Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats
Abstract
:1. Introduction
2. Results
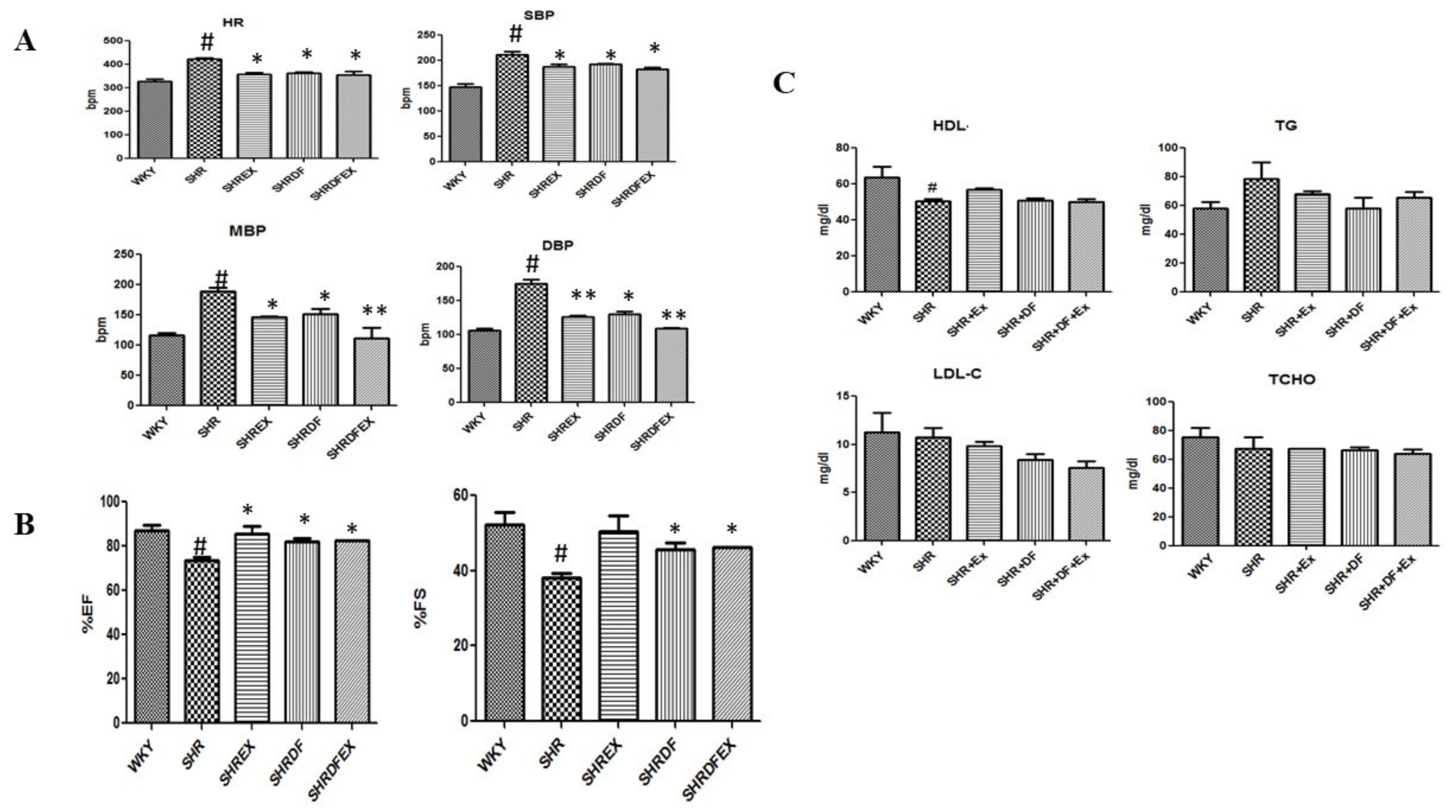
2.1. Effect of DF Bioactive Peptide and a Combination of Exercise (EX) on Whole Heart Weight, Blood Pressure, and Lipid Profile of WKY and SHR Rats
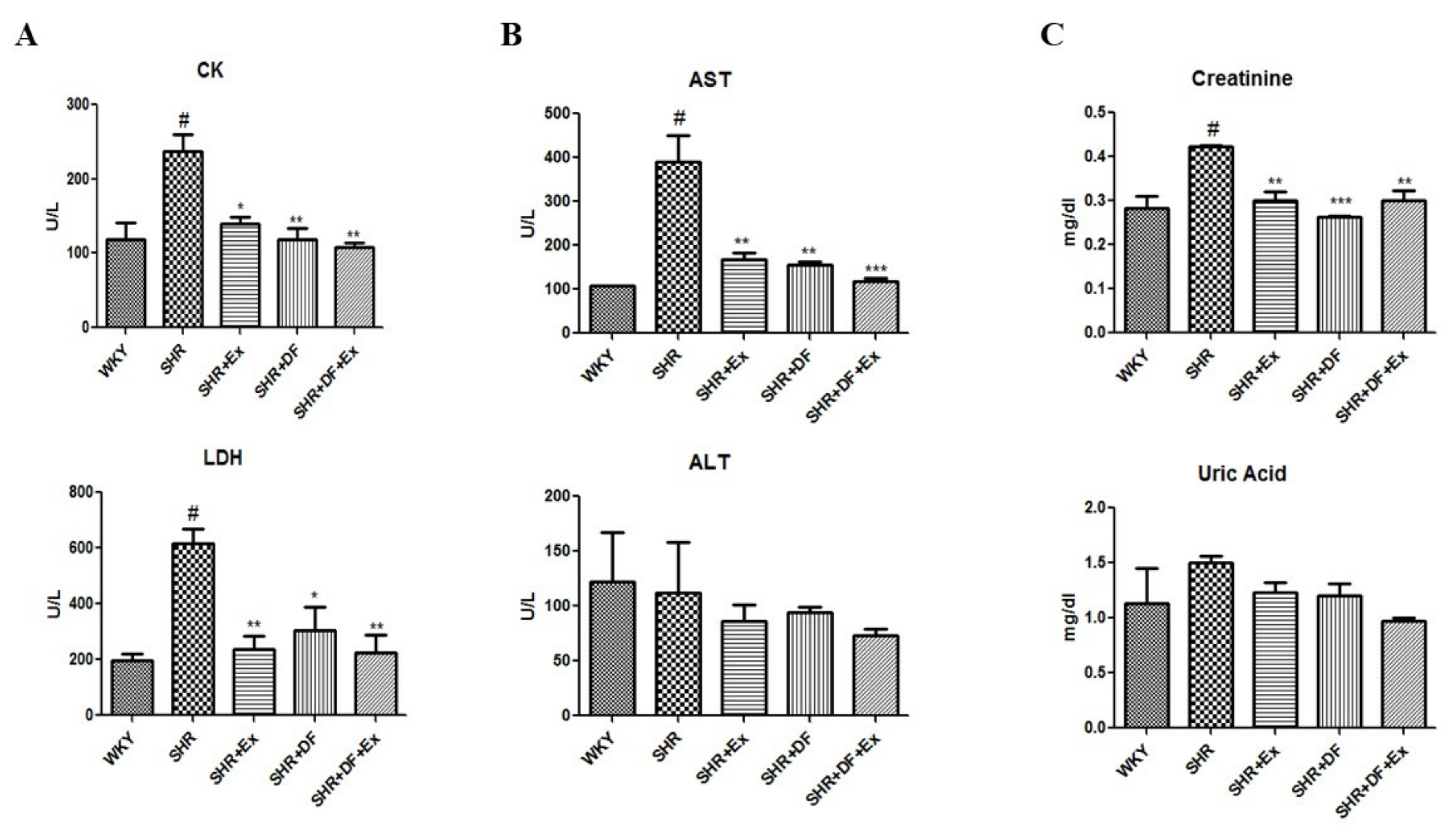
2.2. Effect of DF and Exercise on Myocardial Injury Markers and Cardiac, Hepatic, and Renal Function Markers of WKY and SHR Rats
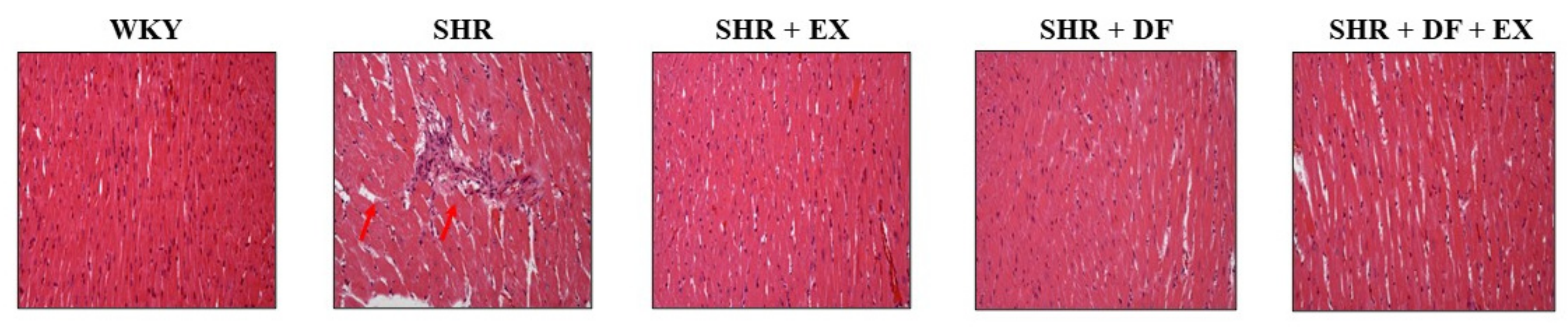
2.3. Effects of DF Peptide and Exercise on Cardiac Structure in Rats
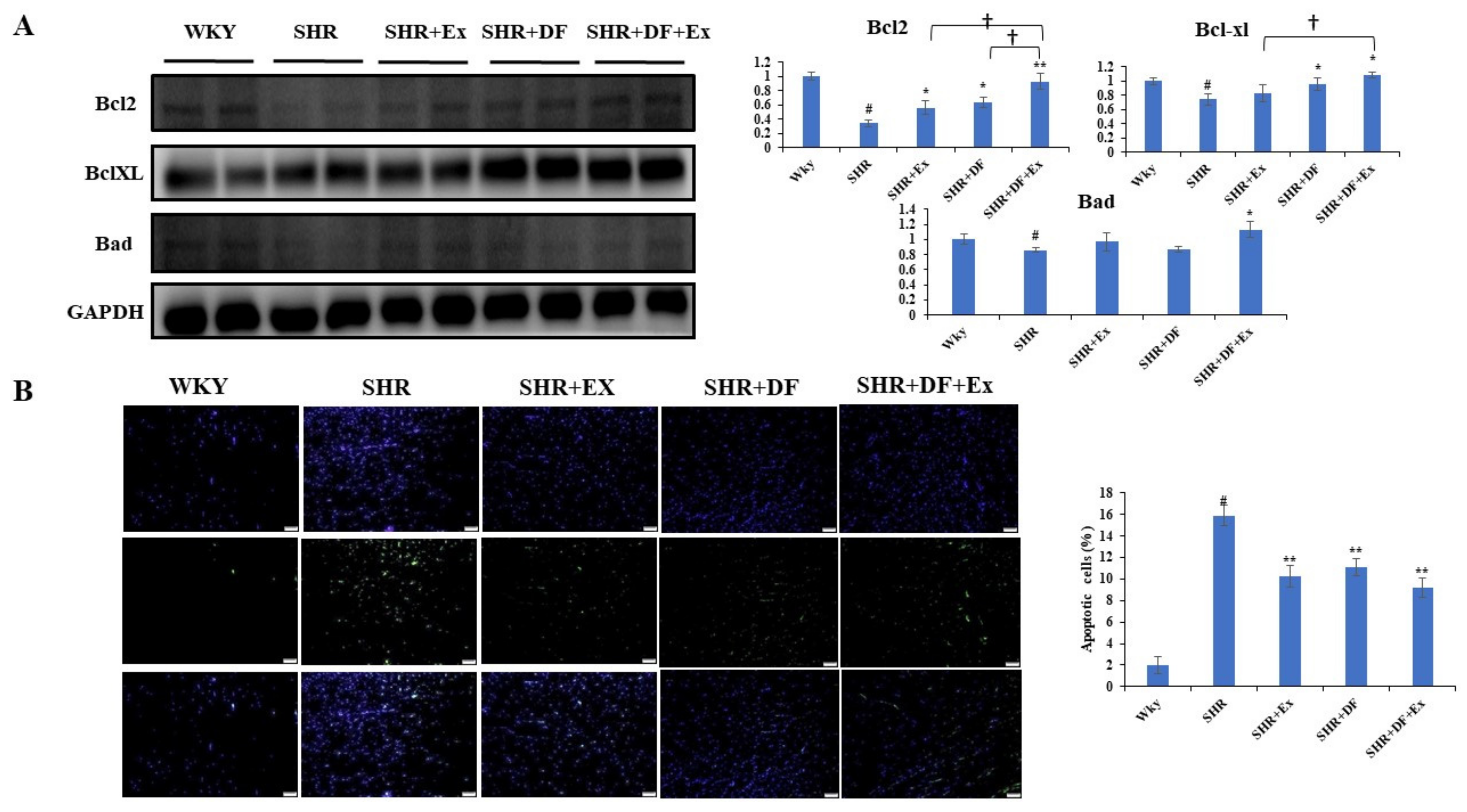
2.4. DF Peptide Administration and Exercise Inhibits Apoptosis in SHR Heart
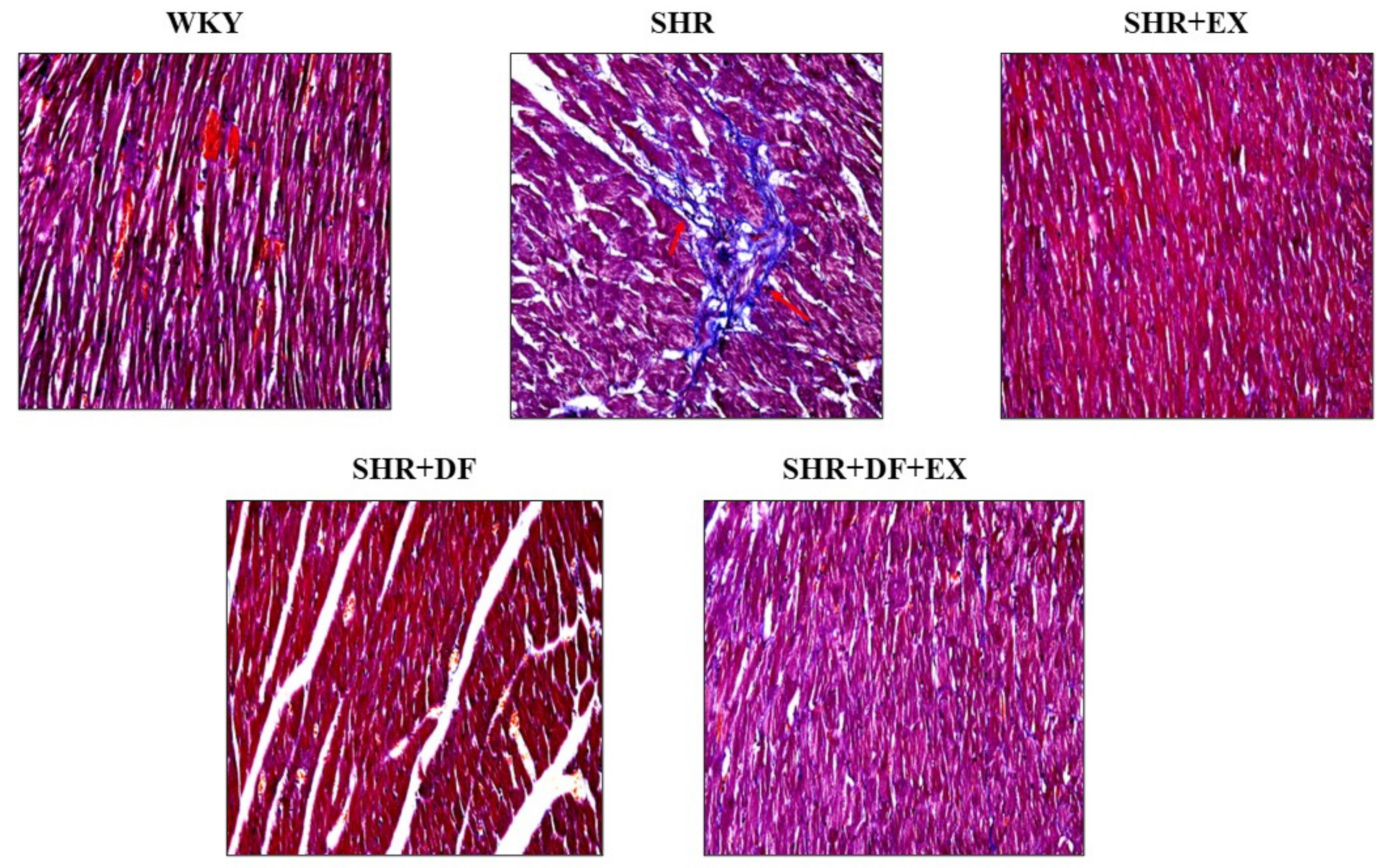
2.5. Effects of DF Peptide Treatment and Exercise in Cardiac Fibrosis
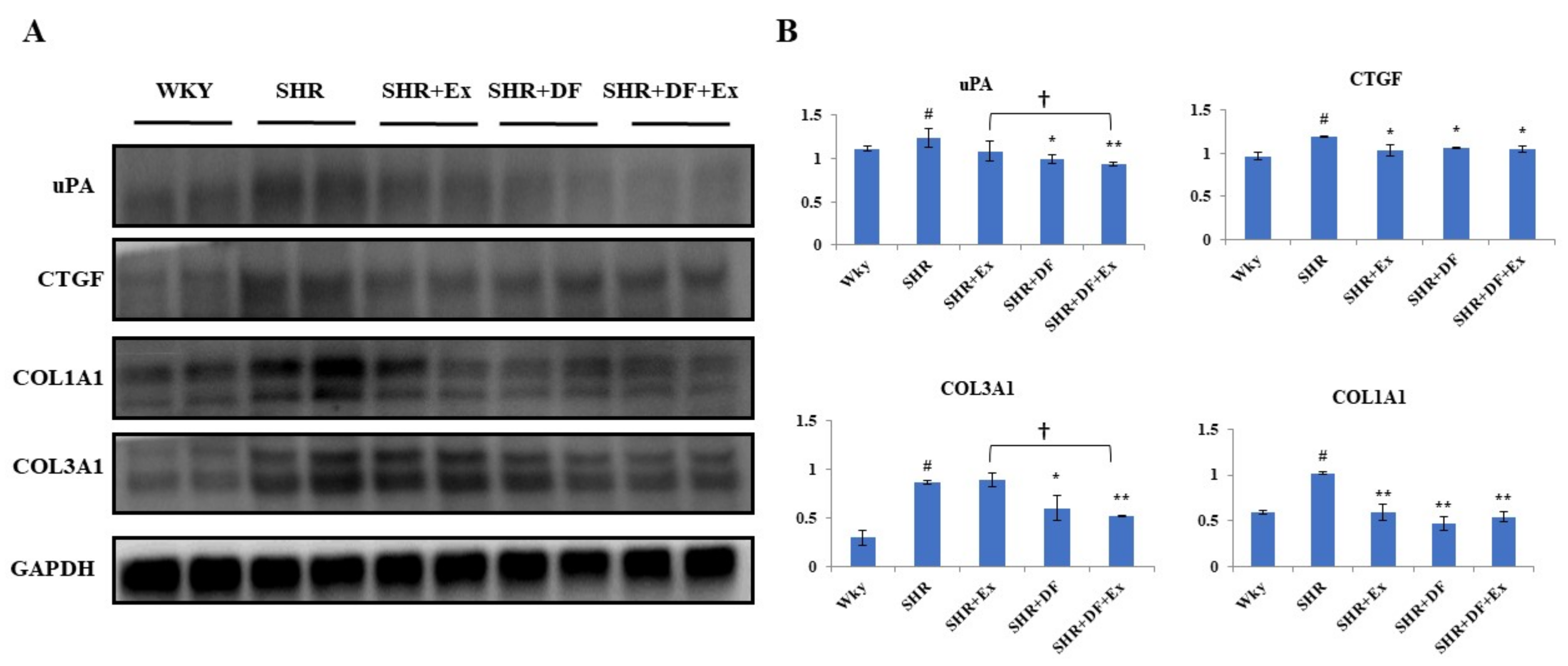
2.6. Effect of DF and Exercise on the Fibrosis-Associated Proteins in the Heart Tissue of SHR Animals
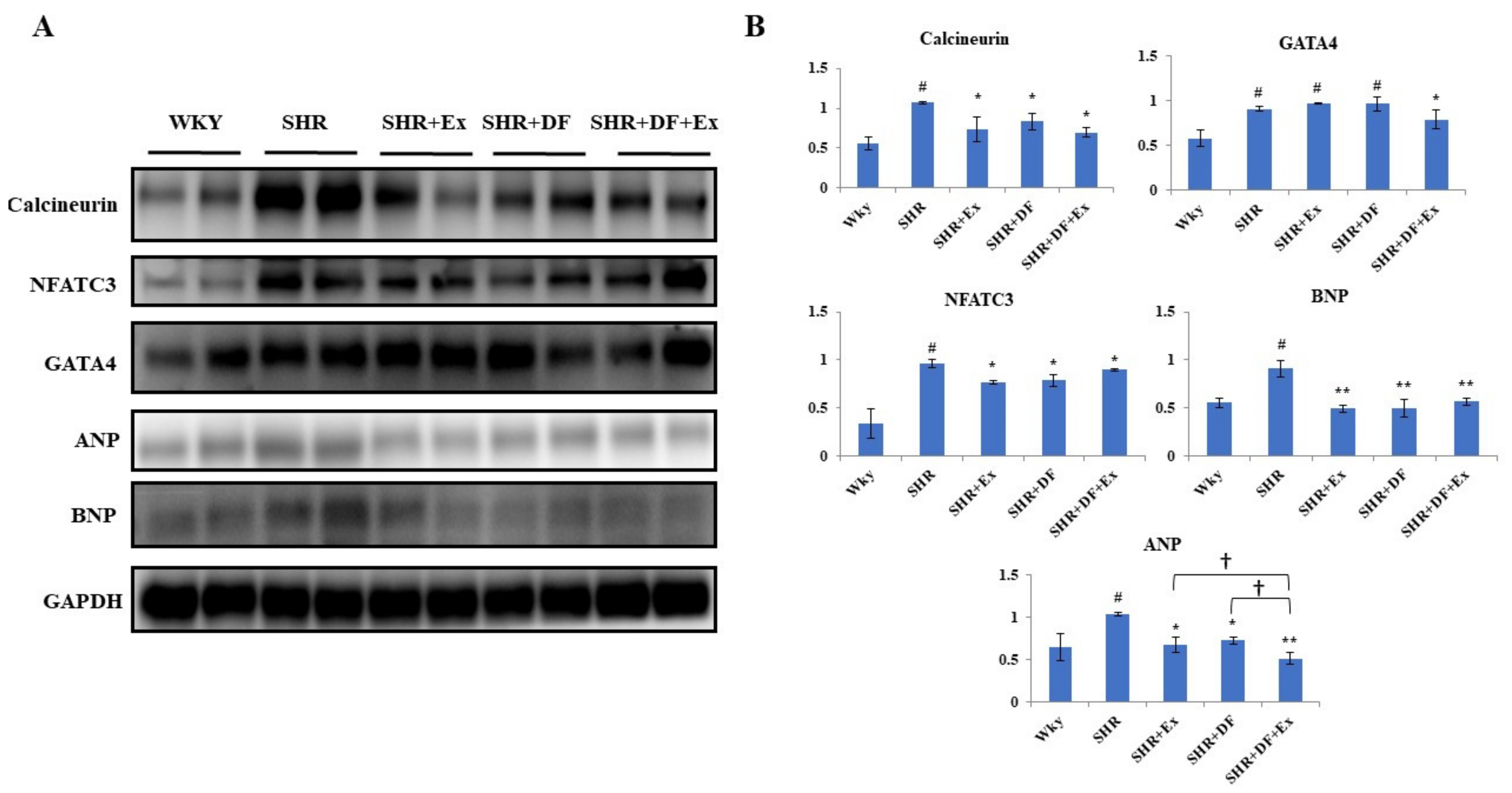
2.7. Effect of DF and Exercise on Cardiac Hypertrophy Markers
2.8. Effect of Bioactive Peptide and Exercise on Survival Protein (PI3K/AKT) Markers and IGF1 Activation
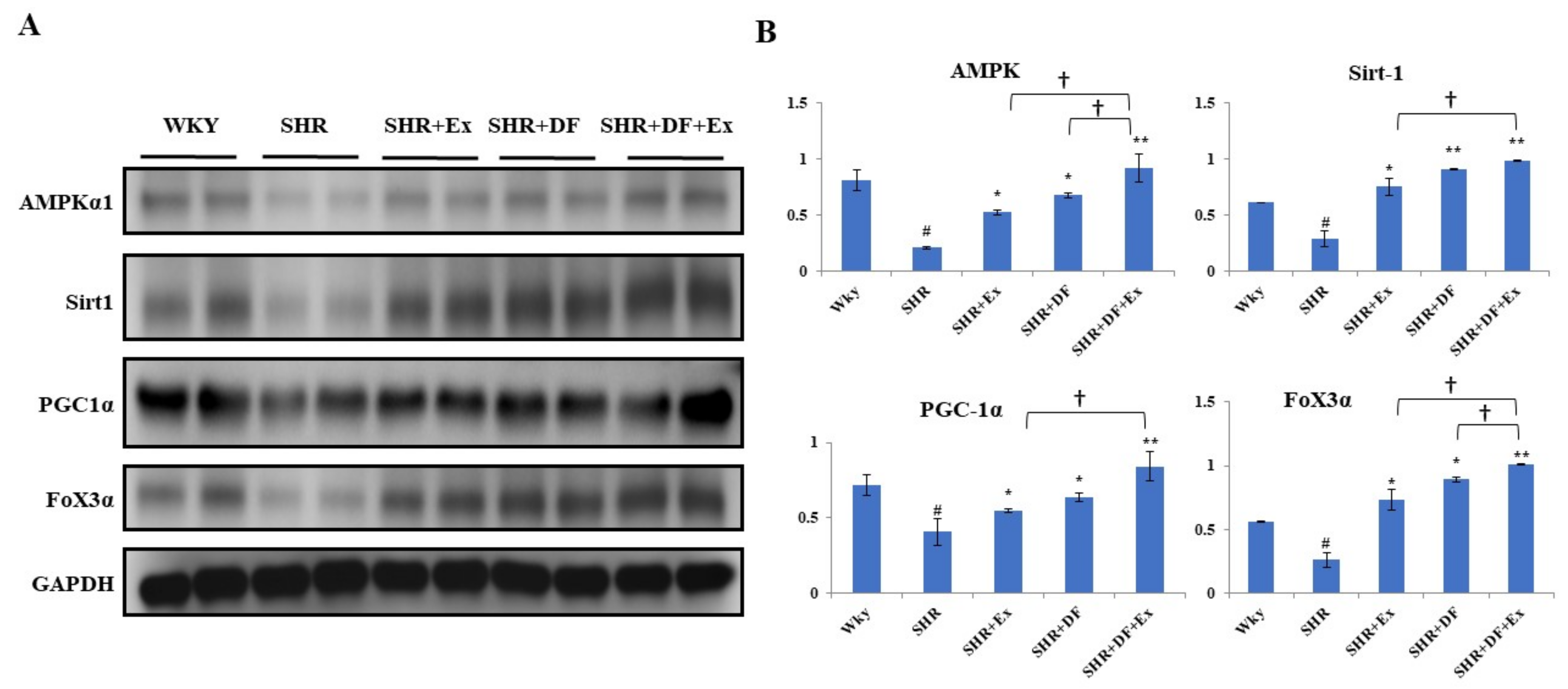
2.9. DF Treatment and Exercise Activates Mitochondrial Biogenesis through Activation of AMPK/SIRT1/PGC-1α/FOXO3 Pathway
3. Discussion
4. Materials and Methods
4.1. Animal Procedure
4.2. Drug Treatment
4.3. Blood Pressure Measurement
4.4. Measurement of Blood Serum Biochemical Parameters
4.5. Tissue Staining
4.6. Tissue Protein Extraction and Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oparil, S.; Schmieder, R.E. New approaches in the treatment of hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, M.; Oktay, A.A.; Stewart, M.H.; Milani, R.V.; Ventura, H.O.; Lavie, C.J. Left ventricular hypertrophy and hypertension. Prog. Cardiovasc. 2020, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tahir, E.; Starekova, J.; Muellerleile, K.; von Stritzky, A.; Münch, J.; Avanesov, M.; Weinrich, J.M.; Stehning, C.; Bohnen, S.; Radunski, U.K. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. JACC Cardiovasc. Imaging 2018, 11, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Dikalova, A.E. Crosstalk between mitochondrial hyperacetylation and oxidative stress in vascular dysfunction and hypertension. Antioxid. Redox Signal. 2019, 31, 710–721. [Google Scholar] [CrossRef]
- Hasan, P.; Saotome, M.; Ikoma, T.; Iguchi, K.; Kawasaki, H.; Iwashita, T.; Hayashi, H.; Maekawa, Y. Mitochondrial fission protein, dynamin-related protein 1, contributes to the promotion of hypertensive cardiac hypertrophy and fibrosis in Dahl-salt sensitive rats. J. Mol. Cell. Cardiol. 2018, 121, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Sádaba, M.; Martín-Estal, I.; Puche, J.; Castilla-Cortázar, I. Insulin-like growth factor 1 (IGF-1) therapy: Mitochondrial dysfunction and diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1267–1278. [Google Scholar] [CrossRef]
- Norling, A.M.; Gerstenecker, A.T.; Buford, T.W.; Khan, B.; Oparil, S.; Lazar, R.M. The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. Geroscience 2020, 42, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Oliveira, G.; Menezes, L.; Nascimento, A.L.; Carvalho, S.; Stumbo, A.C.; Thole, A.; Garcia-Souza, É.; Moura, A.; Carvalho, L. Insulin-like growth factor-1 short-period therapy improves cardiomyopathy stimulating cardiac progenitor cells survival in obese mice. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 151–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, I.; Timotin, A.; Moreno-Corchado, P.; Marsal, D.; Kramar, S.; Loy, H.; Joffre, C.; Boal, F.; Tronchere, H.; Kunduzova, O. Galanin promotes autophagy and alleviates apoptosis in the hypertrophied heart through FoxO1 pathway. Redox Biol. 2021, 40, 101866. [Google Scholar] [CrossRef]
- Carraway, M.S.; Suliman, H.B.; Kliment, C.; Welty-Wolf, K.E.; Oury, T.D.; Piantadosi, C.A. Mitochondrial biogenesis in the pulmonary vasculature during inhalational lung injury and fibrosis. Antioxid. Redox Signal. 2008, 10, 269–276. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Redox regulation of mitochondrial biogenesis. Free Radic. Biol. Med. 2012, 53, 2043–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Dai, W.; Hale, S.L.; Brown, D.A.; Wang, M.; Han, X.; Kloner, R.A. Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sci. 2015, 141, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Predominant expression of Sir2α, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004, 556, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- D′Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Rimbaud, S.; Garnier, A.; Ventura-Clapier, R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol. Rep. 2009, 61, 131–138. [Google Scholar] [CrossRef]
- Khatua, T.N.; Dinda, A.K.; Putcha, U.K.; Banerjee, S.K. Diallyl disulfide ameliorates isoproterenol induced cardiac hypertrophy activating mitochondrial biogenesis via eNOS-Nrf2-Tfam pathway in rats. Biochem. Biophys. Rep. 2016, 5, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Cellular antioxidant effect of bioactive peptides and molecular mechanisms underlying: Beyond chemical properties. Int. J. Food Sci. Technol. 2021, 56, 2193–2204. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Karami, Z.; Pateiro, M.; Lorenzo, J.M. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules 2021, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, D.Y.; Ghoyatul Amin, M.N.; Alamsjah, M.A.; Hsu, J.-L. Marine organisms as potential sources of bioactive peptides that inhibit the activity of angiotensin I-converting enzyme: A review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, T.-T.; Tan, Y.-N.; Ee, K.-Y.; Xiao, J.; Wong, F.-C. Seeds, fermented foods, and agricultural by-products as sources of plant-derived antibacterial peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, S162–S177. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Kudoh, K.; Matsumoto, M.; Onodera, S.; Takeda, Y.; Ando, K.; Shiomi, N. Antioxidative activity and protective effect against ethanol-induced gastric mucosal damage of a potato protein hydrolysate. J. Nutr. Sci. Vitaminol. 2003, 49, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Chiang, W.-D.; Pai, P.; Lin, W.-T. Potato protein hydrolysate attenuates high fat diet-induced cardiac apoptosis through SIRT1/PGC-1a/Akt signalling. J. Funct. Foods 2015, 12, 389–398. [Google Scholar] [CrossRef]
- Asokan, S.M.; Wang, T.; Wang, M.-F.; Lin, W.-T. A novel dipeptide from potato protein hydrolysate augments the effects of exercise training against high-fat diet-induced damages in senescence-accelerated mouse-prone 8 by boosting pAMPK/SIRT1/PGC-1α/pFOXO3 pathway. Aging 2020, 12, 7334. [Google Scholar] [CrossRef]
- Ades, P.A.; Keteyian, S.J.; Balady, G.J.; Houston-Miller, N.; Kitzman, D.W.; Mancini, D.M.; Rich, M.W. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC: Heart Fail. 2013, 1, 540–547. [Google Scholar] [CrossRef]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitao, C.; Gouveia, M.; Figueiredo, D.; Alves, A.J.; Polonia, J.; Oliveira, J. Exercise training reduces arterial stiffness in adults with hypertension: A systematic review and meta-analysis. J. Hypertens. 2021, 39, 214–222. [Google Scholar] [CrossRef]
- Lin, W.T.; Nithiyanantham, S.; Hsieh, D.J.Y.; Chen, R.J.; Day, C.H.; Liao, J.Y.; Kuo, C.H.; Mahalakshmi, B.; Kuo, W.W.; Huang, C.Y. Bioactive peptides attenuate cardiac apoptosis in spontaneously hypertensive rat hearts through activation of autophagy and mitochondrial biogenesis pathway. Environ. Toxicol. 2020, 35, 804–810. [Google Scholar] [CrossRef]
- Nauser, T.D.; Stites, S.W. Diagnosis and treatment of pulmonary hypertension. Am. Fam. Phys. 2001, 63, 1789. [Google Scholar]
- Kundu, B.K.; Zhong, M.; Sen, S.; Davogustto, G.; Keller, S.R.; Taegtmeyer, H. Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: Review of a hypothesis. Cardiology 2015, 130, 211–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Stuewe, S.R.; Gwirtz, P.A.; Agarwal, N.; Mallet, R.T. Exercise training enhances glycolytic and oxidative enzymes in canine ventricular myocardium. J. Mol. Cell. Cardiol. 2000, 32, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Gewehr, D.M.; Giovanini, A.F.; Mattar, B.A.; Agulham, A.P.; Bertoldi, A.D.S.; Nagashima, S.; Kubrusly, F.B.; Kubrusly, L.F. Congestive Hepatopathy Secondary to Right Ventricular Hypertrophy Related to Monocrotaline-Induced Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2021, 22, 11891. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Jackson, K.; Rao, V.S.; Tang, W.W.; Brisco-Bacik, M.A.; Chen, H.H.; Felker, G.M.; Hernandez, A.F.; O’Connor, C.M.; Sabbisetti, V.S. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 2018, 137, 2016–2028. [Google Scholar] [CrossRef]
- Iliev, A.A.; Kotov, G.N.; Dimitrova, I.N.; Landzhov, B.V. Evaluation of structural myocardial changes during chronic hypertensive states in rats. J. Cardiol. Cardiovasc. Sci. 2018, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ju, D.-T.; Kuo, W.-W.; Ho, T.-J.; Chang, R.-L.; Lin, W.-T.; Day, C.H.; Viswanadha, V.V.P.; Liao, P.-H.; Huang, C.-Y. Bioactive peptide VHVV upregulates the long-term memory-related biomarkers in adult spontaneously hypertensive rats. Int. J. Mol. Sci. 2019, 20, 3069. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Weiss, A.; Finkelbrand, S.; Silbermann, M. Age and exercise-related changes in myocardial mitochondria in mice. Acta Histochem. 1988, 83, 81–90. [Google Scholar] [CrossRef]
- Tao, L.; Bei, Y.; Lin, S.; Zhang, H.; Zhou, Y.; Jiang, J.; Chen, P.; Shen, S.; Xiao, J.; Li, X. Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell. Physiol. Biochem. 2015, 37, 162–175. [Google Scholar] [CrossRef]
- Hou, Y.; Yuan, P.; Fu, Y.; Zhang, Q.; Gao, L.; Wei, Y.; Zheng, X.; Feng, W. Geniposide from Gardenia jasminoides var. radicans Makino Attenuates Myocardial Injury in Spontaneously Hypertensive Rats via Regulating Apoptotic and Energy Metabolism Signalling Pathway. Drug Des. Dev. Ther. 2021, 15, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Daseke II, M.J.; Tenkorang, M.A.; Chalise, U.; Konfrst, S.R.; Lindsey, M.L. Cardiac fibroblast activation during myocardial infarction wound healing: Fibroblast polarization after MI. Matrix Biol. 2020, 91, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Wu, S.-B.; Kau, H.-C.; Wei, Y.-H. Essential role of connective tissue growth factor (CTGF) in transforming growth factor-β1 (TGF-β1)-induced myofibroblast transdifferentiation from Graves’ orbital fibroblasts. Sci. Rep. 2018, 8, 7276. [Google Scholar]
- Reginauld, S.H.; Cannone, V.; Iyer, S.; Scott, C.; Bailey, K.; Schaefer, J.; Chen, Y.; Sangaralingham, S.J.; Burnett, J.C. Differential regulation of ANP and BNP in acute decompensated heart failure: Deficiency of ANP. JACC Heart Fail. 2019, 7, 891–898. [Google Scholar] [CrossRef]
- Yoshimura, M.; Yasue, H.; Ogawa, H. Pathophysiological significance and clinical application of ANP and BNP in patients with heart failure. Can. J. Physiol. Pharmacol. 2001, 79, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Intengan, H.D.; Schiffrin, E.L. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension 2001, 38, 581–587. [Google Scholar] [CrossRef]
- Jurasz, P.; Courtman, D.; Babaie, S.; Stewart, D.J. Role of apoptosis in pulmonary hypertension: From experimental models to clinical trials. Pharmacol. Ther. 2010, 126, 1–8. [Google Scholar] [CrossRef]
- Zha, L.H.; Zhou, J.; Li, T.Z.; Luo, H.; Zhang, M.Q.; Li, S.; Yu, Z.X. NLRC3 inhibits MCT-induced pulmonary hypertension in rats via attenuating PI3K activation. J. Cell. Physiol. 2019, 234, 15963–15976. [Google Scholar] [CrossRef]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef]
- Bathina, S.; Gundala, N.K.; Rhenghachar, P.; Polavarapu, S.; Hari, A.D.; Sadananda, M.; Das, U.N. Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 2020, 51, 492–503. [Google Scholar] [CrossRef]
- Hu, M.; Ye, P.; Liao, H.; Chen, M.; Yang, F. Metformin protects H9C2 cardiomyocytes from high-glucose and hypoxia/reoxygenation injury via inhibition of reactive oxygen species generation and inflammatory responses: Role of AMPK and JNK. J. Diabetes Res. 2016, 2016, 2961954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Zhou, T.; Shen, Q.; Qin, X. Exendin-4 promotes the vascular smooth muscle cell re-differentiation through AMPK/SIRT1/FOXO3a signaling pathways. Atherosclerosis 2018, 276, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-Z.; Meng, F.-J.; Cai, H.-Q.; Diao, X.-B.; Zhang, B.; Bai, X.-P. Ginsenoside Rg3 protects heart against isoproterenol-induced myocardial infarction by activating AMPK mediated autophagy. Cardiovasc. Diagn. Ther. 2020, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.C.; Queliconi, B.B.; Bozi, L.H.; Bechara, L.R.; Dourado, P.M.; Andres, A.M.; Jannig, P.R.; Gomes, K.M.; Zambelli, V.O.; Rocha-Resende, C. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 2017, 13, 1304–1317. [Google Scholar] [CrossRef] [Green Version]
- Baskaran, R.; Poornima, P.; Huang, C.Y.; Padma, V.V. Neferine prevents NF-κB translocation and protects muscle cells from oxidative stress and apoptosis induced by hypoxia. Biofactors 2016, 42, 407–417. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, J.-H.; Baskaran, R.; Wang, M.-F.; Yang, H.-S.; Lo, Y.-H.; Mohammedsaleh, Z.M.; Lin, W.-T. Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats. Pharmaceuticals 2022, 15, 819. https://doi.org/10.3390/ph15070819
Ho J-H, Baskaran R, Wang M-F, Yang H-S, Lo Y-H, Mohammedsaleh ZM, Lin W-T. Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats. Pharmaceuticals. 2022; 15(7):819. https://doi.org/10.3390/ph15070819
Chicago/Turabian StyleHo, Jou-Hsuan, Rathinasamy Baskaran, Ming-Fu Wang, Hong-Siang Yang, Yun-Hsin Lo, Zuhair M. Mohammedsaleh, and Wan-Teng Lin. 2022. "Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats" Pharmaceuticals 15, no. 7: 819. https://doi.org/10.3390/ph15070819
APA StyleHo, J.-H., Baskaran, R., Wang, M.-F., Yang, H.-S., Lo, Y.-H., Mohammedsaleh, Z. M., & Lin, W.-T. (2022). Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats. Pharmaceuticals, 15(7), 819. https://doi.org/10.3390/ph15070819






