Understanding the Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter and Its Therapeutic Implications
Abstract
1. Introduction
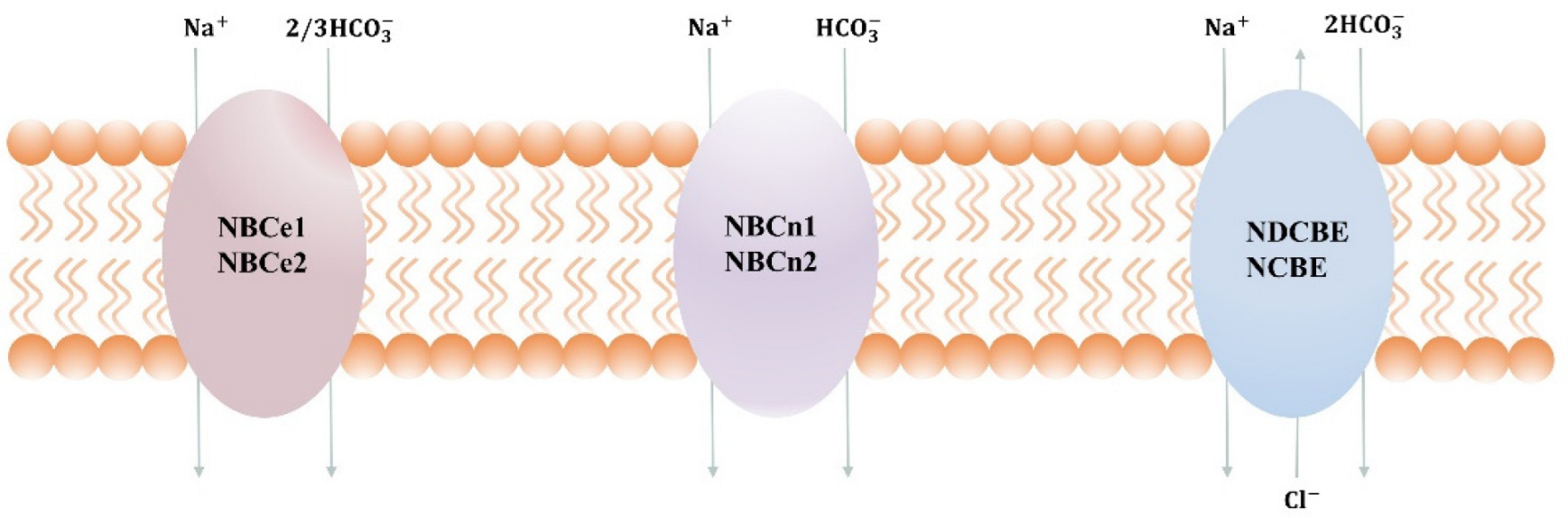
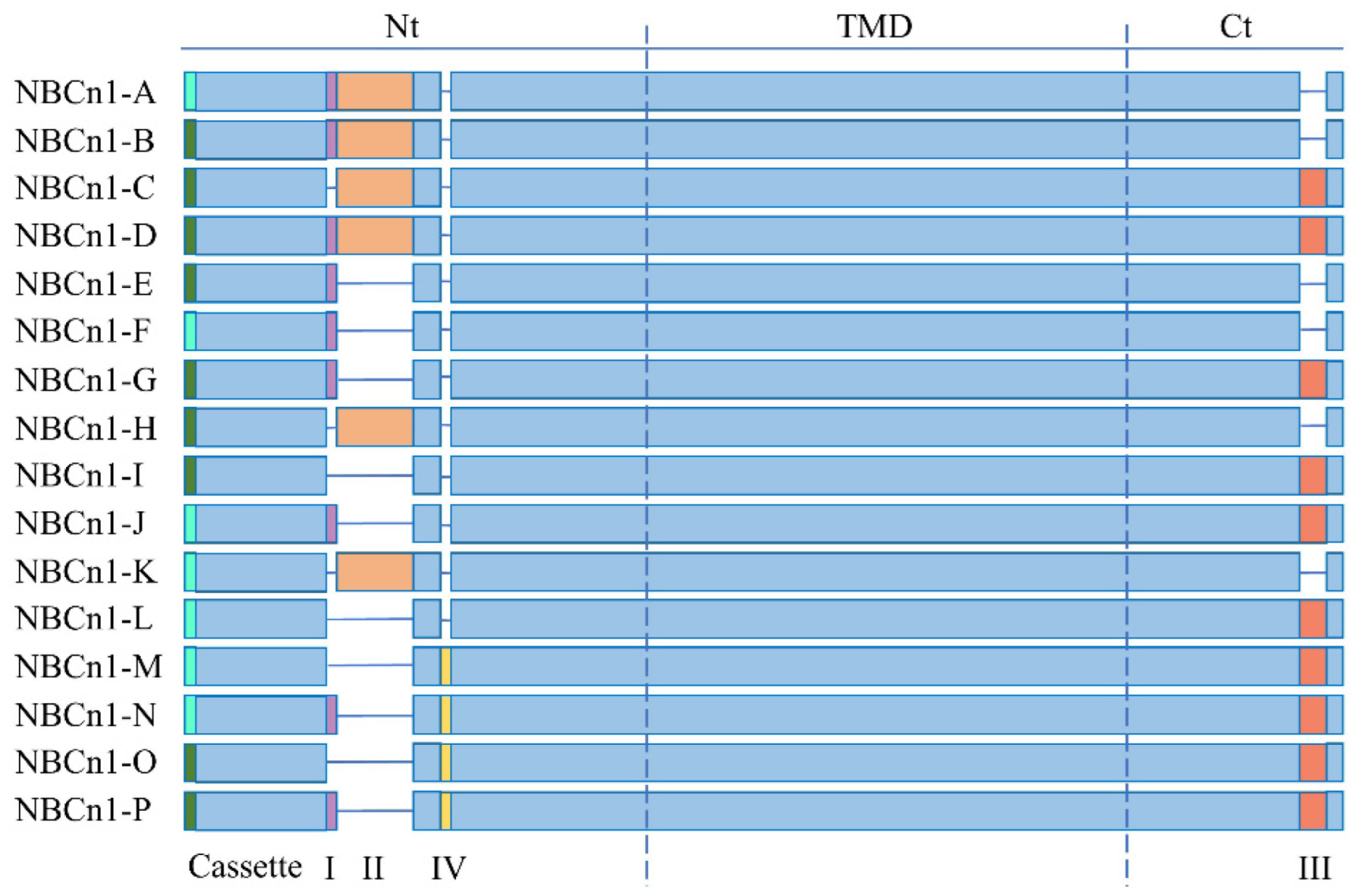
2. NBCn1
3. Function of NBCn1
3.1. Role of NBCn1 in the Central Nervous System
3.2. Role of NBCn1 in the Cardiovascular System
3.3. Role of NBCn1 in the Digestive System
3.4. Role of NBCn1 in the Kidneys
4. Physiological Role of NBCn1 in Breast Cancer
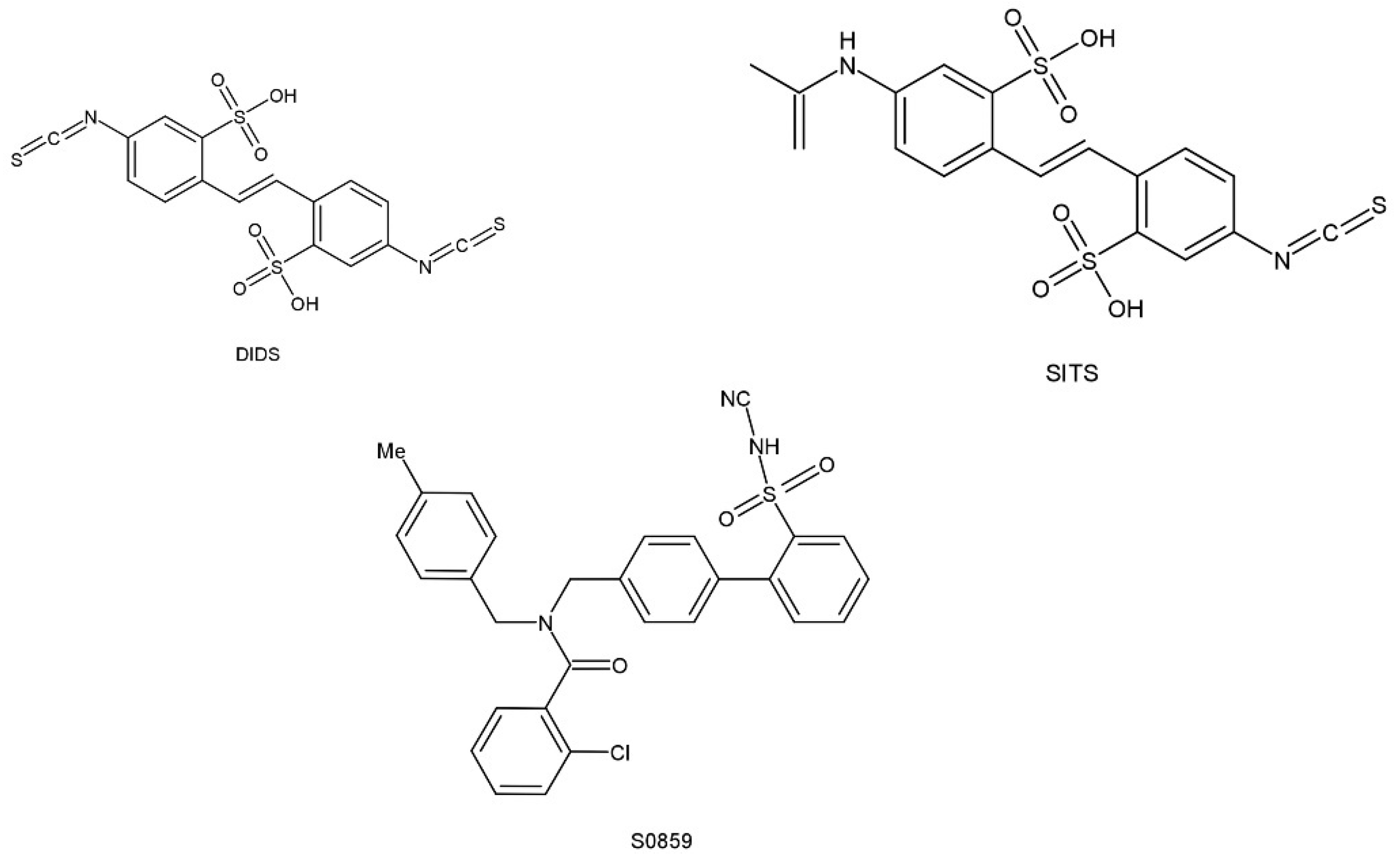
5. Pharmacological Roles of NBCn1
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, S.; Shigekawa, M.; Pouyssegur, J. Molecular Physiology of Vertebrate Na+/H+ Exchangers. Physiol. Rev. 1997, 77, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.F. Molecular pathophysiology of SLC4 bicarbonate transporters. Curr. Opin. Nephrol. Hypertens. 2005, 14, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Chen, L.M. Structure and Function of SLC4 Family HCO3− Transporters. Front. Physiol. 2015, 6, 355. [Google Scholar] [CrossRef]
- Wang, D.K.; Liu, Y.; Myers, E.J.; Guo, Y.M.; Xie, Z.D.; Jiang, D.Z.; Li, J.M.; Yang, J.; Liu, M.; Parker, M.D.; et al. Effects of Nt-truncation and coexpression of isolated Nt domains on the membrane trafficking of electroneutral Na+/HCO3− cotransporters. Sci. Rep. 2015, 5, 12241. [Google Scholar] [CrossRef]
- Romero, M.F.; Fulton, C.M.; Boron, W.F. The SLC4 family of HCO3− transporters. Pflug. Arch.-Eur. J. Physiol. 2004, 447, 495–509. [Google Scholar] [CrossRef]
- Thornell, I.M.; Bevensee, M.O. Regulators of Slc4 bicarbonate transporter activity. Front. Physiol. 2015, 6, 166. [Google Scholar] [CrossRef]
- Aalkjaer, C.; Boedtkjer, E.; Choi, I.; Lee, S. Cation-coupled bicarbonate transporters. Compr. Physiol. 2014, 4, 1605–1637. [Google Scholar] [CrossRef]
- Koltai, T.; Reshkin, S.J.; Harguindey, S. Carbonic anhydrases. In An Innovative Approach to Understanding and Treating Cancer: Targeting pH; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–176. [Google Scholar]
- Kurtz, I.; Zhu, Q. Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr. Opin. Nephrol. Hypertens. 2013, 22, 572–583. [Google Scholar] [CrossRef]
- Gil-Perotin, S.; Jaijo, T.; Verdu, A.G.; Rubio, P.; Mazon, M.; Gasque-Rubio, R.; Diaz, S. Epilepsy, status epilepticus, and hemiplegic migraine coexisting with a novel SLC4A4 mutation. Neurol. Sci. 2021, 42, 3647–3654. [Google Scholar] [CrossRef]
- Collin, G.B.; Shi, L.; Yu, M.; Akturk, N.; Charette, J.R.; Hyde, L.F.; Weatherly, S.M.; Pera, M.F.; Naggert, J.K.; Peachey, N.S.; et al. A Splicing Mutation in Slc4a5 Results in Retinal Detachment and Retinal Pigment Epithelium Dysfunction. Int. J. Mol. Sci. 2022, 23, 2220. [Google Scholar] [CrossRef]
- Groger, N.; Vitzthum, H.; Frohlich, H.; Kruger, M.; Ehmke, H.; Braun, T.; Boettger, T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum. Mol. Genet. 2012, 21, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Chiang, J.; Gorin, M.B. Novel mutation in SLC4A7 gene causing autosomal recessive progressive rod-cone dystrophy. Ophthalmic Genet. 2020, 41, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Liu, Y.; Boron, W.F. Role of an extracellular loop in determining the stoichiometry of Na+-HCO(3)(-) cotransporters. J. Physiol. 2011, 589, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; van Dam, D.; Audenaert, K.; de Deyn, P.P. Genes involved in cerebrospinal fluid production as candidate genes for late-onset Alzheimer’s disease: A hypothesis. J. Neurogenet. 2011, 25, 195–200. [Google Scholar] [CrossRef]
- Bernardo, A.A.; Bernardo, C.M.; Espiritu, D.J.; Arruda, J.A. The sodium bicarbonate cotransporter: Structure, function, and regulation. Semin. Nephrol. 2006, 26, 352–360. [Google Scholar] [CrossRef]
- Pushkin, A.; Abuladze, N.; Lee, I.; Newman, D.; Hwang, J.; Kurtz, I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J. Biol. Chem. 1999, 274, 16569–16575. [Google Scholar] [CrossRef]
- DiMarco, S.P.; Glover, T.W.; Miller, D.E.; Reines, D.; Warren, S.T. Mapping of the Human NBC3 (SLC4A7) Gene to Chromosome 3p22. Genomics 1996, 15, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Rosner, H.I.; Kragelund, B.B. Structure and dynamic properties of membrane proteins using NMR. Compr. Physiol. 2012, 2, 1491–1539. [Google Scholar] [CrossRef]
- Choi, I.; Aalkjaer, C.; Boulpaep, E.L.; Boron, W.F. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 2000, 405, 571–575. [Google Scholar] [CrossRef]
- Boron, W.F.; Chen, L.; Parker, M.D. Modular structure of sodium-coupled bicarbonate transporters. J. Exp. Biol. 2009, 212, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Pushkin, A.; Abuladze, N.; Fedotoff, O.; Kurtz, I. Regulation of the sodium bicarbonate cotransporter kNBC1 function: Role of Asp(986), Asp(988) and kNBC1-carbonic anhydrase II binding. J. Physiol. 2002, 544, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, F.B.; Jaschke, P.; Casey, J.R. Structural and functional characterization of the human NBC3 sodium/bicarbonate co-transporter carboxyl-terminal cytoplasmic domain. Mol. Membr. Biol. 2003, 20, 307–317. [Google Scholar] [CrossRef]
- Kurtz, I. NBCe1 as a model carrier for understanding the structure-function properties of Na+-coupled SLC4 transporters in health and disease. Pflug. Arch. 2014, 466, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, X.; Wang, D.K.; Guo, Y.M.; Gill, H.S.; Morris, N.; Parker, M.D.; Chen, L.M.; Boron, W.F. Effects of optional structural elements, including two alternative amino termini and a new splicing cassette IV, on the function of the sodium-bicarbonate cotransporter NBCn1 (SLC4A7). J. Physiol. 2013, 591, 4983–5004. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.H.; Nielsen, S.; Praetorius, J. An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H172–H180. [Google Scholar] [CrossRef]
- Cooper, D.S.; Saxena, N.C.; Yang, H.S.; Lee, H.J.; Moring, A.G.; Lee, A.; Choi, I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J. Biol. Chem. 2005, 280, 17823–17830. [Google Scholar] [CrossRef]
- Danielsen, A.A.; Parker, M.D.; Lee, S.; Boron, W.F.; Aalkjaer, C.; Boedtkjer, E. Splice cassette II of Na+,HCO3- cotransporter NBCn1 (slc4a7) interacts with calcineurin A: Implications for transporter activity and intracellular pH control during rat artery contractions. J. Biol. Chem. 2013, 288, 8146–8155. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Praetorius, J.; Fuchtbauer, E.M.; Aalkjaer, C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (slc4a7) in mice. Am. J. Physiol. Cell Physiol. 2008, 294, C591–C603. [Google Scholar] [CrossRef]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef]
- Parker, M.D.; Boron, W.F. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol. Rev. 2013, 93, 803–959. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Rajbhandari, I.; Yang, H.S.; Lee, S.; Cucoranu, D.; Cooper, D.S.; Klein, J.D.; Sands, J.M.; Choi, I. Neuronal expression of sodium/bicarbonate cotransporter NBCn1 (SLC4A7) and its response to chronic metabolic acidosis. Am. J. Physiol. Cell Physiol. 2010, 298, C1018–C1028. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, J.; Nejsum, L.N.; Nielsen, S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am. J. Physiol. Cell Physiol. 2004, 286, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.K.; Ha, H.T.T.; Nguyen, T.H.; Nguyen, L.N. The role of SLC transporters for brain health and disease. Cell Mol. Life Sci. 2021, 79, 20. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Candy, S.G. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J. Physiol. 1991, 433, 727–763. [Google Scholar] [CrossRef]
- Kwon, T.H.; Christiaan Fulton, C.; Wang, W.; Kurtz, I.; Frøkiaer, J.; Aalkjaer, C.; Nielsen, S. Chronic metabolic acidosis upregulates rat kidney Na+-HCO3− cotransporters NBCn1 and NBC3 but not NBC1. Am. J. Physiol. Renal. Physiol. 2002, 282, 341–351. [Google Scholar] [CrossRef]
- Pasternack, M.; Voipio, J.; Kaila, K. Intracellular carbonic anhydrase activity and its role in GABA-induced acidosis in isolated rat hippocampal pyramidal neurones. Acta Physiol. Scand. 1993, 148, 229–231. [Google Scholar] [CrossRef]
- Iijima, T.; Ciani, S.; Hagiwara, S. Effects of the external pH on Ca channels: Experimental studies and theoretical considerations using a two-site, two-ion model. Proc. Natl. Acad. Sci. USA 1986, 83, 654–658. [Google Scholar] [CrossRef]
- Cooper, D.S.; Yang, H.S.; He, P.; Kim, E.; Rajbhandari, I.; Yun, C.C.; Choi, I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur. J. Neurosci. 2009, 29, 437–446. [Google Scholar] [CrossRef]
- McKee, J.A.; Brewer, R.P.; Macy, G.E.; Borel, C.O.; Reynolds, J.D.; Warner, D.S. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit. Care 2005, 2, 342–351. [Google Scholar] [CrossRef]
- Park, H.J.; Gonzalez-Islas, C.E.; Kang, Y.; Li, J.M.; Choi, I. Deletion of the Na/HCO3 Transporter NBCn1 Protects Hippocampal Neurons from NMDA-induced Seizures and Neurotoxicity in Mice. Sci. Rep. 2019, 9, 15981. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; McHugh, T.J.; Wilson, M.A.; Tonegawa, S. NMDA receptors, place cells and hippocampal spatial memory. Nat. Rev. Neurosci. 2004, 5, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Barkus, C.; McHugh, S.B.; Sprengel, R.; Seeburg, P.H.; Rawlins, J.N.; Bannerman, D.M. Hippocampal NMDA receptors and anxiety: At the interface between cognition and emotion. Eur. J. Pharmacol. 2010, 626, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Heine, C.; Browning, C.J. Mental health and dual sensory loss in older adults: A systematic review. Front. Aging Neurosci. 2014, 6, 83. [Google Scholar] [CrossRef]
- Bok, D.; Lopez, I.; Woodruff, M.; Nusinowitz, S.; BeltrandelRio, H.; Huang, W. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat. Genet. 2003, 34, 313–319. [Google Scholar] [CrossRef]
- Lopez, I.A.; Acuna, D.; Galbraith, G.; Bok, D.; Ishiyama, A.; Liu, W.; Kurtz, I. Time course of auditory impairment in mice lacking the electroneutral sodium bicarbonate cotransporter NBC3 (slc4a7). Dev. Brain Res. 2005, 160, 63–77. [Google Scholar] [CrossRef]
- Choi, I.; Beedholm, K.; Dam, V.S.; Bae, S.H.; Noble, D.J.; Garraway, S.M.; Aalkjaer, C.; Boedtkjer, E. Sodium bicarbonate cotransporter NBCn1/Slc4a7 affects locomotor activity and hearing in mice. Behav. Brain Res. 2021, 401, 113065. [Google Scholar] [CrossRef]
- Schank, J.R.; Lee, S.; Gonzalez-Islas, C.E.; Nennig, S.E.; Fulenwider, H.D.; Chang, J.; Li, J.M.; Kim, Y.; Jeffers, L.A.; Chung, J.; et al. Increased Alcohol Consumption in Mice Lacking Sodium Bicarbonate Transporter NBCn1. Sci. Rep. 2020, 10, 11017. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.; Ju, E.; Jones, J.A.; Choi, I. Alternative transcription of sodium/bicarbonate transporter SLC4A7 gene enhanced by single nucleotide polymorphisms. Physiol. Genom. 2017, 49, 167–176. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, Y.; Vairamani, K.; Shull, G.E. Critical role of bicarbonate and bicarbonate transporters in cardiac function. World J. Biol. Chem. 2014, 5, 334–345. [Google Scholar] [CrossRef]
- Garciarena, C.D.; Ma, Y.L.; Swietach, P.; Huc, L.; Vaughan-Jones, R.D. Sarcolemmal localisation of Na+/H+ exchange and Na+-HCO3− co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. J. Physiol. 2013, 591, 2287–2306. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Matchkov, V.V.; Boedtkjer, D.M.; Aalkjaer, C. Negative News: Cl− and HCO3− in the Vascular Wall. Physiology 2016, 31, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Bril, A. Transporteurs ioniques dans les pathologies cardiovasculaires: Contrôle du pH ou régulation de la concentration calcique intracellulaire. Ann. Cardiol. D’angéiologie 2003, 52, 41–51. [Google Scholar] [CrossRef]
- Boedtkjer, D.M.; Matchkov, V.V.; Boedtkjer, E.; Nilsson, H.; Aalkjaer, C. Vasomotion has chloride-dependency in rat mesenteric small arteries. Pflug. Arch. 2008, 457, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Praetorius, J.; Matchkov, V.V.; Stankevicius, E.; Mogensen, S.; Fuchtbauer, A.C.; Simonsen, U.; Fuchtbauer, E.M.; Aalkjaer, C. Disruption of Na+,HCO3− cotransporter NBCn1 (slc4a7) inhibits NO-mediated vasorelaxation, smooth muscle Ca2+ sensitivity, and hypertension development in mice. Circulation 2011, 124, 1819–1829. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Aalkjaer, C. Intracellular pH in the resistance vasculature: Regulation and functional implications. J. Vasc. Res. 2012, 49, 479–496. [Google Scholar] [CrossRef]
- Parker, M.D. Mouse models of SLC4-linked disorders of HCO3--transporter dysfunction. Am. J. Physiol. Cell Physiol. 2018, 314, C569–C588. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Praetorius, J.; Aalkjaer, C. NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ. Res. 2006, 98, 515–523. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Bentzon, J.F.; Dam, V.S.; Aalkjaer, C. Na+, HCO3−-cotransporter NBCn1 increases pHi gradients, filopodia, and migration of smooth muscle cells and promotes arterial remodelling. Cardiovasc. Res. 2016, 111, 227–239. [Google Scholar] [CrossRef]
- Thomsen, A.B.; Kim, S.; Aalbaek, F.; Aalkjaer, C.; Boedtkjer, E. Intracellular acidification alters myogenic responsiveness and vasomotion of mouse middle cerebral arteries. J. Cereb. Blood Flow Metab. 2014, 34, 161–168. [Google Scholar] [CrossRef]
- Ito, K.; Hirooka, Y.; Kishi, T.; Kimura, Y.; Kaibuchi, K.; Shimokawa, H.; Takeshita, A. Rho/Rho-kinase pathway in the brainstem contributes to hypertension caused by chronic nitric oxide synthase inhibition. Hypertension 2004, 43, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Quissell, D.O.; Reyland, M.E.; Grichtchenko, I.I. Electrogenic NBCe1 (SLC4A4), but not electroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. Am. J. Physiol. Cell Physiol. 2008, 295, C1385–C1398. [Google Scholar] [CrossRef] [PubMed]
- Gresz, V.; Kwon, T.H.; Vorum, H.; Zelles, T.; Kurtz, I.; Steward, M.C.; Aalkjaer, C.; Nielse, S. Immunolocalization of electroneutral Na+-HCO3− cotransporters in human and rat salivary glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA. 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Xia, W.; Riederer, B.; Juric, M.; Li, J.; Zheng, W.; Cinar, A.; Xiao, F.; Bachmann, O.; Song, P.; et al. Essential role of the electroneutral Na+-HCO3− cotransporter NBCn1 in murine duodenal acid-base balance and colonic mucus layer build-up in vivo. J. Physiol. 2013, 591, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Praetorius, J.; Zheng, W.; Xiao, F.; Riederer, B.; Singh, A.K.; Stieger, N.; Wang, J.; Shull, G.E.; Aalkjaer, C.; et al. The electroneutral Na+:HCO3− cotransporter NBCn1 is a major pHi regulator in murine duodenum. J. Physiol. 2012, 590, 3317–3333. [Google Scholar] [CrossRef] [PubMed]
- Odgaard, E.; Jakobsen, J.K.; Frische, S.; Praetorius, J.; Nielsen, S.; Aalkjaer, C.; Leipziger, J. Basolateral Na+-dependent HCO3− transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J. Physiol. 2004, 555, 205–218. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.J.; Yang, H.S.; Thornell, I.M.; Bevensee, M.O.; Choi, I. Na/Bicarbonate Cotransporter NBCn1 in the Kidney Medullary Thick Ascending Limb Cell Line is Upregulated under Acidic Conditions and Enhances Ammonium Transport. Exp. Physiol. 2010, 95, 926–937. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Bunch, L.; Pedersen, F.D. Physiology, Pharmacology and Pathophysiology of the pH Regulatory Transport Proteins NHE1 and NBCn1: Similarities, Differences, and Implications for Cancer Therapy. Curr. Pharm. Des. 2012, 18, 1345–1371. [Google Scholar] [CrossRef]
- Lee, S.; Choi, I. Sodium-bicarbonate cotransporter NBCn1/Slc4a7 inhibits NH4Cl-mediated inward current in Xenopus oocytes. Exp. Physiol. 2011, 96, 745–755. [Google Scholar] [CrossRef][Green Version]
- Jakobsen, J.K.; Odgaard, E.; Wang, W.; Elkjaer, M.L.; Nielsen, S.; Aalkjaer, C.; Leipziger, J. Functional up-regulation of basolateral Na+-dependent HCO3− transporter NBCn1 in medullary thick ascending limb of K+-depleted rats. Pflug. Arch. 2004, 448, 571–578. [Google Scholar] [CrossRef]
- Lauritzen, G.; Jensen, M.B.; Boedtkjer, E.; Dybboe, R.; Aalkjaer, C.; Nylandsted, J.; Pedersen, S.F. NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: Contributions to pHi regulation and chemotherapy resistance. Exp. Cell Res. 2010, 316, 2538–2553. [Google Scholar] [CrossRef] [PubMed]
- Gorbatenko, A.; Olesen, C.W.; Morup, N.; Thiel, G.; Kallunki, T.; Valen, E.; Pedersen, S.F. ErbB2 upregulates the Na+,HCO3--cotransporter NBCn1/SLC4A7 in human breast cancer cells via Akt, ERK, Src, and Kruppel-like factor 4. FASEB J. 2014, 28, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Thomas, G.; Ghoussaini, M.; Healey, C.S. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat. Genet. 2009, 41, 585–590. [Google Scholar] [CrossRef]
- Long, J.; Shu, X.O.; Cai, Q.; Gao, Y.T.; Zheng, Y.; Li, G.; Li, C.; Gu, K.; Wen, W.; Xiang, Y.B.; et al. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Woo, J.H.; Yu, J.H.; Lee, M.J.; Moon, H.G.; Kang, D.; Noh, D.Y. Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol. Biomark. Prev. 2011, 20, 793–798. [Google Scholar] [CrossRef]
- Chen, Y.; Choong, L.Y.; Lin, Q.; Philp, R.; Wong, C.H.; Ang, B.K.; Tan, Y.L.; Loh, M.C.; Hew, C.L.; Shah, N.; et al. Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol. Cell Proteomics. 2007, 6, 2072–2087. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Yang, H.; Kim, E.; Lee, S. Bicarbonate-Independent Sodium Conductance of Na/HCO3 Cotransporter NBCn1 Decreases NMDA Receptor Function. Curr. Issues Mol. Biol. 2022, 44, 1284–1293. [Google Scholar] [CrossRef]
- Ch’en, F.F.; Villafuerte, F.C.; Swietach, P.; Cobden, P.M.; Vaughan-Jones, R.D. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br. J. Pharmacol. 2008, 153, 972–982. [Google Scholar] [CrossRef]
- Khandoudi, N.; Albadine, J.; Robert, P.; Krief, S.; Berrebi, I.; Martin, X.; Bevensee, M.O.; Boron, W.F.; Bril, A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc. Res. 2001, 52, 387–396. [Google Scholar] [CrossRef]
- De Giusti, V.C.; Orlowski, A.; Villa-Abrille, M.C.; de Cingolani, G.E.; Casey, J.R.; Alvarez, B.V.; Aiello, E.A. Antibodies against the cardiac sodium/bicarbonate co-transporter (NBCe1) as pharmacological tools. Br. J. Pharmacol. 2011, 164, 1976–1989. [Google Scholar] [CrossRef] [PubMed]


| Gene | Mutant Types | Function | Disease |
|---|---|---|---|
| SLC4A4(NBCe1) [11] | Nonsense mutation; missense mutation; frame-shift mutation; 3′UTR mutation; heterozygous mutation | Intracellular retention and truncation of all NBCe variants |
|
| SLC4A5(NBCe2) [12,13] | Splicing mutation | Involved in the regulation of acid–base balance and the regulation of blood pressure |
|
| SLC4A7(NBCn1) [14,15] | Homozygous frame-shift mutation | Modulates pHi and bicarbonate levels |
|
| SLC4A10(NBCn2) [16] | Nonsense mutation | Control of neuronal excitability |
|
| SLC4A8(NDCBE) [8] | ND | NA |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zahra, A.; Wang, Y.; Wu, J. Understanding the Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter and Its Therapeutic Implications. Pharmaceuticals 2022, 15, 1082. https://doi.org/10.3390/ph15091082
Wang J, Zahra A, Wang Y, Wu J. Understanding the Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter and Its Therapeutic Implications. Pharmaceuticals. 2022; 15(9):1082. https://doi.org/10.3390/ph15091082
Chicago/Turabian StyleWang, Jingjing, Aqeela Zahra, YunFu Wang, and Jianping Wu. 2022. "Understanding the Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter and Its Therapeutic Implications" Pharmaceuticals 15, no. 9: 1082. https://doi.org/10.3390/ph15091082
APA StyleWang, J., Zahra, A., Wang, Y., & Wu, J. (2022). Understanding the Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter and Its Therapeutic Implications. Pharmaceuticals, 15(9), 1082. https://doi.org/10.3390/ph15091082





