Augmentation of Pectoral Fin Teratogenicity by Thalidomide in Human Cytochrome P450 3A-Expressing Zebrafish
Abstract
1. Introduction
2. Results
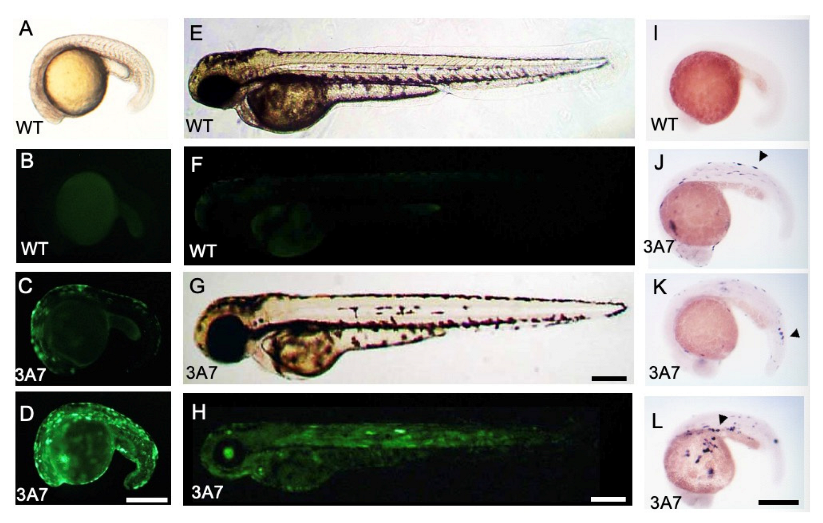
2.1. EGFP and hCYP3A7 Expression in hCYP3A7 Plasmid-Injected Zebrafish
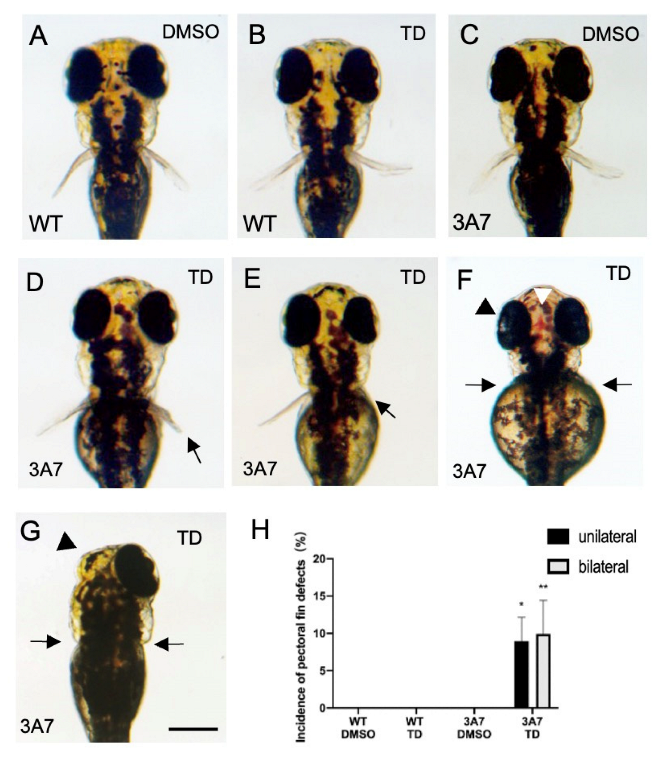
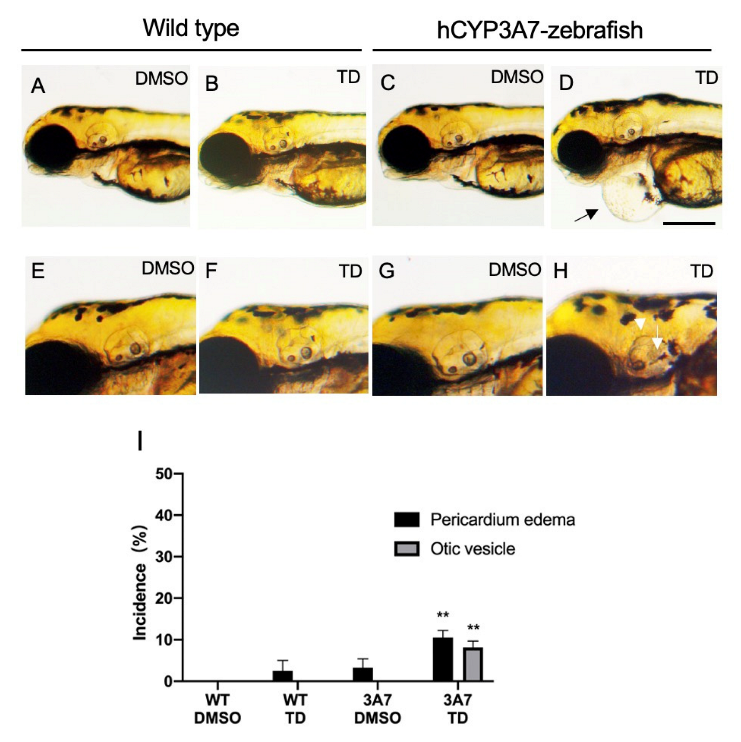
2.2. Effects of Thalidomide on hCYP3As-Expressing Zebrafish during Development
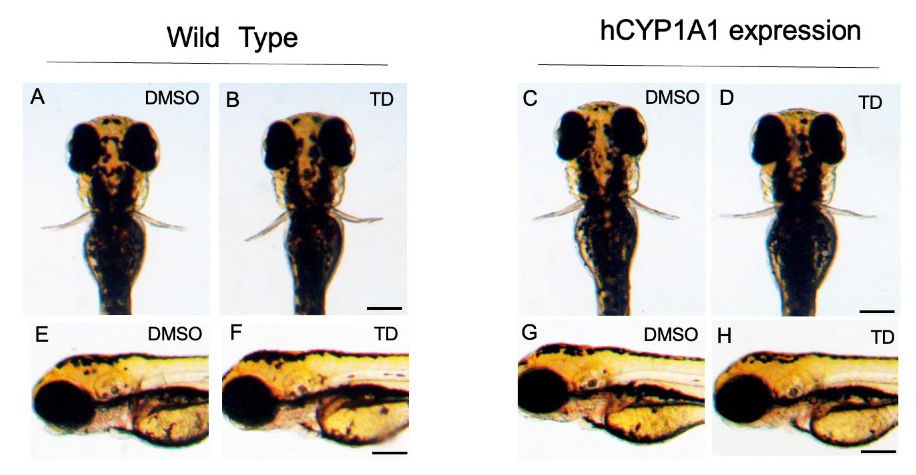
2.3. Effects of Thalidomide on hCYP1A1-Expressing Zebrafish during Development
2.4. Effects of Thalidomide on the Expression of Some Genes Related to Pectoral Fin Development in hCYP3A7-Expressing Zebrafish
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of pT2A Vector and Capped RNA for Expression of Human CYPs
4.3. Zebrafish Culture, Injection of a Vector Mixture and Thalidomide Exposure
4.4. Whole-Mount In Situ Hybridization (WISH)
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, J.A.; Teraoka, H.; Heideman, W.; Peterson, E.R. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Directive 2010/63/EU. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 26 November 2022).
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gamse, J.T.; Gorelick, D.A. Mixtures, Metabolites, and Mechanisms: Understanding Toxicology Using Zebrafish. Zebrafish 2016, 13, 377–378. [Google Scholar] [CrossRef]
- Ioannides, C.; Lewis, D.F.V. Cytochromes P450 in the bioactivation of chemicals. Curr. Top. Med. Chem. 2004, 4, 1767–1788. [Google Scholar] [CrossRef]
- He, X.; Feng, S. Role of Metabolic Enzymes P450 (CYP) on Activating Procarcinogen and their Polymorphisms on the Risk of Cancers. Curr. Drug Metab. 2015, 16, 850–863. [Google Scholar] [CrossRef]
- Wells, P.G.; Winn, L.M. The Role of Biotransformation in Developmental Toxicity. Compr. Toxicol. 2010, 12, 95–116. [Google Scholar]
- Kammala, A.K.; Lintao, R.C.V.; Vora, N.; Mosebarger, A.; Khanipov, K.; Golovko, G.; Yaklic, J.L.; Peltier, M.R.; Conrads, T.P.; Menon, R. Expression of CYP450 Enzymes in Human Fetal Membranes and Its Implications in Xenobiotic Metabolism during Pregnancy. Life Sci. 2022, 307, 120867. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism-A Brief Review on a Fascinating Enzyme Family. J. Xenobiot. 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Franks, M.E.; Macpherson, G.R.; Figg, W.D. Thalidomide. Lancet. 2004, 363, 1802–1811. [Google Scholar] [CrossRef]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Kohler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers. Arzneim. -Forsch. 1979, 29, 1640–1642. [Google Scholar]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the Thalidomide Chirality in Biological Processes by the Self-Disproportionation of Enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef]
- Hansen, J.M.; Harris, K.K.; Philbert, M.A.; Harris, C. Thalidomide Modulates Nuclear Redox Status and Preferentially Depletes Glutathione in Rabbit Limb versus Rat Limb. J. Pharmacol. Exp. Ther. 2002, 300, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Handa, H. Molecular mechanisms of thalidomide and its derivatives. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Kuwagata, M.; Hasegawa, T.; Takashima, H.; Shimizu, M.; Kitajima, S.; Yamazaki, H. Pharmacokinetics of primary metabolites 5-hydroxythalidomide and 5′-hydroxythalidomide formed after oral administration of thalidomide in the rabbit, a thalidomide-sensitive species. J. Toxicol. Sci. 2021, 46, 553–560. [Google Scholar] [CrossRef]
- Yamazaki, H.; Suemizu, H.; Shimizu, M.; Igaya, S.; Shibata, N.; Nakamura, M.; Chowdhury, G.; Guengerich, P.F. In Vivo Formation of Dihydroxylated and Glutathione Conjugate Metabolites Derived from Thalidomide and 5-Hydroxythalidomide in Humanized TK-NOG Mice. Chem. Res. Toxicol. 2012, 25, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, G.; Murayama, N.; Okada, Y.; Uno, Y.; Shimizu, M.; Shibata, N.; Guengerich, F.P.; Yamazaki, H. Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formation of a glutathione conjugate. Chem. Res. Toxicol. 2010, 23, 1018–1024. [Google Scholar] [CrossRef]
- Murayama, N.; Kazuki, Y.; Satoh, D.; Arata, K.; Harada, T.; Shibata, N.; Guengerich, P.F.; Yamazaki, H. Induction of human cytochrome P450 3A enzymes in cultured placental cells by thalidomide and relevance to bioactivation and toxicity. J. Toxicol. Sci. 2017, 42, 343–348. [Google Scholar] [CrossRef]
- Ando, Y.; Fuse, E.; Figg, W.D. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 2002, 8, 1964–1973. [Google Scholar]
- Stevens, J.C.; Hines, R.N.; Gu, C.; Koukouritaki, S.B.; Manro, J.R.; Tandler, P.J.; Zaya, M.J. Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther. 2003, 307, 573–582. [Google Scholar] [CrossRef]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin. Pharm. 2004, 43, 487–514. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Asatsuma-Okumura, T.; Ito, T.; Handa, H. Molecular Mechanisms of the Teratogenic Effects of Thalidomide. Pharmaceuticals 2020, 13, 95. [Google Scholar] [CrossRef]
- Yamanaka, S.; Hidetaka Murai, H.; Saito, D.; Abe, G.; Tokunaga, E.; Iwasaki, T.; Takahashi, H.; Takeda, H.; Suzuki, T.; Shibata, N.; et al. Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neosubstrate PLZF. EMBO J. 2021, 40, e105375. [Google Scholar] [CrossRef]
- Therapontos, C.; Erskine, L.; Gardner, E.R.; Figg, W.D.; Vargesson, N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl. Acad. Sci. USA. 2009, 106, 8573–8578. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Yamashita, T.; Yamauchi, T.; Matsumoto, K.; Sato, K. Effects of thalidomide on Fgf8, Bmp4 and Hoxa11 expression in the limb bud in Kbl:JW rabbit embryos. Congenit. Anom. 2014, 54, 54–62. [Google Scholar] [CrossRef]
- Furihata, H.; Yamanaka, S.; Honda, T.; Miyauchi, Y.; Asano, A.; Shibata, N.; Tanokura, M.; Sawasaki, T.; Miyakawa, T. Structural bases of IMiD selectivity that emerges by 5-hydroxythalidomide. Nat. Commun. 2020, 11, 4578. [Google Scholar] [CrossRef]
- Kazuki, Y.; Akita, M.; Kobayashi, K.; Osaki, M.; Satoh, D.; Ohta, R.; Abe, S.; Takehara, S.; Kazuki, K.; Yamazaki, H.; et al. Thalidomide-Induced Limb Abnormalities in a Humanized CYP3A Mouse Model. Sci. Rep. 2016, 6, 21419. [Google Scholar] [CrossRef]
- Mikami, I.; Yuka Takahashi, Y.; Koiwa, J.; Okamura, M.; Toshio Tanaka, T. Zebrafish yolk sac microinjection of thalidomide for assessment of developmental toxicology. Congenit. Anom. 2019, 60, 71–72. [Google Scholar] [CrossRef]
- Harvey, S.A.; Logan, M.P.O. Sall4 Acts Downstream of Tbx5 and Is Required for Pectoral Fin Outgrowth. Development 2006, 133, 1165–1173. [Google Scholar] [CrossRef]
- Kawakami, K. Transposon tools and methods in zebrafish. Dev. Dyn. 2005, 234, 244–254. [Google Scholar] [CrossRef]
- Poon, K.L.; Wang, X.; Ng, A.S.; Goh, W.H.; McGinnis, C.; Fowler, S.; Carney, T.J.; Wang, H.; Ingham, P.W. Humanizing the Zebrafish Liver Shifts Drug Metabolic Profiles and Improves Pharmacokinetics of CYP3A4 Substrates. Arch. Toxicol. 2017, 91, 1187–1197. [Google Scholar] [CrossRef]
- Lewis, D.F.; Eddershaw, P.J.; Dickins, M.; Tarbit, M.H.; Goldfarb, P.S. Structural determinants of cytochrome P450 substrate specificity, binding affinity and catalytic rate. Chem. Biol. Interact. 1998, 115, 175–199. [Google Scholar] [CrossRef]
- Köster, R.W.; Fraser, S.E. Tracing transgene expression in living zebrafish embryos. Dev. Biol. 2001, 233, 329–346. [Google Scholar] [CrossRef]
- Sato, Y.; Dong, W.; Nakamura, T.; Mizoguchi, N.; Nawaji, T.; Nishikawa, M.; Onaga, T.; Ikushiro, S.; Kobayashi, M.; Teraoka, H. Transgenic Zebrafish Expressing Rat Cytochrome P450 2E1 (CYP2E1): Augmentation of Acetaminophen-Induced Toxicity in the Liver and Retina. Int. J. Mol. Sci. 2023, 24, 4013. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Veeriah, V.; Priya, M.K.; Rajendran, S.; Saran, U.; Sinha, S.; Nagarajan, S.; Pradeep, T.; Chatterjee, S. Nitric oxide rescues thalidomide mediated teratogenicity. Sci. Rep. 2012, 2, 679. [Google Scholar] [CrossRef]
- Gao, X.P.; Feng, F.; Zhang, X.Q.; Liu, X.X.; Wang, Y.B.; She, J.X.; He, Z.H.; He, M.F. Toxicity assessment of 7 anticancer compounds in zebrafish. Int. J. Toxicol. 2014, 33, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Uchida, M.; Terada, A.; Kono, S.; Ishibashi, H.; Arizono, K.; Tominaga, N. Electroporation of thalidomide to medaka (Oryzias latipes) embryo for evaluation of developmental toxicity. Fundam. Toxicol. Sci. 2021, 8, 189–193. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-Induced Teratogenesis: History and Mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.A.; An, J.; Nowak, R.P.; Yuan, J.C.; Fink, E.C.; Berry, B.C.; Ebert, B.L.; Fischer, E.S. Thalidomide Promotes Degradation of SALL4, a Transcription Factor Implicated in Duane Radial Ray Syndrome. ELife 2018, 7, e38430. [Google Scholar] [CrossRef]
- Yabu, T.; Tomimoto, H.; Taguchi, Y.; Yamaoka, S.; Igarashi, Y.; Okazaki, T. Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood 2005, 106, 125–134. [Google Scholar] [CrossRef]
- Paulissen, S.M.; Castranova1, D.M.; Krispin, S.M.; Burns1, M.C.; Menéndez, J.; Vázquez, J.T.; Weinstein, B.M. Anatomy and development of the pectoral fin vascular network in the zebrafish. Development 2022, 149, dev199676. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, C.A.; Kurik, E.M.; Ahlberg, P.E. The Pectoral Fin of Panderichthys and the Origin of Digits. Nature 2008, 456, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Leerberg, D.M.; Hopton, R.E.; Draper, B.W. Fibroblast Growth Factor Receptors Function Redundantly During Zebrafish Embryonic Development. Genetics 2019, 212, 1301–1319. [Google Scholar] [CrossRef] [PubMed]
- Mercader, N. Early steps of paired fin development in zebrafish compared with tetrapod limb development. Develop. Growth Differ. 2007, 49, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Reifers, F.; Böhli, H.; Walsh, E.C.; Crossley, P.H.; Stainier, D.Y.; Brand, M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 1998, 125, 2381–2395. [Google Scholar] [CrossRef]
- Boulet, A.M.; Moon, A.M.; Arenkiel, B.R.; Capecchi, M.R. The Roles of Fgf4 and Fgf8 in Limb Bud Initiation and Outgrowth. Dev. Biol. 2004, 273, 361–372. [Google Scholar] [CrossRef]
- Sun, X.; Mariani, F.V.; Martin, G.R. Functions of FGF Signalling from the Apical Ectodermal Ridge in Limb Development. Nature 2002, 418, 501–508. [Google Scholar] [CrossRef]
- Riddle, R.D.; Johnson, R.L.; Laufer, E.; Tabin, C. Sonic Hedgehog Mediates the Polarizing Activity of the ZPA. Cell 1993, 75, 1401–1416. [Google Scholar] [CrossRef]
- Asatsuma-Okumura, T.; Ando, H.; Simone, M.D.; Yamamoto, J.; Sato, T.; Shimizu, N.; Asakawa, K.; Yamaguchi, Y.; Ito, T.; Guerrini, L.; et al. p63 is a cereblon substrate involved in thalidomide teratogenicity. Nat. Chem. Biol. 2019, 15, 1077–1084. [Google Scholar] [CrossRef]
- Norton, W.H.; Ledin, J.; Grandel, H.; Neumann, C.J. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development 2005, 132, 4963–4973. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef]
- Nawaji, T.; Yamashita, N.; Umeda, H.; Zhang, S.; Mizoguchi, N.; Seki, M.; Kitazawa, T.; Teraoka, H. Cytochrome P450 Expression and Chemical Metabolic Activity before Full Liver Development in Zebrafish. Pharmaceuticals 2020, 13, 456. [Google Scholar] [CrossRef] [PubMed]
- Nijoukubo, D.; Adachi, H.; Kitazawa, T.; Teraoka, H. Blood vessels are primary targets for 2,3,7,8-tetrachlorodibenzo-p-dioxin in pre-cardiac edema formation in larval zebrafish. Chemosphere 2020, 254, 126808. [Google Scholar] [CrossRef]
- Easterbrook, J.; Lu, C.; Sakai, Y.; Li, A.P. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab. Dispos. 2001, 29, 141–144. [Google Scholar]
- Krauss, S.; Concordet, J.P.; Ingham, P.W. A functionally conserved homolog of the Dro sophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 1993, 75, 1431–1444. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Akasaka, I.; Komiyama, A.; Nakamura, T.; Mizoguchi, N.; Nawaji, T.; Ikushiro, S.; Kobayashi, M.; Teraoka, H. Augmentation of Pectoral Fin Teratogenicity by Thalidomide in Human Cytochrome P450 3A-Expressing Zebrafish. Pharmaceuticals 2023, 16, 368. https://doi.org/10.3390/ph16030368
Dong W, Akasaka I, Komiyama A, Nakamura T, Mizoguchi N, Nawaji T, Ikushiro S, Kobayashi M, Teraoka H. Augmentation of Pectoral Fin Teratogenicity by Thalidomide in Human Cytochrome P450 3A-Expressing Zebrafish. Pharmaceuticals. 2023; 16(3):368. https://doi.org/10.3390/ph16030368
Chicago/Turabian StyleDong, Wenjing, Ippo Akasaka, Akifumi Komiyama, Tatsuro Nakamura, Naohiro Mizoguchi, Tasuku Nawaji, Shinichi Ikushiro, Makoto Kobayashi, and Hiroki Teraoka. 2023. "Augmentation of Pectoral Fin Teratogenicity by Thalidomide in Human Cytochrome P450 3A-Expressing Zebrafish" Pharmaceuticals 16, no. 3: 368. https://doi.org/10.3390/ph16030368
APA StyleDong, W., Akasaka, I., Komiyama, A., Nakamura, T., Mizoguchi, N., Nawaji, T., Ikushiro, S., Kobayashi, M., & Teraoka, H. (2023). Augmentation of Pectoral Fin Teratogenicity by Thalidomide in Human Cytochrome P450 3A-Expressing Zebrafish. Pharmaceuticals, 16(3), 368. https://doi.org/10.3390/ph16030368





