Efficacy Evaluation of 10-Hydroxy Chondrofoline and Tafenoquine against Leishmania tropica (HTD7)
Abstract
:1. Introduction
2. Results
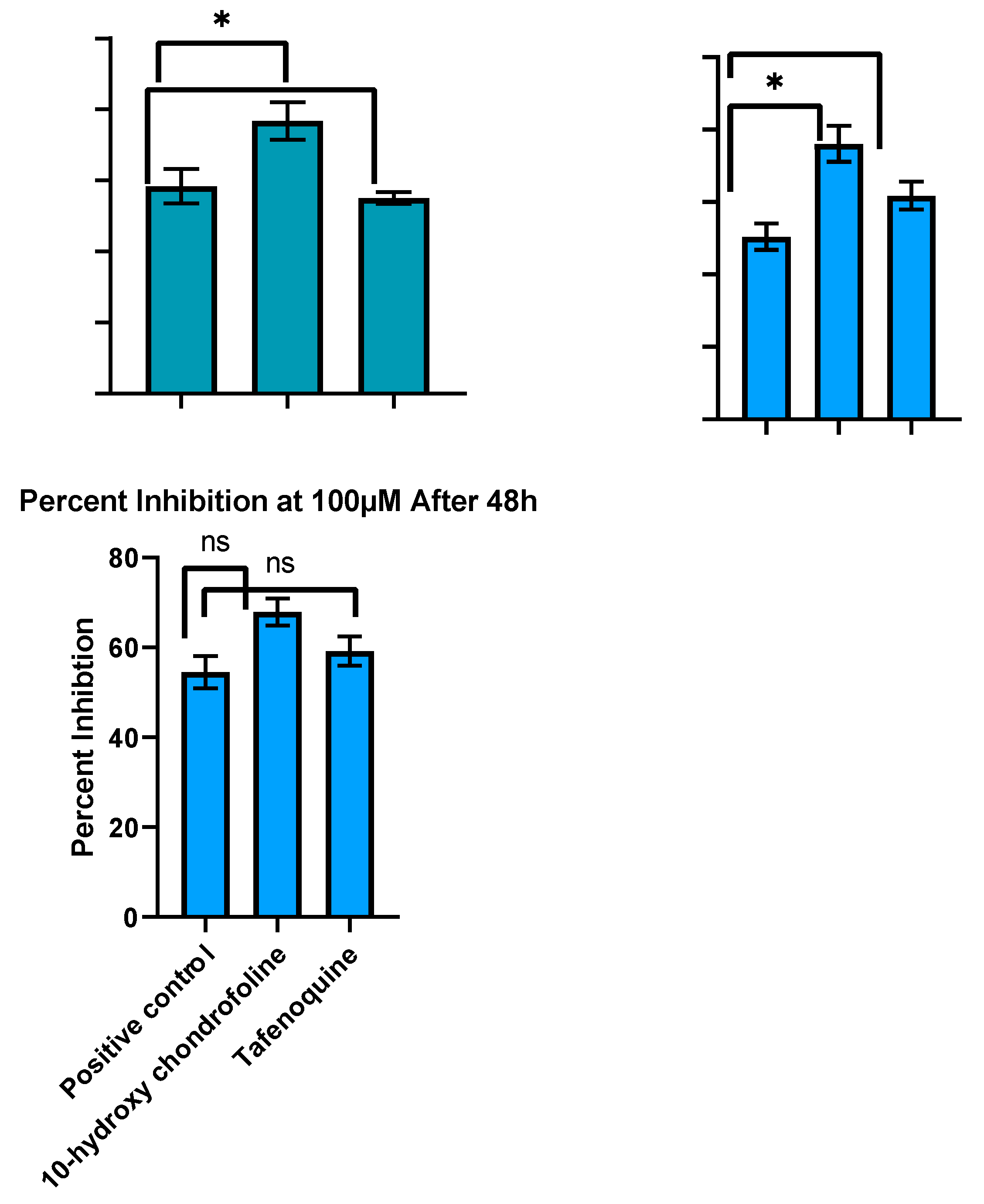
2.1. In Vitro Cytotoxicity of Test Compounds and Standard Drug against THP-1 Cells
2.2. In Vitro Evaluation of Antileishmanial Activity
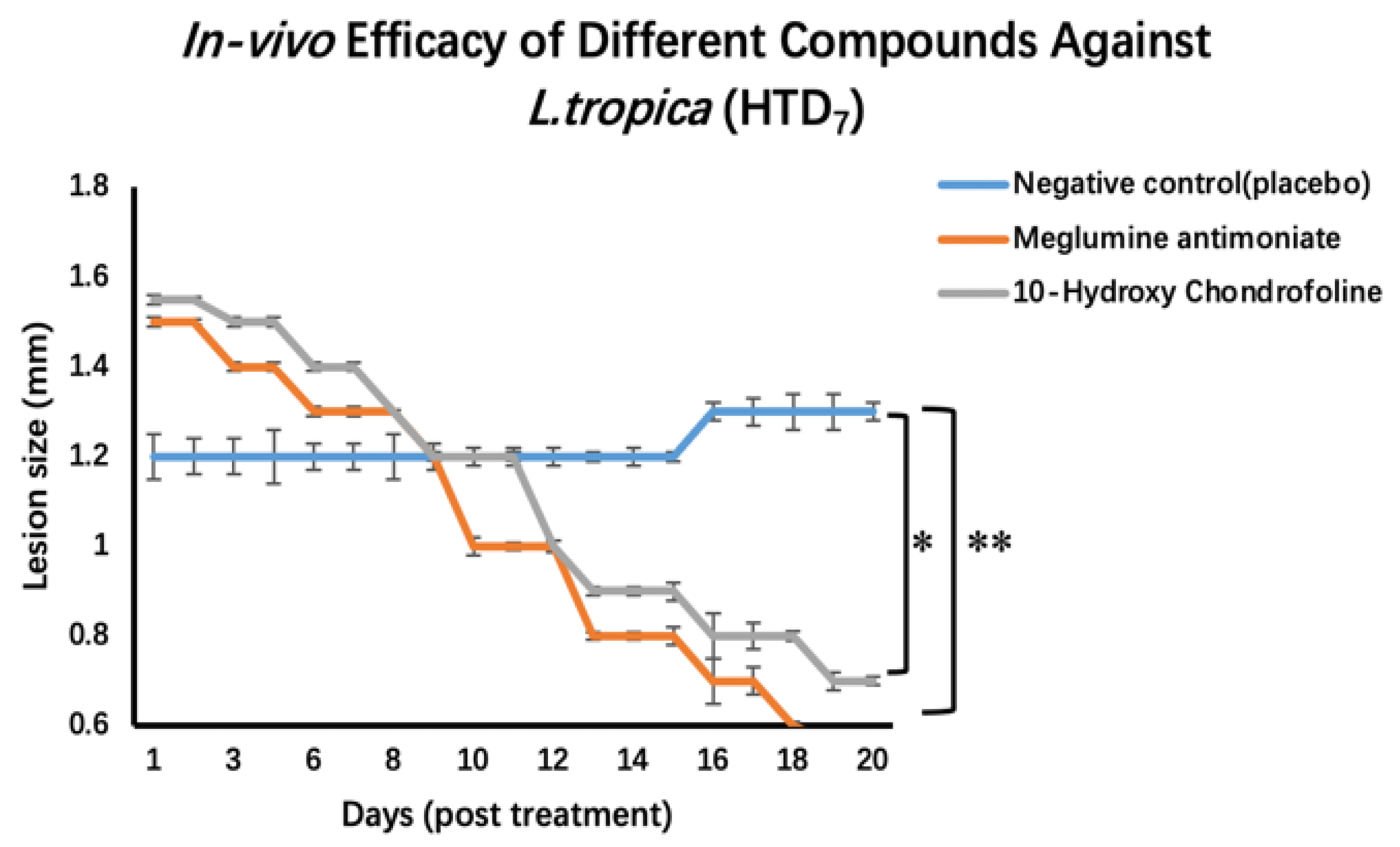
2.3. Testing In Vivo Leishmanicidal Activity Using BALB/c Mice Experimentally Infected with Leishmania Tropica (HTD7)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
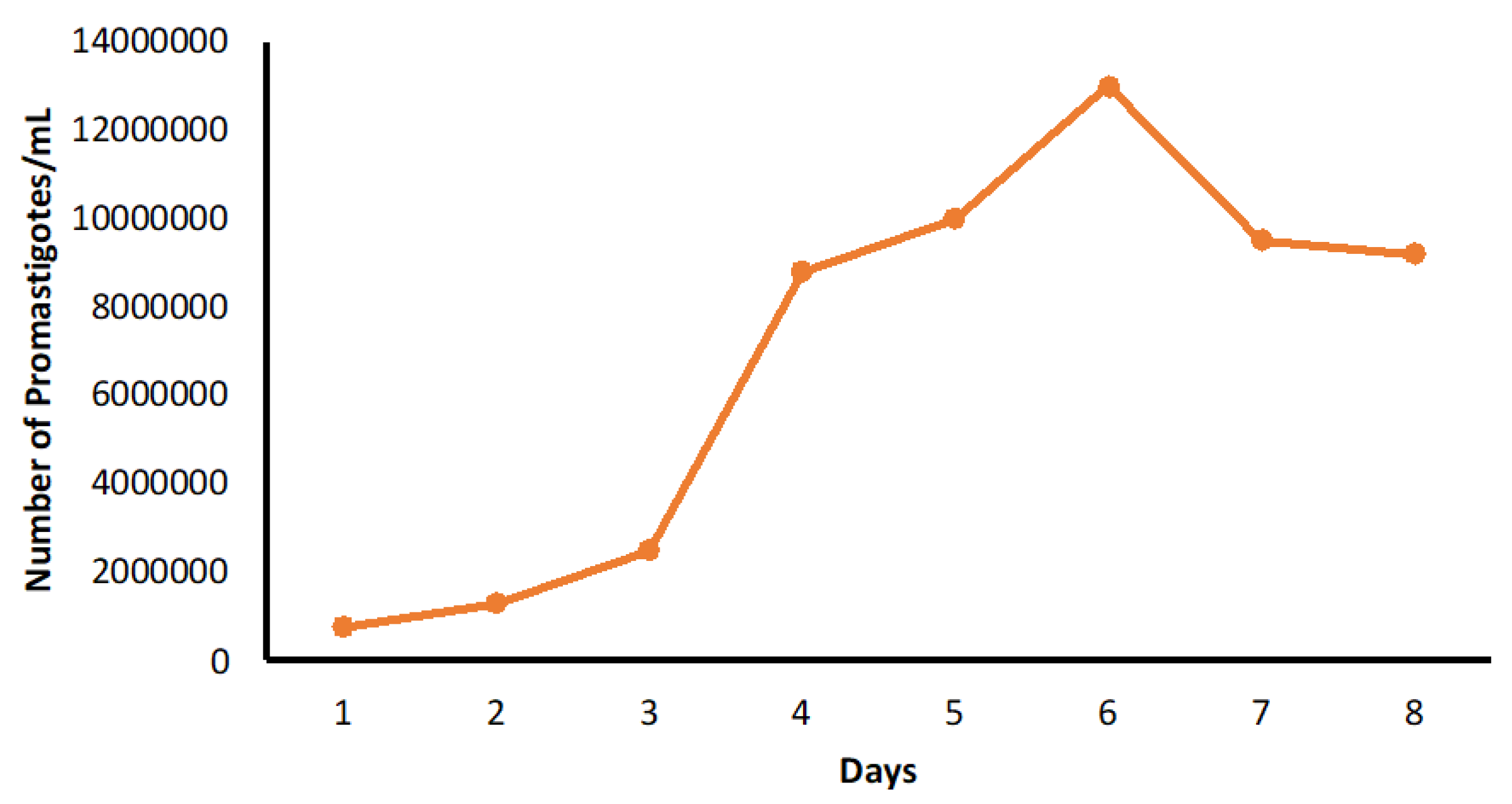
4.2.1. Culture of L. tropica (HTD7)
4.2.2. Infection of THP-1 Macrophages with L. tropica (HTD7)
4.2.3. In Vitro Cytotoxicity of Test Compounds and Standard Drug against THP-1 Cells
total THP-1 cells)] × 100
4.2.4. In Vitro Evaluation of Antileishmanial Activity
4.2.5. Testing In Vivo Leishmanicidal Activity Using BALB/c Mice Experimentally Infected with L. tropica (HTD7)
4.2.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rostamian, M.; Rezaeian, S.; Hamidouche, M.; Bahrami, F.; Ghadiri, K.; Chegeneh Lorestani, R.; Nemati Zargaran, F.; Akya, A. The effects of natural disasters on leishmaniases frequency: A global systematic review and meta-analysis. Acta Trop. 2021, 217, 105855. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Saurabh, S. Leishmaniasis. Lancet 2019, 393, 871. [Google Scholar] [CrossRef]
- World Health Organisation. Leishmaniasis. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 15 June 2022).
- Alvar, J.; Arana, B. Leishmaniasis, impact and therapeutic needs. In Drug Discovery for Leishmaniasis; Rivas, L., Gil, C., Eds.; The Royal Society of Chemistry: Croyden, UK, 2018; Chapter 1; pp. 3–23. [Google Scholar]
- Mendonca, D.V.C.; Martins, V.T.; Lage, D.P.; Dias, D.S.; Ribeiro, P.A.F.; Carvalho, A.; Dias, A.L.T.; Miyazaki, C.K.; Menezes-Souza, D.; Roatt, B.M.; et al. Comparing the therapeutic efficacy of different amphotericin B-carrying delivery systems against visceral leishmaniasis. Exp. Parasitol. 2018, 186, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Gradoni, L. A brief introduction to leishmaniasis epidemiology. In The Leishmaniases: Old Neglected Tropical Diseases; Springer: Cham, Switzerland, 2018; pp. 1–13. [Google Scholar]
- Eroglu, F.; Ozgoztasi, O. The increase in neglected cutaneous leishmaniasis in Gaziantep province of Turkey after mass human migration. Acta Trop. 2019, 192, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, K.; Caridha, D.; Black, C.; Vesely, B.; Leed, S.; Sciotti, R.J.; Wijnant, G.J.; Yardley, V.; Braillard, S.; Mowbray, C.E.; et al. Novel benzoxaborole, nitroimidazole and aminopyrazoles with activity against experimental cutaneous leishmaniasis. Int J. Parasitol. Drugs Drug Resist. 2019, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Garcia, M.; Scull, R.; Cuellar, A.; Setzer, W.N. Antileishmanial activity of the essential oil from Bixa orellana. Phytother. Res. 2014, 28, 753–758. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discov. Today 2017, 22, 1516–1531. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome(®)): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef]
- Saha, A.; Basu, M.; Ukil, A. Recent advances in understanding Leishmania donovani infection: The importance of diverse host regulatory pathways. IUBMB Life 2018, 70, 593–601. [Google Scholar] [CrossRef]
- Carvalho, L.; Martínez-García, M.; Pérez-Victoria, I.; Manzano, J.I.; Yardley, V.; Gamarro, F.; Pérez-Victoria, J.M. The oral antimalarial drug tafenoquine shows activity against Trypanosoma brucei. Antimicrob. Agents Chemother. 2015, 59, 6151–6160. [Google Scholar] [CrossRef]
- Carvalho, L.; Luque-Ortega, J.R.; Manzano, J.I.; Castanys, S.; Rivas, L.; Gamarro, F. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets leishmania respiratory complex III and induces apoptosis. Antimicrob. Agents Chemother. 2010, 54, 5344–5351. [Google Scholar] [CrossRef]
- Manzano, J.I.; Carvalho, L.; García-Hernández, R.; Poveda, J.A.; Ferragut, J.A.; Castanys, S.; Gamarro, F. Uptake of the antileishmania drug tafenoquine follows a sterol-dependent diffusion process in Leishmania. J. Antimicrob. Chemother. 2011, 66, 2562–2565. [Google Scholar] [CrossRef]
- Alamzeb, M.; Ali, S.; Mamoon-Ur-Rashid; Khan, B.; Ihsanullah; Adnan; Omer, M.; Ullah, A.; Ali, J.; Setzer, W.N.; et al. Antileishmanial Potential of Berberine Alkaloids From Berberis glaucocarpa Roots: Molecular Docking Suggests Relevant Leishmania Protein Targets. Nat. Prod. Commun. 2021, 16, 1934578X211031148. [Google Scholar] [CrossRef]
- Koko, W.S.; Al Nasr, I.S.; Khan, T.A.; Schobert, R.; Biersack, B. An Update on Natural Antileishmanial Treatment Options from Plants, Fungi and Algae. Chem Biodivers 2022, 19, e202100542. [Google Scholar] [CrossRef]
- Torres Suarez, E.; Granados-Falla, D.S.; Robledo, S.M.; Murillo, J.; Upegui, Y.; Delgado, G. Antileishmanial activity of synthetic analogs of the naturally occurring quinolone alkaloid N-methyl-8-methoxyflindersin. PLoS ONE 2020, 15, e0243392. [Google Scholar] [CrossRef]
- Azadbakht, M.; Davoodi, A.; Hosseinimehr, S.J.; Keighobadi, M.; Fakhar, M.; Valadan, R.; Faridnia, R.; Emami, S.; Azadbakht, M.; Bakhtiyari, A. Tropolone alkaloids from Colchicum kurdicum (Bornm.) Stef. (Colchicaceae) as the potent novel antileishmanial compounds; purification, structure elucidation, antileishmanial activities and molecular docking studies. Exp. Parasitol 2020, 213, 107902. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Cell Culture Basics Handbook; Gibco: Paisley, UK, 2015; Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/brochures-and-catalogs/guides/gibco-cell-culture-basics-handbook-guide.pdf (accessed on 1 March 2016).
- Efstathiou, A.; Smirlis, D. Leishmania protein kinases: Important regulators of the parasite life cycle and molecular targets for treating leishmaniasis. Microorganisms 2021, 9, 691. [Google Scholar] [CrossRef]
- De Toledo, J.S.; Ambrósio, S.R.; Borges, C.H.; Manfrim, V.; Cerri, D.G.; Cruz, A.K.; Da Costa, F.B. In vitro leishmanicidal activities of sesquiterpene lactones from Tithonia diversifolia against Leishmania braziliensis promastigotes and amastigotes. Molecules 2014, 19, 6070–6079. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, 70, 4054. [Google Scholar] [CrossRef]
- Shokri, A.; Sharifi, I.; Khamesipour, A.; Nakhaee, N.; Fasihi Harandi, M.; Nosratabadi, J.; Hakimi Parizi, M.; Barati, M. The effect of verapamil on in vitro susceptibility of promastigote and amastigote stages of Leishmania tropica to meglumine antimoniate. Parasitol. Res. 2012, 110, 1113–1117. [Google Scholar] [CrossRef]
- Gómez-Pérez, V.; Manzano, J.I.; García-Hernández, R.; Castanys, S.; Campos Rosa, J.M.; Gamarro, F. 4-amino bis-pyridinium derivatives as novel antileishmanial agents. Antimicrob. Agents Chemother. 2014, 58, 4103–4112. [Google Scholar] [CrossRef]
- Wijnant, G.J.; Croft, S.L.; de la Flor, R.; Alavijeh, M.; Yardley, V.; Braillard, S.; Mowbray, C.; Van Bocxlaer, K. Pharmacokinetics and Pharmacodynamics of the Nitroimidazole DNDI-0690 in Mouse Models of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2019, 63, e00829-19. [Google Scholar] [CrossRef]
- Rodriguez, F.; John, S.F.; Iniguez, E.; Montalvo, S.; Michael, K.; White, L.; Liang, D.; Olaleye, O.A.; Maldonado, R.A. In Vitro and In Vivo Characterization of Potent Antileishmanial Methionine Aminopeptidase 1 Inhibitors. Antimicrob. Agents Chemother. 2020, 64, e01422-19. [Google Scholar] [CrossRef]
- Sirak, B.; Asres, K.; Hailu, A.; Dube, M.; Arnold, N.; Häberli, C.; Keiser, J.; Imming, P. In Vitro Antileishmanial and Antischistosomal Activities of Anemonin Isolated from the Fresh Leaves of Ranunculus multifidus Forsk. Molecules 2021, 26, 7473. [Google Scholar] [CrossRef]
- Fernández, M.; Ríos-Vásquez, L.A.; Ocampo-Cardona, R.; Flórez, O.; Cedeño, D.L.; Garrigues, T.M.; Almeida, A.J.; Velez, I.D.; Robledo, S.M. Physicochemical and Biopharmaceutical Characterization of N-Iodomethyl-N,N-Dimethyl-N-(6,6-1 Diphenylhex-5-En-1-Yl) Ammonium Iodide and A Promising Antileishmania Delivery System. Int. Arch. Med. Microbiol. 2018, 1, 007. [Google Scholar] [CrossRef]
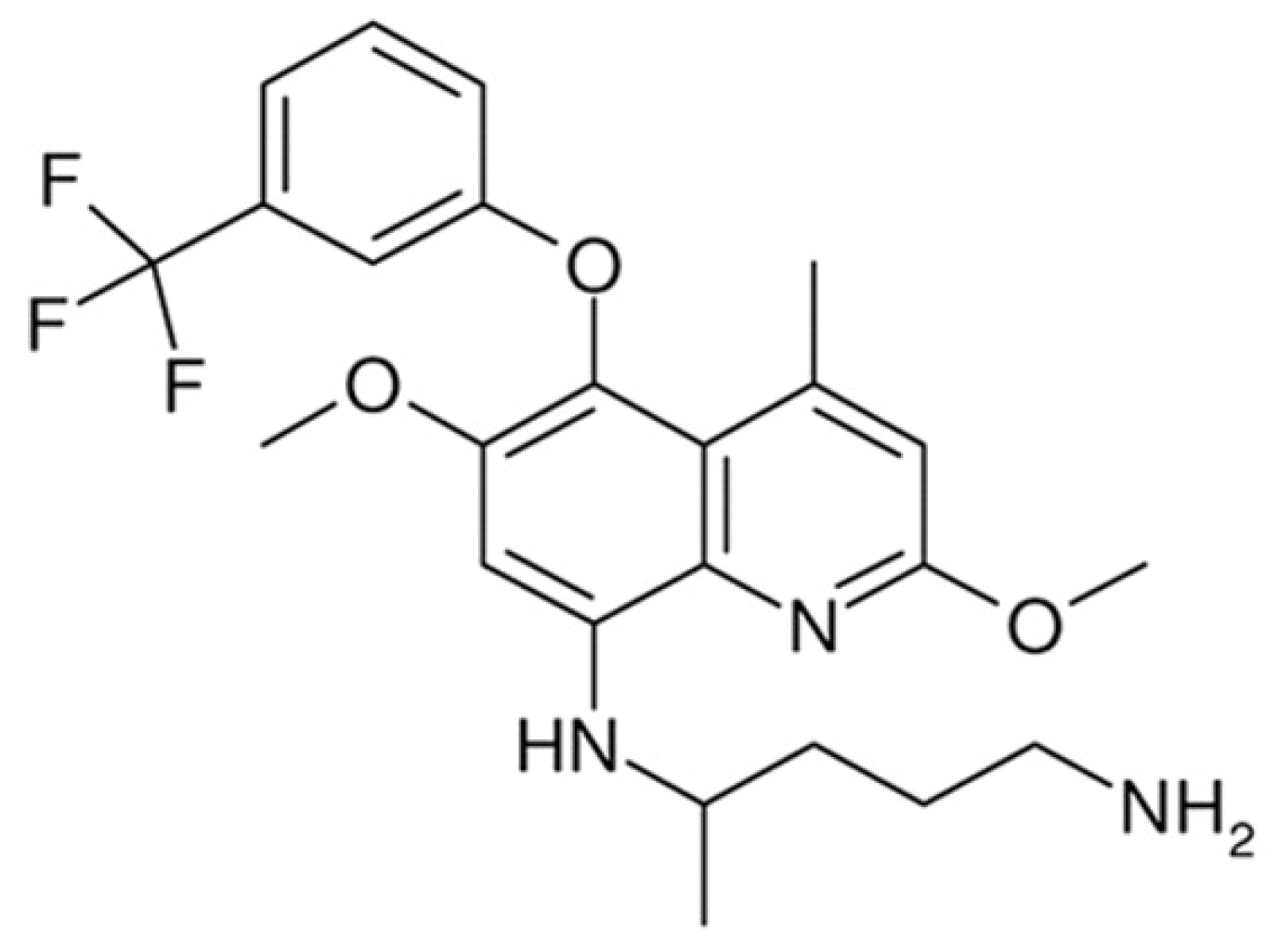
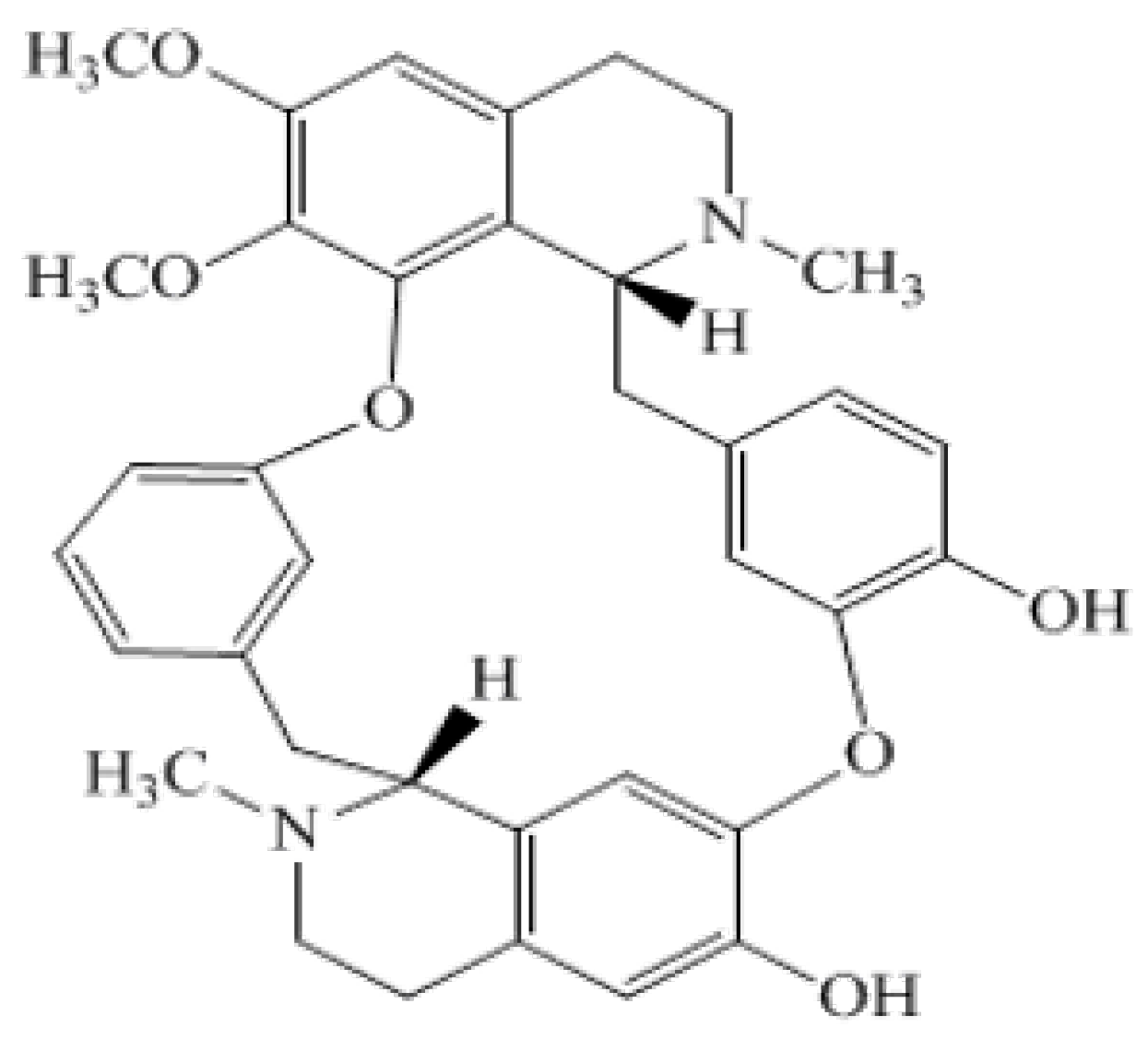
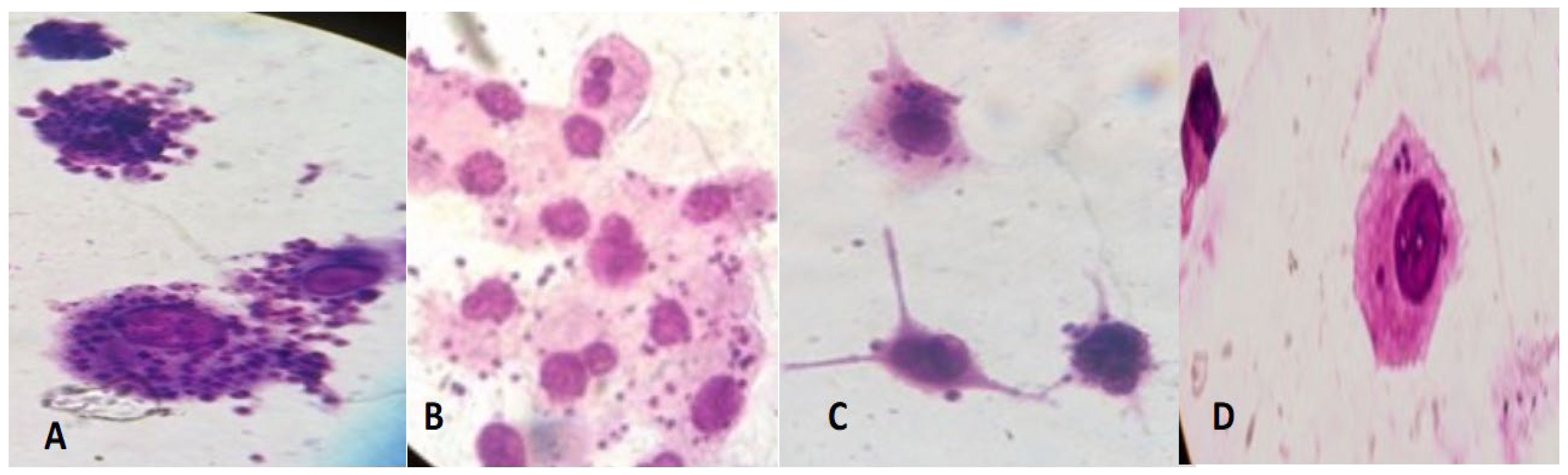

| Compound | Mean Percent Viability ± SD |
|---|---|
| Control THP-1 cells only | 97.1% ± 1.1 |
| Meglumine antimonate + THP-1 cells | 94.84% ± 2.2 |
| 10-Hydroxy Choline + THP-1 cells | 96.62% ± 1.5 |
| Tafenoquine + THP-1 cells | 97.02% ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.I.; Nasir, F.; Malik, N.S.; Alamzeb, M.; Abbas, M.; Rehman, I.U.; Khuda, F.; Shah, Y.; Goh, K.W.; Zeb, A.; et al. Efficacy Evaluation of 10-Hydroxy Chondrofoline and Tafenoquine against Leishmania tropica (HTD7). Pharmaceuticals 2022, 15, 1005. https://doi.org/10.3390/ph15081005
Shah SI, Nasir F, Malik NS, Alamzeb M, Abbas M, Rehman IU, Khuda F, Shah Y, Goh KW, Zeb A, et al. Efficacy Evaluation of 10-Hydroxy Chondrofoline and Tafenoquine against Leishmania tropica (HTD7). Pharmaceuticals. 2022; 15(8):1005. https://doi.org/10.3390/ph15081005
Chicago/Turabian StyleShah, Sayyed Ibrahim, Fazli Nasir, Nadia Shamshad Malik, Muhammad Alamzeb, Muhammad Abbas, Inayat Ur Rehman, Fazli Khuda, Yasir Shah, Khang Weh Goh, Alam Zeb, and et al. 2022. "Efficacy Evaluation of 10-Hydroxy Chondrofoline and Tafenoquine against Leishmania tropica (HTD7)" Pharmaceuticals 15, no. 8: 1005. https://doi.org/10.3390/ph15081005
APA StyleShah, S. I., Nasir, F., Malik, N. S., Alamzeb, M., Abbas, M., Rehman, I. U., Khuda, F., Shah, Y., Goh, K. W., Zeb, A., & Ming, L. C. (2022). Efficacy Evaluation of 10-Hydroxy Chondrofoline and Tafenoquine against Leishmania tropica (HTD7). Pharmaceuticals, 15(8), 1005. https://doi.org/10.3390/ph15081005








