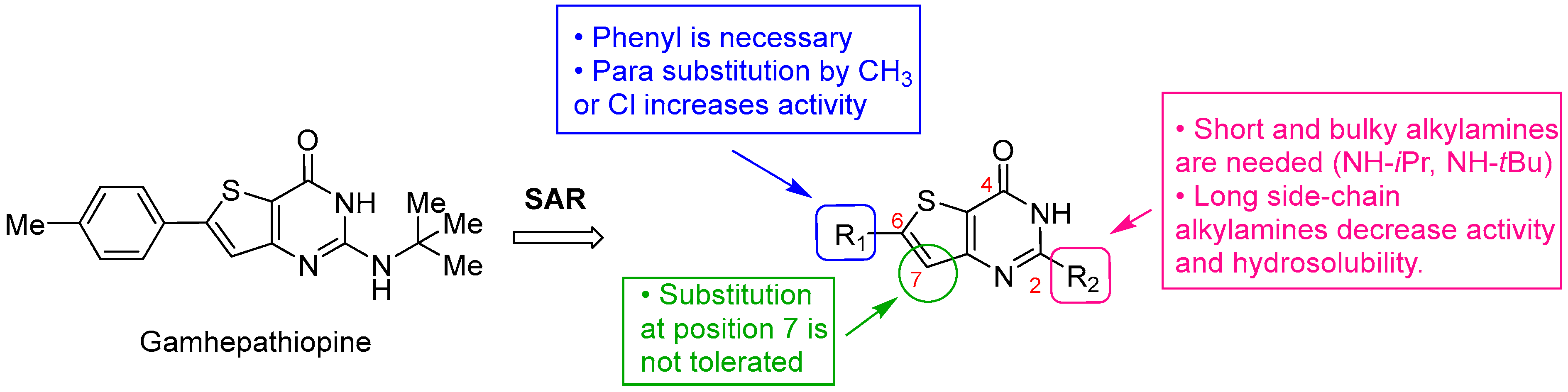
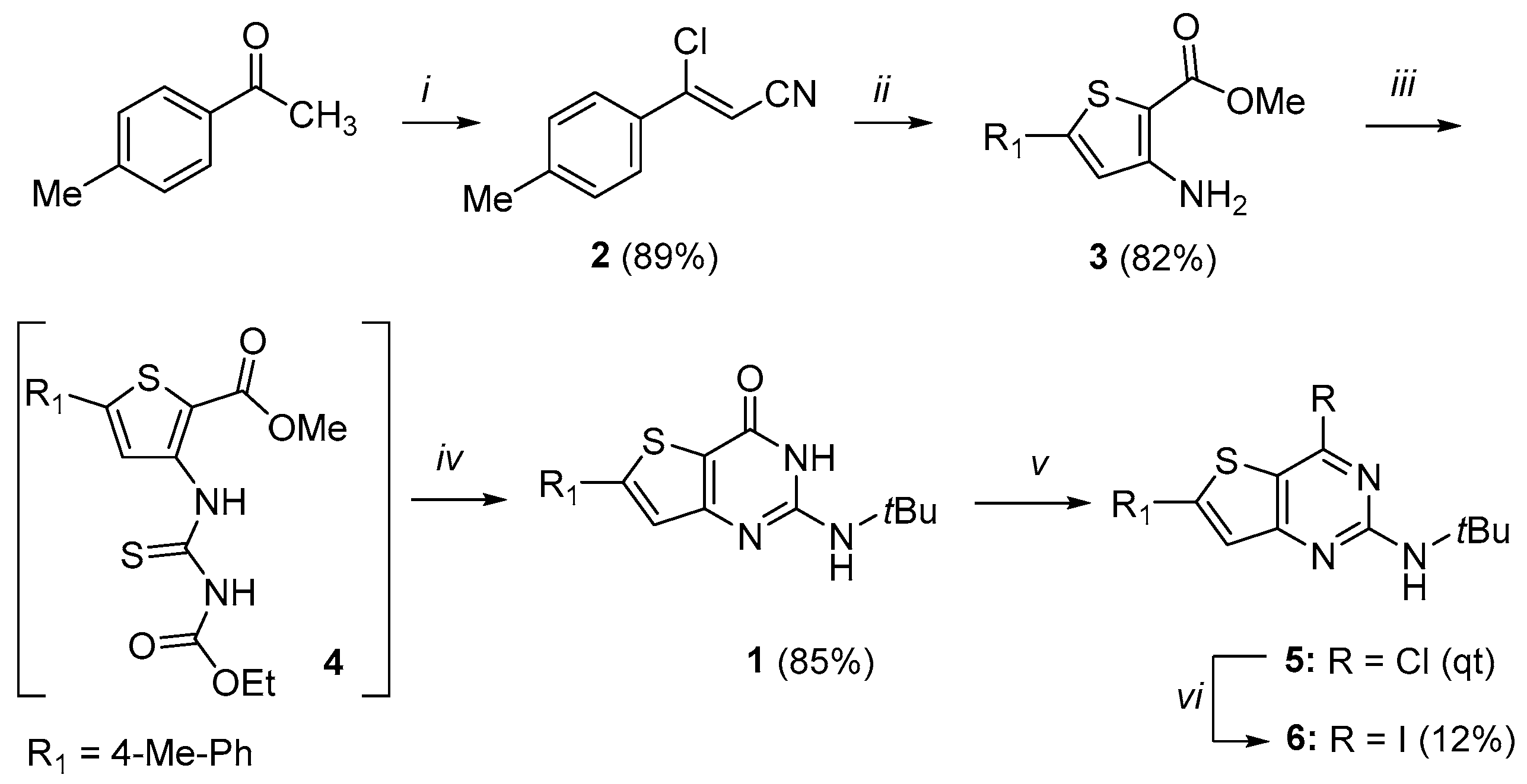
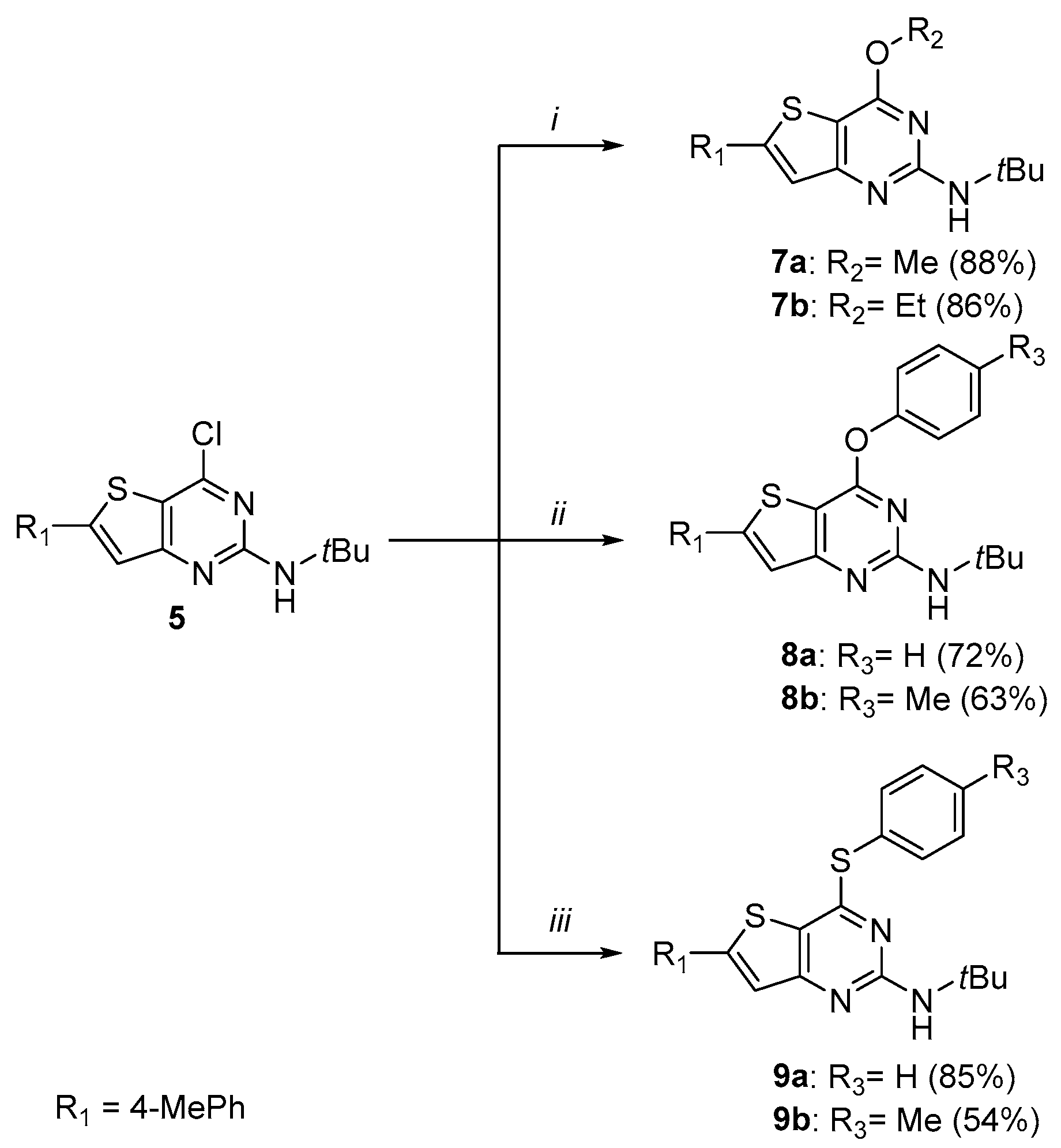
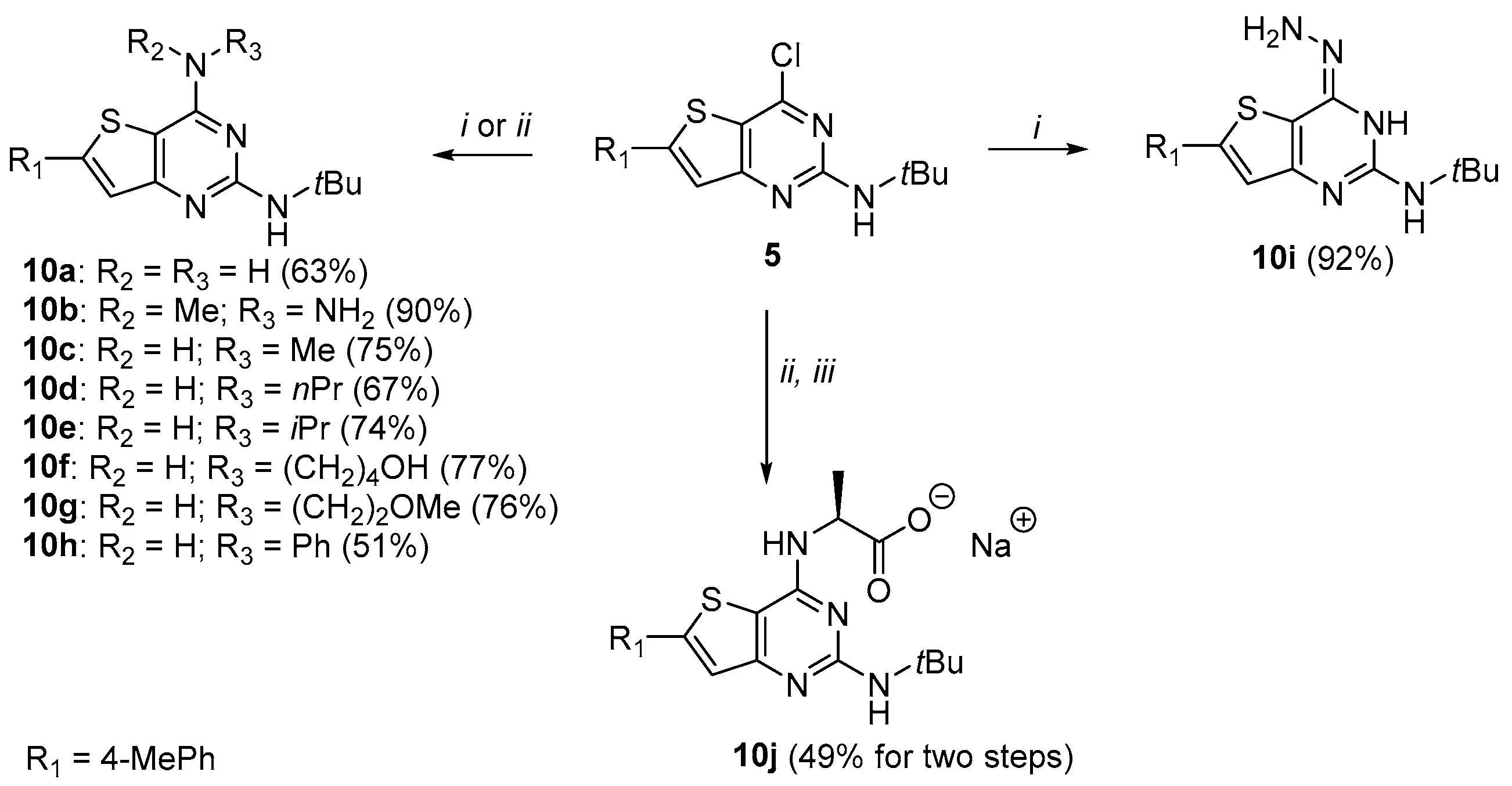
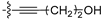3.1. Chemistry
3.1.1. Materials and Methods
Commercial reagents and solvents were used without further purification. The synthesized products were purified by chromatography on silica gel (40–63 μm) or by preparative HPLC on an apparatus equipped with a Delta Pack C18 radial compression column (100 mm × 40 mm, 15 μm, 100 Å) at a wavelength of 214 nm, at a flow rate of 28 mL/min with a variable elution gradient from X % A (H
2O + 0.1 TFA) to X + 30% B (ACN + 0.1% TFA) in 30 min. The purest fractions are pooled, freeze-dried, and lyophilized to give the final compounds. LC/MS analyses were recorded on a Quattro microTM ESI triple quadrupole mass spectrometer (ESI+ electrospray ionization mode) or on a Micromass ZQ spectrometer (ESI+ electrospray ionization mode), coupled to an Alliance HPLC system (Waters, Milford, CT, USA) equipped with a Chromolith High Resolution RP-18e column (25 × 4.6 mm), with the samples being previously separated using a gradient from 100% (H
2O + 0.1% HCO
2H) to 100% (ACN + 0.1% HCO
2H) in 3 min at a flow rate of 3 mL/min and with UV detection at 214 nm. UPLC/MS analyses were recorded with an Acquity H-Class UPLC system, coupled to a Waters SQDetector-2 mass spectrometer (Waters, Milford, CT, USA). Chromatographic separation was carried out under the same conditions as above using a Waters Acquity UPLC BEC C18 column (100 × 2.1 mm, 1.5 μm). High-resolution mass spectrometry (HRMS) analyses were performed with a time-of-flight (TOF) spectrometer coupled to a positive electrospray ionization (ESI) source. The elemental analysis was realized with a Elementar Vario Micro Cube. The NMR spectra were recorded on a Brüker 400, 500, or 600 spectrometer. Chemical shifts δ are expressed in parts per million (ppm) relative to the residual signal of the deuterated solvent used (CDCl
3, DMSO-d
6, MeOH-d
3, ACN-d
3), and the coupling constants
J are expressed in Hertz. The multiplicities are designated as singlet (s), broad singlet (bs) doublet (d), doublet of doublet (dd), triplet (t), quadruplet (q), quintuplet (qt), sextuplet (st), or multiplet (m). Compound
1 was synthesized according to the previous reported procedure and its physical characteristics agreed with the published data [
18].
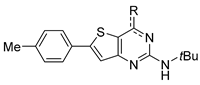
3.1.2. Synthesis of N-(Tert-butyl)-4-chloro-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine 5
Phosphorus oxychloride (5.5 eq., 8.37 g, 54.59 mmol, 5.1 mL) was slowly added to a cold solution of compound 1 (1 eq, 3.11 g, 9.93 mmol) and N,N-dimethylaniline (0.7 eq, 842 mg, 6.95 mmol) in CH3CN (156 mL) for 2 h at 0 °C. The mixture was then heated to 80–85 °C and was stirred for 18 h. The reaction mixture was cooled to 40 °C and then was quenched into water (400 mL) for 2 h. The precipitate was filtered and washed with water (200 mL). The desired product was dried under vacuum at 50 °C for 48 h to afford 5 as a green/yellow solid (3.26 g, qt). 1H NMR (500 MHz, DMSO-d6): δ 7.64 (d, 2H, CHar, J = 8.0 Hz), 7.37 (s, 1H, H-thiophene), 7.28 (d, 2H, CHar, J = 8.0 Hz), 2.36 (s, 3H, CH3-tolyl), 1.46 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 158.8, 157.1, 151.9, 149.9, 138.4, 130.0, 129.2, 125.3, 119.1, 111.0, 50.7, 28.3, 20.2; HR-MS (ESI) calculated for C17H18ClN3S: 332.0988 [M + H]+, found: 332.0988.
3.1.3. Synthesis of N-(Tert-butyl)-4-iodo-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine 6
Under argon atmosphere, compound 5 (100 mg, 0.3013 mmol) was dissolved in anhydrous dioxane (7 mL/mmol) and sodium iodide was added (6 eq., 338 mg, 1.808 mmol, 0.09 mL). The reaction mixture was stirred under reflux for 72 h. The solvent was removed under reduced pressure. The crude was dissolved in sodium thiosulfate solution (10%, 25 mL). The aqueous layer was washed three times with EtOAc. Organic layers were combined, washed with water and brine, dried with MgSO4, and filtered and concentrated under vacuum. The crude was purified by preparative HPLC (ACN/H2O + 0.1% HCO2H) to afford the desired compound as yellow/brown solid (17 mg, 12%); 1H NMR (500 MHz, CDCl3): δ 7.60 (d, 2H, CHar, J = 8.0 Hz), 7.50 (s, 1H, H-thiophene), 7.26 (d, 2H, CHar, J = 8.0 Hz), 5.22 (bs, 1H, NH), 2.40 (s, 3H, CH3-tolyl), 1.47 (s, 9H, C(CH3)3).; 13C NMR (125 MHz, DMSO-d6): δ 160.3, 158.6, 154.1, 140.4, 130.6, 130.0 (2C), 128.9, 126.5 (2C), 122.1, 119.2, 51.4, 28.9, 21.5; LC/MS: tr = 2.71 min, [M + H]+ = 424.0; HR-MS (ESI) calculated for C17H18IN3S: 424.0339 [M + H]+, found: 424.0352.
3.1.4. Synthesis of 4-Arylether-thieno[3,2-d]pyrimidines 7
Sodium (2.5 eq.) was dissolved in the desired alcohol (10 mL/mmol) under argon at 0 °C. Then, compound 5 (1 eq.) was added slowly and the reaction mixture was stirred at 60 °C under argon. After completion of the reaction, the medium was evaporated to dryness. A total of 50 mL of water was added and then extracted with diethyl ether (3 × 25 mL). The organic layer was washed with brine, dried with MgSO4, and filtered and concentrated under reduced pressure. The crude was recrystallized from ACN.
N-(tert-butyl)-4-methoxy-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (7a)
Yellow crystals (354 mg, 88%); 1H NMR (500 MHz, DMSO-d6): δ 7.71 (d, 2H, CHar, J = 8.0 Hz), 7.53 (s, 1H, H-thiophene), 7.28 (d, 2H, CHar, J = 8.0 Hz), 6.57 (bs, 1H, NH), 4.01 (s, 3H, CH3O), 2.34 (s, 3H, CH3-tolyl), 1.43 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 164.2, 163.2, 160.4, 150.4, 139.3, 130.2, 129.8, 126.0, 118.6, 104.6, 53.6, 50.2, 28.9, 20.9; HR-MS (ESI) calculated for C18H22N3OS: 328.1484 [M + H]+, found: 328.1485.
N-(tert-butyl)-4-ethoxy-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (7b)
Yellow crystals (231 mg, 86%); 1H NMR (500 MHz, DMSO-d6): δ 7.72 (d, 2H, CHar, J = 8.0 Hz), 7.52 (s, 1H, H-thiophene), 7.28 (d, 2H, CHar, J = 8.0 Hz), 6.54 (bs, 1H, NH), 4.49 (q, 2H, CH2, J = 7.1 Hz), 2.34 (s, 3H, CH3-tolyl), 1.43 (s, 9H, C(CH3)3), 1.38 (t, 3H, CH3, J = 7.1 Hz); 13C NMR (125 MHz, DMSO-d6): δ 164.2, 162.8, 160.5, 150.3, 139.3, 130.2, 129.8, 126.0, 118.5, 104.6, 62.0, 50.2, 28.9, 20.9, 14.5; HR-MS (ESI) calculated for C19H24N3OS: 342.1635 [M + H]+, found: 342.1638.
3.1.5. Synthesis of 4-Arylether-thieno[3,2-d]pyrimidines 8
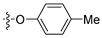
The appropriate phenol (1 eq., 0.753 mmol) and K2CO3 (1.1 eq., 0829 mmol) were dissolved in DMF (5 mL) and the reaction mixture was stirred for 15 min. Then, compound 5 (250 mg, 0.753 mmol) was added and the reaction mixture was heated at 130 °C for 3 h. Once the reaction finished, the reaction mixture returned to room temperature and was poured in water. The aqueous layer was extracted four times with EtOAc. The organic layers were combined, washed with brine, dried with MgSO4, and filtered and concentrated under vacuo. The crude product was purified by chromatography on silica gel (eluent: 9/1 Hexane/EtOAc then 8/2) and was recrystallized in ACN.
N-(tert-butyl)-4-phenoxy-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (8a)
Yellow crystals; (212 mg, 72%); 1H NMR (500 MHz, DMSO-d6): δ 7.76 (d, 2H, CHar, J = 8.2 Hz), 7.59 (s, 1H, H-thiophene), 7.48–7.45 (m, 2H, CHar), 7.32–7.28 (m, 5H, CHar), 6.59 (bs, 1H, NH), 2.36 (s, 3H, CH3-tolyl), 1.18 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 165.5, 163.0, 160.4, 152.2, 151.5, 139.6, 130.0, 129.9, 129.6, 126.2, 125.7, 122.2, 118.4, 104.3, 50.2, 28.6, 20.9; HR-MS (ESI) calculated for C23H24N3OS: 390.1635 [M + H]+, found: 390.1644.
N-(tert-butyl)-6-(p-tolyl)-4-(p-tolyloxy)thieno[3,2-d]pyrimidin-2-amine (8b)
Yellow crystals (192 mg, 63%); 1H NMR (500 MHz, DMSO-d6): δ 7.75 (d, 2H, CHar, J = 8.0 Hz), 7.59 (s, 1H, H-thiophene), 7.31 (d, 2H, CHar, J = 8.0 Hz), 7.25 (d, 2H, CHar, J = 8.4 Hz), 7.25 (d, 2H, CHar, J = 8.4 Hz), 6.52 (bs, 1H, NH), 2.36 (s, 3H, CH3-tolyl), 2.33 (s, 3H, CH3-tolyl), 1.22 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 165.4, 163.1, 160.3, 151.4, 149.9, 139.6, 134.7, 130.1, 129.9, 129.8, 126.1, 121.9, 118.5, 104.4, 50.2, 28.6, 20.9, 20.5; HR-MS (ESI) calculated for C24H26N3OS: 404.1791 [M + H]+, found: 404.1796.
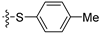
3.1.6. Synthesis of 4-Thioether-thieno[3,2-d]pyrimidines 9
Compound 5 was dissolved in DMF (5mL/mmol). Then, the appropriate thiophenol (10 eq.) and K2CO3 (1 eq.) were added. The reaction mixture was stirred at room temperature for 1 to 1h 30. Once the reaction was completed, the reaction mixture was concentrated under reduced pressure and water was added. The aqueous layer was extracted three times with DCM. Organic layers were washed with brine, dried with MgSO4, and filtered and concentrated under vacuo. The crude was purified by chromatography on silica gel (eluent: 8/2 Hexane/EtOAc for compound 9a and 9/1 Hexane/EtOAc then 7/3 for compound 9b). The isolated product was recrystallized in ACN.
N-(tert-butyl)-6-(p-tolyl)-4-(phenylthio)thieno[3,2-d]pyrimidin-2-amine (9a)
Yellow crystals (365 mg, 85%); 1H NMR (500 MHz, DMSO-d6): δ 7.72 (d, 2H, CHar, J = 7.9 Hz), 7.68–7.68 (m, 2H, CHar), 7.55–7.48 (m, 4H, CHar + H-thiophene), 7.30 (d, 2H, CHar, J = 8.2 Hz), 6.61 (bs, 1H, NH), 2.35 (s, 3H, CH3-tolyl), 1.11 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 161.7, 160.6, 158.9, 150.3, 138.9, 135.1, 129.0, 128.9, 128.8, 128.6, 125.8, 125.3, 117.1, 114.2, 49.0, 27.5, 20.0; HR-MS (ESI) calculated for C23H24N3S2: 406.1406 [M + H]+, found: 406.1398.
N-(tert-butyl)-6-(p-tolyl)-4-(p-tolylthio)thieno[3,2-d]pyrimidin-2-amine (9b)
Yellow crystals (137 mg, 54%); 1H NMR (500 MHz, DMSO-d6): δ 7.71 (d, 2H, CHar, J = 7.9 Hz), 7.55–7.53 (m, 3H, CHar + H-thiophene), 7.32–7.29 (m, 4H, CHar), 6.58 (bs, 1H, NH), 2.37 (s, 3H, CH3-tolyl), 2.35 (s, 3H, CH3-tolyl), 1.12 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 162.5, 162.0, 159.9, 151.3, 139.9, 139.8, 136.1, 130.2, 129.9, 126.3, 123.3, 118.1, 115.2, 50.1, 28.5, 21.0, 20.9; HR-MS (ESI) calculated for C24H26N3S2: 420.1568 [M + H]+, found: 420.1572.
3.1.7. Synthesis of N2-(Tert-butyl)-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine 10a
A mixture of 5 (0.5 g, 1.507 mmol) in EtOH/NH4OH (35%) in a 1:1 ratio (50 mL) was heated at 120 °C. The reaction mixture was stirred for 14 h in a sealed tube. After completion, the reaction mixture was poured into 50 mL of water and was extracted three times with EtOAc. The combined organic layers were washed three times with brine, dried with MgSO4, and filtered and concentrated under reduced pressure. The crude compound was purified by chromatography on silica gel (eluent: 9/1 to 5/5 Hexane/EtOAc) to afford 10a (295 mg, 63%) as an off-white solid. 1H NMR (500 MHz, DMSO-d6): δ 7,66 (d, 2H, CHar, J = 8.0 Hz), 7,37 (s, 1H, H-thiophene), 7,27 (d, 2H, CHar, J = 8.0 Hz), 6,76 (bs, 2H, NH2), 5,64 (bs, 1H, NH), 2.34 (s, 3H, CH3-tolyl), 1.40 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 162.4, 160.9, 157.6, 147.8, 138.7, 130.7, 129.7, 125.7, 119.0, 109.3, 49.8, 29.1, 20.8; HR-MS (ESI) calculated for C17H21N4S: 313.1487 [M + H]+, found: 313.1504.
3.1.8. Synthesis of 4-Hydrazino-thieno[3,2-d]pyrimidines 10b and 10i
A mixture of compound 5 (1.0 eq.) and the appropriate hydrazine (10 eq.) was stirred and heated under EtOH reflux (3 mL/mmol) in a sealed tube. The reaction mixture was stirred for 4 to 12 h. After completion, the reaction mixture returned to room temperature and was concentrated under reduced pressure. The crude was dissolved in EtOAc and the organic layer was washed three times with brine. The organic layer was dried with MgSO4 and filtered and concentrated under reduced pressure. The crude was purified by recrystallization in ACN.
N-(tert-butyl)-4-(1-methylhydrazinyl)-6-(p-tolyl)-3,4-dihydrothieno[3,2-d]pyrimidin-2-amine (10b)
Brown solid (209 mg, 90%); 1H NMR (500 MHz, DMSO-d6): δ 7.65 (d, 2H, CHar, J = 8.1 Hz), 7.26 (s, 1H, H-thiophene), 7.25 (d, 2H, CHar, J = 8.1 Hz), 5.76 (s, 1H, NH), 5.09 (bs, 2H, NH2), 3.31 (bs, 3H, CH3N), 2.33 (s, 3H, CH3-tolyl), 1.40 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 162.3, 160.0, 159.4, 150.2, 138.3, 131.2, 129.7, 125.7, 117.9, 104.0, 49.8, 38.9, 29.2, 20.9; HR-MS (ESI) calculated for C18H24N5S: 342.1747 [M + H]+, found: 342.1756.
N-(tert-butyl)-4-hydrazino-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (10i)
Brown solid (94 mg, 92%); 1H NMR (500 MHz, DMSO-d6): δ 8.30 (bs, 1H, NH), 7.66 (d, 2H, CHar, J = 8.1 Hz), 7.30 (s, 1H, H-thiophene), 7.26 (d, 2H, CHar, J = 8.0 Hz), 5.62 (bs, 1H, NH), 4.67 (bs, 2H, NH2), 2.33 (s, 3H, CH3-tolyl), 1.39 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 162.6, 160.4, 160.3, 150.3, 138.4, 131.1, 129.7, 125.7, 118.1, 102.5, 49.8, 30.8, 29.3, 20.9; HR-MS (ESI) calculated for C17H22N5S: 328.1596 [M + H]+, found: 328.1599.
3.1.9. General Procedure for the Synthesis of 4-Amino-thieno[3,2-d]pyrimidines 10c–h
Compound 5 (1 eq.) was dissolved in EtOH (22 mL/mmol). The appropriate amine (3 eq.) and Na2CO3 (2 eq.) were added to the solution. The reaction mixture was heated at 35 °C for compound 10c or under reflux for other amines. If the reaction was not completed after 72 h, another portion of the appropriate amine was added, and the heating was continued until completion of the reaction (1 eq. was added for compound 10h; 15 eq. for compound 10c; and 4.5 eq. for other compounds). Then, the mixture returned to room temperature and was concentrated under reduced pressure. The mixture was dissolved in water. The aqueous layer was extracted three times with EtOAc. Organic layers were washed with brine, dried with MgSO4, and filtered and concentrated under vacuo. The crude was purified by the appropriate method.
N2-(tert-butyl)-N4-methyl-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine (10c)
Following the general procedure, using 5 (237 mg, 0.742 mmol) and methylamine. The crude was purified by chromatography on silica gel (eluent: 5/5 Hexane/AcOEt) to afford 10c as a yellow solid (175 mg, 75%); 1H NMR (500 MHz, DMSO-d6): δ 7.63 (d, 2H, CHar, J = 8.1 Hz), 7.29 (s, 1H, H-thiophene), 7.28 (d, 2H, CHar, J = 7.9 Hz), 5.76 (bs, 1H, NH), 3.31 (s, 3H, CH3N), 2.33 (s, 3H, CH3-tolyl), 1.40 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 161.3, 160.4, 156.8, 147.0, 138.1, 130.4, 129.2, 125.4, 118.6, 104.4, 49.5, 28.9, 26.9, 20.2; HR-MS (ESI) calculated for C18H22N4S: 327.1643 [M + H]+, found: 4327.1643.
N2-(tert-butyl)-N4-propyl-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine (10d)
Following the general procedure, using 5 (300 mg, 0.904 mmol) and propylamine. The crude compound was purified by chromatography on silica gel (eluent: 9/1 to 7/3 Hexane/EtOAc) to afford 10d as a yellow solid (214 mg, 67%); 1H NMR (400 MHz, DMSO-d6): δ 7.65 (d, 2H, CHar, J = 8.0 Hz), 7.33–7.31 (m, 2H, H-thiophène + NHCH2), 7.28 (d, 2H, CHar, J = 8.0 Hz), 5.79 (bs, 1H, NH), 3.41–3.36 (m, 2H, CH2N), 2.34 (s, 3H, CH3-tolyl), 1.65–1.56 (m, 2H, CH2), 1.40 (s, 9H, C(CH3)3), 0.91 (t, 3H, CH3CH2, J = 7.4 Hz); 13C NMR (400 MHz, DMSO-d6): δ 161.8, 160.7, 155.8, 147.2, 138.6, 130.7, 129.7, 125.7, 118.8, 104.2, 49.8, 41.5, 29.2, 22.4, 20.8; HR-MS (ESI) calculated for C20H27N4S: 355.1956 [M + H]+, found: 355.1960.
N2-(tert-butyl)-N4-isopropyl-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine (10e)
Following the general procedure, using 5 (300 mg, 0.904 mmol) and isopropylamine. The crude was purified by a manual chromatographic column of silica gel (eluent: 9/1 Hexane/AcOEt to 6/4). Compound 10e was obtained as a white solid (238 mg, 74%); 1H NMR (600 MHz, DMSO-d6): δ 7.64 (d, 2H, CHar, J = 8.0 Hz), 7.32 (s, 1H, H-thiophene), 7.28 (d, 2H, CHar, J = 8.0 Hz), 7.06 (d, 1H, NHCH, J = 7.6 Hz), 5.76 (bs, 1H, NH), 4.42–4.34 (m, 1H, CHN), 2.34 (s, 3H, CH3-tolyl), 1.40 (s, 9H, C(CH3)3), 1.21 (d, 6H, C(CH3)2, J = 6.5 Hz); 13C NMR (150 MHz, DMSO-d6): δ 161.8, 160.7, 155.8, 147.2, 138.6, 130.7, 129.7, 125.7, 118.8, 104.2, 49.8, 41.5, 29.2, 22.4, 20.8; HR-MS (ESI) calculated for C20H27N4S: 355.1956 [M + H]+, found: 355.1956.
4-((2-(tert-butylamino)-6-(p-tolyl)thieno[3,2-d]pyrimidin-4-yl)amino)butan-1-ol (10f)
Following the general procedure, using 5 (150 mg, 0.45.2 mmol) and 4-amino-1-butanol. The crude compound was purified by recrystallization to afford 10f as pale yellow crystals (134 mg, 77%); 1H NMR (500 MHz, DMSO-d6): δ 7.65 (d, 2H, CHar, J = 8.0 Hz), 7.36–7.34 (m, 2H, H-thiophene + NHCH2), 7.27 (d, 2H, CHar, J = 8.0 Hz), 5.82 (bs, 1H, NH), 4.45 (t, 1H, OH, J = 5.0 Hz), 3.45–3.40 (m, 4H, CH2N + CH2O), 2.34 (s, 3H, CH3-tolyl), 1.64–1.58 (m, 2H, CH2), 1.52–1.46 (m, 2H, CH2), 1.40 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 161.7, 160.7, 156.5, 147.3, 138.7, 130.7, 129.8, 125.7, 118.9, 104.2, 60.7, 49.9, 40.1, 30.2, 29.3, 25.9, 20.9; HR-MS (ESI) calculated for C21H29N4OS: 385.2062 [M + H]+, found: 385.2068.
N2-(tert-butyl)-N4-(2-methoxyethyl)-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine (10g)
Following the general procedure, using 5 (150 mg, 0.45.2 mmol) and 2-methoxyethylamine. The crude compound was purified by a silica gel chromatography (eluent: 8/2 to 6/4 Hexane/EtOAc) to afford 10g as a yellowish solid (127 mg, 76%); 1H NMR (500 MHz, DMSO-d6): δ 7.65 (d, 2H, CHar, J = 8.2 Hz), 7.40 (t, 1H, NHCH2, J = 4.7 Hz), 7.35 (s, 1H, H-thiophene), 7.28 (d, 2H, CHar, J = 8.2 Hz), 5.88 (bs, 1H, NH), 3.61–3.58 (m, 2H, CH2N), 3.53–3.50 (m, 2H, CH2O), 3.27 (s, 3H, CH3O), 2.34 (s, 3H, CH3-tolyl), 1.40 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 161.9, 160.6, 156.5, 147.5, 138.7, 130.7, 129.8, 125.8, 118.9, 104.2, 70.6, 58.0, 49.9, 39.76, 29.2, 20.9; HR-MS (ESI) calculated for C20H27N4OS: 371.1906 [M + H]+, found: 371.1904.
N2-(tert-butyl)-N4-phenyl-6-(p-tolyl)thieno[3,2-d]pyrimidine-2,4-diamine (10h)
Following the general procedure, using 5 (250 mg, 0.753 mmol) and aniline. The crude was then purified by chromatography on silica gel (eluent: 9/1 Hexane/AcOEt to 8/2). Compound 10h was obtained as a brown solid (149 mg, 51%); 1H NMR (500 MHz, DMSO-d6): δ 9.01 (bs, 1H, NH), 7.83 (d, 2H, CHar, J = 7.5 Hz), 7.70 (d, 2H, CHar, J = 8.2 Hz), 7.47 (s, 1H, H-thiophene), 7.34–7.29 (m, 4H, CHar), 7.05–7.03 (m, 1H, CHar), 6.61 (bs, 1H, NH), 2.35 (s, 3H, CH3-tolyl), 1.40 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 162.8, 160.2, 154.6, 148.5, 139.8, 139.0, 130.5, 129.9, 128.4, 125.8, 122.6, 121.5, 119.1, 105.0, 50.0, 29.0, 20.9; LC/MS: tr = 1.68 min, [M + H]+ = 389.2; HR-MS (ESI) calculated for C23H25N4S: 389.1794 [M + H]+, found: 389.1811.
3.1.10. Synthesis of Sodium (S)-2-((2-(Tert-butylamino)-6-(p-tolyl)thieno[3,2-d]pyrimidin-4-yl) amino)-propanoate trihydrate 10j
Compound 5 (200 mg, 0.603 mmol) was dissolved in EtOH (17 mL/mmol). Alanine (3 eq., 161 mg, 1.808 mmol) and Na2CO3 (2 eq., 192 mg, 1.808 mmol) were added to the solution. The reaction mixture was heated under reflux for 8 days. Then, the mixture returned to room temperature and was concentrated under reduce pressure. Water (50 mL) was added, and the precipitate was filtered and dried under vacuum at 50 °C overnight. The desired compound was obtained without further purification as a white powder (129 mg, 56%); 1H NMR (500 MHz, DMSO-d6): δ 7.64 (d, 2H, CHar, J = 8.1 Hz), 7.31–7.28 (m, 3H, H-thiophene + CHar), 7.08–7.07 (m, 1H, NHCH), 5.43 (bs, 1H, NH), 4.66–4.61 (m, 1H, CH), 2.37 (s, 3H, CH3-tolyl), 1.47 (d, 3H, CH3, J = 7.2 Hz), 1.42 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 173.7, 161.7, 160.1, 155.7, 147.5, 138.2, 130.4, 129.2, 125.4, 118.5, 104.3, 49.5, 48.6, 28.9, 20.2, 17.2; UPLC/MS: tr = 2.58 min, [M + H]+ = 385.3; HR-MS (ESI) C20H25N4O2S calculated: 385.1698 [M + H]+, found: 385.1700. This compound (79 mg, 0.2055 mmol) was dissolved in anhydrous EtOH. Sodium ethoxide, 21% w/w in ethanol (1.1 eq., 73 mg, 0.226 mmol) was added and the mixture was stirred at room temperature for 7h. The volatiles were removed under reduce pressure. The desired product was obtained as a yellow powder (82 mg, 87%); 1H NMR (400 MHz, DMSO-d6): δ 7.68 (d, 2H, CHar, J = 8.0 Hz), 7.35 (s, 1H, H-thiophene), 7.27 (d, 2H, CHar, J = 8.0 Hz), 6.65 (d, 1H, NHCH, J = 5.1 Hz), 5.77 (bs, 1H, NH), 4.08–4.01 (m, 1H, CH), 2.34 (s, 3H, CH3-tolyl), 1.41 (s, 9H, C(CH3)3), 1.36 (d, 3H, J = 7.2 Hz); 13C NMR (100 MHz, DMSO-d6): δ 174.1, 161.6, 161.0, 155.4, 146.8, 138.7, 130.5, 129.7, 125.8, 119.1, 104.1, 50.9, 49.8, 29.3, 20.8, 19.7.LC/MS: tr = 1.41 min, [M + H]+ = 385.1; HR-MS (ESI) C20H25N4O2S calculated: 385.1693 [M + H]+, found: 385.1695.Elemental analysis calculated for the trihydrate salt: C20H29N4NaO5S: C, 52.16; H, 6.35; N, 12.17; S, 6.96; found: C, 52.07; H, 5.99; N, 11.82; S, 6.83.
3.1.11. General Procedure for the Synthesis of 4-Aryl-thieno[3,2-d]pyrimidines 11a–f
Compound 5 (1 eq.) was dissolved in THF (15 mL/mmol) (for compound 12a–d) or 1,4-dioxane (for compounds 12e–f). The mixture was degassed, filled with argon. Then, the appropriate boronic acid (2 eq.) was added and the mixture was heated (30 to 40 °C) to solubilize the starting material. A solution of 1 M potassium carbonate in water (2.6 eq.) and Pd(PPh3)4 (0.02 eq.) were added. The reaction mixture was stirred and heated under reflux until completion of the reaction. The resulting mixture was partitioned between DCM and water. The organic layer was washed with a saturated aqueous solution of sodium bicarbonate, dried with MgSO4, and filtered and concentrated under vacuum. The residue was purified by column chromatography.
N-(tert-butyl)-4,6-di-p-tolylthieno[3,2-d]pyrimidin-2-amine (11a)
Following the general procedure, starting from 5 (300 mg, 0.904 mmol) and 4-tolylboronic acid (246 mg, 1.808 mmol). The crude was purified by column chromatography (eluent: 98/02 Cyclohexane/EtOAc to 80/20). A yellow solid was collected (236 mg, 67%); 1H NMR (500 MHz, DMSO-d6): δ 8.02 (d, 2H, CHar, J = 8.1 Hz), 7.78 (d, 2H, CHar, J = 8.2 Hz), 7.66 (s, 1H, H-thiophene), 7.42 (d, 2H, CHar, J = 8.0 Hz), 7.30 (d, 2H, CHar, J = 7.9 Hz), 6.73 (bs, 1H, NH), 2.41 (s, 3H, CH3-tolyl), 2.35 (s, 3H, CH3-tolyl), 1.47 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 164.4, 160.9, 158.5, 152.1, 140.6, 139.8, 134.7, 129.9, 129.5, 127.8, 126.2, 118.7, 115.5, 50.3, 28.8, 21.1, 20.9; LC/MS: tr = 2.65 min, [M + H]+ = 388.4; HR-MS (ESI) calculated for C24H26N3S: 388.1842 [M + H]+, found: 388.1835.
N-(tert-butyl)-4-(m-tolyl)-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (11b)
Following the general procedure, starting from 5 (300 mg, 0.904 mmol) and 3-tolylboronic acid (246 mg, 1.808 mmol). The crude was purified by column chromatography (eluent: 80/20 Hexane/EtOAc to 60/40) to offer a yellow solid (252 mg, 72%); 1H NMR (500 MHz, DMSO-d6): δ 7.90–7.88 (m, 2H, CHar), 7.80 (d, 2H, CHar, J = 8.0 Hz), 7.68 (s, 1H, H-thiophene), 7.51 (t, 1H, CHar, J = 7.6 Hz), 7.41 (d, 1H, CHar, J = 7.6 Hz), 7.31 (d, 2H, CHar, J = 8.0 Hz), 6.77 (bs, 1H, NH), 2.44 (s, 3H, CH3-tolyl), 2.36 (s, 3H, CH3-tolyl), 1.47 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 164.4, 160.9, 158.8, 152.3, 138.3, 137.5, 131.3, 129.9, 128.9, 128.4, 126.3, 125.0, 118.7, 115.8, 50.3, 28.8, 21.2, 20.9; LC/MS: tr = 2.68 min, [M + H]+ = 388.3; HR-MS (ESI) calculated for C24H26N3S: 388.1842 [M + H]+, found: 388.1841.
N-(tert-butyl)-4-phenyl-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (11c)
Following the general procedure, starting from 5 (300 mg, 0.904 mmol) and phenylboronic acid (246 mg, 1.808 mmol). The crude was purified by column chromatography (eluent: 90/10 Hexane/EtOAc) to offer a yellow solid (268 mg, 79%); 1H NMR (500 MHz, DMSO-d6): δ 8.12–8.10 (m, 2H, CHar), 7.80 (d, 2H, CHar, J = 8.2 Hz), 7.68 (s, 1H, H-thiophene), 7.65–7.58 (m, 3H, CHar), 7.31 (d, 2H, CHar, J = 7.9 Hz), 6.80 (bs, 1H, NH), 2.35 (s, 3H, CH3-tolyl), 1.48 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 164.5, 160.9, 158.6, 152.4, 139.9, 137.5, 130.7, 129.9, 129.9, 129.0, 127.9, 126.3, 118.7, 115.7, 50.3, 28.8, 20.9; LC/MS: tr = 2.52 min, [M + H]+ = 374.1; HR-MS (ESI) calculated for C23H24N3S: 374.1685 [M + H]+, found: 374.1680.
N-(tert-butyl)-4-(thiophen-3-yl)-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (11d)
Following the general procedure, starting from 5 (300 mg, 0.904 mmol) and 3-thiopheneboronic acid (246 mg, 1.808 mmol). The crude was purified by column chromatography (eluent: 90/10 Hexane/EtOAc to 85/15) to offer a brown solid (273 mg, 80%); 1H NMR (500 MHz, DMSO-d6): δ 8.39–8.38 (m, 1H, H-thiophene), 7.86–7.85 (m, 1H, H-thiophene), 7.81–7.79 (m, 3H, H-thiophene + CHar), 7.66 (s, 1H, H-thiophene), 7.32 (d, 2H, CHar, J = 8.0 Hz), 6.70 (bs, 1H, NH), 2.36 (s, 3H, CH3-tolyl), 1.47 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 164.4, 160.6, 153.7, 152.0, 139.9, 139.7, 129.9, 128.3, 127.7, 126.9, 126.3, 118.6, 114.9, 50.3, 28.8, 21.0; LC/MS: tr = 2.51 min, [M + H]+ = 380.2; HR-MS (ESI) calculated for C21H22N3S2: 380.1250 [M + H]+, found: 380.1254.
N-(tert-butyl)-4-(pyridin-4-yl)-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (11e)
Following the general procedure, starting from 5 (177 mg, 0.5345 mmol) and 4-pyridinylboronic acid (146 mg, 1.069 mmol). The crude was purified by column chromatography (eluent: 60/40 Hexane/EtOAc to 100% EtOAc) to offer a yellow solid (133 mg, 66%); 1H NMR (500 MHz, CDCl3): δ 8.83 (d, 2H, CHpyridinyl, J = 5.5 Hz), 8.03 (d, 2H, CHpyridinyl, J = 5.5 Hz), 7.65 (d, 2H, CHar, J = 8.2 Hz), 7.43 (s, 1H, H-thiophene), 7.28–7.26 (m, 2H, CHar), 5.37 (bs, 1H, NH), 2.41 (s, 3H, CH3-tolyl), 1.56 (s, 9H, C(CH3)3); 13C NMR (125 MHz, CDCl3): δ 165.0, 160.8, 157.0, 154.4, 150.7, 145.4, 140.5, 130.5, 130.0, 126.7, 122.4, 118.4, 111.7, 51.4, 29.8, 21.5; LC/MS: tr = 2.47 min, [M + H]+ = 375.0; HR-MS (ESI) calculated for C22H23N4S: 375.1638 [M + H]+, found: 375.1641.
N-(tert-butyl)-4-(pyridin-3-yl)-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine (11f)
Following the general procedure, starting from 5 (200 mg, 0.6027 mmol) and 3-pyridinylboronic acid (148 mg, 1.205 mmol). The crude was purified by column chromatography (eluent: 70/30 Hexane/EtOAc to 100% EtOAc) to offer a yellow solid (171 mg, 76%); 1H NMR (500 MHz, DMSO-d6): δ 9.41 (d, 1H, CHpyridinyl, J = 1.8 Hz), 8.76 (dd, 1H, CHpyridinyl, J = 4.8 and 1.8 Hz), 8.45–8.43 (m, 1H, CHpyridinyl), 7.65 (d, 2H, J = 8.2 Hz, CHar), 7.50–7.47 (m, 1H, CHpyridinyl), 7.41 (s, 1H, H-thiophene), 7.27–7.26 (m, 2H, CHar), 5.21 (bs, 1H, NH), 2.41 (s, 3H, CH3-tolyl), 1.55 (s, 9H, CH3-tolyl); 13C NMR 125 MHz, DMSO-d6): δ 165.2, 161.1, 156.8, 153.9, 151.3, 149.7, 140.3, 135.7, 134.0, 130.6, 130.0, 126.6, 123.7, 118.6, 117.7, 51.2, 29.3, 21.5; LC/MS: tr = 2.41 min, [M + H]+ = 375.1; HR-MS (ESI) calculated for C22H23N4S: 375.1638 [M + H]+, found: 375.1637.
3.1.12. General Procedure for the Synthesis of 4-Alkynyl-thieno[3,2-d]pyrimidines 12a–c
Under nitrogen atmosphere, compound 5, the appropriate alkyne, copper iodide (16% mol), bis(triphenylphosphine)palladium(II) dichloride (4% mol), and triethylamine (10 eq.) were dissolved in dry ACN (1:1 v:v with triethylamine) in a sealed vial. The obtained suspension was stirred for 10 min under microwave irradiation at 100 °C. Water (20 times the quantity of ACN) was added to the reaction mixture, which was then extracted with dichloromethane. The organic layer was washed with water and the excess was removed under reduced pressure. The obtained crude was purified via the appropriate method.
N-tert-butyl-6-(4-methylphenyl)-4-(phenylethynyl)thieno[3,2-d]pyrimidin-2-amine (12a)
Following the general procedure, starting from 5 (0.26 g, 0.78 mmol) and phenylacetylene (103.2 µL, 0.94 mmol). The obtained crude was purified via chromatography (cyclohexane/DCM). Fractions of interest were triturated in n-pentane, affording 12a as a yellow solid (109 mg, 35%); 1H NMR (DMSO-d6, 400 MHz): δ 7.91 (d, 2H, CHar, J = 7.9 Hz), 7.73–7.65 (m, 3H, CHar), 7.60–7.49 (m, 3H, CHar), 7.32 (d, 2H, J = 7.9 Hz, CHar), 6.96 (bs, 1H, NH), 2.36 (s, 3H, CH3-tolyl), 1.44 (s, 9H, C(CH3)3); 13C NMR (DMSO-d6, 100 MHz) δ 163.1, 160.7, 152.8, 143.6, 140.0, 132.1 (2C), 130.5, 129.9 (2C), 129.8, 129.1 (2C), 126.3 (2C), 120.9, 120.2, 118.8, 94.3, 85.3, 50.3, 28.6 (3C), 20.9. HR-MS (ESI) calculated for C25H24N3S: 398.1685 [M + H]+, found 398.1678.
N-tert-butyl-4-(cyclopropylethynyl)-6-(4-methylphenyl)thieno[3,2-d]pyrimidin-2-amine (12b)
Starting from 5 (0.32 g, 0.96 mmol) and ethynylcyclopropane (409 µL, 4.82 mmol). The obtained crude was purified via flash chromatography (cyclohexane/EtOAc), affording 12b as a dark brown solid (165 mg, 47% yield); 1H NMR (DMSO-d6, 400 MHz) δ 7.77 (d, 2H, CHar, J = 8.2 Hz), 7.61 (s, 1H, H-thiophene), 7.30 (d, 2H, CHar, J = 7.9 Hz), 6.79 (bs, 1H, NH), 2.36 (s, 3H, CH3-tolyl), 1.76–1.68 (m, 1H, CH), 1.40 (s, 9H, C(CH3)3), 1.09–1.01 (m, 2H, CH2), 0.91–0.82 (m, 2H, CH2); 13C NMR (DMSO-d6, 100 MHz) δ 162.7, 160.1, 152.4, 144.3, 139.9, 129.9, 129.8 (2C), 126.2 (2C), 118.7, 101.1, 72.4, 50.2, 28.6 (3C), 26.3, 20.9, 9.33 (2C), −0.3. HRMS (ESI) m/z calculated for C22H24N3S [M + H]+ 362.1685, found 362.1685.
4-[2-tert-butylamino-6-(4-methylphenyl)thieno[3,2-d]pyrimidin-4-yl]but-3-yn-1-ol (12c)
Starting from 5 (0.32 g, 0.96 mmol) and but-3-yn-1-ol (219 µL, 2.89 mmol), the obtained crude was purified via two successive flash chromatography (DCM/MeOH and then cyclohexane/EtOAc), affording 12c as a yellow solid (24 mg, 7% yield); 1H NMR (DMSO-d6, 400 MHz): δ 7.77 (d, 2H, CHar, J = 8.2 Hz), 7.62 (s, 1H, H-thiophene), 7.31 (d, 2H, CHar, J = 8.2 Hz), 6.81 (bs, 1H, NH), 5.01 (t, 1H, OH, J = 5.5 Hz), 3.69–3.61 (m, 2H, CH2O), 2.71 (t, 2H, CH2, J = 6.6 Hz), 2.36 (s, 3H, CH3-tolyl), 1.41 (s, 9H, C(CH3)3); 13C NMR (DMSO-d6, 100 MHz): δ 162.8, 160.7, 152.6, 144.3, 139.9, 129.9 (3C), 126.3 (2C), 120.8, 118.7, 95.8, 77.9, 59.3, 50.2, 28.6 (3C), 23.2, 20.9; HRMS (ESI) calculated for C21H24N3OS [M + H]+ 366.1635, found 366.1636.
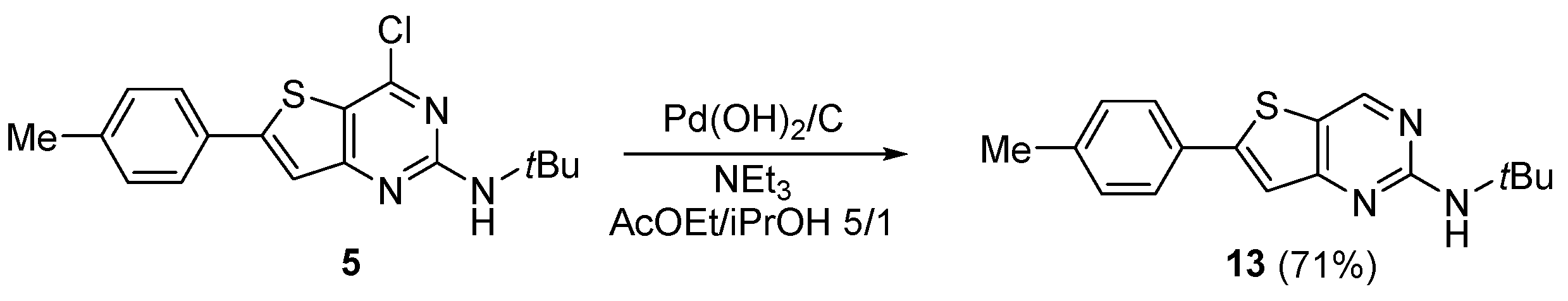
3.1.13. Synthesis of N-(Tert-butyl)-6-(p-tolyl)thieno[3,2-d]pyrimidin-2-amine 13
Compound 5 (250 mg, 0.7533 mmol) was dissolved in a solution of ethyl acetate/propan-2-ol, 5/1 (16 mL/mmol). Then, Pd(OH)2 (125 mg, 20% on carbon, wetted with ca.50% water) and triethylamine (2.3 eq., 175 mg, 1.733 mmol, 0.24 mL) were added. The suspension was placed under a stream of hydrogen and was stirred at room temperature until total consumption of the starting material (72 h). Three portions of Pd(OH)2 were added during the experiment. The reaction mixture was filtered on Celite® and the filtrate was concentrated. The crude was dissolved in EtOAc and washed with a saturated aqueous solution of NaHCO3. The aqueous layer was extracted twice with EtOAc. Combined organic layers were washed with brine, dried over MgSO4, and filtered and concentrated under reduced pressure. The crude was purified with chromatography of silica (eluent: Hexane/EtOAc 90/10 to 80/20) to afford 14 as an off-white solid (159 mg, 71%); 1H NMR (500 MHz, DMSO-d6): δ 8.88 (s, 1H, H-pyrimidine), 7.75 (d, 2H, CHar, J = 8.2 Hz), 7.60 (s, 1H, H-thiophene), 7.31 (d, 2H, CHar, J = 8.0 Hz), 6.70 (bs, 1H, NH), 2.35 (s, 3H, CH3-tolyl), 1.42 (s, 9H, C(CH3)3); 13C NMR (125 MHz, DMSO-d6): δ 162.4, 160.4, 152.5, 152.2, 139.7, 130.1, 129.9 (2C), 126.2 (2C), 118.9, 118.3, 50.1, 28.7, 20.9; LC/MS: tr = 2.19 min, [M + H]+ = 298.3; HR-MS (ESI) C17H20N3S calculated: 298.1372 [M + H]+, found: 298.1384.