Study of Orally Disintegrating Tablets Using Erythritol as an Excipient Produced by Moisture-Activated Dry Granulation (MADG)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Visual Observation of ODTs
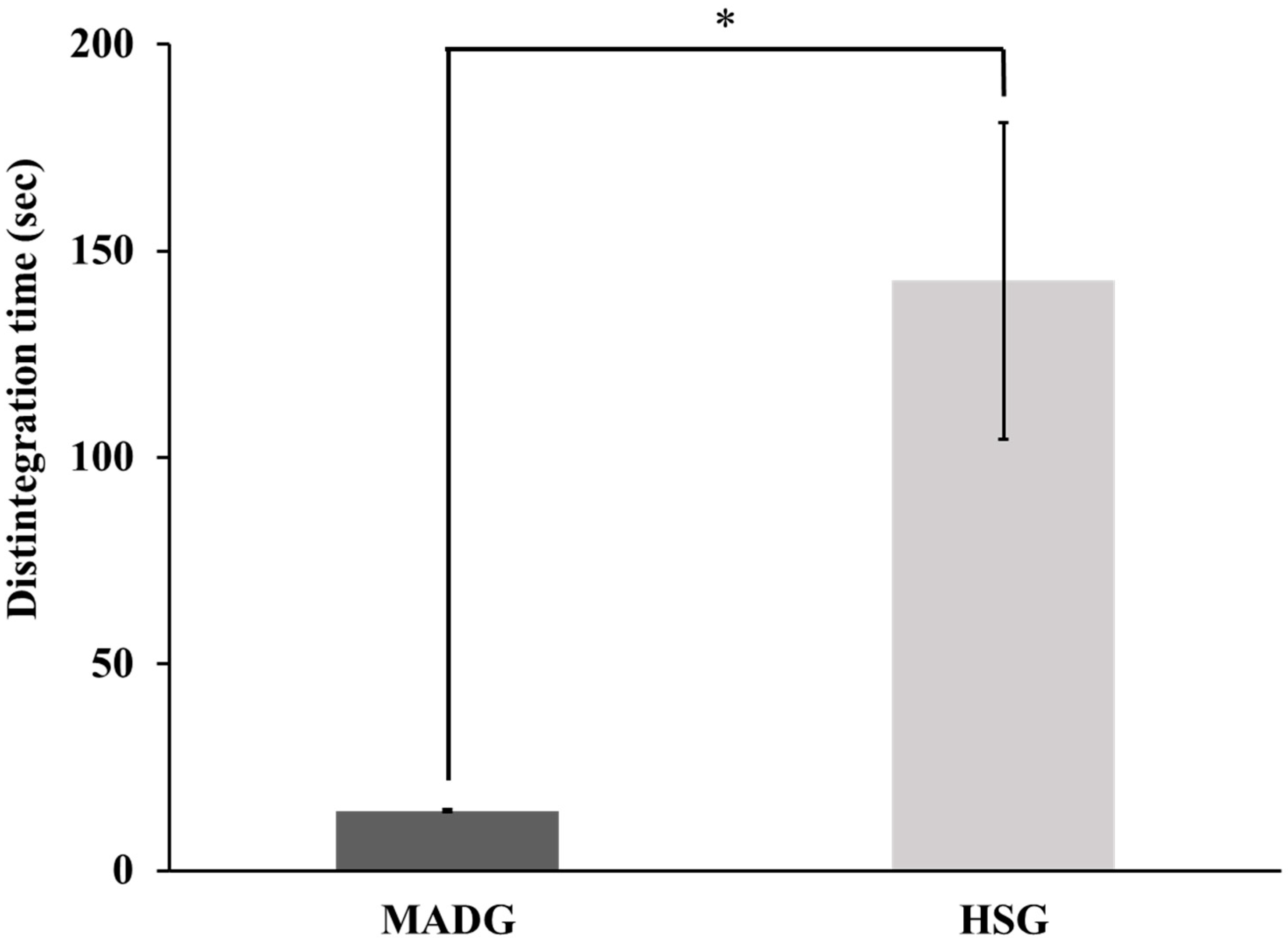
2.2. Tablet Disintegration Time and Scanning Electron Microscope Photographs
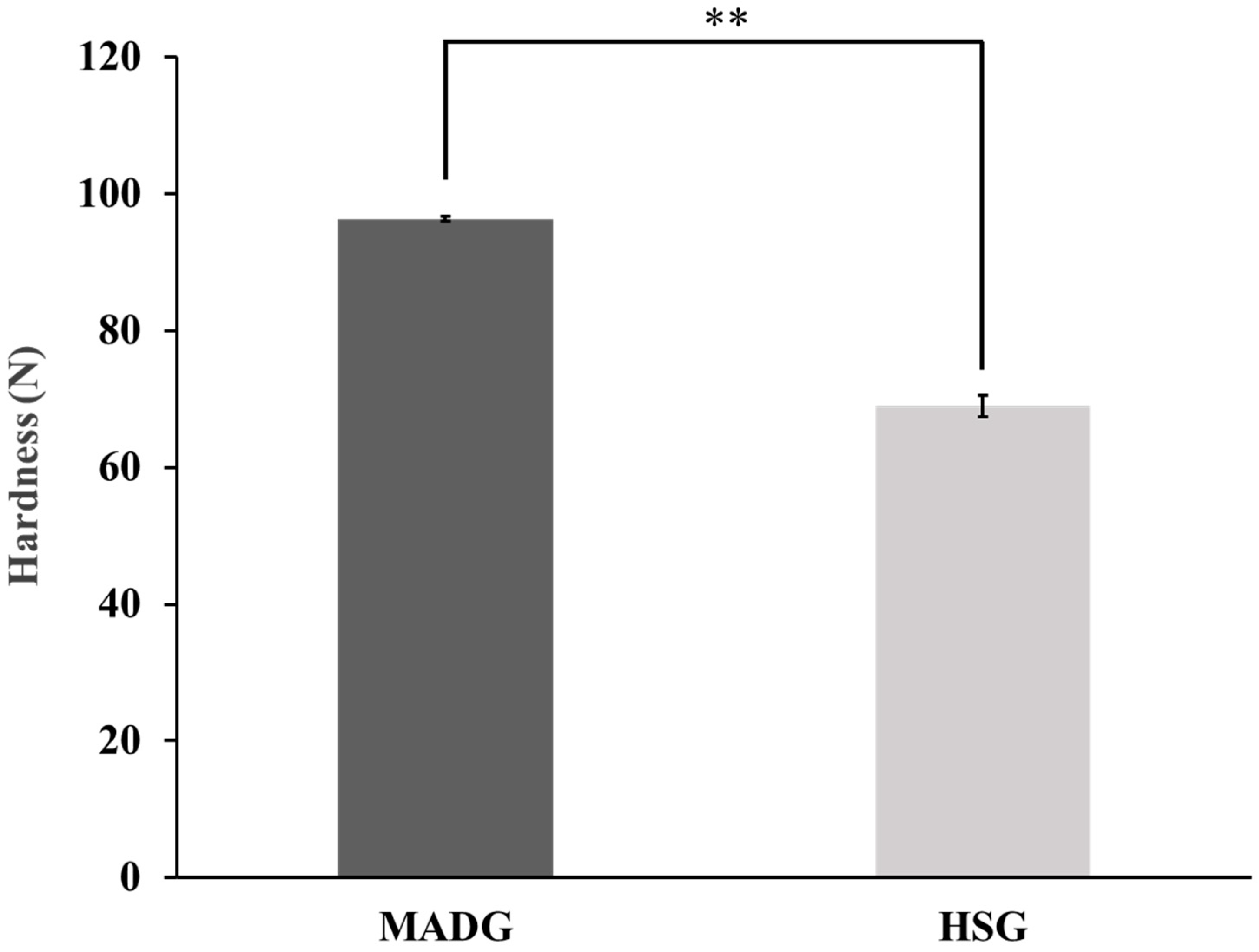
2.3. Tablet Hardness
2.4. Effect of Water Content on the Water Activity of Granules Produced Using MADG
2.5. Effect of Water Content on the Tabletability of ODTs Produced Using MADG
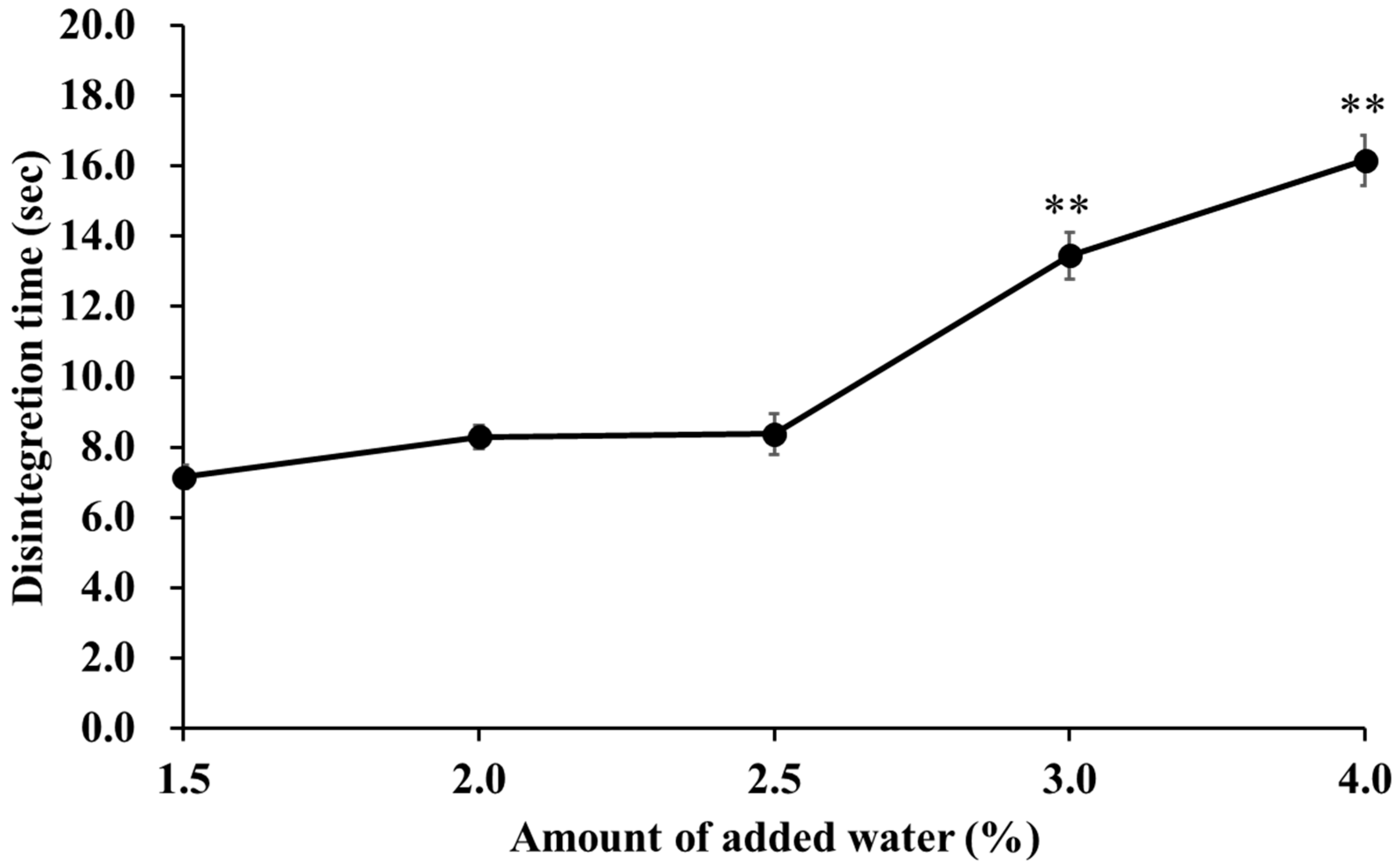
2.6. Effect of Water Addition on Tablet Disintegration Time
2.7. Tableting Properties
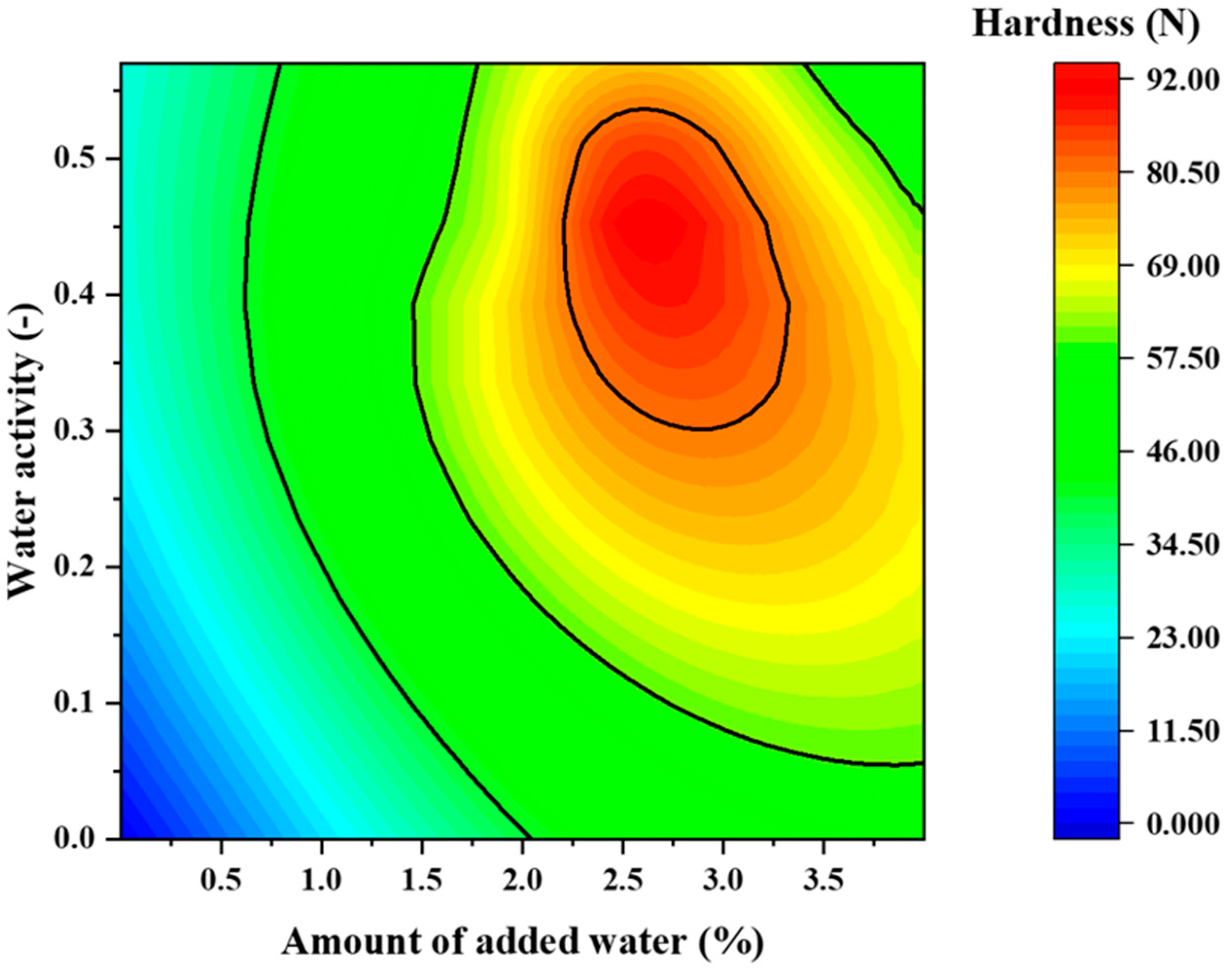
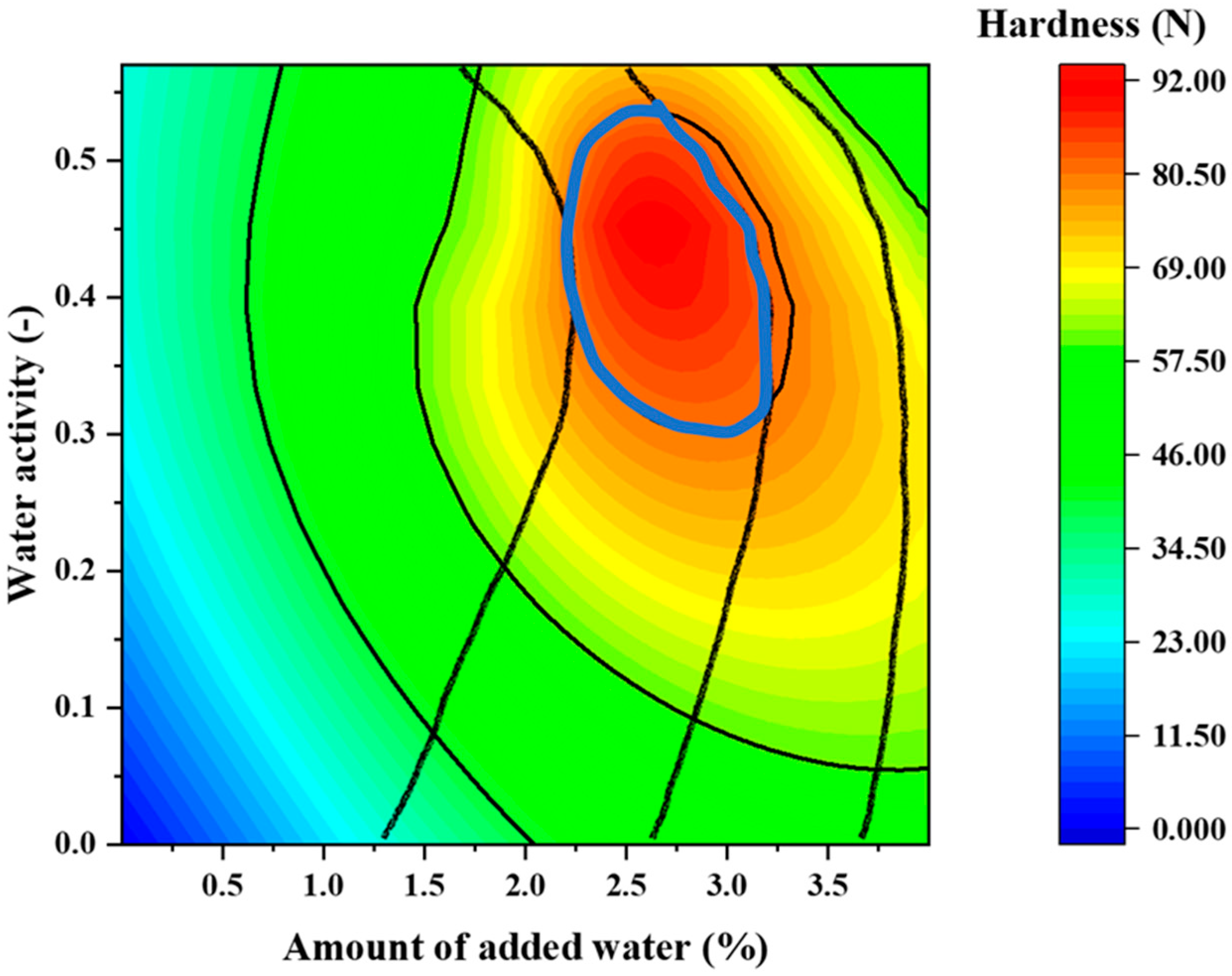
2.8. Effect of the Amount of Added Water and Water Activity on the Disintegration Time and Tablet Hardness
3. Materials and Methods
3.1. Materials
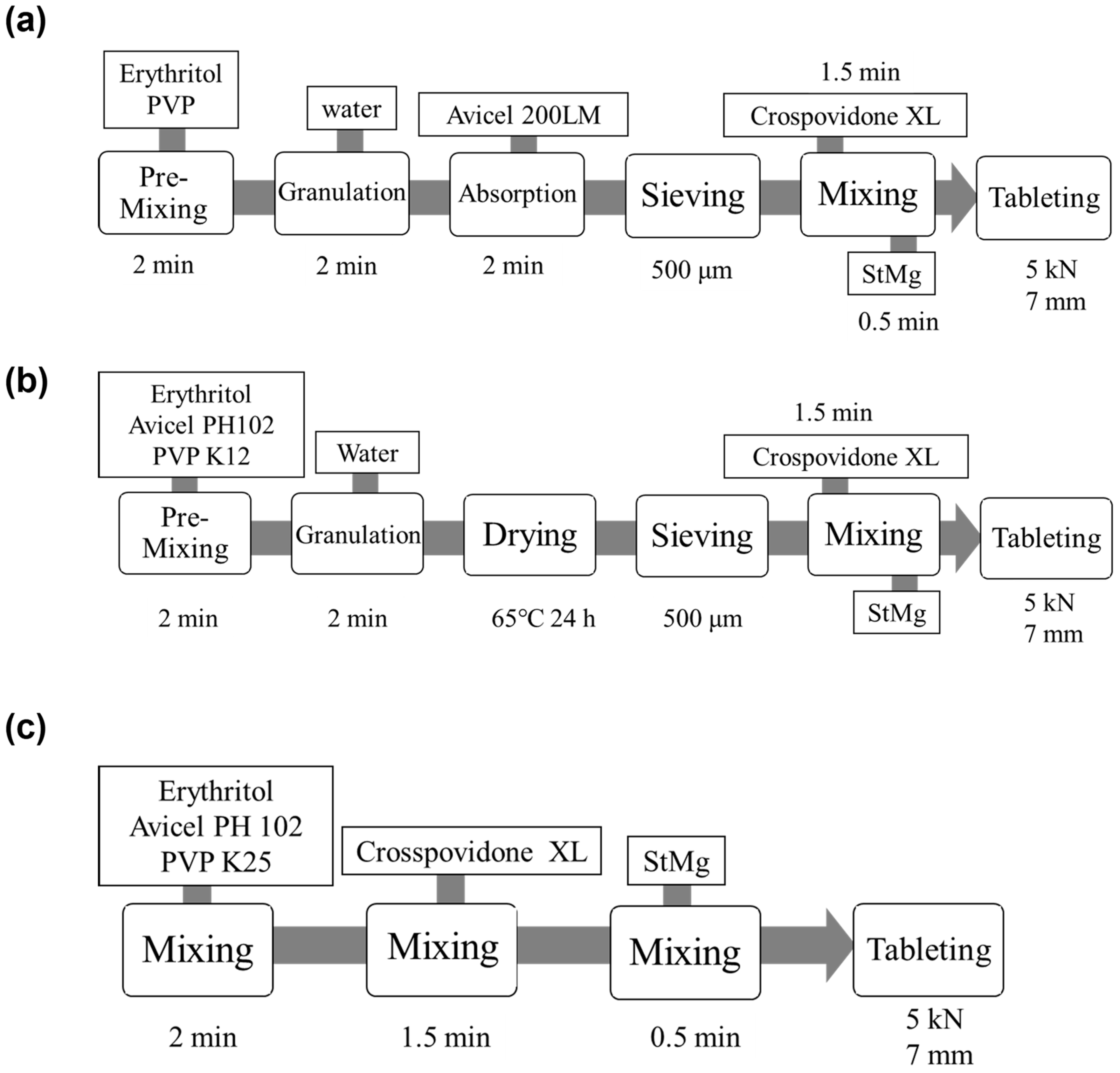
3.2. Preparation of ODTs Using MADG, HSG, and DC
3.3. Water Activity
3.4. Tablet Disintegration Time
3.5. Tablet Friability
3.6. Scanning Electron Microscopy (SEM)
3.7. Tablet Hardness
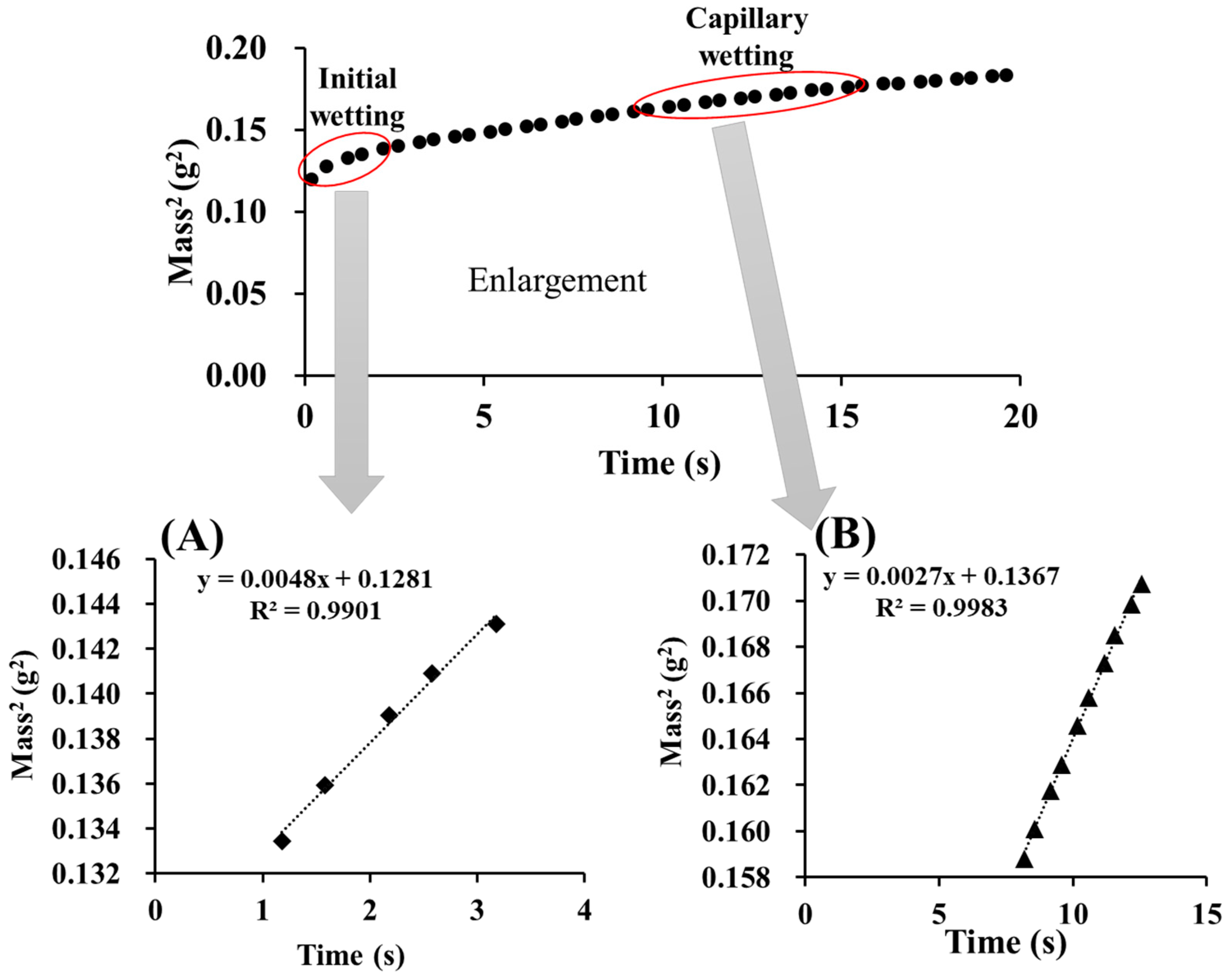
3.8. Wettability of Moisture Absorbents and Tablets
3.9. Tableting Properties
3.10. Effect of the Amount of Added Water and Water Activity on the Disintegration Time and Tablet Hardness
3.11. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindgren, S.; Janzon, L. Prevalence of swallowing complaints and clinical findings among 50–79-year-old men and women in an urban population. Dysphagia 1991, 6, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.J.; Kahrilas, P.J. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology 1999, 116, 455–478. [Google Scholar] [CrossRef]
- Barczi, S.R.; Sullivan, P.A.; Robbins, J. How should dysphagia care of older adults differ? Establishing optimal practice patterns. Semin. Speech Lang. 2000, 21, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Schiele, J.T.; Quinzler, R.; Klimm, H.D.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalence, causes, and relationship to dosage forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Habib, W.; Khankari, R.; Hontz, J. Fast-dissolve drug delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2000, 17, 61–72. [Google Scholar] [CrossRef]
- Sugimoto, M.; Matsubara, K.; Koida, Y.; Kobayashi, M. The preparation of rapidly disintegrating tablets in the mouth. Pharm. Dev. Technol. 2001, 6, 487–493. [Google Scholar] [CrossRef]
- Almukainzi, M.; Araujo, G.L.B.; Löbenberg, R. Orally disintegrating dosage forms. J. Pharm. Investig. 2018, 49, 229–243. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry Orally Disintegrating Tablets; Office of Pharmaceutical Science in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration: Silver Spring, MD, USA, 2008.
- Badawy, S.I.; Menning, M.M.; Gorko, M.A.; Gilbert, D.L. Effect of process parameters on compressibility of granulation manufactured in a high-shear mixer. Int. J. Pharm. 2000, 198, 51–61. [Google Scholar] [CrossRef]
- Badawy, S.I.; Narang, A.S.; LaMarche, K.; Subramanian, G.; Varia, S.A. Mechanistic basis for the effects of process parameters on quality attributes in high shear wet granulation. Int. J. Pharm. 2012, 439, 324–333. [Google Scholar] [CrossRef]
- Pandey, P.; Tao, J.; Chaudhury, A.; Ramachandran, R.; Gao, J.Z.; Bindra, D.S. A combined experimental and modeling approach to study the effects of high-shear wet granulation process parameters on granule characteristics. Pharm. Dev. Technol. 2013, 18, 210–224. [Google Scholar] [CrossRef]
- Shi, L.; Feng, Y.; Sun, C.C. Roles of granule size in over-granulation during high shear wet granulation. J. Pharm. Sci. 2010, 99, 3322–3325. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.A. Selection of excipients for direct compression tablet formulation. Pharm. Technol. Eur. 1997, 9, 24–30. [Google Scholar]
- Rubinstein, M.H. Tablets. In Pharmaceutics: The Science of Dosage Form Design; Churchill Livingstone: Edinburgh, UK, 1988; pp. 304–321. [Google Scholar]
- Jivraj, I.I.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Jarvinen, M.A.; Paaso, J.; Paavola, M.; Leiviska, K.; Juuti, M.; Muzzio, F.; Jarvinen, K. Continuous direct tablet compression: Effects of impeller rotation rate, total feed rate and drug content on the tablet properties and drug release. Drug Dev. Ind. Pharm. 2013, 39, 1802–1808. [Google Scholar] [CrossRef]
- Augsburger, L.L.; Zellhofer, M.J. Tablet formulation. In Encyclopedia of Pharmaceutical Science and Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 3511–3521. [Google Scholar]
- Garg, N.; Pandey, P.; Kaushik, D.; Dureja, H. Development of novel multifunction directly compressible co-processed excipient by melt granulation technique. Int. J. Pharm. Investig. 2015, 5, 266–274. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Morton, D.; Larson, I. Particle Engineering of Excipients for Direct Compression: Understanding the Role of Material Properties. Curr. Pharm. Des. 2015, 21, 5877–5889. [Google Scholar] [CrossRef]
- Kunnath, K.; Huang, Z.; Chen, L.; Zheng, K.; Dave, R. Improved properties of fine active pharmaceutical ingredient powder blends and tablets at high drug loading via dry particle coating. Int. J. Pharm. 2018, 543, 288–299. [Google Scholar] [CrossRef]
- Otsuka, A. Adhesive properties and related phenomena for powdered pharmaceuticals. Yakugaku Zasshi 1998, 118, 127–142. [Google Scholar] [CrossRef]
- Ullah, I.; Corrao, R.; Wiley, G.; Lipper, R. Moisture activated dry granulation: A general process. Pharm. Technol. 1987, 11, 48–54. [Google Scholar]
- Ullah, I.; Chang, J.W.Y.; Wiley, G.J.; Jain, N.B.; Kiang, S. Moisture-activated dry granulation part I: A guide to excipient and equipment selection and-formulation development. Pharm. Technol. 2009, 33, 62–70. [Google Scholar]
- Ullah, I.; Wang, J.; Chang, S.Y.; Guo, H.; Kiang, S.; Jain, N.B. Moisture-activated dry granulation part II: The effects of formulation ingredients and manufacturing-process variables on granulation quality attributes. Pharm. Technol. 2009, 33, 42–49. [Google Scholar]
- Takasaki, H.; Yonemochi, E.; Messerschmid, R.; Ito, M.; Wada, K.; Terada, K. Importance of excipient wettability on tablet characteristics prepared by moisture activated dry granulation (MADG). Int. J. Pharm. 2013, 456, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Lee, A.R.; Choi, D.H.; Jeong, S.H. Effects of moisture content and compression pressure of various deforming granules on the physical properties of tablets. Powder Technol. 2017, 310, 92–102. [Google Scholar] [CrossRef]
- Christensen, L.H.; Johansen, H.E.; Schaefer, T. Moisture-Activated dry Granulation in a high Shear Mixer. Drug Dev. Ind. Pharm. 2008, 20, 2195–2213. [Google Scholar] [CrossRef]
- Malamataris, S.; Goidas, P.; Dimitriou, A. Moisture sorption and tensile strength of some tableted direct compression excipients. Int. J. Pharm. 1991, 68, 51–60. [Google Scholar] [CrossRef]
- Railkar, A.M.; Schwartz, J.B. Evaluation and comparison of a moist granulation technique to conventional methods. Drug Dev. Ind. Pharm. 2000, 26, 885–889. [Google Scholar] [CrossRef]
- Railkar, A.M.; Schwartz, J.B. The effects of formulation factors on the moist granulation technique for controlled-release tablets. Drug Dev. Ind. Pharm. 2001, 27, 893–898. [Google Scholar] [CrossRef]
- Chen, C.-M.; Alli, D.; Igga, M.R.; Czeisler, J.L. Comparison of Moisture-Activated Dry Granulation Profess with Conventional Granulation Methods for Sematilide Hydrochloride Tablets. Drug Dev. Ind. Pharm. 2008, 16, 379–394. [Google Scholar] [CrossRef]
- Moravkar, K.K.; Ali, T.M.; Pawar, J.N.; Amin, P.D. Application of moisture activated dry granulation (MADG) process to develop high dose immediate release (IR) formulations. Adv. Powder Technol. 2017, 28, 1270–1280. [Google Scholar] [CrossRef]
- Takasaki, H.; Yonemochi, E.; Ito, M.; Wada, K.; Terada, K. The effect of water activity on granule characteristics and tablet properties produced by moisture activated dry granulation (MADG). Powder Technol. 2016, 294, 113–118. [Google Scholar] [CrossRef]
- Aodah, A.H.; Fayed, M.H.; Alalaiwe, A.; Alsulays, B.B.; Aldawsari, M.F.; Khafagy, E.S. Design, Optimization, and Correlation of in vitro/in vivo Disintegration of Novel Fast Orally Disintegrating Tablet of High Dose Metformin Hydrochloride Using Moisture Activated Dry Granulation Process and Quality by Design Approach. Pharmaceutics 2020, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Kuno, Y.; Kojima, M.; Ando, S.; Nakagami, H. Effect of preparation method on properties of orally disintegrating tablets made by phase transition. Int. J. Pharm. 2008, 355, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Tahara, K.; Takeuchi, H. Spray-dried composite particles of erythritol and porous silica for orally disintegrating tablets prepared by direct tableting. Powder Technol. 2015, 286, 444–450. [Google Scholar] [CrossRef]
- Okuda, Y.; Irisawa, Y.; Okimoto, K.; Osawa, T.; Yamashita, S. A new formulation for orally disintegrating tablets using a suspension spray-coating method. Int. J. Pharm. 2009, 382, 80–87. [Google Scholar] [CrossRef]
- Mizumoto, T.; Masuda, Y.; Yamamoto, T.; Yonemochi, E.; Terada, K. Formulation design of a novel fast-disintegrating tablet. Int. J. Pharm. 2005, 306, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.X.; Sunada, H.; Yonezawa, Y.; Danjo, K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method. Drug Dev. Ind. Pharm. 1999, 25, 571–581. [Google Scholar] [CrossRef]
- Takasaki, H.; Sakurai, A.; Katayama, T.; Matsuura, Y.; Ohyagi, N.; Wada, K.; Ishikawa, A.; Yonemochi, E. Novel, lean and environment-friendly granulation method: Green fluidized bed granulation (GFBG). Int. J. Pharm. 2019, 557, 18–25. [Google Scholar] [CrossRef]
- Fukami, J.; Yonemochi, E.; Yoshihashi, Y.; Terada, K. Evaluation of rapidly disintegrating tablets containing glycine and carboxymethylcellulose. Int. J. Pharm. 2006, 310, 101–109. [Google Scholar] [CrossRef]
- Badawy, S.I.; Gray, D.B.; Hussain, M.A. A study on the effect of wet granulation on microcrystalline cellulose particle structure and performance. Pharm. Res. 2006, 23, 634–640. [Google Scholar] [CrossRef]
- Snider, B.; Liang, P.; Pearson, N. Implementation of water-activity testing to replace Karl Fischer water testing. Pharm. Technol. 2007, 31, 56. [Google Scholar]
- Abdel-Hamid, S.; Betz, G. Radial die-wall pressure as a reliable tool for studying the effect of powder water activity on high speed tableting. Int. J. Pharm. 2011, 411, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Roopwani, R.; Buckner, I.S. Co-Processed Particles: An Approach to Transform Poor Tableting Properties. J. Pharm. Sci. 2019, 108, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Law, Y.; Chakrabarti, S. Physical properties and compact analysis of commonly used direct compression binders. AAPS PharmSciTech 2003, 4, E62. [Google Scholar] [CrossRef] [PubMed]
- Osamura, T.; Takeuchi, Y.; Onodera, R.; Kitamura, M.; Takahashi, Y.; Tahara, K.; Takeuchi, H. Characterization of tableting properties measured with a multi-functional compaction instrument for several pharmaceutical excipients and actual tablet formulations. Int. J. Pharm. 2016, 510, 195–202. [Google Scholar] [CrossRef]
- Medarevic, D.; Djuris, J.; Krkobabic, M.; Ibric, S. Improving Tableting Performance of Lactose Monohydrate by Fluid-Bed Melt Granulation Co-Processing. Pharmaceutics 2021, 13, 2165. [Google Scholar] [CrossRef] [PubMed]
- Dular Vovko, A.; Hodžić, B.; Brec, T.; Hudovornik, G.; Vrečer, F. Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets. Pharmaceutics 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]




| Process | MADG | HSG | |
|---|---|---|---|
| Process Stage | Composition | - | - |
| Add Water (%) a | 1–5 | 15 | |
| 1 | Erythritol | 70.0 | 70.0 |
| Povidone K25 | - | 4.0 | |
| Povidone K12 | 4.0 | - | |
| Avicel PH102 | - | 20.0 | |
| 2 | Avicel 200LM b | 20.0 | - |
| 3 | Crospovidone XL | 5.0 | 5.0 |
| Magnesium stearate | 1.0 | 1.0 | |
| Total | 100.0 | 100.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, M.; Ishikawa, A.; Muramatsu, S.; Furuishi, T.; Onuki, Y.; Fukuzawa, K.; Yonemochi, E. Study of Orally Disintegrating Tablets Using Erythritol as an Excipient Produced by Moisture-Activated Dry Granulation (MADG). Pharmaceuticals 2022, 15, 1004. https://doi.org/10.3390/ph15081004
Yamada M, Ishikawa A, Muramatsu S, Furuishi T, Onuki Y, Fukuzawa K, Yonemochi E. Study of Orally Disintegrating Tablets Using Erythritol as an Excipient Produced by Moisture-Activated Dry Granulation (MADG). Pharmaceuticals. 2022; 15(8):1004. https://doi.org/10.3390/ph15081004
Chicago/Turabian StyleYamada, Mizuki, Agata Ishikawa, Shun Muramatsu, Takayuki Furuishi, Yoshinori Onuki, Kaori Fukuzawa, and Etsuo Yonemochi. 2022. "Study of Orally Disintegrating Tablets Using Erythritol as an Excipient Produced by Moisture-Activated Dry Granulation (MADG)" Pharmaceuticals 15, no. 8: 1004. https://doi.org/10.3390/ph15081004
APA StyleYamada, M., Ishikawa, A., Muramatsu, S., Furuishi, T., Onuki, Y., Fukuzawa, K., & Yonemochi, E. (2022). Study of Orally Disintegrating Tablets Using Erythritol as an Excipient Produced by Moisture-Activated Dry Granulation (MADG). Pharmaceuticals, 15(8), 1004. https://doi.org/10.3390/ph15081004








