmethane photostability. Eur. J. Pharm. Biopharm. 2006, 63, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Chand, P.; Kumar, H.; Badduri, N.; Gupta, N.V.; Bettada, V.G.; Madhunapantula, S.V.; Kesharwani, S.S.; Dey, S.; Jain, V. Design and evaluation of cabazitaxel loaded NLCs against breast cancer cell lines. Colloids Surfaces B Biointerfaces 2021, 199, 111535. [Google Scholar] [CrossRef]
- Aboud, H.M.; Mahmoud, M.O.; Mohammed, M.A.; Awad, M.S.; Sabry, D. Preparation and appraisal of self-assembled valsartan-loaded amalgamated Pluronic F127/Tween 80 polymeric micelles: Boosted cardioprotection via regulation of Mhrt/Nrf2 and Trx1 pathways in cisplatin-induced cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef]
- Hanson, L.R.; Fine, J.M.; Svitak, A.L.; Faltesek, K.A. Intranasal Administration of CNS Therapeutics to Awake Mice. J. Vis. Exp. 2013, 74, e4440. [Google Scholar] [CrossRef]
- Kanazawa, T.; Fukuda, M.; Suzuki, N.; Suzuki, T. Novel Methods for Intranasal Administration Under Inhalation Anesthesia to Evaluate Nose-to-Brain Drug Delivery. J. Vis. Exp. 2018, 141, e58485. [Google Scholar] [CrossRef]
- Marks, D.R.; Tucker, K.; Cavallin, M.A.; Mast, T.G.; Fadool, D.A. Awake Intranasal Insulin Delivery Modifies Protein Complexes and Alters Memory, Anxiety, and Olfactory Behaviors. J. Neurosci. 2009, 29, 6734–6751. [Google Scholar] [CrossRef]
- Ezzeldin, E.; Asiri, Y.A.; Iqbal, M. Effects of Green Tea Extracts on the Pharmacokinetics of Quetiapine in Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 615285. [Google Scholar] [CrossRef]
- Koehl, N.J.; Holm, R.; Kuentz, M.; Griffin, B.T. Chase Dosing of Lipid Formulations to Enhance Oral Bioavailability of Nilotinib in Rats. Pharm. Res. 2020, 37, 124. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Feng, M.; Swalve, N.; Davis, C.; Sui, N.; Li, M. Effects of repeated quetiapine treatment on conditioned avoidance responding in rats. Eur. J. Pharmacol. 2015, 769, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, J.B. Todd, Sanford, Davidson Clinical Diagnosis and Management by Laboratory Methods; Saunders: Philadelphia, PA, USA, 2001. [Google Scholar]
- Wu, B.; Hu, M. A Useful Microsoft Excel Add-in Program for Pharmacokinetic Analysis. Pharm. Anal. Acta 2012, S11, 2. [Google Scholar] [CrossRef]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: Formulation, optimization and in vivo pharmacokinetics. Drug Des. Dev. Ther. 2017, 11, 1815–1825. [Google Scholar] [CrossRef] [Green Version]
- Shadab; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar]
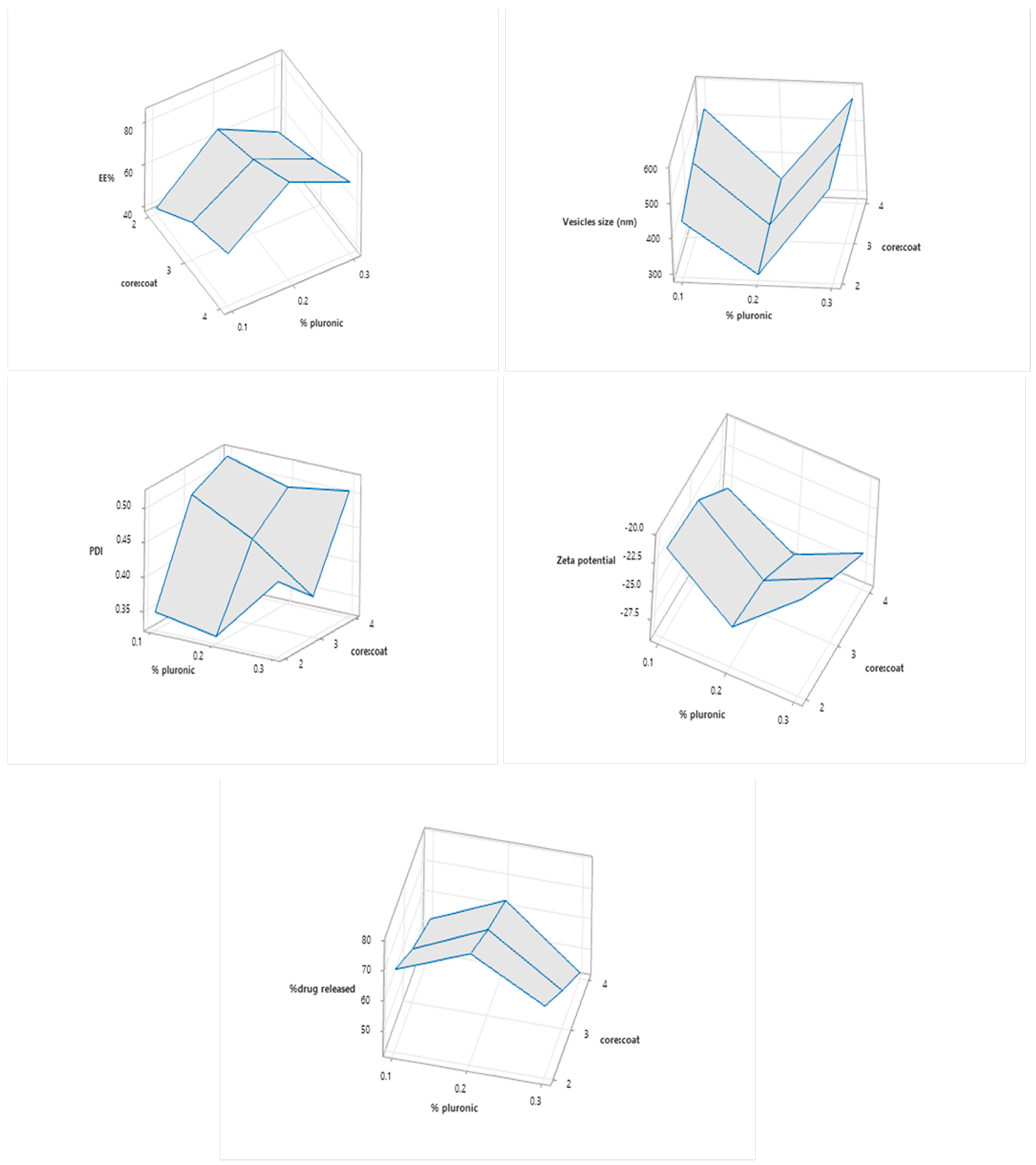
| Formulations | Drug:Lipid | Core:Coat | %Pluronic | EE% | Vesicles Size (nm) | Zeta Potential | PDI |
|---|---|---|---|---|---|---|---|
| TB1 | 1:2 | 2:1 | 0.2 | 21.790 ± 2.0482 | 233.75 ± 6.8589 | −24.1 ± 1.0748 | 0.2735 ± 0.0671 |
| TB2 | 1:4 | 2:1 | 0.2 | 35.888 ± 1.6683 | 259.7 ± 7.4953 | −19.45 ± 1.7111 | 0.542 ± 0.1555 |
| TB3 | 1:6 | 2:1 | 0.2 | 41.670 ± 1.6187 | 275.9 ± 14.2835 | −18.73 ± 0.5515 | 0.3025 ± 0.0615 |
| TB4 | 1:8 | 2:1 | 0.2 | 65.331 ± 0.3006 | 294.4 ± 18.2433 | −25.76 ± 0.4737 | 0.333 ± 0.1697 |
| TB5 | 1:10 | 2:1 | 0.2 | 50.313 ± 1.5196 | 578.9 ± 34.2239 | −24.05 ± 0.4101 | 0.824 ± 0.0721 |
| Formulations | Drug:Lipid | Core:Coat | %Pluronic | EE% | Vesicles Size (nm) | Zeta Potential | PDI | %Drug Released |
|---|---|---|---|---|---|---|---|---|
| F1 | 1:8 | 2:1 | 0.1 | 40.046 ± 1.800 | 436.35 ± 25.5265 | −21.35 ± 2.8991 | 0.349 ± 0.1605 | 69.1164 |
| F2 | 1:8 | 3:1 | 0.1 | 55.186 ± 0.964 | 489.95 ± 17.7483 | −21.85 ± 0.6363 | 0.490 ± 0.0332 | 59.4658 |
| F3 | 1:8 | 4:1 | 0.1 | 62.334 ± 0.660 | 528.75 ± 20.4353 | −25.5 ± 1.2727 | 0.516 ± 0.0876 | 52.8374 |
| F4 | 1:8 | 2:1 | 0.2 | 65.284 ± 0.366 | 294.4 ± 18.2433 | −25.76 ± 0.4737 | 0.333 ± 0.1697 | 78.6064 |
| F5 | 1:8 | 3:1 | 0.2 | 72.675 ± 0.498 | 321.8 ± 16.2634 | −26.35 ± 1.3435 | 0.445 ± 0.1393 | 70.1822 |
| F6 | 1:8 | 4:1 | 0.2 | 83.841 ± 1.086 | 338.45 ± 19.7282 | −28.8 ± 1.9798 | 0.491 ± 0.1336 | 63.3056 |
| F7 | 1:8 | 2:1 | 0.3 | 51.469 ± 1.437 | 546.4 ± 7.49533 | −20.67 ± 1.3010 | 0.432 ± 0.1187 | 65.5978 |
| F8 | 1:8 | 3:1 | 0.3 | 60.444 ± 1.192 | 563.8 ± 82.5900 | −23.57 ± 2.5102 | 0.381 ± 0.0509 | 54.312 |
| F9 | 1:8 | 4:1 | 0.3 | 71.409 ± 0.294 | 575.8 ± 46.6690 | −26.09 ± 2.4607 | 0.505 ± 0.1873 | 43.8146 |
| Formula Code | Zero Order | First Order | Higuchi Diffusion Model | Kors–Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | Eq | R2 | Eq | R2 | Eq | R2 | Eq | |
| F1 | 0.8942 | y = 11.392x + 10.299 | 0.9599 | y = −0.0871x + 1.9678 | 0.9677 | y = 30.459x − 2.6565 | 0.9297 | y = 78.795x + 12.312 |
| F2 | 0.9242 | y = 9.7096x + 7.2996 | 0.9697 | y = −0.0651x + 1.9779 | 0.97217 | y = 25.592x − 3.1733 | 0.9455 | y = 66.616x + 9.2364 |
| F3 | 0.8981 | y = 8.7721x + 7.5736 | 0.9397 | y = −0.0557x + 1.9715 | 0.96613 | y = 23.382x − 2.2916 | 0.9331 | y = 60.653x + 9.1322 |
| F4 | 0.8666 | y = 12.799x + 13.598 | 0.9585 | y = −0.1142x + 1.9584 | 0.96266 | y = 34.668x − 1.6512 | 0.9083 | y = 88.879x + 15.715 |
| F5 | 0.8909 | y = 11.509x + 11.037 | 0.9598 | y = −0.0896x + 1.9647 | 0.9711 | y = 30.881x − 2.2225 | 0.9233 | y = 79.472x + 13.123 |
| F6 | 0.9121 | y = 10.658x + 8.0304 | 0.9593 | y = −0.0756x + 1.9766 | 0.9643 | y = 28.165x − 3.5793 | 0.9455 | y = 73.606x + 9.9578 |
| F7 | 0.9074 | y = 11.091x + 8.5314 | 0.9578 | y = −0.0811x + 1.9756 | 0.9626 | y = 29.36x − 3.6261 | 0.943 | y = 76.696x + 10.499 |
| F8 | 0.9197 | y = 9.2261x + 6.3518 | 0.9568 | y = −0.0593x + 1.9798 | 0.9626 | y = 24.259x − 3.5081 | 0.9525 | y = 63.691x + 8.0321 |
| F9 | 0.9010 | y = 7.5196x + 5.2225 | 0.9277 | y = −0.0439x + 1.9804 | 0.9487 | y = 19.831x − 2.9051 | 0.9467 | y = 52.287x + 6.4384 |
| Responses | Fresh | After 7 Days | After 30 Days |
|---|---|---|---|
| Particle size (nm) | 294.4 ± 18.243 | 296.53 ± 10.32 | 301.14 ± 9.27 |
| Zeta potential (mV) | −25.76 ± 0.4737 | −25.54 ± 0.98 | −25.27 ± 1.04 |
| EE% | 65.284 ± 0.366 | 64.85 ± 1.25 | 63.96 ± 1.87 |
| Pharmacokinetic Parameter | QTF Lipospheres IN Suspension | QTF IN Suspension | QTF Oral Suspension | |||
|---|---|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | Plasma | Brain | |
| Cmax (µg/mL) | 22.08 ± 10.23 | 237.86 ± 34.01 | 6.67 ± 1.37 | 132.37 ± 17.24 | 10.87 ± 0.93 | 190.14 ± 42.30 |
| tmax (hr) | 6 ± 0.25 | 4 ± 0.01 | 10 ± 0.05 | 4 ± 2 | 7.33 ± 2.31 | 6 ± 0.01 |
| AUC0-24hr (µg hr/g) | 133.65 ± 16.5 | 2361.04 ± 279.46 | 79.09 ± 12.52 | 1733.93 ± 182.37 | 122.65 ± 9.96 | 1098.05 ± 39.72 |
| AUC0-∞ (µg hr/g) | 235.85 ± 78.53 | 2478.14 ± 291.32 | 101.64 ± 18.80 | 1778.05 ± 178.50 | 158.82 ± 23.66 | 1147.40 ± 68.09 |
| MRT (hr) | 29.61 ± 10.56 | 9.41 ± 0.47 | 16.99 ± 2.64 | 9.14 ± 0.34 | 16.33 ± 5.32 | 7.42 ± 0.50 |
| t1/2 (hr) | 22.07 ± 10.22 | 4.87 ± 1.19 | 8.44 ± 1.84 | 3.67 ± 0.54 | 11.32 ± 5.17 | 7.56 ± 2.82 |
| DTE % | 228.36 | 169.66 | ||||
| DPT % | 51.72 | 48.82 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaki, R.M.; Aldawsari, M.F.; Alossaimi, M.A.; Alzaid, S.F.; Devanathadesikan Seshadri, V.; Almurshedi, A.S.; Aldosari, B.N.; Yusif, R.M.; Sayed, O.M. Brain Targeting of Quetiapine Fumarate via Intranasal Delivery of Loaded Lipospheres: Fabrication, In-Vitro Evaluation, Optimization, and In-Vivo Assessment. Pharmaceuticals 2022, 15, 1083. https://doi.org/10.3390/ph15091083
Zaki RM, Aldawsari MF, Alossaimi MA, Alzaid SF, Devanathadesikan Seshadri V, Almurshedi AS, Aldosari BN, Yusif RM, Sayed OM. Brain Targeting of Quetiapine Fumarate via Intranasal Delivery of Loaded Lipospheres: Fabrication, In-Vitro Evaluation, Optimization, and In-Vivo Assessment. Pharmaceuticals. 2022; 15(9):1083. https://doi.org/10.3390/ph15091083
Chicago/Turabian StyleZaki, Randa Mohammed, Mohammed F. Aldawsari, Manal A. Alossaimi, Shaikah F. Alzaid, Vidya Devanathadesikan Seshadri, Alanood S. Almurshedi, Basmah Nasser Aldosari, Rehab Mohammad Yusif, and Ossama M. Sayed. 2022. "Brain Targeting of Quetiapine Fumarate via Intranasal Delivery of Loaded Lipospheres: Fabrication, In-Vitro Evaluation, Optimization, and In-Vivo Assessment" Pharmaceuticals 15, no. 9: 1083. https://doi.org/10.3390/ph15091083
APA StyleZaki, R. M., Aldawsari, M. F., Alossaimi, M. A., Alzaid, S. F., Devanathadesikan Seshadri, V., Almurshedi, A. S., Aldosari, B. N., Yusif, R. M., & Sayed, O. M. (2022). Brain Targeting of Quetiapine Fumarate via Intranasal Delivery of Loaded Lipospheres: Fabrication, In-Vitro Evaluation, Optimization, and In-Vivo Assessment. Pharmaceuticals, 15(9), 1083. https://doi.org/10.3390/ph15091083






