Adverse Drug Reactions in Relation to Clozapine Plasma Levels: A Systematic Review
Abstract
1. Introduction
2. Results
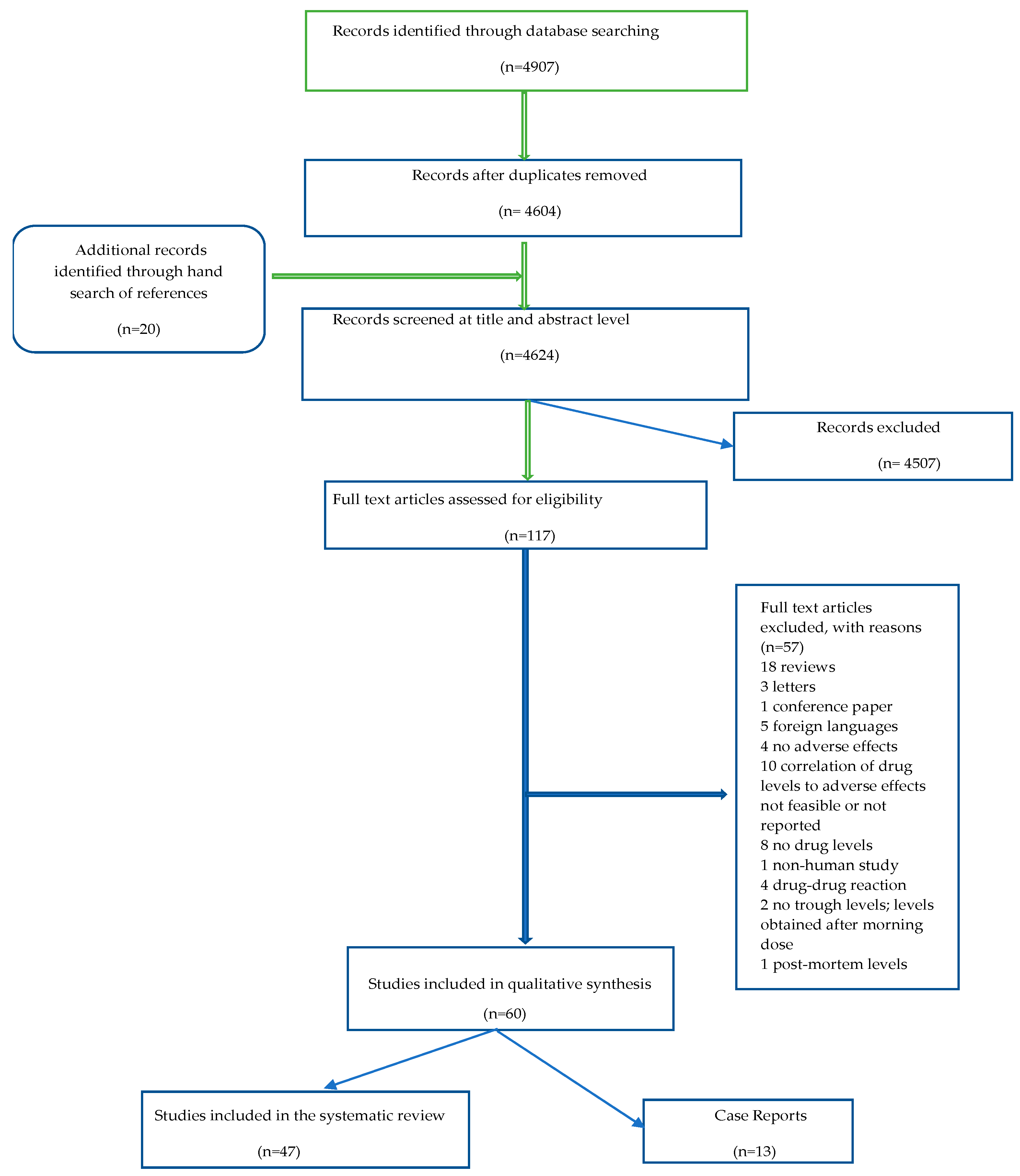
2.1. General Considerations during Literature Search and Evaluation
2.2. Method of Plasma Clozapine Measurement
2.3. Overall or Combined Adverse Effects
2.4. Nervous System Adverse Effects
2.4.1. Εlectroencephalographic (EEG) Abnormalities and Seizures
2.4.2. Cognition
2.4.3. Extrapyramidal Side Effects
2.5. Psychiatric Adverse Effects
2.6. Cardiovascular System
2.6.1. QTc Prolongation
2.6.2. Sudden Death, Myocardial Function, Myocarditis, and Pericarditis
2.6.3. Pulse and Blood Pressure Irregularities
2.7. Metabolic Adverse Effects
2.7.1. Weight Gain
2.7.2. Dyslipidemia
2.7.3. Hyperglycemia, Hyperinsulinemia, Insulin Resistance
2.8. Endocrine System
2.9. Gastrointestinal System
2.9.1. Hypersalivation, Nocturnal Sialorrhea, Drooling
2.9.2. Hepatotoxicity
2.9.3. Constipation
2.10. Hematological System
2.11. Genitourinary System
2.12. Other Adverse Effects
3. Discussion
4. Materials and Methods
4.1. Protocol and Registration
4.2. Eligibility Criteria
4.3. Search Strategy and Study Selection
4.4. Data Collection Process
4.5. Outcomes
4.6. Evaluation of Study Quality Using the Jadad Scoring System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miles, S.H. The Hippocratic Oath and the Ethics of Medicine; Oxford University Press: Oxford, UK, 2005; 232p. [Google Scholar]
- Grandjean, P. Paracelsus Revisited: The Dose Concept in a Complex World. Basic Clin. Pharmacol. Toxicol. 2016, 119, 126. [Google Scholar] [CrossRef]
- Hayes, R.D.; Downs, J.; Chang, C.K.; Jackson, R.G.; Shetty, H.; Broadbent, M.; Hotopf, M.; Stewart, R. The effect of clozapine on premature mortality: An assessment of clinical monitoring and other potential confounders. Schizophr. Bull. 2015, 41, 644–655. [Google Scholar] [CrossRef]
- Cho, J.; Hayes, R.D.; Jewell, A.; Kadra, G.; Shetty, H.; MacCabe, J.H.; Downs, J. Clozapine and all-cause mortality in treatment-resistant schizophrenia: A historical cohort study. Acta Psychiatr. Scand. 2019, 139, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Bente, D.; Engelmeier, M.P.; Heinrich, K.; Hippius, H.; Schmitt, W. Clinical studies with a neuroleptically active dibenzothiazepine derivative. Arzneim.-Forsch. Drug Res. 1966, 16, 314–316. [Google Scholar]
- Ereshefsky, L.; Watanabe, M.D.; Tran-Johnson, T.K. Clozapine: An atypical antipsychotic agent. Clin. Pharm. 1989, 8, 691–709. [Google Scholar] [PubMed]
- Shen, W.W. A history of antipsychotic drug development. Compr. Psychiatry 1999, 40, 407–414. [Google Scholar] [CrossRef]
- Lobos, C.A.; Komossa, K.; Rummel-Kluge, C.; Hunger, H.; Schmid, F.; Schwarz, S.; Leucht, S. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2010, 83, CD006633. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; De Bartolomeis, A.; Van Beveren, N.J.M.; Birnbaum, M.L.; Bloomfield, M.A.P.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-ResistantSchizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef]
- Kane, J.M.; Agid, O.; Baldwin, M.L.; Howes, O.; Lindenmayer, J.P.; Marder, S.; Olfson, M.; Potkin, S.G.; Correll, C.U. Clinical Guidance on the Identification and Management of Treatment-Resistant Schizophrenia. J. Clin. Psychiatry 2019, 80, 2783. [Google Scholar] [CrossRef]
- Rubio, J.M.; Kane, J.M. How and when to use clozapine. Acta Psychiatr. Scand. 2020, 141, 178–189. [Google Scholar] [CrossRef]
- WHOCC—ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 20 June 2022).
- Daskalakis, Z.J.; George, T.P. Clozapine, GABAB, and the treatment of resistant schizophrenia. Clin. Pharmacol. Ther. 2009, 86, 442–446. [Google Scholar] [CrossRef]
- Woods, A.S. The dopamine D4 receptor, the ultimate disordered protein. J. Recept. Signal Transduct. Res. 2010, 30, 331. [Google Scholar] [CrossRef]
- Nair, P.C.; McKinnon, R.A.; Miners, J.O.; Bastiampillai, T. Binding of clozapine to the GABA B receptor: Clinical and structural insights. Mol. Psychiatry 2020, 25, 1910–1919. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant properties of second-generation antipsychotics: Focus on microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef]
- Young, C.R.; Bowers, M.B.; Manure, C.M. Management of the Adverse Effects of Clozapine. Schizophr. Bull. 1998, 24, 381–390. [Google Scholar] [CrossRef]
- Pirmohamed, M.; Williams, D.; Madden, S.; Templeton, E.; Park, B.K. Metabolism and bioactivation of clozapine by human liver in vitro. J. Pharmacol. Exp. Ther. 1995, 272, 984–990. [Google Scholar]
- Dragovic, S.; Gunness, P.; Ingelman-Sundberg, M.; Vermeulen, N.P.E.; Commandeur, J.N.M. Characterization of human cytochrome P450s involved in the bioactivation of clozapines. Drug Metab. Dispos. 2013, 41, 651–658. [Google Scholar] [CrossRef]
- Tóth, K.; Csukly, G.; Sirok, D.; Belic, A.; Kiss, Á.; Háfra, E.; Déri, M.; Menus, Á.; Bitter, I.; Monostory, K. Potential Role of Patients’ CYP3A-Status in Clozapine Pharmacokinetics. Int. J. Neuropsychopharmacol. 2017, 20, 529–537. [Google Scholar] [CrossRef]
- Brandl, E.J.; Chowdhury, N.I.; Tiwari, A.K.; Lett, T.A.P.; Meltzer, H.Y.; Kennedy, J.L.; Müller, D.J. Genetic variation in CYP3A43 is associated with response to antipsychotic medication. J. Neural Transm. 2015, 122, 29–34. [Google Scholar] [CrossRef]
- Zhang, W.V.; D’Esposito, F.; Edwards, R.J.; Ramzan, I.; Murray, M. Interindividual variation in relative CYP1A2/3A4 phenotype influences susceptibility of clozapine oxidation to cytochrome P450-specific inhibition in human hepatic microsomes. Drug Metab. Dispos. 2008, 36, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Olesen, O.V.; Linnet, K. Contributions of five human cytochrome P450 isoforms to the N-demethylation of clozapine in vitro at low and high concentrations. J. Clin. Pharmacol. 2001, 41, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, R.; Lamnisos, D.; Giannakou, K. Anticholinergic Burden and Cognitive Performance in Patients with Schizophrenia: A Systematic Literature Review. Front. Psychiatry 2021, 12, 779607. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Edman, G.; Bertilsson, L.; Hukic, D.S.; Lavebratt, C.; Eriksson, S.V.; Ösby, U. Genetic and clinical factors affecting plasma clozapine concentration. Prim. Care Companion J. Clin. Psychiatry 2015, 17. [Google Scholar] [CrossRef]
- Albitar, O.; Harun, S.N.; Zainal, H.; Ibrahim, B.; Maisharah, S.; Ghadzi, S.; Baynes, R.E. Population Pharmacokinetics of Clozapine: A Systematic Review. BioMed Res. Int. 2020, 2020, 9872936. [Google Scholar] [CrossRef]
- Suzuki, T.; Uchida, H.; Watanabe, K.; Kashima, H. Factors associated with response to clozapine in schizophrenia: A review. Psychopharmacol. Bull. 2011, 44, 32–60. [Google Scholar]
- Greenwood-Smith, C.; Lubman, D.I.; Castle, D.J. Serum clozapine levels: A review of their clinical utility. J. Psychopharmacol. 2003, 17, 234–238. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Honarparvar, F.; Falconer, J.R.; Parekh, H.S.; Pandey, P.; Siskind, D.J. A systematic review and meta-analysis of the association between clozapine and norclozapine serum levels and peripheral adverse drug reactions. Psychopharmacology 2021, 238, 615–637. [Google Scholar] [CrossRef]
- Myles, N.; Myles, H.; Xia, S.; Large, M.; Kisely, S.; Galletly, C.; Bird, R.; Siskind, D. Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr. Scand. 2018, 138, 101–109. [Google Scholar] [CrossRef]
- Varma, S.; Bishara, D.; Besag, F.M.C.; Taylor, D. Clozapine-related EEG changes and seizures: Dose and plasma-level relationships. Ther. Adv. Psychopharmacol. 2011, 1, 47–66. [Google Scholar] [CrossRef]
- De Leon, J.; Ruan, C.J.; Schoretsanitis, G.; De Las Cuevas, C. A Rational Use of Clozapine Based on Adverse Drug Reactions, Pharmacokinetics, and Clinical Pharmacopsychology. Psychother. Psychosom. 2020, 89, 200–214. [Google Scholar] [CrossRef]
- Freudenreich, O.; Weiner, R.D.; McEvoy, J.P. Clozapine-induced electroencephalogram changes as a function of clozapine serum levels. Biol. Psychiatry 1997, 42, 132–137. [Google Scholar] [CrossRef]
- Olesen, O.V.; Thomsen, K.; Jensen, P.N.; Rosenberg, R.; Wulff, C.H.; Rasmussen, N.A.; Refshammer, C.; Bysted, M.; Sørensen, J.; Christensen, J. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: A cross-sectional study. Psychopharmacology 1995, 117, 371–378. [Google Scholar] [CrossRef]
- Combs, M.D.; Perry, P.J.; Bever, K.A. N-desmethylclozapine, an insensitive marker of clozapine-induced agranulocytosis and granulocytopenia. Pharmacotherapy 1997, 17, 1300–1304. [Google Scholar] [CrossRef]
- Kola Oyewumi, L.; Cernovsky, Z.Z.; Freeman, D.J.; Streiner, D.L. Relation of blood counts during clozapine treatment to serum concentrations of clozapine and nor-clozapine. Can. J. Psychiatry 2002, 47, 257–261. [Google Scholar] [CrossRef]
- Mauri, M.C.; Rudelli, R.; Bravin, S.; Gianetti, S.; Giuliani, E.; Guerrini, A.; Orlandi, R.; Invernizzi, G. Clozapine metabolism rate as a possible index of drug-induced granulocytopenia. Psychopharmacology 1998, 137, 341–344. [Google Scholar] [CrossRef]
- VanderZwaag, C.; McGee, M.; McEvoy, J.P.; Freudenreich, O.; Wilson, W.H.; Cooper, T.B. Response of patients with treatment-refractory schizophrenia to clozapine within three serum level ranges. Am. J. Psychiatry 1996, 153, 1579–1584. [Google Scholar] [CrossRef]
- Sporn, A.L.; Vermani, A.; Greenstein, D.K.; Bobb, A.J.; Spencer, E.P.; Clasen, L.S.; Tossell, J.W.; Stayer, C.C.; Gochman, P.A.; Lenane, M.C.; et al. Clozapine treatment of childhood-onset schizophrenia: Evaluation of effectiveness, adverse effects, and long-term outcome. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1349–1356. [Google Scholar] [CrossRef]
- Centorrino, F.; Baldessarini, R.J.; Kando, J.C.; Frankenburg, F.; Volpicelli, S.A.; Flood, J.G. Clozapine and metabolites: Concentrations in serum and clinical findings during treatment of chronically psychotic patients. J. Clin. Psychopharmacol. 1994, 14, 119–125. [Google Scholar] [CrossRef]
- Oi-Yin Wong, J.; Leung, S.P.; Mak, T.; Man-Kin Ng, R.; Chan, K.T.; Hon-Kee Cheung, H.; Choi, W.K.; Lai, J.; Wai-Kiu Tsang, A. Plasma clozapine levels and clinical response in treatment-refractory Chinese schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 251–264. [Google Scholar] [CrossRef]
- Frazier, J.A.; Cohen, L.G.; Jacobsen, L.; Grothe, D.; Flood, J.; Baldessarini, R.J.; Piscitelli, S.; Kim, G.S.; Rapoport, J.L. Clozapine pharmacokinetics in children and adolescents with childhood-onset schizophrenia. J. Clin. Psychopharmacol. 2003, 23, 87–91. [Google Scholar] [CrossRef]
- Ackenheil, M. Clozapine—Pharmacokinetic investigations and biochemical effects in man. Psychopharmacology 1989, 99, S32–S37. [Google Scholar] [CrossRef]
- Melkersson, K.I.; Scordo, M.G.; Gunes, A.; Dahl, M.L. Impact of CYP1A2 and CYP2D6 polymorphisms on drug metabolism and on insulin and lipid elevations and insulin resistance in clozapine-treated patients. J. Clin. Psychiatry 2007, 68, 697–704. [Google Scholar] [CrossRef]
- Spina, E.; Avenoso, A.; Facciolà, G.; Scordo, M.G.; Ancione, M.; Madia, A.G.; Ventimiglia, A.; Perucca, E. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology 2000, 148, 83–89. [Google Scholar] [CrossRef]
- Kim, H.S.; Youn, T.; Kim, S.H.; Jeong, S.H.; Jung, H.Y.; Jeong, S.W.; Kim, K.K.; Kim, Y.S.; Chung, I.W. Association between electroencephalogram changes and plasma clozapine levels in clozapine-treated patients. Int. Clin. Psychopharmacol. 2019, 34, 131–137. [Google Scholar] [CrossRef]
- Nilsson, B.M.; Lindström, L.; Mohsen, I.; Holmlöv, K.; Bodén, R. Persistent tachycardia in clozapine treated patients: A 24-hour ambulatory electrocardiogram study. Schizophr. Res. 2018, 199, 403–406. [Google Scholar] [CrossRef]
- Melkersson, K.I.; Hulting, A.L.; Brismar, K.E. Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. J. Clin. Psychiatry 1999, 60, 783–791. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Perry, E.; Jayathilake, K. Clozapine-induced weight gain predicts improvement in psychopathology. Schizophr. Res. 2003, 59, 19–27. [Google Scholar] [CrossRef]
- Vasudev, K.; Choi, Y.H.; Norman, R.; Kim, R.B.; Schwarz, U.I. Genetic Determinants of Clozapine-Induced Metabolic Side Effects. Can. J. Psychiatry. 2017, 62, 138–149. [Google Scholar] [CrossRef]
- Hummer, M.; Kurz, M.; Kurzthaler, I.; Oberbauer, H.; Miller, C.; Wolfgang Fleischhacker, W. Hepatotoxicity of clozapine. J. Clin. Psychopharmacol. 1997, 17, 314–317. [Google Scholar] [CrossRef]
- Every-Palmer, S.; Nowitz, M.; Stanley, J.; Grant, E.; Huthwaite, M.; Dunn, H.; Ellis, P.M. Clozapine-treated Patients Have Marked Gastrointestinal Hypomotility, the Probable Basis of Life-threatening Gastrointestinal Complications: A Cross Sectional Study. EBioMedicine 2016, 5, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Varma, S.; Ahmad, N.; Gee, S.; Taylor, D.M. Factors predicting use of laxatives in outpatients stabilized on clozapine. Ther. Adv. Psychopharmacol. 2015, 5, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, R.; Xiao, H.; Zhou, Q.; Cui, Q.; Chen, J. Higher serum clozapine level is associated with increased antiphospholipid antibodies in schizophrenia patients. J. Psychiatr. Res. 2009, 43, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Yusufi, B.; Mukherjee, S.; Flanagan, R.; Paton, C.; Dunn, G.; Page, E.; Barnes, T.R.E. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int. Clin. Psychopharmacol. 2007, 22, 238–243. [Google Scholar] [CrossRef]
- Hummer, M.; Kemmler, G.; Kurz, M.; Kurzthaler, I.; Oberbauer, H.; Fleischhacker, W.W. Sexual disturbances during clozapine and haloperidol treatment for schizophrenia. Am. J. Psychiatry 1999, 156, 631–633. [Google Scholar] [CrossRef]
- Haring, C.; Neudorfer, C.; Schwitzer, J.; Hummer, M.; Saria, A.; Hinterhuber, H.; Fleischhacker, W.W. EEG alterations in patients treated with clozapine in relation to plasma levels. Psychopharmacology 1994, 114, 97–100. [Google Scholar] [CrossRef]
- Rajji, T.K.; Uchida, H.; Ismail, Z.; Ng, W.; Mamo, D.C.; Remington, G.; Pollock, B.G.; Mulsant, B.H. Clozapine and global cognition in schizophrenia. J. Clin. Psychopharmacol. 2010, 30, 431–436. [Google Scholar] [CrossRef]
- Lin, S.K.; Su, S.F.; Pan, C.H. Higher plasma drug concentration in clozapine-treated schizophrenic patients with side effects of obsessive/compulsive symptoms. Ther. Drug Monit. 2006, 28, 303–307. [Google Scholar] [CrossRef]
- Pinto, D.; Pagnamenta, A.T.; Klei, L.; Anney, R.; Merico, D.; Regan, R.; Conroy, J.; Magalhaes, T.R.; Correia, C.; Abrahams, B.S.; et al. Functional Impact of Global Rare Copy Number Variation in Autism Spectrum Disorder. Nature 2010, 466, 368. [Google Scholar] [CrossRef]
- De Leon, J.; Odom-White, A.; Josiassen, R.C.; Diaz, F.J.; Cooper, T.B.; Simpson, G.M. Serum antimuscarinic activity during clozapine treatment. J. Clin. Psychopharmacol. 2003, 23, 336–341. [Google Scholar] [CrossRef]
- De Leon, J.; Diaz, F.J.; Josiassen, R.C.; Cooper, T.B.; Simpson, G.M. Weight gain during a double-blind multidosage clozapine study. J. Clin. Psychopharmacol. 2007, 27, 22–27. [Google Scholar] [CrossRef]
- Oyewumi, L.K.; Cernovsky, Z.Z.; Freeman, D.J. Autonomic signs and dosing during the initial stages of clozapine therapy. Med. Sci. Monit. 2004, 10, 19–23. [Google Scholar]
- Subramaniam, M.; Ng, C.; Chong, S.-A.; Mahendran, R.; Lambert, T.; Pek, E.; Huak, C.Y. Metabolic differences between Asian and Caucasian patients on clozapine treatment. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 217–222. [Google Scholar] [CrossRef]
- De Leon, J.; Diaz, F.J.; Josiassen, R.C.; Simpson, G.M. Possible individual and gender differences in the small increases in plasma prolactin levels seen during clozapine treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 318–325. [Google Scholar] [CrossRef]
- Vaquero-Baez, M.; Diáz-Ruíz, A.; Tristán-López, L.; Avinã-Cervantes, C.; Torner, C.; Ramírez-Bermúdez, J.; Montes, S.; Riós, C. Clozapine and desmethylclozapine: Correlation with neutrophils and leucocytes counting in Mexican patients with schizophrenia. BMC Psychiatry 2019, 19, 295. [Google Scholar] [CrossRef]
- Carceller-Sindreu, M.; Portella, M.J.; Carmona, C.; Rametti, G.; Puigdemont, D.; Figueras, M.; Fernández-Vidal, A.; Villalta, L.; Alvarez, E. Neuropsychological effects of maintenance treatment with Clozapine in treatment-resistant psychotic disorder. Actas Esp. Psiquiatr. 2014, 42, 68–73. [Google Scholar]
- Hummer, M.; Kurz, M.; Barnas, C.; Saria, A.; Fleischhacker, W.W. Clozapine-induced transient white blood count disorders. J. Clin. Psychiatry 1994, 55, 429–432. [Google Scholar]
- Melkersson, K.I.; Dahl, M.L. Relationship between levels of insulin or triglycerides and serum concentrations of the atypical antipsychotics clozapine and olanzapine in patients on treatment with therapeutic doses. Psychopharmacology 2003, 170, 157–166. [Google Scholar] [CrossRef]
- Lu, M.L.; Lane, H.Y.; Lin, S.K.; Chen, K.P.; Chang, W.H. Adjunctive fluvoxamine inhibits clozapine-related weight gain and metabolic disturbances. J. Clin. Psychiatry 2004, 65, 766–771. [Google Scholar] [CrossRef]
- Rechlin, T.; Beck, G.; Weis, M.; Kaschka, W.P. Correlation between plasma clozapine concentration and heart rate variability in schizophrenic patients. Psychopharmacology 1998, 135, 338–341. [Google Scholar] [CrossRef]
- Eschweiler, G.W.; Bartels, M.; Längle, G.; Wild, B.; Gaertner, I.; Nickola, M. Heart-rate variability (HRV) in the ECG trace of routine EEGs: Fast monitoring for the anticholinergic effects of clozapine and olanzapine? Pharmacopsychiatry 2002, 35, 96–100. [Google Scholar] [CrossRef]
- Anderson, S.G.; Livingston, M.; Couchman, L.; Smith, D.J.; Connolly, M.; Miller, J.; Flanagan, R.J.; Heald, A.H. Sex differences in plasma clozapine and norclozapine concentrations in clinical practice and in relation to body mass index and plasma glucose concentrations: A retrospective survey. Ann. Gen. Psychiatry 2015, 14, 39. [Google Scholar] [CrossRef]
- Seppälä, N.; Leinonen, E.; Viikki, M.; Solismaa, A.; Nuolivirta, T.; Kampman, O. Factors associated with subjective side-effects during clozapine treatment. Nord. J. Psychiatry 2015, 69, 161–166. [Google Scholar] [CrossRef]
- Curto, M.; Comparelli, A.; Ciavarella, G.M.; Gasperoni, C.; Lionetto, L.; Corigliano, V.; Uccellini, A.; Mancinelli, I.; Ferracuti, S.; Girardi, P.; et al. Impairment of left ventricular function early in treatment with clozapine: A preliminary study. Int. Clin. Psychopharmacol. 2015, 30, 282–289. [Google Scholar] [CrossRef]
- Kamil Gharab, K.M.; Onmaz, D.E.; Abusoglu, S.; Aydin, M.; Sivrikaya, A.; Tok, O.; Abusoglu, G.; Unlu, A. The relationship between serum clozapine concentrations and hematological parameters by a validated mass spectrometric method. J. Pharm. Biomed. Anal. 2020, 180, 113056. [Google Scholar] [CrossRef]
- Smith, R.L.; Haslemo, T.; Andreassen, O.A.; Eliasson, E.; Dahl, M.L.; Spigset, O.; Molden, E. Correlation between Serum Concentrations of N-Desmethylclozapine and Granulocyte Levels in Patients with Schizophrenia: A Retrospective Observational Study. CNS Drugs 2017, 31, 991–997. [Google Scholar] [CrossRef]
- Grande, I.; Pons, A.; Baeza, I.; Torras, Ú.; Bernardo, M. QTc prolongation: Is clozapine safe? Study of 82 cases before and after clozapine treatment. Hum. Psychopharmacol. 2011, 26, 397–403. [Google Scholar] [CrossRef]
- Khan, A.A.; Ashraf, A.; Baker, D.; Al-Omary, M.S.; Savage, L.; Ekmejian, A.; Singh, R.S.H.; Brienesse, S.; Majeed, T.; Gordon, T.; et al. Clozapine and incidence of myocarditis and sudden death—Long term Australian experience. Int. J. Cardiol. 2017, 238, 136–139. [Google Scholar] [CrossRef]
- Lally, J.; Gallagher, A.; Bainbridge, E.; Avalos, G.; Ahmed, M.; McDonald, C. Increases in triglyceride levels are associated with clinical response to clozapine treatment. J. Psychopharmacol. 2013, 27, 401–403. [Google Scholar] [CrossRef]
- Liu, H.C.; Chang, W.H.; Wei, F.C.; Lin, S.K.; Lin, S.K.; Jann, M.W. Monitoring of plasma clozapine levels and its metabolites in refractory schizophrenic patients. Ther. Drug Monit. 1996, 18, 200–207. [Google Scholar] [CrossRef]
- Lieberman, J.; Johns, C.; Cooper, T.; Pollack, S.; Kane, J. Clozapine pharmacology and tardive dyskinesia. Psychopharmacology 1989, 99, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Aymard, N.; Baldacci, C.; Leyris, A.; Smagghe, P.O.; Tribolet, S.; Vacheron, M.N.; Viala, A.; Caroli, F. Neuroleptic-resistant schizophrenic patients treated by clozapine: Clinical evolution, plasma and red blood cell clozapine and desmethylclozapine levels. Therapie 1997, 52, 227–232. [Google Scholar] [PubMed]
- Fitzsimons, J.; Berk, M.; Lambert, T.; Bourin, M.; Dodd, S. A review of clozapine safety. Expert Opin. Drug Saf. 2005, 4, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Prescriber’s Guide: Stahl’s Essential Psychopharmacology, 6th ed.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Rechlin, T.; Claus, D.; Weis, M. Heart rate variability in schizophrenic patients and changes of autonomic heart rate parameters during treatment with clozapine. Biol. Psychiatry 1994, 35, 888–892. [Google Scholar] [CrossRef]
- Davydov, L.; Botts, S.R. Clozapine-induced hypersalivation. Ann. Pharmacother. 2000, 662. [Google Scholar] [CrossRef]
- Pirmohamed, M.; Park, K. Mechanism of clozapine-induced agranulocytosis: Current status of research and implications for drug development. CNS Drugs 1997, 7, 139–158. [Google Scholar] [CrossRef]
- Bergemann, N.; Abu-Tair, F.; Aderjan, R.; Kopitz, J. High clozapine concentrations in leukocytes in a patient who developed leukocytopenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1068–1071. [Google Scholar] [CrossRef]
- Kim, D.D.; Barr, A.M.; White, R.F.; Honer, W.G.; Procyshyn, R.M. Clozapine-induced obsessive-compulsive symptoms: Mechanisms and treatment. J. Psychiatry Neurosci. 2019, 44, 71–72. [Google Scholar] [CrossRef]
- Citrome, L.; McEvoy, J.P.; Saklad, S.R. A guide to the management of clozapine-related tolerability and safety concerns. Clin. Schizophr. Relat. Psychoses 2016, 10, 163–177D. [Google Scholar] [CrossRef]
- Wiciński, M.; Wȩclewicz, M.M. Clozapine-induced agranulocytosis/granulocytopenia: Mechanisms and monitoring. Curr. Opin. Hematol. 2018, 25, 22–28. [Google Scholar] [CrossRef]
- Mann, C.J. Observational research methods. Research design II: Cohort, cross sectional, and case-control studies. Emerg. Med. J. 2003, 20, 54–60. [Google Scholar] [CrossRef]
- Verster, J.C.; van de Loo, A.J.A.E.; Adams, S.; Stock, A.K.; Benson, S.; Scholey, A.; Alford, C.; Bruce, G. Advantages and Limitations of Naturalistic Study Designs and Their Implementation in Alcohol Hangover Research. J. Clin. Med. 2019, 8, 2160. [Google Scholar] [CrossRef]
- Whitley, H.P. Sex-Based Differences in Drug Activity. Am. Fam. Physician 2009, 80, 1254–1258. [Google Scholar]
- Lavan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11. [Google Scholar] [CrossRef]
- Kim, K.; Johnson, J.A.; Derendorf, H. Differences in Drug Pharmacokinetics Between East Asians and Caucasians and the Role of Genetic Polymorphisms. J. Clin. Pharmacol. 2004, 44, 1083–1105. [Google Scholar] [CrossRef]
- Siskind, D.; Sidhu, A.; Cross, J.; Chua, Y.T.; Myles, N.; Cohen, D.; Kisely, S. Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy. Aust. N. Z. J. Psychiatry 2020, 54, 467–481. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Simpson, G.M.; Cooper, T.A. Clozapine Plasma Levels and Convulsions. Am. J. Psychiatry 1978, 135, 99–100. [Google Scholar] [CrossRef]
- Khan, A.Y.; Preskorn, S.H. Examining Concentration-Dependent Toxicity of Clozapine: Role of Therapeutic Drug Monitoring. J. Psychiatr. Pract. 2005, 11, 289–301. [Google Scholar] [CrossRef]
- McCollum, B.; Barclay, J.; de Leon, J. Unexpected Falls during Clozapine Treatment Explained by Myoclonus. Prim. Care Companion CNS Disord. 2018, 20, 26969. [Google Scholar] [CrossRef]
- Zink, M.; Knopf, U.; Kuwilsky, A. Management of Clozapine-Induced Obsessive-Compulsive Symptoms in a Man with Schizophrenia. Aust. N. Z. J. Psychiatry 2007, 41, 293–294. [Google Scholar] [CrossRef]
- Cadeddu, G.; Deidda, A.; Stochino, M.E.; Velluti, N.; Burrai, C.; Del Zompo, M. Clozapine Toxicity Due to a Multiple Drug Interaction: A Case Report. J. Med. Case Rep. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Pieroni, M.; Cavallaro, R.; Chimenti, C.; Smeraldi, E.; Frustaci, A. Clozapine-Induced Hypersensitivity Myocarditis. Chest 2004, 126, 1703–1705. [Google Scholar] [CrossRef]
- Flanagan, R.J.; Ball, R.Y. Gastrointestinal Hypomotility: An under-Recognised Life-Threatening Adverse Effect of Clozapine. Forensic Sci. Int. 2011, 206, e31–e36. [Google Scholar] [CrossRef]
- Chopra, A.; Rai, A.; Philbrick, K.; Das, P. A Dangerous GI Complication. Curr. Psychiatr. 2011, 10, 68–72. [Google Scholar]
- Pelizza, L.; De Luca, P.; La Pesa, M.; Borella, D. Clozapine-Induced Intestinal Occlusion: A Serious Side Effect. Acta Biomed. l’Ateneo Parm. 2007, 78, 144–148. [Google Scholar]
- Abou Farha, K.; van Vliet, A.; Knegtering, H.; Bruggeman, R. The Value of Desmethylclozapine and Serum CRP in Clozapine Toxicity: A Case Report. Case Rep. Psychiatry 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Haack, S.; Seeringer, A.; Thürmann, P.A.; Becker, T.; Kirchheiner, J. Sex-Specific Differences in Side Effects of Psychotropic Drugs: Genes or Gender? Pharmacogenomics 2009, 10, 1511–1526. [Google Scholar] [CrossRef]
- Yadav, D.; Burton, S.; Sehgal, C. Clozapine-Induced Neutropenia Reversed by Lithium. Prog. Neurol. Psychiatry 2016, 20, 13–15. [Google Scholar] [CrossRef]
| Methods for Measurement of Plasma CLOZ Levels | References |
|---|---|
| High-Performance Liquid Chromatography (HPLC) | Combs et al. [36], Carceller-Sindreu et al. [68], Olesen et al. [35], Oyewumi et al. [37], Hummer et al. [69], Rajii et al. [59], Wong et al. [42], Oyewumi et al. [64], Melkersson et al. [49], Yusufi et al. [56], Spina et al. [46], Melkersson and Dahl [70], Melkersson et al. [45], Lu et al. [71], Rechlin et al. [72], Eschweiler et al. [73], Mauri et al. [38], Vaquero-Baez et al. [67], Anderson et al. [74] |
| Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) | Haring et al. [58], Lin et al. [60], Shen et al. [55] |
| Liquid Chromatography (LC) | Seppala et al. [75], Kim et al. [47] |
| Reversed-Phase Liquid Chromatography | Centorrino et al. [41], Frazier et al. [43] |
| Gas Chromatography (GC) | Ackenheil [44], De Leon et al. [66], De Leon et al. [62], De Leon et al. [63], Freudenreich et al. [34], VanderZwaag et al. [39] |
| Liquid Chromatography coupled with Mass Spectrometry (LC-MS) | Curto et al. [76], Vasudev et al. [51] |
| Liquid Chromatography coupled with tandem Mass Spectrometry (LC-MS/MS) | Gharab et al. [77], Smith et al. [78] |
| Not specified | Hummer et al. [52], Every-Palmer et al. [53], Grande et al. [79], Sporn et al. [40], Khan et al. [80], Subramaniam et al. [65], Lally et al. [81], Nilsson et al. [48], Meltzer et al. [50], Bailey et al. [54], Hummer et al. [57] |
| Reference | Type of Study | Age (Mean) (Years) | Comedication | Averaged Serum Levels of Clozapine (ng/mL) | Duration of CLOZ Exposure | Reported Side Effects | Correlation to CLOZ Plasma Levels | Jadad Score |
|---|---|---|---|---|---|---|---|---|
| [56] | Cross-sectional | 39.3 ± 8.8 | Mood stabilizer (31%), Anticholinergic (18%), antidepressant (16%), other antipsychotic (5%), anxiolytic or hypnotic (5%) | CLOZ: 530 ± 370, NCLOZ: 310 ± 190 | Median (range): 30 (3–156) months | Parkinsonism, akathisia, tardive dyskinesia, non-neurological side effects including cardiovascular, gastrointestinal, sexual genitourinary and others | CLOZ levels vs. total ANSSERS score: r = 0.29, p < 0.004 CLOZ levels vs. moderate and severe ADRs: r = 0.23, p < 0.03 | 1 |
| [43] | Open-label | 13.3 ± 2.7 (range 9–16) | No concomitant medications | Crude (ng/mL): 289 ± 116, Normalized (ng/mL-mg-Kg): 99 ± 37.3 | 6 weeks | Adverse effects included sedation, enuresis, tachycardia, sialorrhea, reduced neutrophil count, increased hepatic transaminases | Moderate and severe side effects vs. CLOZ + NCLOZ: r = 0.4, p = 0.002) Moderate and severe side effects vs. NCLOZ: r = 0.6, p = 0.002 Moderate and severe side effects vs. CLOZ + NCLOZ + NOX levels: r = 0.4, p = 0.03 | 0 |
| [46] | Prospective, open follow-up | 19–65 | No | CLOZ: 385 ± 183 (range 147–974) NCLOZ: 174 ± 84 (range: 43–445) | 12 weeks | Hypersalivation, constipation, tachycardia, dizziness, sedation, weight gain | No | 1 |
| [41] | Cross-sectional | 36.6 ± 9.1 (range 20–54) | Benzodiazepines, lithium, antidepressants | CLOZ: mean = 297 (median: 291), among 68 samples. Subsample not exposed to fluoxetine or valproate (n = 27): 239 ± 159 | 2.15 ± 2.30 years | SAFTEE scale ADRs | No | 0 |
| Reference | Type of Study | Age (Mean) (Years) | Comedication | Averaged Serum Levels of Clozapine (ng/mL) | Duration | Reported Side Effects | Correlation to Clozapine Plasma Levels | Jadad Score |
|---|---|---|---|---|---|---|---|---|
| [34] | Prospective, randomized | 38 (range: 21–56) | Rarely given doses of haloperidol or fluphenazine | group I (n = 16): 50–150 group II (n = 22): 200–300 group III (n = 12): 350–450 | 12 weeks | Seizures, EEG changes, sleepiness | EEG abnormalities, more severe than borderline, rate: group III: 73% vs. group I: (20%) and group II: 21% (p = 0.006) Severity: group I 0.9 ± 1.8, group II 1.0 ± 1.5, group III 3.4 ± 1.9 (p < 0.001) Spike/sharp activity: no correlation Slowing: more slowing in group III vs. group II and I (p = 0.049), positive correlation with levels (r = 0.44, p = 0.002), possible cutoff: 300 ng/mL Sleepiness: positive correlation with levels (r = 0.33, p = 0.029). The EEG slowing correlated with observed sleepiness | 2 |
| [58] | Prospective, observational | 31.7 ± 10.2 | No | Whole sample (n = 29): 161.3 ± 150.0 group 1 (n = 14): 81.6 ± 64.6, group 2 (n = 15): 235.7 ± 169.8 | 20.2 ± 16.8 days | EEG changes | Plasma levels significantly different among groups according to severity of EEG changes. Group 1 (n = 14): degree 0–1, plasma levels: 81.6 ± 64.6 ng/mL (95% CI = 44.3–118.9). Group 2 (n = 15): degree 2–4, plasma levels: 235.7 ± 169.8 mg.mL (95% CI: 141.7–329.7) (p = 0.0009) | 0 |
| [35] | Cross-sectional, blinded for EEG measures | 37.6 ± 1.67 | Levomepromazine or chlorprothixene for sedation up to 100 mg/day. Other medication as usual | Median CLOZ: 351 (231–615) (range: 64–1824) | 2.5 (1.0–9.0) years | EEG changes | Severity correlated to plasma CLOZ (r = 0.43; p < 0.05) but not NCLOZ levels. CLOZ concentrations ≥1306 ng/mL lead to progressive gradual EEG changes | 0 |
| [39] | Prospective, randomized, double-blind | 3 (range 21–56) | All psychoactive medication tapered off. Valproate in two patients with a history of seizures | Low: 91 ± 15 (50–150), medium: 251 ± 13 (200–300), high: 396 ± 16 (350–450) | 12 weeks | Sleepiness, EPS | Trend of sleepiness and serum level to correlate at week 6 (p = 0.08), but no significance at week 12. EPS improved over time, with no group-by-time interactions. | 2 |
| [40] | Prospective, longitudinal, observational, partly double-blind (n = 22) partly open-label (n = 32) | Range: 8–18 | No | Week 6: CLOZ = 455 ± 285.1, NCLOZ + CLOZ 302.4 ± 142.2 | 6 week treatment at first and then 2–6 years follow-up | EEG changes seizures, akathisia | Rates of side effects were not directly associated with CLOZ or nor CLOZ blood levels or their ratio | 0 |
| [41] | Cross-sectional | 36.6 ± 9.1 (range 20–54) | Benzodiazepines, lithium, antidepressants, other medically indicated agents | CLOZ: mean = 297 (median: 291), among 68 samples. Subsample not exposed to fluoxetine or valproate (n = 27): 239 ± 159 | 2.15 ± 2.30 years | Sedation | No | 0 |
| [42] | Prospective, non-randomized, double-blind, observational | 37.61 ± 8.68 (range 21–63) | Chloral hydrate, Lorazepam, Valproate | Week 6: cloz: 470.20 ± 234.2, range 100–1220 norclozapine: 233.06 ± 105.56, range 70–670, Week 12: cloz 681 ± 390.71, range 220–1920, NCLOZ: 297.8 ± 146.49 range 8–720 | 12 weeks | EEG changes, akathisia, EPS | No significant correlation between plasma levels and EEG abnormalities on week 6, BARS, AIMS, SAS scores on weeks 6 and 12, negative correlation with sedation at week 6, which was clinically implausible | 0 |
| [47] | Retrospective, observational | 37.7 ± 11.7 | Valproate (n = 25, 35.2%) Mood stabilizers (lamotrigine, valproate, lithium, topiramate) (n = 31) Antipsychotics, antidepressants, benzodiazepines, or mood stabilizers in combination (n = 68) | CLOZ: 429.4 ± 264.1 NCLOZ: 197.8 ± 132.6 | 4.6 ± 4.9 years | EEG changes | Positive correlation with CLOZ levels (p = 0.008). No correlation with NCLOZ levels (p = 0.12). Patients with CLOZ levels > 600 ng/mL had higher rate of EEG abnormalities (93.8%) than those with levels <600 ng/mL (65.5%) (p = 0.02) | 0 |
| [75] | Cross-sectional | Age: 42.5 (range: 20–65) | Clozapine monotherapy: 65.4%, CLOZ + atypical: 22.5%, ClOZ + typical: 9.7%, CLOZ + typical + atypical: 1.7% | Men: 722 ± 366, Women: 886 ± 480 (p = 0.03) Total sample: 778 ± 444.57 | 3–12 months (1.7%), 1–5 years (32.5%), 5 years (57.8%), not specified (8%) | Difficulty in concentrating, tension, difficulty remembering things, depression, restlessness, difficulty getting to sleep | Yes, only with the Depression/Anxiety score | 0 |
| [68] | Single-blind, cross-sectional | Group I: 45 ± 10.3, Group II 47.2 ± 7.5 | No | Group I: CLOZ ≥300 Group II: CLOZ <300 | ≥5 years | Cognitive performance | No relationship between clozapine plasma levels and cognitive performance. Tendency to significance regarding the executive test (31% of variability of number of attempts in the WCST was explained by clozapine plasma levels) | 0 |
| [43] | Open-label | 13.3 ± 2.7 (range 9–16) | No | Crude (ng/mL): 289 ± 116, normalized (ng/mL-mg-Kg): 99 ± 37.3 | 6 weeks | Sedation | No | 0 |
| [59] | Retrospective analysis of clinically collected cross-sectional data. | 41.6 ± 12.0 | Not reported | All subjects (n = 73): 458.5 ± 248.8 Low cognitive impairment (n = 57): 437.1 ± 249.6 High cognitive impairment (n = 16): 534.7 ± 237.7 | ≥3 months | Cognitive impairment | Sixteen subjects (21.9%) had high cognitive impairment and the rest had low cognitive impairment. Age and clozapine levels were associated with high cognitive impairment, as well as clozapine/desmethylclozapine raitio (OR: 7.3). Yes | 0 |
| [56] | Cross-sectional | 39.3 ± 8.8 | Mood stabilizer (31%), Anticholinergic (18%), antidepressant (16%) other antipsychotic (5%), anxiolytic or hypnotic (5%) | CLOZ: 530 ± 370, NCLOZ: 310 ± 190 | Median (range): 30 (3–156) months | Memory and concentration problems, night-time sleep problems, Parkinsonism, akathisia, tardive dyskinesia | No correlation | 1 |
| [62] | Double-blind, prospective. Randomized to clozapine doses | Not reported | Haloperidol | End of first trial (16 weeks): 335 ± 340 | First trial: 16 weeks, second trial:16 weeks | Drowsiness, sedation | Clozapine levels were very good predictors of serum antimuscarinic activity in doses of 300 mg/d or higher. Sedation showed no significant association with serum antimuscarinic activity. | 2 |
| [60] | Retrospective, naturalistic, 1-year study/cross-sectional | 37.9 ± 9.3 | Fluvoxamine (n = 8), fluoxetine (n = 2), sertraline (n = 2), paroxetine (n = 1), valproate (n = 15) | Patients with OCS: 595.1 ± 364.9 (range 84–1491) Patients without OCS: 433.5 ± 252.8 (range 82–1273) | Patients with OCS: 81.8 ± 32.2 months, Patients without OCS: 56.1 ± 40.6 months | OCS | Plasma concentration of clozapine was significantly higher in patients with OCS than in those without (p = 0.001) | 0 |
| Reference | Type of Study | Age (Mean) | Comedication | Averaged Serum Levels of Clozapine (ng/mL) | Duration | Reported Side Effects | Correlation to Clozapine Plasma Level | Jadad Score |
|---|---|---|---|---|---|---|---|---|
| [35] | Cross-sectional | 37.6 ± 1.67 | Levomepromazine or chlorprothixene. Other medication as usual | Median S-CLOZ: 351 (231–615) (range: 64–1824) | 2.5 (1.0–9.0) years | Increased pulse rate (>80 bpm), tachycardia (>100 bpm), orthostatic hypotension | No | 0 |
| [39] | Prospective, randomized, double-blind | 38 (range 21–56) | Haloperidol, phluphenazine, valproate | Low: 91 ± 15 (50–150), medium: 251 ± 13 (200–300), high: 396 ± 16 (350–450) | 12 weeks | Tachycardia: Orthostaic hypotension: | No | 2 |
| [40] | Prospective, longitudinal, observational, partly double-blind (n = 22) partly open-label (n = 32) | Range: 8–18 | No | Week 6: CLOZ 455 ± 285.1, NCLOZ 302.4 ± 142.2 | 6 week treatment at first and then 2–6 years follow-up | Hypertension (>140/90 mmHg), orthostatic hypotension, tachycardia (>120 bpm) | No | 0 |
| [42] | Prospective, non-randomized, double-blind, observational | 37.61 ± 8.68 (range 21–63) | Chloral hydrate. Lorazepam. Valproate | Week 6: Clozapine: 470.20 ± 234.23, range 100–1220 Norclozapine: 233.06 ± 105.56, range 70–670, Week 12: clozapine 681 ± 390.71 ng/mL (range 220–1920 ng/mL), NCLOZ 297.8 ± 146.49 ng/mL (range 8–720 ng/mL) | 12 weeks | QTc alterations | No | 0 |
| [44] | Prospective, open-label | Not reported | Not reported | 200–300 ng/mL (divided into three groups I: <30 ng/mL, II: 30–100 ng/mL, III: >100 ng/mL for paranoid-hallucinatory schizophrenic patients and manic syndrome) | 30 days | Orthostatic hypontension, temperature | Yes, with severity of orthostatic dysregulation | 0 |
| [43] | Open-label | 13.3 ± 2.7 (range 9–16) | No | Crude (ng/mL): 289 ± 116, Normalized (ng/mL-mg-Kg): 99 ± 37.3 | 6 weeks | Tachycardia | No | 0 |
| [64] | Prospective longitudinal | Not reported | Lorazepam allowed | Mean serum CLOZ level by week (1–8): 63.5 ± 46.0 199.3 ± 149.1 251.9 ± 178.0 300.7 ± 200.2 316.2 ± 189.4 364.7 ± 195.6 351.9 ± 176.2 379.5 ± 156.5 | First 8 weeks of administration | Hypotension, temperature, pulse | The blood pressure and pulse did not change significantly from baseline to week 8. Temperature was inversely related to clozapine dose (p < 0.003) Higher norclozapine to clozapine ratios were associated with higher BP measures (p = 0.002). The magnitude of these relationships is weak (r < 0.30). There is a tendency to autonomic dysregulation during clozapine use | 0 |
| [80] | Cohort, prospective study, open | 44 ± 12 | Not reported | 475 ± 236 | Mean follow-up of 9 ± 6 years | Myocarditis, sudden death | No | 0 |
| [79] | Retrospective, review of case records | 31.21 ± 9.59 | None: 52.44% Antidepressants: 19.51% Mood stabilizers:31.71% Stimulants: 1.22% | CLOZ: 305.56 ± 299.64, nor CLZ: 160.23 ± 105.12 | 18 weeks | - | No | 1 |
| [76] | Preliminary prospective study | 35.5 ± 11.0 | One other antipsychotic, one mood-stabilizing drug, or a benzodiazepine | CLOZ: 124 ± 70.8 nor CLOZ: 52.3 ± 35.7 | 4 weeks | Increased Heart Rate, Myocardial Performance Index (MPI) > 0.44. Reduced LV functioning | No | 0 |
| [72] | Cross-sectional | Patients: 40.7 (range: 21–68) Controls: 41.2 (range: 20–64) | No | 290 | >8 weeks | HRV | Yes, negative (inverse) correlation | 0 |
| [73] | Retrospective | 42.1 (range: 19–73) | Haloperidol, benzodiazepines, SSRIs, Carbamazepine | 331 ± 294 (range: 65–1475) | >1 week | HRV | Yes, negative (inverse) correlation | 0 |
| [48] | Cross-sectional | 33.5 (range: 26–41) | Not reported | 451 (range: 337–569) (n = 29) | 7 (range: 3–13) | Persistent tachycardia | No | 0 |
| Reference | Type of Study | Age (Mean) (Years) | Comedication | Averaged Serum Levels of Clozapine (ng/mL) | Duration | Reported Side Effects | Correlation to Clozapine Plasma Levels | Jadad Score |
|---|---|---|---|---|---|---|---|---|
| [63] | Prospective, double-blind, randomized | 44.8 ± 9.6 | No | CLOZ: >350 in responders | 29.7 ± 13.2 weeks (16 weeks, 32 weeks, 48 weeks, based on response status) | Weight gain | In nonsmokers (n = 8) r = 0.89 (p = 0.046) | 2 |
| [81] | Prospective, observational, open-label | 37.4 ± 9.3, (range 22–57) | Not reported | 500 ± 280 (range 70–1360) | 32.6 ± 6.6 weeks (23–62 weeks) | Weight gain, waist circumference | No (r = −0.04, p = 0.77) at follow-up | 0 |
| [65] | Cross-sectional | 38.2 ± 11.3 (range 22–74); Caucasians: 40.2 ± 8.6, Asians: 36.3 ± 13.4 | Not reported | Caucasians: 415.3 ± 185.8 Asians: 417.1 ± 290.8 | ≥6 months | Lipid profiles, fasting glucose levels | No | 0 |
| [70] | Cross-sectional | 46 (29–63) | Not reported | Median: 359.1 (range 60.5–810.46) | At least 6 months Median: 5.3 years (range 0.5–16.3 years) | Fasting insulin, C-peptide, insulin-like growth factor I, insulin-like growth factor binding protein-1, leptin, glucose and lipids | CLOZ vs. insulin: r = 0.51, p = 0.03 CLOZ vs. C-peptide: r = 0.48, p = 0.04 CLOZ vs. triglycerides: r = 0.50, p = 0.03) | 0 |
| [49] | Cross-sectional | 35 (26–47) | No | 28.8–721 | 2.7 (range 0.5–7.3 years) | Fasting glucose, insulin, growth hormone (GH)-dependent insulin-like growth factor I (IGF-I), and insulin-dependent insulin-like growth factor binding protein-1 | CLOZ vs. insulin levels | 0 |
| [50] | Open, prospective | Males: 34.7 ± 8.1 Females: 36.2 ± 11.6 | Benztropine (n = 6), diphenylhydantoin (n = 2), fluoxetine (n = 1), divalproate (n = 1) | 6 weeks: 388 ± 242 6 months: 444 ± 355 | 6 months | Weight gain | No | 0 |
| [51] | Cross-sectional | 36.5 ± 11.3 | Antipsychotics (61.9%), antidepressants (14.3%), mood stabilizers (21.4%) | CLOZ: 1613.57 ± 976.05 NCLOZ: 964.60 ± 976.05 | Stable clozapine therapy for at least 6 months | Metabolic syndrome BMI | CLOZ vs. metabolic syndrome | 0 |
| [74] | Retrospective | Males: 36.9 (95% CI: 33.9–39.8) Females: 39 (95% CI: 35.4–42.7) | Aripiprazole (n = 6) Amisulpride (n = 4) Haloperidol (n = 2) For the females: estrogen-containing contraceptive pill (n = 5), estrogen-containing hormone replacement treatment | CLOZ: Males: 440 (10th–90th percentile: 260–700) Females: 490 (10th–90th percentile: 270–790) NCLOZ: Males: 310 (10th–90th percentile: 260–350) Females: 310 (10th–90th percentile: 270–340 | Males: 4.4 (95% CI: 1.2–10.3) years Females: 5.1 (95% CI: 2.3–7.9) years | BMI Fasting blood glucose HDL | CLOZ vs. BMI CLOZ vs. fasting blood glucose | 0 |
| [45] | Cross-sectional | Median: 41 (range: 29–36) | Benzodiazepines (n = 4), and/or levomepromazine (n = 3) and/or lithium (n = 1) | CLOZ: 392(69–918) NCLOZ: 288 (88–641) | 6.9 years (range: 0.7–16.3 years) | Elevated blood glucose, elevated levels of insulin, elevated levels of C-peptide, elevated triglycerides, cholesterol, HOMA-IR | CLOZ vs. insulin: r = 0.53, p = 0.03, CLOZ vs. C-peptide r = 0.51, p = 0.04 CLOZ vs. triglyceride levels: r = 0.46, p = 0.06 | 0 |
| [71] | Prospective, randomized | Coadministration group: 32.9 ± 8.5 Monotherapy group: 35.1 ± 9.4 | No | Coadministration group: CLOZ: 509.8 ± 281.1 NCLOZ: 179.0 ± 95.8 Monotherapy group: CLOZ: 502.0 ± 220.6 NCLOZ: 242.8 ± 100.3 | 12 weeks | Serum glucose, cholesterol, and TRG levels, weight gain | NCLOZ vs. weight gain: r = 0.27, p = 0.026 NCLOZ vs. blood sugar: r = 0.34, p = 0.005 NCLOZ vs. Triglycerides: r = 0.27, p = 0.028 | 2 |
| [77] | Cross-sectional, controlled | Patients: 40.94 ± 10.15 Controls: 40.09 ± 1.67 | Not reported | CLOZ: 594.90 ± 492.90 NCLOZ: 220.33 ± 182.55 | At least 4 months | Blood measures | CLOZ vs. total cholesterol: r = 0.34, p = 0.04 | 0 |
| [41] | Cross-sectional | 36.6 ± 9.1 (range 20–54) | Benzodiazepines, lithium, antidepressants, other medically indicated agents | CLOZ: mean = 297 (median: 291), among 68 samples. Subsample not exposed to fluoxetine or valproate (n = 27): 239 ± 159 | 2.15 ± 2.30 years | Excess weight | No | 0 |
| [66] | Double-blind dose–response | 49 females (32–60 years old), 42 males (31–58 years old) | No | 400–1600 | 16-week | Hyperprolactinemia | For every 100 ng/mL increase in plasma clozapine levels, average increments in prolactin levels of 0.45 ng/mL in females and 0.15 ng/mL in males were recorded | 2 |
| [75] | Cross-sectional | 42.5 (20–65) | CLOZ Monotherapy: 65.4%, CLOZ + atypical: 22.5%, CLOZ + typical: 9.7%, CLOZ +typical + atypical: 1.7% | Men (722 ± 366) and women (886 ± 480) | 3–12 months (1.7%), 1–5 years (32.5%). 5 years (57.8%), unspecified (8%) | Menstrual problems | No correlation with CLOZ + NCLOZ concentration | 1 |
| [77] | Cross-sectional, controlled | Patients: 40.94 ± 10.15 Controls: 40.09 ± 1.67 | Not reported | 594.90 ± 492.90 220.33 ± 182.55 | At least 4 months | Blood measures | No correlation with TSH, FT4, PRL. CLOZ vs. FT3: r = −0.373, p = 0.021 | 0 |
| Reference | Type of Study | Age (Mean) (Years) | Comedication | Averaged Serum Levels of Clozapine (ng/mL) | Duration | Reported Side Effects | Correlation to Clozapine Plasma Levels | Jadad Score |
|---|---|---|---|---|---|---|---|---|
| [41] | Cross-sectional | 36.6 ± 9.1 (range 20–54) | Benzodiazepines, lithium, antidepressants, other medically indicated agents | Median 291, range: 15–726 | 2.15 ± 2.30 years | Nocturnal sialorrhea, drooling, parotid swelling, constipation | No | 0 |
| [46] | Prospective, observational follow-up | 19–65 | Benzodiazepines | CLOZ: 385 ± 183 (range 147–974) NCLOZ: 174 ± 84 (range: 43–445) | 12 weeks | Hypersalivation, constipation | No | 1 |
| [52] | Prospective | CLOZAPINE subsample: 31.37 ± 11.8 | Not reported | 165.4 ± 163.4 | 18 weeks | Pathologic liver function tests (LFTs): SGOT, SGPT, GGT, ALP, bilirubin | Yes, for SGPT only | 0 |
| [35] | Cross-sectional, naturalistic | Range 22–55 | Nortriptyline, levomepromazine, clonazepam, hyoscyamine, oxazepam, chlorprothixene, phenobarbital, nitrazepam, biperiden, orphenadrine, benztropine, diazepam, piroxicam, disulfiram | Median: 1076 (range 706–1882) NCLOZ/CLOZ ratio: 0.77 ± 0.17 | Median: 2.5 (range 1.0–9.0) years | Increased liver enzyme activity (increased GGT, ALP, AST, ALT) | No | 0 |
| [77] | Cross-sectional, controlled | Patients: 40.94 ± 10.15 Controls: 40.09 ± 1.67 | Not reported | 594.90 ± 492.90 220.33 ± 182.55 | At least 4 months | AST, ALT | No | |
| [65] | Cross-sectional | 38.2 ± 11.3 (range 22–74); Caucasians: 40.2 ± 8.6, Asians: 36.3 ± 13.4 | Not reported | Caucasians: 415.3 ± 185.8, Asians: 417.1 ± 290.8 | ≥6 months | Elevated levels of alanine (ALT) and aspartate (AST) transferases | No | 0 |
| [43] | Open-label trial | 13.3 ± 2.7 (range 9–16) | No | 289 ± 116 | 6 weeks | Increased hepatic transaminase | No | 0 |
| [40] | Data from double-blind and open-label clozapine trials | 13.5 ± 2.5 (range 7.0–19.1) | Not reported | 455.6 ± 285.1 (n = 46) | 6 weeks | Elevated liver enzymes (AST, ALP, ALT) | No | 0 |
| [53] | Cross-sectional | 39.3 ± 9.8 (range 20–61) (CLOZAPINE group: 37 ± 8.2, NON CLOZAPINE group: | Laxatives (laxsol, polyethylene glycol, lactulose), antipsychotics (risperidone, aripiprazole, haloperidol, amisulpride, aripiprazole + quetiapine) omeprazole, metformin, cholecalciferol | 489 ± 137 (range 284–885) | At least 3 months | Colonic hypomotility | Positive correlation Clear colonic hypomotility in 80% of CLOZAPINE patients, with Colonic Transit Time (CCT) four times longer than population morms (p < 0.0001) and NON-CLOZAPINE patients (p < 0.0001) | 0 |
| [54] | Retrospective, collection of records | Males: 43.5 ± 10.1 Females: 47.2 ± 11.2 | Anticholinergic agents | CLOZ: Laxative users: 533 ± 0.29 Non-laxative users: 486 ± 30 NCLOZ: Laxative users: 337 ± 0.19 Non-laxative users: 269 ± 0.18 | >3 months | Constipation | NCLOZ vs. laxative use, p = 0.046) | 0 |
| Reference | Type of Study | Age (Mean) (Years) | Comedication | Averaged Serum Levels of Clozapine (ng/mL) | Duration | Reported Side Effects | Correlation to Clozapine Plasma Levels | Jadad Score |
|---|---|---|---|---|---|---|---|---|
| Hematological System | ||||||||
| [41] | Cross-sectional | 35.6 ± 9.3 (range 17–54) | Not reported | CLOZ: 304 ± 174 NCLOZ: 216 ± 133 | 2.16 ± 0.35 years | WBC Neutrophil Count | No | 0 |
| [35] | Cross-sectional | 37.6 ± 1.67 | Levomepromazine or chlorprothixene | Median 430.4 (282.4–752.8) | At least 3 months (range: 1–17 years) | Mild leukocytosis and increased erythrocyte folate | No | 0 |
| [37] | Prospective longitudinal | 35.2 ± 10.2 (range: 18–57) | Lorazepam | 379.5 ± 156 | 4 to 8 weeks | Hematological parameters (WBC, red blood count, neutrophils, platelets, and lymphocytes counts or hemoglobin and hematocrit) | CLOZ vs. platelets count r = 0.32, p = 0.042 | 0 |
| [40] | Prospective, double-blind (n = 22), open-label (n = 32) | 13.5 ± 2.5 (range 7.0–19.1) | Mood stabilizers and antidepressants | CLOZ: 455 ± 285.1 NCLOZ: 302.4 ± 142.2 | 6 week treatment (2–6 years follow-up) | Neutropenia | No | 0 |
| [43] | Prospective, open-label/double-blind | 13.3 ± 2.7 (range 9–16) | No | CLOZ: 289 ± 116 NCLOZ: 410 ± 190 | 6 weeks | Moderate neutropenia | No | 0 |
| [36] | Retrospective chart review | 36.4 ± 10.4 | Not reported | CLOZ: 389 ± 386 NCLOZ: 199 ± 216 | Mean duration not reported; samples for measuring drug levels drawn at least once during the first 3 months and then randomly after | WBC or granulocyte counts | No | 0 |
| [78] | Retrospective, observational | 34 (median) 20–84 (range) | Not reported | CLOZ: 1068 (26–3955) NCLOZ: 712 (31–5813) | Not reported | Absolute neutrophil count (ANC) | NCLOZ vs. ANC p = 0.002) | 0 |
| [77] | Cross-sectional, controlled | Patients: 40.94 ± 10.15 Controls: 40.09 ± 1.67 | Not reported | 594.90 ± 492.90 220.33 ± 182.55 | At least 4 months | Hematological parameters | NCLOZ vs. PLT: r = −0.362, p = 0.025 NCLOZ vs. HGB: r = −0.342, p = 0.025, NCLOZ vs. NEU: r = −0.385, p = 0.017 | 0 |
| [38] | Prospective, open-label | 34.62 ± 7.56 (range: 25–48) | No | CLOZ: 266.27 ± 197.44 (25–1270) NCLOZ: 169.0 ± 127.94 (25–1280) | 9 weeks | Leucocyte count Neutrophil count | CLOZ vs. NEU: r = 026, p = 0.001 NCLOZ vs. NEU r = 0.20, p = 0.01 .NCLOZ/CLOZ ratio vs. NEU: r = −0.26, p = 0.002 | 0 |
| [69] | Prospective, open | Males: 28.9 ± 9.7 Females: 34.2 ± 10.7 | Not reported | 145.8 ± 160.1 (3.1–1571.0) | 16.7 ± 24.7 weeks | WBC disorders: Transient neutropenia, eosinophilia, and leukocytosis. Progressive neutropenia, chronic leukocytosis | No | 0 |
| [67] | Cross-sectional | Males: 34.68 ± 1.58 Females: 31.58 ± 1.29 | Clonazepam, Fluoxetine, Paroxetine, Lithium Mirtazapine, Metformin, Sulpiride, valproate, venlafaxine, Duloxetine, escitalopram, imipramine, losartan, omeprazole, pregabalin | Males: 290.11 ± 51.56 Females: 336.36 ± 29.16 | Males: 10.05 ± 1.74 months Females: 6.63 ± 1.15 months | Leukocyte count Neutrophil count | CLOZ vs. NEU: r: 0.631, p = 0.004 CLOZ vs. leukocyte count r: −0.725, p = 0.001 | 0 |
| Geniturinary Side Effects | ||||||||
| [62] | Prospective, double-blind | Not reported | Not reported | CLOZ: 325 ± 199 NCLOZ: 576 ± 326 (dose-dependent) | 16 weeks | Urinary disturbances | No | 2 |
| [40] | Prospective, double-blind (n = 22), open-label (n = 32) | 13.5 ± 2.5 (range 7.0–19.1) | Mood stabilizers and antidepressants | CLOZ: 455 ± 285.1 NCLOZ: 302.4 ± 142.2 | 6 week treatment (2–6 years follow-up) | Enuresis | No | 0 |
| [43] | Prospective, open-label/double-blind | 13.3 ± 2.7 (range 9–16) | No | CLOZ: 289 ± 116 NCLOZ: 410 ± 190 | 6 weeks | Enuresis | No | 0 |
| [41] | Cross-sectional | 35.6 ± 9.3 (range 17–54) | Not reported | CLOZ: 304 ± 174 NCLOZ: 216 ± 133 | 2.16 ± 0.35 years | Nocturnal enuresis | No | 0 |
| [57] | Prospective | 28.6 ± 9.5 | Benzodiazepines, anticholinergic drugs, b-blockers, antidepressants, anticonvulsants | 183.3 ± 150.2 | 18 weeks | Sexual disturbances | CLOZ vs. sexual desire (p = 0.02) CLOZ and sexual functional disturbances (p = 0.008) | 0 |
| Other | ||||||||
| [55] | Clinical study | Group I: 38.8 (23–58), group II: 41.5 (21–58), group III: 43.9 (22–60) | Not mentioned | 221.4 ± 109.6 | 38.3 ± 6.3 days (CLOZ group) | Serum anticardiolipin antibodies (aCL) IgM, IgG | CLOZ vs. aCL IgM: r = 0.461, p = 0.001 | 1 |
| [77] | Cross-sectional, controlled | Patients: 40.94 ± 10.15 Controls: 40.09 ± 1.67 | Not reported | CLOZ: 594.90 ± 4 NCLOZ: 92.90 220.33 ± 182.55 | At least 4 months | B12, Na, K, ure, cre | No | 0 |
| System | Side Effect | ||
|---|---|---|---|
| Positive Correlation with Plasma Clozapine Levels | No Significant Correlation with Plasma Clozapine Levels | Controversial | |
| Nervous system | Εlectroencephalographic abnormalities, EEG slowing | Seizures, sedation | |
| Impaired cognitive performance (incl. memory problems and lack of concentration) | |||
| Extrapyramidal symptoms and tardive dyskinesia | |||
| Psychiatric | Obsessive-compulsive symptoms | ||
| Depression/anxiety factor score (incl. difficulty in concentrating, tension, difficulty remembering things, depression, restlessness, difficulty getting to sleep) | |||
| Cardiovascular | Heart rate variability | QTc prolongation | Myocarditis, pericarditis |
| Μyocardial function | |||
| Sudden death | |||
| Autonomic Dysregulation | Body temperature dysregulation | Hypertension | Orthostatic hypotension |
| Τachycardia | |||
| Metabolic | Hyperinsulinemia, C-peptide | Weight gain | |
| Hyperlipidemia | Hyperglycemia | ||
| Endocrine | Inverse correlation with fT3 | Hyperprolactinemia | |
| Gastrointestinal | Paralytic ileus, constipation, colonic hypomotilityElevation of serum liver enzymes | Sialorrhea, drooling | |
| Geniturinary | Enuresis | Sexual disturbances | |
| Other | Elevation of IgM anticardiolipin antibodies |
| Question | Yes/No |
|---|---|
| Was the study described as random? | 1/0 |
| Was the randomization scheme described and appropriate? | 1/0 |
| Was the study described as double-blind? | 1/0 |
| Was the method of double-blinding appropriate? | 1/0 |
| Was there a description of dropouts and withdrawals? | 1/0 |
| Quality Assessment Based on Total Score | |
| Jadad Score | Quality of evidence |
| 0–2 | Low |
| 3–5 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skokou, M.; Karavia, E.A.; Drakou, Z.; Konstantinopoulou, V.; Kavakioti, C.-A.; Gourzis, P.; Kypreos, K.E.; Andreopoulou, O. Adverse Drug Reactions in Relation to Clozapine Plasma Levels: A Systematic Review. Pharmaceuticals 2022, 15, 817. https://doi.org/10.3390/ph15070817
Skokou M, Karavia EA, Drakou Z, Konstantinopoulou V, Kavakioti C-A, Gourzis P, Kypreos KE, Andreopoulou O. Adverse Drug Reactions in Relation to Clozapine Plasma Levels: A Systematic Review. Pharmaceuticals. 2022; 15(7):817. https://doi.org/10.3390/ph15070817
Chicago/Turabian StyleSkokou, Maria, Eleni A. Karavia, Zoi Drakou, Vassiliki Konstantinopoulou, Christina-Anna Kavakioti, Philippos Gourzis, Kyriakos E. Kypreos, and Ourania Andreopoulou. 2022. "Adverse Drug Reactions in Relation to Clozapine Plasma Levels: A Systematic Review" Pharmaceuticals 15, no. 7: 817. https://doi.org/10.3390/ph15070817
APA StyleSkokou, M., Karavia, E. A., Drakou, Z., Konstantinopoulou, V., Kavakioti, C.-A., Gourzis, P., Kypreos, K. E., & Andreopoulou, O. (2022). Adverse Drug Reactions in Relation to Clozapine Plasma Levels: A Systematic Review. Pharmaceuticals, 15(7), 817. https://doi.org/10.3390/ph15070817







