Discovering a Multi-Component Combination against Vascular Dementia from Danshen-Honghua Herbal Pair by Spectrum-Effect Relationship Analysis
Abstract
:1. Introduction
2. Results
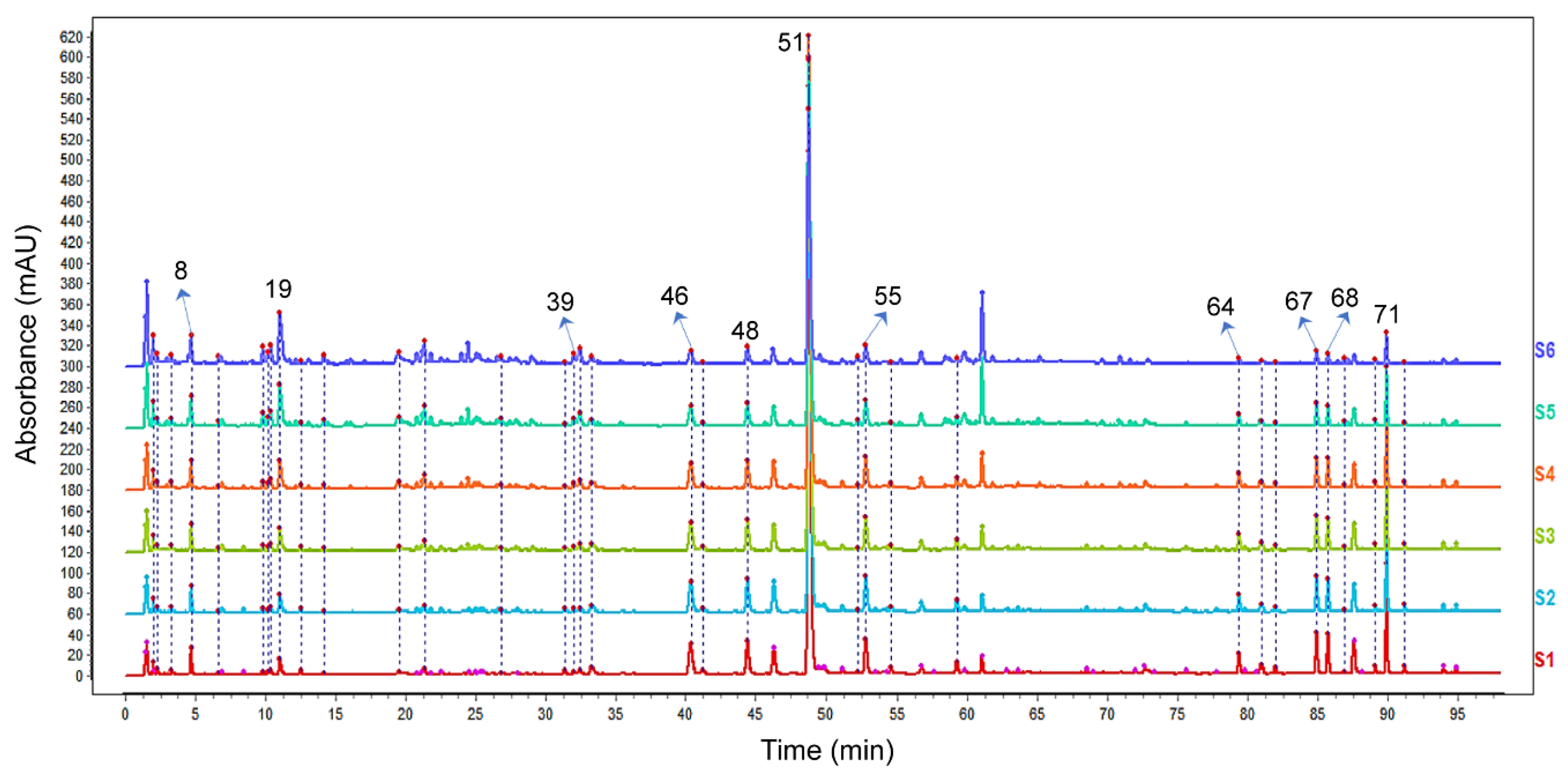
2.1. Characterization of the Chemical Constituents in the DH Herbal Pair
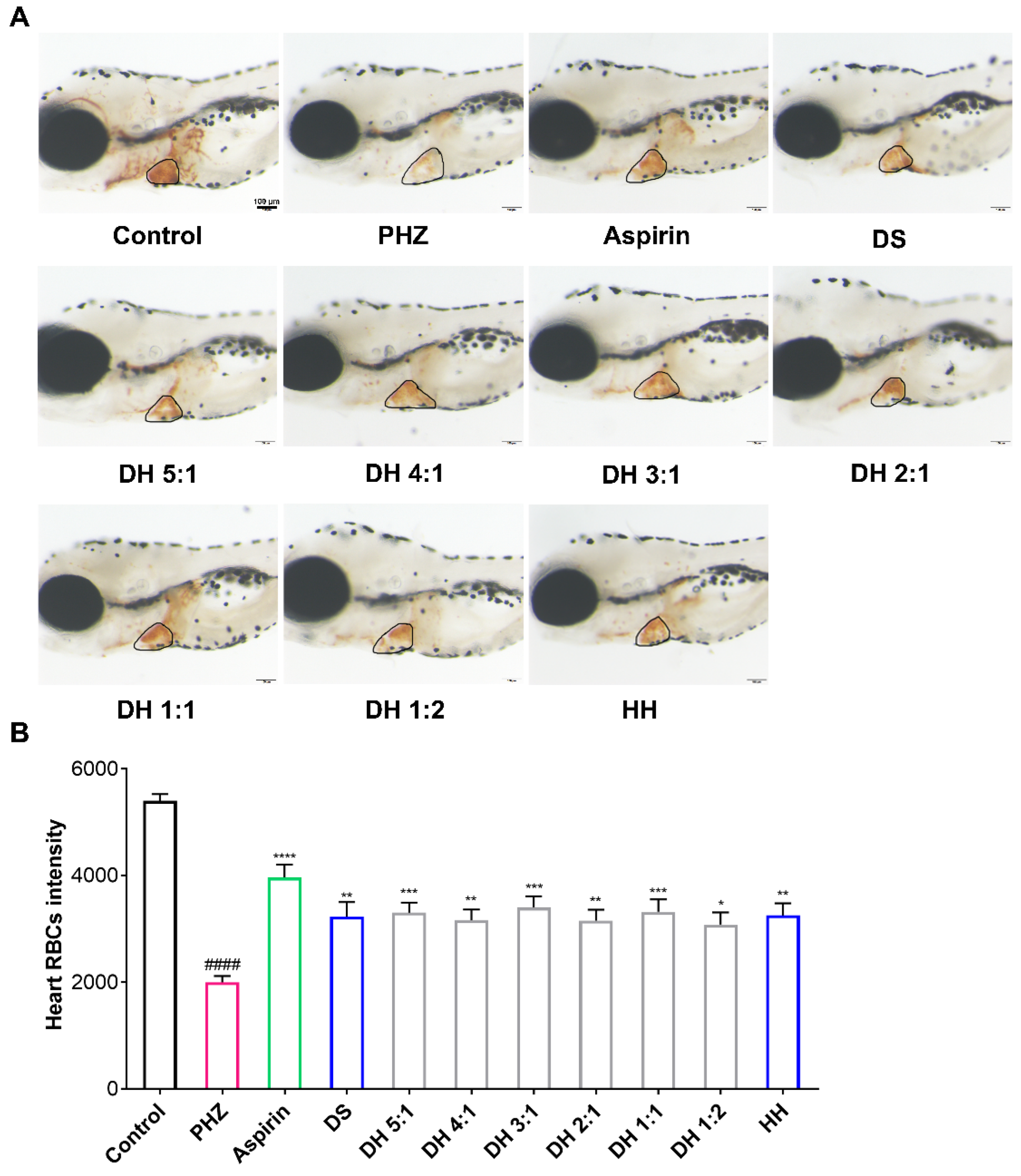
2.2. The DH Herbal Pair Alleviated PHZ-Induced Thrombosis in Zebrafish
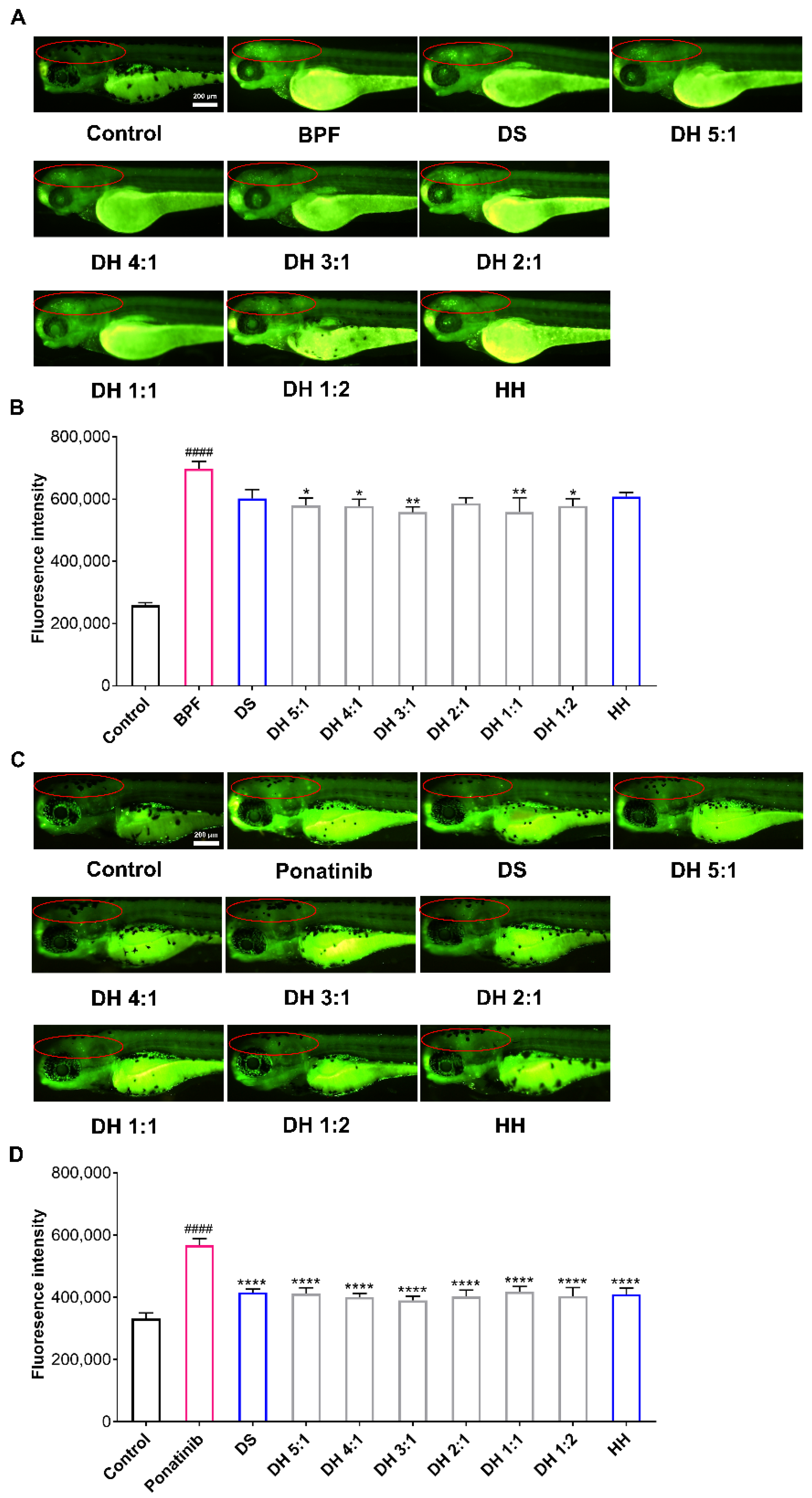
2.3. The DH Herbal Pair Improved Brain Injury of Zebrafish
2.4. Discovering Potential Bioactive Components of the DH Herbal Pair Based on Spectrum-Effect Relationship Analysis
2.5. Determination and Preparation of a Multi-Component Combination
2.6. The MCC of the DH Herbal Pair Improved Thrombosis and Neuronal Damage in Zebrafish
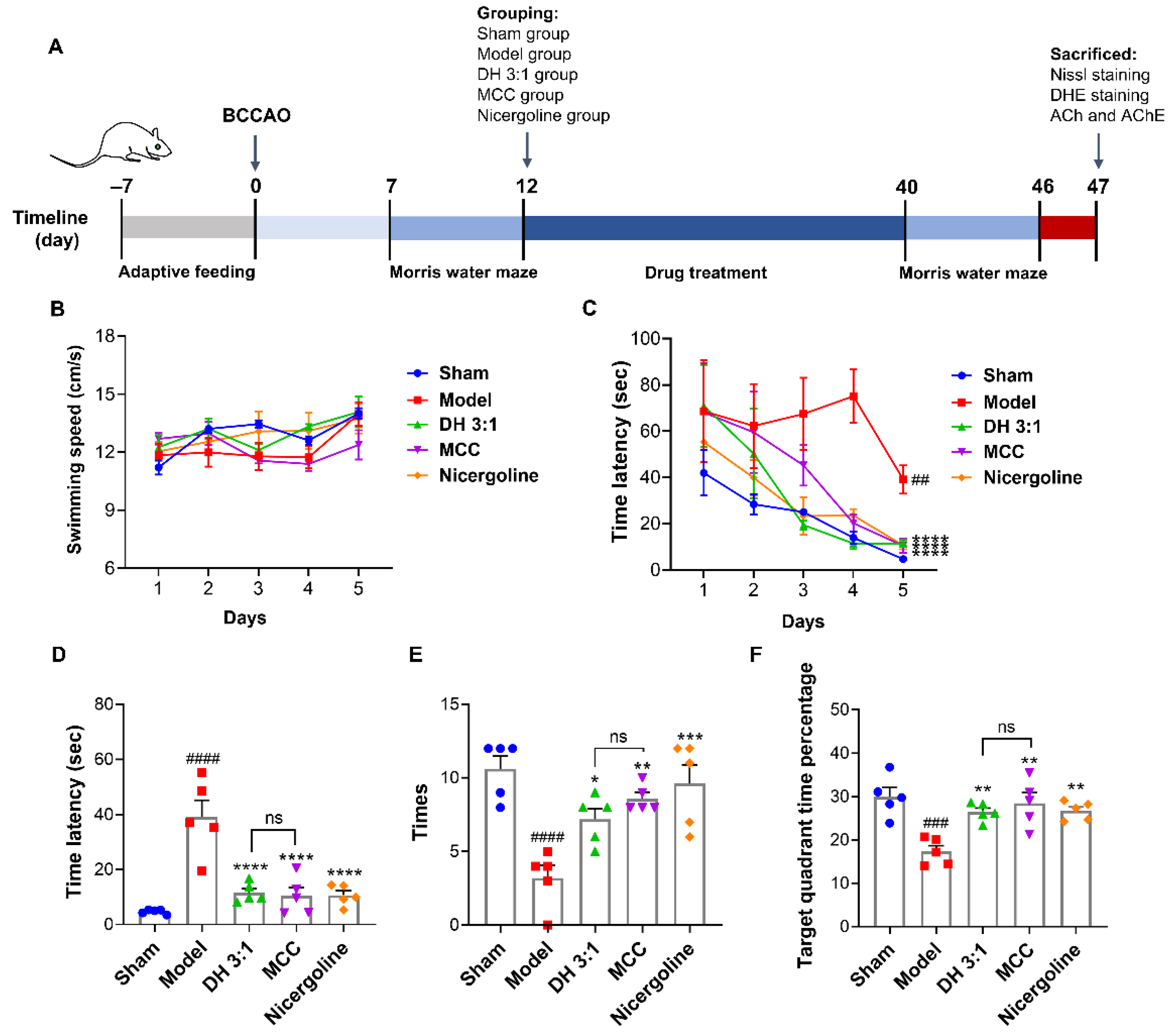
2.7. The MCC of the DH Herbal Pair Improved Cognitive Impairment in VaD Rats
2.8. The MCC Showed an Equivalent Neuroprotective Effect to the DH Herbal Pair in VaD Rats
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Sample Solutions
4.3. HPLC Analysis
4.4. Qualitative Analysis of the DH Herbal Pair
4.5. Quantitative Analysis of DH Herbal Pair
4.6. Method Validation
4.7. Zebrafish Husbandry and Model Construction
4.8. Development of the Rat BCCAO Model and Drug Treatments
4.9. Morris Water Maze Test (MWM)
4.10. Measurement of ACh Content and AChE Activity
4.11. Nissl Staining and Dihydroethidium (DHE) Staining
4.12. The Spectrum-Effect Relationship Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VaD | Vascular dementia |
| AD | Alzheimer’s disease |
| ROS | Reactive oxygen species |
| TCMCR | Traditional Chinese medicine compound recipe |
| DS | Danshen |
| HH | Honghua |
| DH | Danshen-Honghua |
| DHI | Danhong injection |
| BCCAO | Bilateral common carotid artery occlusion |
| UHPLC-QTOF MS | Ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry |
| TIC | Total ion chromatograms |
| MS | Mass Spectrometry |
| PHZ | Phenylhydrazine |
| MNLC | Maximum nonlethal concentration |
| RBCs | Red blood cells |
| BPF | Bisphenol F |
| PLS | Partial least squares |
| HPLC | High performance liquid chromatography |
| VIP | Variable importance in projection |
| MCC | Multi-component combination |
| r2 | Correlation coefficients |
| LODs | Limits of detection |
| LOQs | Limits of quantification |
| RSD | Relative standard deviations |
| MWM | Morris water maze |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| DHE | Dihydroethidium |
| TCM | Traditional Chinese medicine |
| BECCs | Bioactive equivalent combinatorial components |
| DMSO | Dimethyl sulfoxide |
| FI | Fluorescence intensity |
| SI | Staining intensity |
| ESI | Electrospray ionization |
| ICH | International Conference on Harmonization |
| S/NCNS | Signal-to-noise ratiosCentral nervous system |
References
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging Concepts in Vascular Dementia: A Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Hong, F.F.; Yang, S.L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhang, B.; Xia, R.; Jia, Q.Y. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9601–9614. [Google Scholar] [PubMed]
- Wang, P.; Wang, F.; Ni, L.; Wu, P.; Chen, J. Targeting redox-altered plasticity to reactivate synaptic function: A novel therapeutic strategy for cognitive disorder. Acta Pharm. Sin. B 2021, 11, 599–608. [Google Scholar] [CrossRef]
- Kua, E.H.; Ho, E.; Tan, H.H.; Tsoi, C.; Thng, C.; Mahendran, R. The natural history of dementia. Psychogeriatrics 2014, 14, 196–201. [Google Scholar] [CrossRef]
- Buckley, J.S.; Salpeter, S.R. A Risk-Benefit Assessment of Dementia Medications: Systematic Review of the Evidence. Drugs Aging 2015, 32, 453–467. [Google Scholar] [CrossRef]
- Sinha, K.; Sun, C.; Kamari, R.; Bettermann, K. Current status and future prospects of pathophysiology-based neuroprotective drugs for the treatment of vascular dementia. Drug Discov. Today 2020, 25, 793–799. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Q.; Yin, S.J.; Cai, L.; Yang, Y.X.; Liu, W.J.; Hu, Y.J.; Chen, H.; Yang, F.Q. Screening of blood-activating active components from Danshen-Honghua herbal pair by spectrum-effect relationship analysis. Phytomedicine 2019, 54, 149–158. [Google Scholar] [CrossRef]
- Liang, J.; He, X.; Zhou, H.; Liang, P. Effects of Danhong injection on cardiac function and blood lipid in patients with angina pectoris of coronary heart disease: A protocol for randomized, double-blind, placebo-controlled clinical trial. Medicine (Baltimore) 2021, 100, e27479. [Google Scholar] [CrossRef]
- Gao, J.; Shao, X.; Guan, Y.; Mei, J. Effect of Danhong injection on neurological recovery and adverse events in patients with acute ischemic stroke: A protocol for a randomized, double-blind, placebo-controlled clinical study. Medicine (Baltimore) 2021, 100, e27683. [Google Scholar] [CrossRef]
- Orgah, J.O.; He, S.; Wang, Y.; Jiang, M.; Wang, Y.; Orgah, E.A.; Duan, Y.; Zhao, B.; Zhang, B.; Han, J.; et al. Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res. 2020, 153, 104654. [Google Scholar] [CrossRef] [PubMed]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.D.; Mezzomo, N.J.; Kalueff, A.V.; Rosemberg, D.B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 2018, 299, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Liu, H.C.; Guo, S.Y.; Xia, B.; Song, R.S.; Lao, Q.C.; Xuan, Y.X.; Li, C.Q. A Zebrafish Thrombosis Model for Assessing Antithrombotic Drugs. Zebrafish 2016, 13, 335–344. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Yan, X.L.; Guo, Z.N.; Yang, Y. Pathological changes in neurovascular units: Lessons from cases of vascular dementia. CNS Neurosci. Ther. 2021, 27, 17–25. [Google Scholar] [CrossRef]
- Yuan, L.; Qian, L.; Qian, Y.; Liu, J.; Yang, K.; Huang, Y.; Wang, C.; Li, Y.; Mu, X. Bisphenol F-Induced Neurotoxicity toward Zebrafish Embryos. Environ. Sci. Technol. 2019, 53, 14638–14648. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Xia, B.; Ye, T.; Dai, M.Z.; Yang, H.; Li, C.Q.; Li, P. Ponatinib-induced ischemic stroke in larval zebrafish for drug screening. Eur. J. Pharmacol. 2020, 889, 173292. [Google Scholar] [CrossRef]
- Solari, N.; Hangya, B. Cholinergic modulation of spatial learning, memory and navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef]
- Winek, K.; Soreq, H.; Meisel, A. Regulators of cholinergic signaling in disorders of the central nervous system. J. Neurochem. 2021, 158, 1425–1438. [Google Scholar] [CrossRef]
- Varshney, V.V.; Garabadu, D. Naringin Exhibits Mas Receptor-Mediated Neuroprotection Against Amyloid Beta-Induced Cognitive Deficits and Mitochondrial Toxicity in Rat Brain. Neurotox. Res. 2021, 39, 1023–1043. [Google Scholar] [CrossRef]
- Yu, H.; Chen, T.; Zhou, L.; Tang, J. Effect of Selective 5-HT6R Agonist on Expression of 5-HT Receptor and Neurotransmitter in Vascular Dementia Rats. Med. Sci. Monit. 2017, 23, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, H.; Long, F.; Hao, H.P.; Xu, X.; Liu, Y.; Shi, X.W.; Zhang, D.D.; Zheng, H.C.; Wen, Q.Y. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharm. Res. 2014, 31, 1788–1800. [Google Scholar] [CrossRef]
- Long, F.; Yang, H.; Xu, Y.; Hao, H.; Li, P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci. Rep. 2015, 5, 12361. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Lu, J.; Liu, J.; Yuan, Y.; Yu, Y.; Wang, P.; Zhao, X.; Wang, Z. Evaluating the effects of Danhong injection in treatment of acute ischemic stroke: Study protocol for a multicenter randomized controlled trial. Trials 2015, 16, 561. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.D.; Dong, W.; Liu, Y.Q.; Wu, Y.; Tang, D.X.; Zhang, F.C.; Qiu, M.; Hua, Q.; He, J.Y.; et al. Detection of an anti-angina therapeutic module in the effective population treated by a multi-target drug Danhong injection: A randomized trial. Signal Transduct. Target. Ther. 2021, 6, 329. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yang, Y.; Li, Z.; Cheng, L.; Ding, Z.; Wan, H.; Yang, J.; Zhou, H. Compatibility of ingredients of Danshen (Radix Salviae Miltiorrhizae) and Honghua (Flos Carthami) and their protective effects on cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2021, 22, 849. [Google Scholar] [CrossRef]
- Yu, L.; Wan, H.; Jin, W.; Yang, J.; Li, C.; Dai, L.; Ge, L.; Zhou, H.; Wan, H.; He, Y. Protective effects of effective ingredients of Danshen (Radix Salviae Miltiorrhizae) and Honghua (Flos Carthami) compatibility after rat hippocampal neurons induced by hypoxia injury. J. Tradit. Chin. Med. 2018, 38, 685–697. [Google Scholar]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef]
- Staudt, D.; Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef]
- Xu, G.L.; Xie, M.; Yang, X.Y.; Song, Y.; Yan, C.; Yang, Y.; Zhang, X.; Liu, Z.Z.; Tian, Y.X.; Wang, Y.; et al. Spectrum-effect relationships as a systematic approach to traditional chinese medicine research: Current status and future perspectives. Molecules 2014, 19, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2007; ISBN 9789994860579. [Google Scholar]
- Chen, Y.; Chen, P.D.; Bao, B.H.; Shan, M.Q.; Zhang, K.C.; Cheng, F.F.; Cao, Y.D.; Zhang, L.; Ding, A.W. Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J. Ethnopharmacol. 2018, 219, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Yuan, X.; Chu, S.F.; Chen, C.; Ren, Q.; Luo, P.; Lin, M.Y.; Wang, S.S.; Zhu, T.B.; Ai, Q.D.; et al. CZ-7, a new derivative of Claulansine F, ameliorates 2VO-induced vascular dementia in rats through a Nrf2-mediated antioxidant responses. Acta Pharmacol. Sin. 2019, 40, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J. Morris Water Maze Experiment. J. Vis. Exp. 2008, 19, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| No. | TR (min) | Ion Mode | m/z | Calculate (m/z) | Error (ppm) | Fragment Ions (m/z) | Formula | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.04 | [M−H]− | 128.0363 | 128.0353 | −5.30 | 128.04, 82.03 | C5H7NO3 | L-Pyroglutamic acid |
| 2 | 2.28 | [M+H]+ | 268.1035 | 268.1040 | 1.99 | 136.06, 119.03 | C10H13N5O4 | Adenosine |
| 3 | 2.73 | [M−H]− | 117.0196 | 117.0193 | −2.27 | 117.02, 99.01, 73.03 | C4H6O4 | Succinic acid |
| 4 | 3.53 | [M−H]− | 299.0766 | 299.0772 | 2.14 | 137.03, 93.04 | C13H16O8 | 4-Hydroxybenzoic acid 4-O-glucoside |
| 5 | 3.96 | [M+H]+ | 166.0860 | 166.0863 | 1.55 | 120.08, 103.05, 91.06, 77.04 | C9H11NO2 | Phenylalanine |
| 6 | 4.49 | [M+H]+ | 127.0388 | 127.0390 | 1.35 | 109.03, 81.03, 53.04 | C6H6O3 | 5-Hydroxymethylfurfural |
| 7 | 4.80 | [M−H]− | 611.1604 | 611.1618 | 2.22 | 491.12, 473.11, 403.10, 325.08, 283.06, 163.01, 119.05 | C27H32O16 | Hydroxysafflor yellow A isomer |
| 8 | 5.02 | [M−H]− | 197.0452 | 197.0455 | 1.75 | 179.04, 135.05, 109.03 | C9H10O5 | Danshensu a |
| 9 | 5.91 | [M−H]− | 153.0198 | 153.0193 | −3.04 | 109.03, 91.02, 53.05 | C7H6O4 | Protocatechuic acid a |
| 10 | 6.86 | [M−H]− | 353.0878 | 353.0878 | 0.02 | 191.05, 179.03, 173.04, 161.03, 135.04 | C16H18O9 | Neochlorogenic acid a |
| 11 | 7.14 | [M+H]+ | 205.0965 | 205.0972 | 3.20 | 188.07, 146.06, 118.06 | C11H12N2O2 | L-Tryptophan |
| 12 | 7.62 | [M−H]− | 181.0506 | 181.0506 | 0.18 | 163.04, 135.04, 117.03, 92.92, 53.75 | C9H10O4 | 3,4-Dihydroxy benzenepropionic acid |
| 13 | 8.85 | [M−H]− | 137.0239 | 137.0244 | 3.75 | 119.01, 109.03, 108.02, 91.02 | C7H6O3 | Protocatechualdehyde a |
| 14 | 9.37 | [M−H]− | 137.0243 | 137.0244 | 1.58 | 125.35, 94.04, 93.03, 88.20, 81.53 | C7H6O3 | p-Hydroxybenzoic acid a |
| 15 | 9.91 | [M−H]− | 153.0186 | 153.0193 | 4.75 | 109.03, 79.02 | C7H6O4 | 3,5-Dihydroxybenzonic acid/isomer |
| 16 | 10.13 | [M−H]− | 341.0876 | 341.0878 | 0.60 | 179.04, 135.04 | C15H18O9 | Caffeic acid-O-hexoside |
| 17 | 10.52 | [M−H]− | 787.1937 | 787.1938 | 0.19 | 625.14, 463.09, 301.03 | C33H40O22 | 6-Hydroxykaempferol-3,6,7-triglucoside |
| 18 | 10.72 | [M−H]− | 353.0882 | 353.0878 | −1.33 | 353.09, 191.05, 85.03 | C16H18O9 | Chlorogenic acid a |
| 19 | 11.33 | [M−H]− | 611.1625 | 611.1618 | −1.21 | 611.16, 491.12, 473.11, 403.10, 325.07, 313.07, 295.06, 283.06, 207.05, 205.01, 163.01, 119.05 | C27H32O16 | Hydroxysafflor yellow A a |
| 20 | 11.46 | [M−H]− | 325.0938 | 325.0929 | −2.79 | 163.04, 119.05 | C15H18O8 | Coumaric acid-O-hexoside |
| 21 | 12.36 | [M−H]− | 353.0876 | 353.0878 | 0.58 | 191.05, 179.03, 173.04, 135.04 | C16H18O9 | Cryptochlorogenic acid a |
| 22 | 12.96 | [M−H]− | 179.0347 | 179.0350 | 1.57 | 179.03, 135.04, 89.04 | C9H8O4 | Caffeic acid a |
| 23 | 13.32 | [M+H]+ | 627.1540 | 627.1556 | 2.52 | 465.10, 303.05 | C27H30O17 | 6-Hydroxykaempferol-di-O-glucoside |
| 24 | 14.77 | [M−H]− | 595.1664 | 595.1668 | 0.74 | 505.14, 385.09, 313.07 | C27H32O15 | Eriocitrin |
| 25 | 14.82 | [M+H]+ | 627.1566 | 627.1556 | 0.12 | 465.10, 303.05 | C27H30O17 | 6-Hydrokaempferol-di-O-glucoside |
| 26 | 16.09 | [M+COOH]− | 431.1925 | 431.1923 | −0.53 | 385.19, 223.13, 205.12, 153.09 | C19H30O8 | Roseoside |
| 27 | 16.54 | [M−H]− | 357.1189 | 357.1191 | 0.57 | 195.07, 119.05 | C16H22O | Sweroside |
| 28 | 19.48 | [M−H]− | 503.1779 | 503.1770 | −1.76 | 355.09, 337.08, 193.04, 89.02 | C22H32O13 | Tinosinen |
| 29 | 20.01 | [M−H]− | 163.0405 | 163.0401 | −2.64 | 119.05, 93.04, 63.90 | C9H8O3 | p-coumaric acid a |
| 30 | 20.39 | [M−H]− | 611.1635 | 611.1618 | −2.84 | 521.123, 491.11, 448.10, 313.07, 207.05, 119.05 | C27H32O16 | Hydroxysafflor yellow B |
| 31 | 21.19 | [M−H]− | 325.0937 | 325.0929 | −2.48 | 163.04, 119.05 | C15H18O8 | (2Z)-2-(Glucopyranosyloxy)-3-phenyl-2-propenoic acid |
| 32 | 21.54 | [M−H]− | 771.2003 | 771.1989 | −1.77 | 609.15, 301.04 | C33H40O21 | 6-Hydroxykaempferol-3-O-rutinoside-6-O-glucoside |
| 33 | 21.70 | [M−H]− | 625.1424 | 625.1410 | −2.20 | 463.09, 301.03 | C27H30O17 | 6-Hydroxykaempferol-3,6-di-O-glucoside |
| 34 | 25.24 | [M+H]+ | 611.1603 | 611.1607 | 0.59 | 449.10, 287.05 | C27H30O16 | kaempferol 3-O-sophoroside a |
| 35 | 26.99 | [M−H]− | 609.1472 | 609.1461 | −1.79 | 301.04, 255.03 | C27H30O16 | Rutin a |
| 36 | 27.51 | [M−H]− | 463.0886 | 463.0882 | −0.86 | 301.04, 185.87 | C21H20O12 | Quercetin-7-O-glucoside a |
| 37 | 29.54 | [M−H]− | 449.1102 | 449.1089 | −2.81 | 329.06, 287.06 | C21H22O11 | Neocarthamin |
| 38 | 31.89 | [M−H]− | 521.1324 | 521.1301 | −4.47 | 359.08, 323.08, 197.04, 179.04, 161.03 | C24H26O13 | Salviaflaside |
| 39 | 32.54 | [M−H]− | 593.1529 | 593.1512 | −2.87 | 473.09, 285.04, 255.03, 227.03 | C27H30O15 | Kaempferol 3-O-rutinoside a |
| 40 | 32.87 | [M−H]− | 1043.2690 | 1043.2674 | −1.53 | 1025.26, 923.23, 863.20, 593.15, 449.11, 407.10, 287.06 | C48H52O26 | Anhydrosafflor yellow B a |
| 41 | 33.71 | [M−H]− | 537.1047 | 537.1038 | −1.58 | 493.09, 339.05, 321.04, 313.07, 295.06, 277.05, 269.09, 267.07, 253.05, 185.02, 179.04, 135.05 | C27H22O12 | Salvianolic acid H |
| 42 | 33.92 | [M−H]− | 623.1627 | 623.1618 | −1.51 | 315.05, 271.02 | C28H32O16 | Isorhamnetin-3-O-rutinoside |
| 43 | 34.43 | [M−H]− | 447.0933 | 447.0933 | −0.03 | 284.03, 151.00 | C21H20O11 | Luteoloside |
| 44 | 36.03 | [M−H]− | 477.1034 | 477.1038 | 0.94 | 462.08, 315.05, 300.03, 271.03, 209.01, 170.05, 106.04 | C22H22O12 | Safloroside |
| 45 | 40.26 | [M−H]− | 537.1018 | 537.1038 | 3.81 | 339.05, 321.05, 295.06, 277.06, 185.01, 159.04 | C27H22O12 | Salvianolic acid I |
| 46 | 41.11 | [M−H]− | 359.0773 | 359.0722 | −0.16 | 197.04, 179.03, 161.02, 135.04, 133.03, 123.04 | C18H16O8 | Rosmarinic acid a |
| 47 | 41.80 | [M−H]− | 717.1456 | 717.1461 | 0.71 | 537.11, 519.09, 493.12, 339.05, 321.04, 295.06, 279.03, 185.03 | C36H30O16 | Salvianolic acid E |
| 48 | 45.04 | [M−H]− | 537.1037 | 537.1038 | 0.28 | 493.11, 313.07, 295.06, 277.06, 269.07, 267.09, 203.04, 197.04, 185.02, 135.04, 109.03 | C27H22O12 | Lithospermic acid a |
| 49 | 46.23 | [M−H]− | 613.1561 | 613.1563 | 0.29 | 551.16, 361.11, 287.06 | C30H30O14 | Safflomin C |
| 50 | 48.15 | [M−H]− | 613.1570 | 613.1563 | −1.17 | 551.15, 361.11, 287.06 | C30H30O14 | Safflomin C isomer |
| 51 | 49.39 | [M−H]− | 717.1470 | 717.1461 | −1.24 | 717.14, 673.16, 537.10, 519.09, 493.11, 475.10, 457.09, 377.09, 339.05, 321.04, 295.06, 293.05, 197.05, 185.02, 135.05, 109.03 | C36H30O16 | Salvianolic acid B a |
| 52 | 50.40 | [M−H]− | 717.1469 | 717.1461 | −1.10 | 519.09, 339.05, 321.04, 295.06, 185.02, 135.04 | C36H30O16 | Salvianolic acid L |
| 53 | 51.70 | [M−H]− | 373.0923 | 373.0929 | 1.58 | 179.03, 135.05 | C19H18O8 | Rosmarinic acid methyl ester |
| 54 | 52.63 | [M+H]+ | 311.1481 | 311.1489 | 2.63 | 149.09, 127.07, 85.03 | C16H22O16 | (2Z)-2-decene-4,6-diyn-1-yl-Glucopyranoside |
| 55 | 53.38 | [M−H]− | 493.1155 | 493.1140 | −2.99 | 383.10, 313.07, 295.06, 277.05, 267.07, 249.06, 203.04, 197.05, 185.03, 159.05, 135.05, 109.03 | C26H22O10 | Salvianolic acid A a |
| 56 | 55.17 | [M−H]− | 731.1623 | 731.1618 | −0.74 | 533.11, 515.10, 507.1310, 353.07, 335.06, 327.08, 320.04, 309.07, 197.05, 179.03, 135.04 | C37H32O16 | 3′-methyl Salvianolic acid B |
| 57 | 59.79 | [M−H]− | 565.1338 | 565.1351 | 2.38 | 385.09, 367.08, 321.04, 293.05, 277.05, 257.05, 245.05, 231.07, 179.04 | C29H26O12 | Dimethyl lithospermic acid |
| 58 | 62.75 | [M−H]− | 491.0991 | 491.0984 | −1.48 | 311.0556, 293.0447, 267.07, 265.06, 197.04, 135.04 | C26H20O10 | Salvianolic acid C a |
| 59 | 72.96 | [M+H]+ | 311.1268 | 311.1278 | 3.18 | 293.12, 278.09, 275.11, 265.10, 251.10, 247.11, 232.08, 219.11, 204.09 | C19H18O4 | Tanshinone IIB |
| 60 | 75.73 | [M+H]+ | 341.1377 | 341.1384 | 1.91 | 341.1391, 281.1161, 263.11, 235.11, 192.08 | C20H20O5 | Trijuganone C |
| 61 | 77.24 | [M+H]+ | 293.0797 | 293.0808 | 3.89 | 293.08, 275.76, 266.09, 265.08, 250.06, 247.07, 237.08, 222.07, 219.08, 209.09, 194.08, 191.08 | C18H12O4 | Monohydroxytanshinone I |
| 62 | 78.28 | [M+H]+ | 281.1536 | 281.1536 | 0.02 | 266.12, 263.14, 253.13, 252.12, 239.11, 238.10, 235.16, 222.26, 211.10, 208.09, 197.13 | C19H20O2 | Sibiriquinone A |
| 63 | 78.46 | [M+H]+ | 293.1174 | 293.1172 | −0.61 | 278.09, 275.10, 263.07, 251.07, 247.11, 235.07, 232.08, 229.11, 219.12, 204.09, 193.10, 189.07, 179.09, 167.08 | C19H16O3 | 1,2-Didehydrotanshinone IIA |
| 64 | 79.74 | [M+H]+ | 279.1011 | 279.1016 | 1.69 | 261.09, 251.09, 246.07, 233.10, 223.08, 218.07, 205.10, 190.08, 169.06, 141.07 | C18H14O3 | Dihydrotanshinone I a |
| 65 | 81.26 | [M+H]+ | 281.1179 | 281.1172 | −2.42 | 281.12, 263.11, 248.08, 235.11, 220.09, 217.10, 207.11, 202.07, 192.09, 179.09, 169.06, 165.07 | C18H16O3 | Trijuganone B |
| 66 | 82.31 | [M+H]+ | 339.1213 | 339.1217 | 4.14 | 311.13, 279.10, 261.09, 233.10, 190.08 | C20H18O5 | Methyl tanshinonate |
| 67 | 85.24 | [M+H]+ | 297.1479 | 297.1485 | 2.10 | 282.13, 279.14, 268.11, 264.11, 254.09, 251.14, 237.10, 236.11, 233.13, 223.14, 208.11, 197.09, 195.08, 193.10, 181.10 | C19H20O3 | Cryptotanshinone a |
| 68 | 86.08 | [M+H]+ | 277.0860 | 277.0859 | 0.29 | 259.07, 249.09, 234.07, 231.08, 221.10, 206.07, 203.08, 193.10, 178.08 | C18H12O3 | Tanshinone I a |
| 69 | 87.13 | [M+H]+ | 265.1215 | 265.1223 | 3.05 | 265.12, 247.11, 232.09, 223.08, 219.12, 204.09, 194.11, 179.08, 167.09 | C18H16O2 | R0-090680 |
| 70 | 89.40 | [M+H]+ | 281.1534 | 281.1536 | 0.47 | 266.13, 263.14, 253.16, 248.11, 239.13, 238.12, 235.15, 233.09, 225.09, 211.13, 221.09, 193.10, 149.02 | C19H20O2 | 1,2-Didehydromiltirone |
| 71 | 90.20 | [M+H]+ | 295.1321 | 295.1329 | 2.62 | 280.11, 277.12, 262.10, 253.08, 252.08, 249.13, 235.08, 234.10, 231.12, 221.13, 207.09, 206.11, 191.08 | C19H18O3 | Tanshinone IIA a |
| 72 | 91.14 | [M+H]+ | 283.1689 | 283.1693 | 1.26 | 268.15, 265.16, 250.13, 241.12, 240.11, 237.16, 225.10, 223.11, 208.09, 195.11, 180.10, 167.08 | C19H22O2 | Miltirone |
| Sample | Antithrombotic Rate (PHZ) | Neuroprotection Rate (BPF) | Neuroprotection Rate (Ponatinib) |
|---|---|---|---|
| DS | 36.36% a,** | 21.54% b,ns | 64.60% b,**** |
| DH 5:1 | 38.49% *** | 26.75% * | 66.14% **** |
| DH 4:1 | 34.38% ** | 27.33% * | 71.06% **** |
| DH 3:1 | 41.20% *** | 31.65% ** | 74.99% **** |
| DH 2:1 | 34.09% ** | 25.34% ns | 70.01% **** |
| DH 1:1 | 39.04% *** | 31.57% ** | 63.45% **** |
| DH 1:2 | 31.92% * | 27.09% * | 69.44% **** |
| HH | 36.97% ** | 20.17% ns | 67.16% **** |
| Model | Peak Number | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| PHZ | 0.7521 | 0.5154 | 0.5134 | 0.2658 | 0.5549 | 0.1648 | 0.4273 | 1.1379 * | 0.1276 | 0.4321 | 0.5204 | 0.1416 | 0.2747 | 0.1825 | 0.1582 |
| BPF | 0.7342 | 0.4950 | 0.4993 | 0.2660 | 0.5378 | 0.2254 | 0.4101 | 1.1467 * | 0.1744 | 0.4127 | 0.5037 | 0.1939 | 0.3141 | 0.1898 | 0.1578 |
| Ponatinib | 0.7621 | 0.5218 | 0.5166 | 0.2721 | 0.5558 | 0.1571 | 0.4324 | 1.1388 * | 0.1217 | 0.4414 | 0.5217 | 0.1351 | 0.2739 | 0.1948 | 0.1748 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | |
| PHZ | 0.6944 | 0.5726 | 0.8449 | 1.7532 * | 0.2560 | 0.2815 | 0.5518 | 0.4626 | 0.5997 | 0.3404 | 0.2235 | 0.3138 | 0.2133 | 0.8976 | 0.3111 |
| BPF | 0.6975 | 0.5483 | 0.8080 | 1.6917 * | 0.2505 | 0.2788 | 0.5500 | 0.4359 | 0.5930 | 0.3138 | 0.2394 | 0.3115 | 0.2913 | 0.9546 | 0.4250 |
| Ponatinib | 0.7035 | 0.5802 | 0.8547 | 1.7750 * | 0.2610 | 0.2860 | 0.5511 | 0.4675 | 0.6078 | 0.3391 | 0.2351 | 0.3208 | 0.2034 | 0.9323 | 0.2967 |
| 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | |
| PHZ | 0.5635 | 0.5113 | 0.8562 | 0.7700 | 0.3947 | 0.3917 | 0.5786 | 0.4836 | 1.0373 * | 0.7676 | 0.6828 | 0.6586 | 0.3385 | 0.4838 | 0.2182 |
| BPF | 0.5463 | 0.5035 | 0.8407 | 0.7447 | 0.3976 | 0.3769 | 0.5505 | 0.4848 | 1.0025 * | 0.7343 | 0.6842 | 0.6492 | 0.3354 | 0.4696 | 0.2980 |
| Ponatinib | 0.5686 | 0.5140 | 0.8634 | 0.7773 | 0.4000 | 0.3983 | 0.5819 | 0.4819 | 1.0441 * | 0.7737 | 0.6773 | 0.6722 | 0.3451 | 0.4898 | 0.2081 |
| 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | |
| PHZ | 1.6341 * | 0.6784 | 1.4893 * | 0.3097 | 0.3815 | 5.9960 * | 0.7086 | 0.5442 | 0.6152 | 1.5265 * | 0.3003 | 0.3915 | 0.8039 | 0.5504 | 0.3459 |
| BPF | 1.6390 * | 0.6806 | 1.4975 * | 0.2921 | 0.3698 | 6.0138 * | 0.7099 | 0.5456 | 0.5841 | 1.5573 * | 0.3023 | 0.3918 | 0.8083 | 0.5500 | 0.3528 |
| Ponatinib | 1.6305 * | 0.6761 | 1.4866 * | 0.3115 | 0.3863 | 5.9798 * | 0.7083 | 0.5436 | 0.6222 | 1.5242 * | 0.2972 | 0.3884 | 0.8010 | 0.5460 | 0.3411 |
| 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | ||||
| PHZ | 0.3040 | 0.3391 | 0.5084 | 1.0073 * | 0.6035 | 0.4975 | 1.3229 * | 1.2337 * | 0.4814 | 0.5069 | 1.8227 * | 0.5298 | |||
| 0.3217 | 0.3392 | 0.5170 | 1.0088 * | 0.6094 | 0.4992 | 1.3258 * | 1.2423 * | 0.4627 | 0.5218 | 1.8252 * | 0.5309 | ||||
| Ponatinib | 0.3038 | 0.3354 | 0.5062 | 1.0017 * | 0.5979 | 0.4944 | 1.3152 * | 1.2265 * | 0.4812 | 0.5166 | 1.8115 * | 0.5269 | |||
| Peaks | Content (mg/g) | RSD (%) |
|---|---|---|
| Danshensu | 3.03 ± 0.05 | 1.77 |
| Hydroxysafflor yellow A | 15.85 ± 0.19 | 1.19 |
| Kaempferol-3-O-rutinoside | 0.89 ± 0.01 | 1.64 |
| Rosmarinic acid | 2.15 ± 0.02 | 1.11 |
| Lithospermic acid | 2.83 ± 0.04 | 1.51 |
| Salvianolic acid B | 17.31 ± 0.20 | 1.20 |
| Salvianolic acid A | 1.82 ± 0.02 | 1.25 |
| Dihydroisotanshinone I | 0.54 ± 0.01 | 2.20 |
| Cryptotanshinone | 2.76 ± 0.08 | 3.09 |
| Tanshinone I | 0.93 ± 0.02 | 2.59 |
| Tanshinone ⅡA | 2.09 ± 0.07 | 3.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; He, S.; Wu, S.; Li, Y.; Wang, H.; Yan, C.; Yang, H.; Li, P. Discovering a Multi-Component Combination against Vascular Dementia from Danshen-Honghua Herbal Pair by Spectrum-Effect Relationship Analysis. Pharmaceuticals 2022, 15, 1073. https://doi.org/10.3390/ph15091073
Zhang P, He S, Wu S, Li Y, Wang H, Yan C, Yang H, Li P. Discovering a Multi-Component Combination against Vascular Dementia from Danshen-Honghua Herbal Pair by Spectrum-Effect Relationship Analysis. Pharmaceuticals. 2022; 15(9):1073. https://doi.org/10.3390/ph15091073
Chicago/Turabian StyleZhang, Peilin, Shiru He, Siqi Wu, Yi Li, Huiying Wang, Changyang Yan, Hua Yang, and Ping Li. 2022. "Discovering a Multi-Component Combination against Vascular Dementia from Danshen-Honghua Herbal Pair by Spectrum-Effect Relationship Analysis" Pharmaceuticals 15, no. 9: 1073. https://doi.org/10.3390/ph15091073
APA StyleZhang, P., He, S., Wu, S., Li, Y., Wang, H., Yan, C., Yang, H., & Li, P. (2022). Discovering a Multi-Component Combination against Vascular Dementia from Danshen-Honghua Herbal Pair by Spectrum-Effect Relationship Analysis. Pharmaceuticals, 15(9), 1073. https://doi.org/10.3390/ph15091073





