Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective
Abstract
1. Introduction
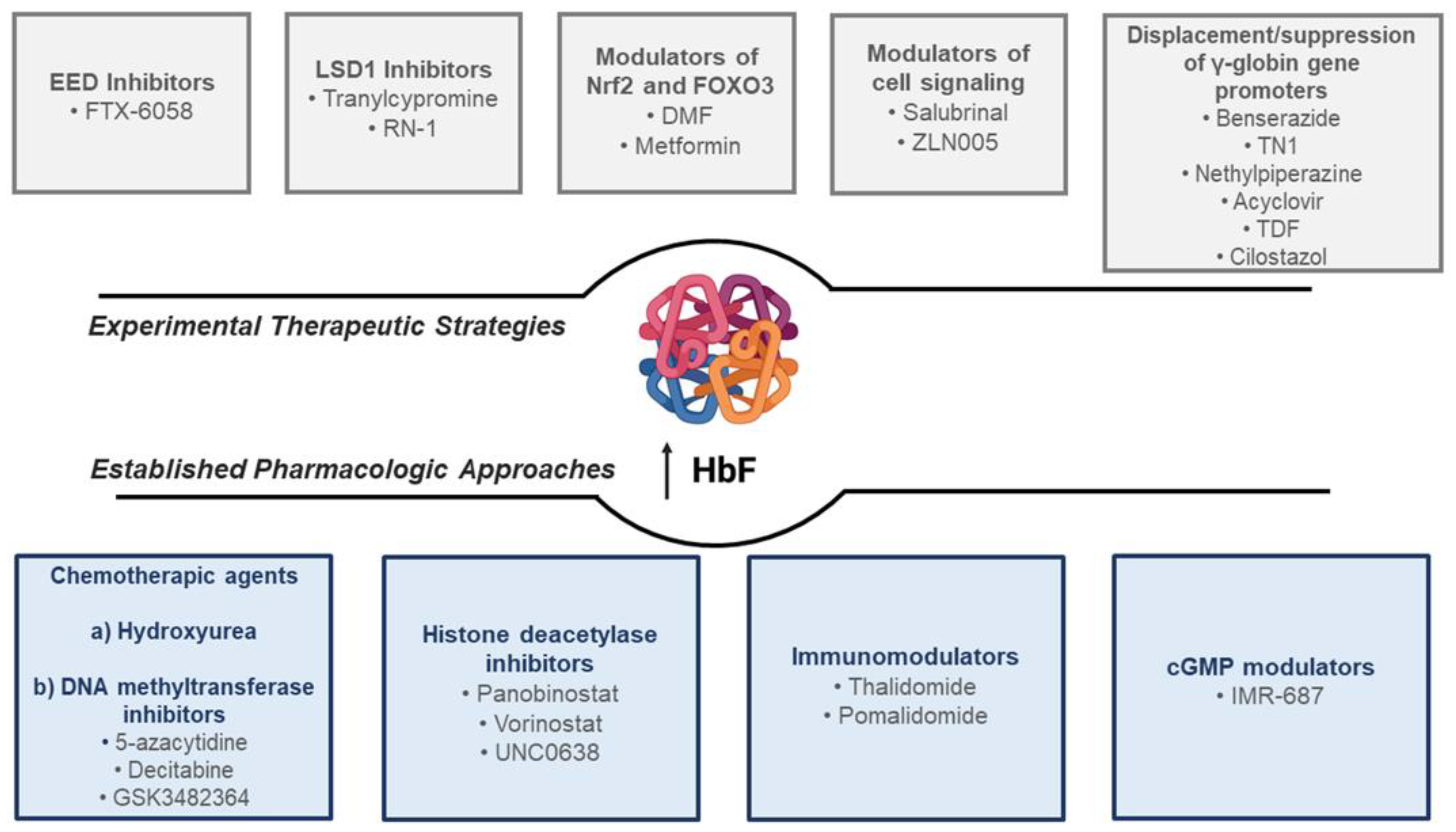
2. Established Pharmacologic Approaches to HbF Induction in Hemoglobinopathies
2.1. Chemotherapeutic Agents
2.1.1. Hydroxyurea
2.1.2. DNA Methyltransferase Inhibitors
2.2. Histone Deacetylase Inhibitors
EHMT1/2 Inhibitors
2.3. Immunomodulators: Thalidomide and Its Derivatives
2.4. cGMP Modulators: Phosphodiesterase-9 Inhibitor
3. Novel Experimental Strategies for the Pharmacologic Induction of HbF in Hemoglobinopathies
3.1. FTX-6058: Embryonic Ectoderm Development (EED) Inhibitor
3.2. Lysine-Specific Histone Demethylase 1 (LSD1) Inhibitors
3.3. Modulators of Redox-Related Transcriptional Factors: Nrf2 or FOXO3
3.3.1. Dimethyl Fumarate
3.3.2. Metformin
3.4. Agents Involved in Displacement/Suppression of γ-Globin Gene Promoters
3.4.1. Benserazide
3.4.2. Purine-Based Fetal Hemoglobin Inducers
3.4.3. Cilostazol (OPC-13013)
3.5. Molecules Targeting Post-Translational Modifications Involved in HbF Expression
3.5.1. Salubrinal (SAL)
3.5.2. PGC-1α Agonist: ZLN005
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. beta-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Weatherall, D.J.; Cappellini, M.D. Thalassaemia. Lancet 2018, 391, 155–167. [Google Scholar] [CrossRef]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Orkin, S.H. The switch from fetal to adult hemoglobin. Cold Spring Harb. Perspect. Med. 2013, 3, a011643. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Cappabianca, M.P.; Perri, M.; Zaghis, I.; Grisanti, P.; Ponzini, D.; Di Biagio, P. Interpreting elevated fetal hemoglobin in pathology and health at the basic laboratory level: New and known γ- gene mutations associated with hereditary persistence of fetal hemoglobin. Int. J. Lab. Hematol. 2014, 36, 13–19. [Google Scholar] [CrossRef]
- Hampl, V.; Bibova, J.; Stranak, Z.; Wu, X.; Michelakis, E.D.; Hashimoto, K.; Archer, S.L. Hypoxic fetoplacental vasoconstriction in humans is mediated by potassium channel inhibition. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2440–H2449. [Google Scholar] [CrossRef]
- Murji, A.; Sobel, M.L.; Hasan, L.; McLeod, A.; Waye, J.S.; Sermer, M.; Berger, H. Pregnancy outcomes in women with elevated levels of fetal hemoglobin. J. Matern. Fetal. Neonatal. Med. 2012, 25, 125–129. [Google Scholar] [CrossRef]
- Sokolova, A.; Mararenko, A.; Rozin, A.; Podrumar, A.; Gotlieb, V. Hereditary persistence of hemoglobin F is protective against red cell sickling. A case report and brief review. Hematol. Oncol. Stem. Cell Ther. 2019, 12, 215–219. [Google Scholar] [CrossRef]
- Alter, B.P.; Rappeport, J.M.; Huisman, T.H.J.; Schroeder, W.A.; Nathan, D.G. Fetal Erythropoiesis Following Bone Marrow Transplantation. Blood 1976, 48, 843–853. [Google Scholar] [CrossRef]
- Nachbaur, D.; Kropshofer, G.; Heitger, A.; Lätzer, K.; Glassl, H.; Ludescher, C.; Nussbaumer, W.; Niederwieser, D. Phenotypic and functional lymphocyte recovery after CD34+-enriched versus non-T cell-depleted autologous peripheral blood stem cell transplantation. J. Hematother. Stem Cell Res. 2000, 9, 727–736. [Google Scholar] [CrossRef]
- Liu, J.W.; Hong, T.; Qin, X.; Liang, Y.M.; Zhang, P. Recent advance on genome editing for therapy of beta-hemoglobinopathies. Yi Chuan 2018, 40, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hargreaves, V.V.; Zhu, Q.; Kurland, J.V.; Hong, J.; Kim, W.; Sher, F.; Macias-Trevino, C.; Rogers, J.M.; Kurita, R.; et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell 2018, 173, 430–442.e417. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.L.; Craig, J.E. Genetics of Hb F/F cell variance in adults and heterocellular hereditary persistence of fetal hemoglobin. Hemoglobin 1998, 22, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H.; Forget, B.G.; Higgs, D.R.; Weatherall, D.J. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Steinberg, M.H. Fetal hemoglobin in sickle cell anemia. Blood 2020, 136, 2392–2400. [Google Scholar] [CrossRef]
- Steinberg, M.H. Fetal Hemoglobin in Sickle Hemoglobinopathies: High HbF Genotypes and Phenotypes. J. Clin. Med. 2020, 9, 3782. [Google Scholar] [CrossRef]
- Mukherjee, M.; Rahaman, M.; Ray, S.K.; Shukla, P.C.; Dolai, T.K.; Chakravorty, N. Revisiting fetal hemoglobin inducers in beta-hemoglobinopathies: A review of natural products, conventional and combinatorial therapies. Mol. Biol. Rep. 2022, 49, 2359–2373. [Google Scholar] [CrossRef]
- Métais, J.-Y.; Doerfler, P.A.; Mayuranathan, T.; Bauer, D.E.; Fowler, S.C.; Hsieh, M.M.; Katta, V.; Keriwala, S.; Lazzarotto, C.R.; Luk, K. Genome editing of HBG1 and HBG2 to induce fetal hemoglobin. Blood Adv. 2019, 3, 3379–3392. [Google Scholar] [CrossRef]
- Magrin, E.; Miccio, A.; Cavazzana, M. Lentiviral and genome-editing strategies for the treatment of β-hemoglobinopathies. Blood J. Am. Soc. Hematol. 2019, 134, 1203–1213. [Google Scholar] [CrossRef]
- Walters, M.C. Induction of Fetal Hemoglobin by Gene Therapy. Mass Med. Soc. 2021, 384, 284–285. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Samuelson, C.; Radtke, S.; Zhu, H.; Llewellyn, M.; Fields, E.; Cook, S.; Huang, M.-L.W.; Jerome, K.R.; Kiem, H.-P.; Humbert, O. Multiplex CRISPR/Cas9 genome editing in hematopoietic stem cells for fetal hemoglobin reinduction generates chromosomal translocations. Mol. Ther. Methods Clin. Dev. 2021, 23, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Breda, L.; Motta, I.; Lourenco, S.; Gemmo, C.; Deng, W.; Rupon, J.W.; Abdulmalik, O.Y.; Manwani, D.; Blobel, G.A.; Rivella, S. Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers. Blood J. Am. Soc. Hematol. 2016, 128, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Rupon, J.W.; Krivega, I.; Breda, L.; Motta, I.; Jahn, K.S.; Reik, A.; Gregory, P.D.; Rivella, S.; Dean, A.; et al. Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell 2014, 158, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Sherkow, J.S. Focus: Genome Editing: CRISPR, Patents, and the Public Health. Yale J. Biol. Med. 2017, 90, 667. [Google Scholar] [PubMed]
- Rigter, T.; Klein, D.; Weinreich, S.S.; Cornel, M.C. Moving somatic gene editing to the clinic: Routes to market access and reimbursement in Europe. Eur. J. Hum. Genet. 2021, 29, 1477–1484. [Google Scholar] [CrossRef]
- Cornel, M.C.; Howard, H.C.; Lim, D.; Bonham, V.L.; Wartiovaara, K. Moving towards a cure in genetics: What is needed to bring somatic gene therapy to the clinic? Eur. J. Hum. Genet. 2019, 27, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Yasara, N.; Premawardhena, A.; Mettananda, S. A comprehensive review of hydroxyurea for β-haemoglobinopathies: The role revisited during COVID-19 pandemic. Orphanet J. Rare Dis. 2021, 16, 114. [Google Scholar] [CrossRef]
- Lemonne, N.; Möckesch, B.; Charlot, K.; Garnier, Y.; Waltz, X.; Lamarre, Y.; Antoine-Jonville, S.; Etienne-Julan, M.; Hardy-Dessources, M.-D.; Romana, M. Effects of hydroxyurea on blood rheology in sickle cell anemia: A two-years follow-up study. Clin. Hemorheol. Microcirc. 2017, 67, 141–148. [Google Scholar] [CrossRef]
- Keikhaei, B.; Yousefi, H.; Bahadoram, M. Hydroxyurea: Clinical and hematological effects in patients with sickle cell anemia. Glob. J. Health Sci. 2016, 8, 252. [Google Scholar] [CrossRef]
- Ballas, S.K.; McCarthy, W.F.; Guo, N.; Brugnara, C.; Kling, G.; Bauserman, R.L.; Waclawiw, M.A. Early detection of response to hydroxyurea therapy in patients with sickle cell anemia. Hemoglobin 2010, 34, 424–429. [Google Scholar] [CrossRef]
- Keikhaei, B.; Yousefi, H.; Bahadoram, M. Clinical and haematological effects of hydroxyurea in β-Thalassemia intermedia patients. J. Clin. Diagn. Res. JCDR 2015, 9, OM01. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.K.; Jena, R.; Chowdhury, D. Red Cell Indices as Predictors of Response to Hydroxyurea Therapy in HbE/Beta Thalassaemia Patients; American Society of Hematology: Washington, DC, USA, 2016. [Google Scholar]
- Cisneros, G.S.; Thein, S.L. Research in sickle cell disease: From bedside to bench to bedside. Hemasphere 2021, 5, e584. [Google Scholar] [CrossRef] [PubMed]
- Italia, K.Y.; Jijina, F.J.; Merchant, R.; Panjwani, S.; Nadkarni, A.H.; Sawant, P.M.; Nair, S.B.; Ghosh, K.; Colah, R.B. Response to hydroxyurea in β thalassemia major and intermedia: Experience in western India. Clin. Chim. Acta 2009, 407, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Watanapokasin, R.; Sanmund, D.; Winichagoon, P.; Muta, K.; Fucharoen, S. Hydroxyurea responses and fetal hemoglobin induction in β-thalassemia/HbE patients’ peripheral blood erythroid cell culture. Ann. Hematol. 2006, 85, 164–169. [Google Scholar] [CrossRef]
- Watanapokasin, Y.; Chuncharunee, S.; Sanmund, D.; Kongnium, W.; Winichagoon, P.; Rodgers, G.P.; Fucharoen, S. In vivo and in vitro studies of fetal hemoglobin induction by hydroxyurea in β-thalassemia/hemoglobin E patients. Exp. Hematol. 2005, 33, 1486–1492. [Google Scholar] [CrossRef]
- Rigano, P.; Pecoraro, A.; Calzolari, R.; Troia, A.; Acuto, S.; Renda, D.; Pantalone, G.R.; Maggio, A.; Marzo, R.D. Desensitization to hydroxycarbamide following long-term treatment of thalassaemia intermedia as observed in vivo and in primary erythroid cultures from treated patients. Br. J. Haematol. 2010, 151, 509–515. [Google Scholar] [CrossRef]
- Singer, S.T.; Vichinsky, E.P.; Larkin, S.; Olivieri, N.; Sweeters, N.; Kuypers, F.A. Hydroxycarbamide-induced changes in E/beta thalassemia red blood cells. Am. J. Hematol. 2008, 83, 842–845. [Google Scholar] [CrossRef][Green Version]
- Musallam, K.M.; Taher, A.T.; Cappellini, M.D.; Sankaran, V.G. Clinical experience with fetal hemoglobin induction therapy in patients with β-thalassemia. Blood J. Am. Soc. Hematol. 2013, 121, 2199–2212. [Google Scholar] [CrossRef]
- Luchtman-Jones, L.; Pressel, S.; Hilliard, L.; Brown, R.C.; Smith, M.G.; Thompson, A.A.; Lee, M.T.; Rothman, J.; Rogers, Z.R.; Owen, W. Effects of hydroxyurea treatment for patients with hemoglobin SC disease. Am. J. Hematol. 2016, 91, 238–242. [Google Scholar] [CrossRef]
- Di Maggio, R.; Hsieh, M.M.; Zhao, X.; Calvaruso, G.; Rigano, P.; Renda, D.; Tisdale, J.F.; Maggio, A. Chronic administration of hydroxyurea (HU) benefits caucasian patients with sickle-beta thalassemia. Int. J. Mol. Sci. 2018, 19, 681. [Google Scholar] [CrossRef]
- Voskaridou, E.; Christoulas, D.; Bilalis, A.; Plata, E.; Varvagiannis, K.; Stamatopoulos, G.; Sinopoulou, K.; Balassopoulou, A.; Loukopoulos, D.; Terpos, E. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: Results of a 17-year, single-center trial (LaSHS). Blood J. Am. Soc. Hematol. 2010, 115, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S. Hydroxyurea for the treatment of sickle cell anemia. N. Engl. J. Med. 2008, 358, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.C.; Zhu, J.; Liu, W.; Chin, K.; Sun, J.; Chen, L.; Hanover, J.A.; Rodgers, G.P. The hydroxyurea-induced small GTP-binding protein SAR modulates gamma-globin gene expression in human erythroid cells. Blood 2005, 106, 3256–3263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, J.; Chin, K.; Aerbajinai, W.; Kumkhaek, C.; Li, H.; Rodgers, G.P. Hydroxyurea-inducible SAR1 gene acts through the Giα/JNK/Jun pathway to regulate γ-globin expression. Blood J. Am. Soc. Hematol. 2014, 124, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Hebert, N.; Rakotoson, M.G.; Bodivit, G.; Audureau, E.; Bencheikh, L.; Kiger, L.; Oubaya, N.; Pakdaman, S.; Sakka, M.; Di Liberto, G. Individual red blood cell fetal hemoglobin quantification allows to determine protective thresholds in sickle cell disease. Am. J. Hematol. 2020, 95, 1235–1245. [Google Scholar] [CrossRef]
- Buchanan, G.R. “Packaging” of fetal hemoglobin in sickle cell anemia. Blood J. Am. Soc. Hematol. 2014, 123, 464–465. [Google Scholar] [CrossRef]
- Steinberg, M.H.; Chui, D.H.; Dover, G.J.; Sebastiani, P.; Alsultan, A. Fetal hemoglobin in sickle cell anemia: A glass half full? Blood J. Am. Soc. Hematol. 2014, 123, 481–485. [Google Scholar] [CrossRef]
- Dong, M.; McGann, P.T. Changing the clinical paradigm of hydroxyurea treatment for sickle cell anemia through precision medicine. Clin. Pharmacol. Ther. 2021, 109, 73–81. [Google Scholar] [CrossRef]
- McGann, P.T.; Niss, O.; Dong, M.; Marahatta, A.; Howard, T.A.; Mizuno, T.; Lane, A.; Kalfa, T.A.; Malik, P.; Quinn, C.T. Robust clinical and laboratory response to hydroxyurea using pharmacokinetically guided dosing for young children with sickle cell anemia. Am. J. Hematol. 2019, 94, 871–879. [Google Scholar] [CrossRef]
- Steinberg, M.H.; McCarthy, W.F.; Castro, O.; Ballas, S.K.; Armstrong, F.D.; Smith, W.; Ataga, K.; Swerdlow, P.; Kutlar, A.; DeCastro, L. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am. J. Hematol. 2010, 85, 403–408. [Google Scholar] [CrossRef]
- Thomas, R.; Dulman, R.; Lewis, A.; Notarangelo, B.; Yang, E. Prospective longitudinal follow-up of children with sickle cell disease treated with hydroxyurea since infancy. Pediatric Blood Cancer 2019, 66, e27816. [Google Scholar] [CrossRef] [PubMed]
- Green, N.S.; Manwani, D.; Qureshi, M.; Ireland, K.; Sinha, A.; Smaldone, A.M. Decreased fetal hemoglobin over time among youth with sickle cell disease on hydroxyurea is associated with higher urgent hospital use. Pediatric Blood Cancer 2016, 63, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Matte, A.; Zorzi, F.; Mazzi, F.; Federti, E.; Olivieri, O.; De Franceschi, L. New Therapeutic Options for the Treatment of Sickle Cell Disease. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019002. [Google Scholar] [CrossRef] [PubMed]
- Matte, A.; Cappellini, M.D.; Iolascon, A.; Enrica, F.; De Franceschi, L. Emerging drugs in randomized controlled trials for sickle cell disease: Are we on the brink of a new era in research and treatment? Expert. Opin. Investig. Drugs 2020, 29, 23–31. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, L.; Cappellini, M.D.; Olivieri, O. Thrombosis and sickle cell disease. Semin. Thromb Hemost. 2011, 37, 226–236. [Google Scholar] [CrossRef]
- De Franceschi, L.; Corrocher, R. Established and experimental treatments for sickle cell disease. Haematologica 2004, 89, 348–356. [Google Scholar]
- Lavelle, D.; Engel, J.D.; Saunthararajah, Y. Fetal hemoglobin induction by epigenetic drugs. Semin Hematol. 2018, 55, 60–67. [Google Scholar] [CrossRef]
- Charache, S.; Dover, G.; Smith, K.; Talbot, C.C.; Moyer, M.; Boyer, S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc. Natl. Acad. Sci. USA 1983, 80, 4842–4846. [Google Scholar] [CrossRef]
- Olivieri, N.F.; Saunthararajah, Y.; Thayalasuthan, V.; Kwiatkowski, J.; Ware, R.E.; Kuypers, F.A.; Kim, H.-Y.; Trachtenberg, F.L.; Vichinsky, E.P.; Network, T.C.R. A pilot study of subcutaneous decitabine in β-thalassemia intermedia. Blood J. Am. Soc. Hematol. 2011, 118, 2708–2711. [Google Scholar] [CrossRef]
- Saunthararajah, Y.; Hillery, C.A.; Lavelle, D.; Molokie, R.; Dorn, L.; Bressler, L.; Gavazova, S.; Chen, Y.-H.; Hoffman, R.; DeSimone, J. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood 2003, 102, 3865–3870. [Google Scholar] [CrossRef]
- Koshy, M.; Dorn, L.; Bressler, L.; Molokie, R.; Lavelle, D.; Talischy, N.; Hoffman, R.; van Overveld, W.; DeSimone, J. 2-deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood J. Am. Soc. Hematol. 2000, 96, 2379–2384. [Google Scholar]
- DeSimone, J.; Koshy, M.; Dorn, L.; Lavelle, D.; Bressler, L.; Molokie, R.; Talischy, N. Maintenance of elevated fetal hemoglobin levels by decitabine during dose interval treatment of sickle cell anemia. Blood J. Am. Soc. Hematol. 2002, 99, 3905–3908. [Google Scholar] [CrossRef] [PubMed]
- Saunthararajah, Y.; Molokie, R.; Saraf, S.; Sidhwani, S.; Gowhari, M.; Vara, S.; Lavelle, D.; DeSimone, J. Clinical effectiveness of decitabine in severe sickle cell disease. Br. J. Haematol. 2008, 141, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Molokie, R.; Lavelle, D.; Gowhari, M.; Pacini, M.; Krauz, L.; Hassan, J.; Ibanez, V.; Ruiz, M.A.; Ng, K.P.; Woost, P. Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study. PLoS Med. 2017, 14, e1002382. [Google Scholar] [CrossRef]
- Gilmartin, A.G.; Groy, A.; Gore, E.R.; Atkins, C.; Long, E.R.; Montoute, M.N.; Wu, Z.; Halsey, W.; McNulty, D.E.; Ennulat, D. In vitro and in vivo induction of fetal hemoglobin with a reversible and selective DNMT1 inhibitor. Haematologica 2021, 106, 1979. [Google Scholar] [CrossRef]
- Ronzoni, L.; Sonzogni, L.; Fossati, G.; Modena, D.; Trombetta, E.; Porretti, L.; Cappellini, M.D. Modulation of gamma globin genes expression by histone deacetylase inhibitors: An in vitro study. Br. J. Haematol. 2014, 165, 714–721. [Google Scholar] [CrossRef]
- Weinberg, R.S.; Ji, X.; Sutton, M.; Perrine, S.; Galperin, Y.; Li, Q.; Liebhaber, S.A.; Stamatoyannopoulos, G.; Atweh, G.F. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood 2005, 105, 1807–1809. [Google Scholar] [CrossRef]
- de Melo, T.R.F.; Dulmovits, B.M.; dos Santos Fernandes, G.F.; de Souza, C.M.; Lanaro, C.; He, M.; Al Abed, Y.; Chung, M.C.; Blanc, L.; Costa, F.F. Synthesis and pharmacological evaluation of pomalidomide derivatives useful for sickle cell disease treatment. Bioorg. Chem. 2021, 114, 105077. [Google Scholar] [CrossRef]
- Bradner, J.E.; Mak, R.; Tanguturi, S.K.; Mazitschek, R.; Haggarty, S.J.; Ross, K.; Chang, C.Y.; Bosco, J.; West, N.; Morse, E. Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease. Proc. Natl. Acad. Sci. USA 2010, 107, 12617–12622. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Mettananda, S.; Yasara, N.; Fisher, C.A.; Taylor, S.; Gibbons, R.; Higgs, D. Synergistic silencing of α-globin and induction of γ-globin by histone deacetylase inhibitor, vorinostat as a potential therapy for β-thalassaemia. Sci. Rep. 2019, 9, 11649. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Weiss, M.J. Anemia: Progress in molecular mechanisms and therapies. Nat. Med. 2015, 21, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hebbel, R.P.; Vercellotti, G.M.; Pace, B.S.; Solovey, A.N.; Kollander, R.; Abanonu, C.F.; Nguyen, J.; Vineyard, J.V.; Belcher, J.D.; Abdulla, F. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathobiology of sickle transgenic mice. Blood J. Am. Soc. Hematol. 2010, 115, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.H.; Li, B.; Zhu, X.; Koti, S.; Cerbone, R.E.; Hendrick, C.L.; Sangerman, J.; Perrine, S.; Pace, B.S. Novel histone deacetylase inhibitor CT-101 induces γ-globin gene expression in sickle erythroid progenitors with targeted epigenetic effects. Blood Cells Mol. Dis. 2022, 93, 102626. [Google Scholar] [CrossRef]
- Krivega, I.; Byrnes, C.; de Vasconcellos, J.F.; Lee, Y.T.; Kaushal, M.; Dean, A.; Miller, J.L. Inhibition of G9a methyltransferase stimulates fetal hemoglobin production by facilitating LCR/γ-globin looping. Blood J. Am. Soc. Hematol. 2015, 126, 665–672. [Google Scholar] [CrossRef]
- Renneville, A.; Van Galen, P.; Canver, M.C.; McConkey, M.; Krill-Burger, J.M.; Dorfman, D.M.; Holson, E.B.; Bernstein, B.E.; Orkin, S.H.; Bauer, D.E. EHMT1 and EHMT2 inhibition induces fetal hemoglobin expression. Blood J. Am. Soc. Hematol. 2015, 126, 1930–1939. [Google Scholar] [CrossRef]
- Chen, X.; Skutt-Kakaria, K.; Davison, J.; Ou, Y.-L.; Choi, E.; Malik, P.; Loeb, K.; Wood, B.; Georges, G.; Torok-Storb, B. G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment. Genes Dev. 2012, 26, 2499–2511. [Google Scholar] [CrossRef]
- Nualkaew, T.; Khamphikham, P.; Pongpaksupasin, P.; Kaewsakulthong, W.; Songdej, D.; Paiboonsukwong, K.; Sripichai, O.; Engel, J.D.; Hongeng, S.; Fucharoen, S. UNC0638 induces high levels of fetal hemoglobin expression in β-thalassemia/HbE erythroid progenitor cells. Ann. Hematol. 2020, 99, 2027–2036. [Google Scholar] [CrossRef]
- Liu, F.; Barsyte-Lovejoy, D.; Li, F.; Xiong, Y.; Korboukh, V.; Huang, X.-P.; Allali-Hassani, A.; Janzen, W.P.; Roth, B.L.; Frye, S.V. Discovery of an in vivo chemical probe of the lysine methyltransferases G9a and GLP. J. Med. Chem. 2013, 56, 8931–8942. [Google Scholar] [CrossRef]
- Dulmovits, B.M.; Appiah-Kubi, A.O.; Papoin, J.; Hale, J.; He, M.; Al-Abed, Y.; Didier, S.; Gould, M.; Husain-Krautter, S.; Singh, S.A. Pomalidomide reverses γ-globin silencing through the transcriptional reprogramming of adult hematopoietic progenitors. Blood J. Am. Soc. Hematol. 2016, 127, 1481–1492. [Google Scholar] [CrossRef]
- Khamphikham, P.; Nualkaew, T.; Pongpaksupasin, P.; Kaewsakulthong, W.; Songdej, D.; Paiboonsukwong, K.; Engel, J.D.; Hongeng, S.; Fucharoen, S.; Sripichai, O. High-level induction of fetal haemoglobin by pomalidomide in β-thalassaemia/HbE erythroid progenitor cells. Br. J. Haematol. 2020, 189, e240–e245. [Google Scholar] [CrossRef] [PubMed]
- Aerbajinai, W.; Zhu, J.; Gao, Z.; Chin, K.; Rodgers, G.P. Thalidomide induces γ-globin gene expression through increased reactive oxygen species–mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood J. Am. Soc. Hematol. 2007, 110, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Chakrabarti, P.; Dolai, T.K.; Ghosh, P.; Mandal, P.K.; Baul, S.N.; De, R. Comparison of efficacy and safety of thalidomide vs hydroxyurea in patients with Hb E-β thalassemia-a pilot study from a tertiary care Centre of India. Blood Cells Mol. Dis. 2021, 88, 102544. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, S.; Liu, Y.; Huang, J.; Hong, W.; Xu, L.; Xu, H.; Fang, J. Efficacy of thalidomide treatment in children with transfusion dependent β-thalassemia: A retrospective clinical study. Front. Pharmacol. Front Pharmacol. 2021, 12, 722502. [Google Scholar] [CrossRef]
- Chen, J.-M.; Zhu, W.-J.; Liu, J.; Wang, G.-Z.; Chen, X.-Q.; Tan, Y.; Xu, W.-W.; Qu, L.-W.; Li, J.-Y.; Yang, H.-J. Safety and efficacy of thalidomide in patients with transfusion-dependent β-thalassemia: A randomized clinical trial. Signal Transduct. Target. Ther. 2021, 6, 405. [Google Scholar] [CrossRef]
- Chandra, J.; Parakh, N.; Singh, N.; Sharma, S.; Goel, M.; Pemde, H. Efficacy and safety of thalidomide in patients with transfusion-dependent thalassemia. Indian Pediatrics 2021, 58, 611–616. [Google Scholar] [CrossRef]
- Yassin, A.K. Promising Response to Thalidomide in Symptomatic beta-Thalassemia. Indian J. Hematol. Blood Transfus. 2020, 36, 337–341. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Y.; Zhou, Y.; Long, B.; Lu, Q.; Zhou, T.; Wang, L.; Geng, Z.; Yin, X. Thalidomide for patients with β-thalassemia: A multicenter experience. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020021. [Google Scholar] [CrossRef]
- Javed, R.; Radhakrishnan, V.; Basu, S.; Chandy, M. Challenges in transfusion and the role of Thalidomide in E-β-Thalassemia—A case report. Clin. Case Rep. 2020, 8, 2208–2210. [Google Scholar] [CrossRef]
- Nag, A.; Radhakrishnan, V.S.; Kumar, J.; Bhave, S.; Mishra, D.K.; Nair, R.; Chandy, M. Thalidomide in patients with transfusion-dependent E-beta thalassemia refractory to hydroxyurea: A single-center experience. Indian J. Hematol. Blood Transfus. 2020, 36, 399–402. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, Z.; Yang, G.; Lai, Y.; Liu, R. Investigating the Efficacy and Safety of Thalidomide for Treating Patients With ss-Thalassemia: A Meta-Analysis. Front. Pharmacol. 2021, 12, 814302. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, W.; Cai, N.; Bu, S.; Li, J.; Huang, L. Thalidomide induces haematologic responses in patients with beta-thalassaemia. Eur. J. Haematol. 2017, 99, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, N.; Lai, Y.; Xu, W.; Li, J.; Huang, L.; Huang, Y.; Hu, M.; Yang, H.; Chen, J. Thalidomide for the Treatment of Thrombocytopenia and Hypersplenism in Patients with Cirrhosis or Thalassemia. Front. Pharmacol. 2020, 11, 1137. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Lopez, L.B.; Delgado-Lamas, J.L.; Rubio-Jurado, B.; Perea, F.J.; Ibarra, B. Thalidomide therapy in a patient with thalassemia major. Blood Cells Mol. Dis. 2008, 41, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Masera, N.; Tavecchia, L.; Capra, M.; Cazzaniga, G.; Vimercati, C.; Pozzi, L.; Biondi, A.; Masera, G. Optimal response to thalidomide in a patient with thalassaemia major resistant to conventional therapy. Blood Transfus. 2010, 8, 63–65. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Q.; Zhou, Y.; Li, P.; Lin, W.; Yin, X. Thalidomide has a significant effect in patients with thalassemia intermedia. Hematology 2018, 23, 50–54. [Google Scholar] [CrossRef]
- Ren, Q.; Zhou, Y.L.; Wang, L.; Chen, Y.S.; Ma, Y.N.; Li, P.P.; Yin, X.L. Clinical trial on the effects of thalidomide on hemoglobin synthesis in patients with moderate thalassemia intermedia. Ann. Hematol. 2018, 97, 1933–1939. [Google Scholar] [CrossRef]
- Meiler, S.E.; Wade, M.; Kutlar, F.; Yerigenahally, S.D.; Xue, Y.; Moutouh-de Parseval, L.A.; Corral, L.G.; Swerdlow, P.S.; Kutlar, A. Pomalidomide augments fetal hemoglobin production without the myelosuppressive effects of hydroxyurea in transgenic sickle cell mice. Blood J. Am. Soc. Hematol. 2011, 118, 1109–1112. [Google Scholar] [CrossRef]
- Moutouh-de Parseval, L.A.; Verhelle, D.; Glezer, E.; Jensen-Pergakes, K.; Ferguson, G.D.; Corral, L.G.; Morris, C.L.; Muller, G.; Brady, H.; Chan, K. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. J. Clin. Investig. 2008, 118, 248–258. [Google Scholar] [CrossRef]
- McArthur, J.G.; Svenstrup, N.; Chen, C.; Fricot, A.; Carvalho, C.; Nguyen, J.; Nguyen, P.; Parachikova, A.; Abdulla, F.; Vercellotti, G.M.; et al. A novel, highly potent and selective phosphodiesterase-9 inhibitor for the treatment of sickle cell disease. Haematologica 2020, 105, 623–631. [Google Scholar] [CrossRef]
- Andemariam, B.; Bronte, L.; Gordeuk, V.; Howard, J.; Kanter, J.; Eleftheriou, P.; Pancham, S.; Hagar, R.; Clarke, L.; Blyden, G.; et al. The safety, pharmacokinetics & pharmacodynamic effects of IMR-687, a highly selective PDE9 inhibitor, in adults with sickle cell disease: Phase-2a placebo-controlled & open-label extension studies [abstract]. Hemasphere 2021, 5, 90. [Google Scholar]
- Andemariam, B.; Mant, T.; Eleftheriou, P.; Lugthart, S.; Bronté-Hall, L.; Barroso, F.; Blyden, G.; Mason, J.; Barysauskas, C.M.; Yen, J. Treatment with IMR-687, a Highly Selective PDE9 Inhibitor, Increases HbF and Reduces VOCs in Adults with Sickle Cell Disease in a Long-Term, Phase 2a, Open-Label Extension Study. Blood 2021, 138, 2046. [Google Scholar] [CrossRef]
- Matson, D.; Xie, K.; Roth, M.; Stuart, B.; Bruno, P.; Efremov, I.; Thompson, L.; Silver, S.; Moxham, C. Ftx-6058 Induces Fetal Hemoglobin Production and Ameliorates Disease Pathology in Sickle Cell Mice. Blood 2021, 138, 2018. [Google Scholar] [CrossRef]
- Higher FTX-6058 Doses Raise Hemoglobin in Healthy Adults in Trial. Sickle Cell Disease News. 16 December 2021. Available online: https://sicklecellanemianews.com/2021/12/16/higher-ftx-6058-doses-raise-hemoglobin-healthy-adults-trial/ (accessed on 15 April 2022).
- Shi, L.; Cui, S.; Engel, J.D.; Tanabe, O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat. Med. 2013, 19, 291–294. [Google Scholar] [CrossRef]
- Lee, M.G.; Wynder, C.; Schmidt, D.M.; McCafferty, D.G.; Shiekhattar, R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem. Biol. 2006, 13, 563–567. [Google Scholar] [CrossRef]
- Xu, J.; Bauer, D.E.; Kerenyi, M.A.; Vo, T.D.; Hou, S.; Hsu, Y.J.; Yao, H.; Trowbridge, J.J.; Mandel, G.; Orkin, S.H. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc. Natl. Acad. Sci. USA 2013, 110, 6518–6523. [Google Scholar] [CrossRef]
- Neelamegam, R.; Ricq, E.L.; Malvaez, M.; Patnaik, D.; Norton, S.; Carlin, S.M.; Hill, I.T.; Wood, M.A.; Haggarty, S.J.; Hooker, J.M. Brain-penetrant LSD1 inhibitors can block memory consolidation. ACS Chem. Neurosci. 2012, 3, 120–128. [Google Scholar] [CrossRef]
- Cui, S.; Lim, K.C.; Shi, L.; Lee, M.; Jearawiriyapaisarn, N.; Myers, G.; Campbell, A.; Harro, D.; Iwase, S.; Trievel, R.C.; et al. The LSD1 inhibitor RN-1 induces fetal hemoglobin synthesis and reduces disease pathology in sickle cell mice. Blood 2015, 126, 386–396. [Google Scholar] [CrossRef]
- Rivers, A.; Vaitkus, K.; Ruiz, M.A.; Ibanez, V.; Jagadeeswaran, R.; Kouznetsova, T.; DeSimone, J.; Lavelle, D. RN-1, a potent and selective lysine-specific demethylase 1 inhibitor, increases γ-globin expression, F reticulocytes, and F cells in a sickle cell disease mouse model. Exp. Hematol. 2015, 43, 546–553.e543. [Google Scholar] [CrossRef]
- Kaewsakulthong, W.; Pongpaksupasin, P.; Nualkaew, T.; Hongeng, S.; Fucharoen, S.; Jearawiriyapaisarn, N.; Sripichai, O. Lysine-specific histone demethylase 1 inhibition enhances robust fetal hemoglobin induction in human beta(0)-thalassemia/hemoglobin E erythroid cells. Hematol. Rep. 2021, 13, 9215. [Google Scholar] [CrossRef]
- Viglietta, V.; Miller, D.; Bar-Or, A.; Phillips, J.T.; Arnold, D.L.; Selmaj, K.; Kita, M.; Hutchinson, M.; Yang, M.; Zhang, R.; et al. Efficacy of delayed-release dimethyl fumarate in relapsing-remitting multiple sclerosis: Integrated analysis of the phase 3 trials. Ann. Clin. Transl. Neurol. 2015, 2, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Arnold, D.L.; Bar-Or, A.; Fox, R.J.; Kappos, L.; Chen, C.; Parks, B.; Miller, C. Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years’ follow-up of DEFINE, CONFIRM, and ENDORSE. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420915005. [Google Scholar] [CrossRef] [PubMed]
- Alroughani, R.; Huppke, P.; Mazurkiewicz-Beldzinska, M.; Blaschek, A.; Valis, M.; Aaen, G.; Pultz, J.; Peng, X.; Beynon, V. Delayed-Release Dimethyl Fumarate Safety and Efficacy in Pediatric Patients with Relapsing-Remitting Multiple Sclerosis. Front. Neurol. 2020, 11, 606418. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Gold, R.; Phillips, J.T.; Okwuokenye, M.; Zhang, A.; Marantz, J.L. Efficacy and Tolerability of Delayed-release Dimethyl Fumarate in Black, Hispanic, and Asian Patients with Relapsing-Remitting Multiple Sclerosis: Post Hoc Integrated Analysis of DEFINE and CONFIRM. Neurol. Ther. 2017, 6, 175–187. [Google Scholar] [CrossRef]
- de Franceschi, L.; Turrini, F.; Honczarenko, M.; Ayi, K.; Rivera, A.; Fleming, M.D.; Law, T.; Mannu, F.; Kuypers, F.A.; Bast, A.; et al. In vivo reduction of erythrocyte oxidant stress in a murine model of beta-thalassemia. Haematologica 2004, 89, 1287–1298. [Google Scholar]
- De Franceschi, L.; Bertoldi, M.; Matte, A.; Santos Franco, S.; Pantaleo, A.; Ferru, E.; Turrini, F. Oxidative stress and beta-thalassemic erythroid cells behind the molecular defect. Oxid. Med. Cell Longev. 2013, 2013, 985210. [Google Scholar] [CrossRef]
- Matte, A.; De Falco, L.; Iolascon, A.; Mohandas, N.; An, X.; Siciliano, A.; Leboeuf, C.; Janin, A.; Bruno, M.; Choi, S.Y. The interplay between peroxiredoxin-2 and nuclear factor-erythroid 2 is important in limiting oxidative mediated dysfunction in β-thalassemic erythropoiesis. Antioxid. Redox Signal. 2015, 23, 1284–1297. [Google Scholar] [CrossRef]
- Brugnara, C.; de Franceschi, L. Effect of cell age and phenylhydrazine on the cation transport properties of rabbit erythrocytes. J. Cell Physiol. 1993, 154, 271–280. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Pace, B.; Gupta, D.; Sturtevant, S.; Li, B.; Makala, L.; Brittain, J.; Moore, N.; Vieira, B.F.; Thullen, T.; et al. Dimethyl fumarate increases fetal hemoglobin, provides heme detoxification, and corrects anemia in sickle cell disease. JCI Insight 2017, 2, e96409. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Gupta, D.; Sturtevant, S.; Li, B.; Makala, L.C.; Hobbs, W.E.; Light, D.R.; Pace, B. Dimethyl fumarate induces fetal hemoglobin in sickle cell disease. Blood 2015, 126, 410. [Google Scholar] [CrossRef]
- Keleku-Lukwete, N.; Suzuki, M.; Otsuki, A.; Tsuchida, K.; Katayama, S.; Hayashi, M.; Naganuma, E.; Moriguchi, T.; Tanabe, O.; Engel, J.D.; et al. Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc. Natl. Acad. Sci. USA 2015, 112, 12169–12174. [Google Scholar] [CrossRef] [PubMed]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Zhang, P.; Abdulla, F.; Nguyen, P.; Killeen, T.; Xu, P.; O’Sullivan, G.; Nath, K.A.; et al. Control of Oxidative Stress and Inflammation in Sickle Cell Disease with the Nrf2 Activator Dimethyl Fumarate. Antioxid. Redox. Signal. 2017, 26, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chung, Y.M.; Guan, M.; Ma, M.; Ma, J.; Berek, J.S.; Hu, M.C. Reprogramming ovarian and breast cancer cells into non-cancerous cells by low-dose metformin or SN-38 through FOXO3 activation. Sci. Rep. 2014, 4, 5810. [Google Scholar] [CrossRef]
- Takayama, H.; Misu, H.; Iwama, H.; Chikamoto, K.; Saito, Y.; Murao, K.; Teraguchi, A.; Lan, F.; Kikuchi, A.; Saito, R.; et al. Metformin suppresses expression of the selenoprotein P gene via an AMP-activated kinase (AMPK)/FoxO3a pathway in H4IIEC3 hepatocytes. J. Biol. Chem. 2014, 289, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.; Chan, D.W.; Liu, V.W.; Yao, K.M.; Ngan, H.Y. Activation of AMPK inhibits cervical cancer cell growth through AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer 2013, 13, 327. [Google Scholar] [CrossRef]
- Zhang, Y.; Paikari, A.; Sumazin, P.; Ginter Summarell, C.C.; Crosby, J.R.; Boerwinkle, E.; Weiss, M.J.; Sheehan, V.A. Metformin induces FOXO3-dependent fetal hemoglobin production in human primary erythroid cells. Blood 2018, 132, 321–333. [Google Scholar] [CrossRef]
- Han, J.; Saraf, S.L.; Molokie, R.E.; Gordeuk, V.R. Use of metformin in patients with sickle cell disease. Am. J. Hematol. 2019, 94, E13–E15. [Google Scholar] [CrossRef]
- Badawy, S.M.; Payne, A.B. Association between clinical outcomes and metformin use in adults with sickle cell disease and diabetes mellitus. Blood Adv. 2019, 3, 3297–3306. [Google Scholar] [CrossRef]
- Pace, B.S.; Perrine, S.; Li, B.; Makala, L.; Xu, H.; Takezaki, M.; Wolf, R.F.; Wang, A.; Xu, X.; Huang, J.; et al. Benserazide racemate and enantiomers induce fetal globin gene expression in vivo: Studies to guide clinical development for beta thalassemia and sickle cell disease. Blood Cells Mol. Dis. 2021, 89, 102561. [Google Scholar] [CrossRef]
- Dai, Y.; Sangerman, J.; Nouraie, M.; Faller, A.D.; Oneal, P.; Rock, A.; Owoyemi, O.; Niu, X.; Nekhai, S.; Maharaj, D.; et al. Effects of hydroxyurea on F-cells in sickle cell disease and potential impact of a second fetal globin inducer. Am. J. Hematol. 2017, 92, E10–E11. [Google Scholar] [CrossRef][Green Version]
- Boosalis, M.S.; Sangerman, J.I.; White, G.L.; Wolf, R.F.; Shen, L.; Dai, Y.; White, E.; Makala, L.H.; Li, B.; Pace, B.S. Novel inducers of fetal globin identified through high throughput screening (HTS) are active in vivo in anemic baboons and transgenic mice. PLoS ONE 2015, 10, e0144660. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Sangerman, J.; Luo, H.Y.; Fucharoen, S.; Chui, D.H.; Faller, D.V.; Perrine, S.P. Therapeutic fetal-globin inducers reduce transcriptional repression in hemoglobinopathy erythroid progenitors through distinct mechanisms. Blood Cells Mol. Dis. 2016, 56, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.E.H.P.; Olops, L.; Vendrame, F.; Tavares, A.H.J.; Leonardo, D.P.; de Azevedo, P.C.; Piovesana, L.G.; Costa, F.F.; Fertrin, K.Y. Benserazide as a potential novel fetal hemoglobin inducer: An observational study in non-carriers of hemoglobin disorders. Blood Cells Mol. Dis. 2021, 87, 102511. [Google Scholar] [CrossRef]
- Nam, T.G.; Lee, J.; Walker, J.R.; Brinker, A.; Cho, C.Y.; Schultz, P.G. Identification and characterization of small-molecule inducers of fetal hemoglobin. ChemMedChem 2011, 6, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.S.; Yeh, T.K.; Chou, Y.C.; Hsu, T.; Lu, C.T.; Kung, F.C.; Hsieh, M.Y.; Lin, C.H.; Chen, C.T.; James Shen, C.K.; et al. Potent and orally active purine-based fetal hemoglobin inducers for treating beta-thalassemia and sickle cell disease. Eur. J. Med. Chem. 2021, 209, 112938. [Google Scholar] [CrossRef] [PubMed]
- Elion, G. Acyclovir: Discovery, mechanism of action, and selectivity. J. Med. Virol. 1993, 41 (Suppl. 1), 2–6. [Google Scholar] [CrossRef]
- Ali, H.; Khan, F.; Musharraf, S.G. Acyclovir induces fetal hemoglobin via downregulation of γ-globin repressors, BCL11A and SOX6 trans-acting factors. Biochem. Pharmacol. 2021, 190, 114612. [Google Scholar] [CrossRef]
- Khan, F.; Ali, H.; Musharraf, S.G. Tenofovir disoproxil fumarate induces fetal hemoglobin production in K562 cells and beta-YAC transgenic mice: A therapeutic approach for gamma-globin induction. Exp. Cell Res. 2020, 394, 112168. [Google Scholar] [CrossRef]
- Liu, Y.; Shakur, Y.; Yoshitake, M.; Kambayashi, J.i. Cilostazol (Pletal®): A dual inhibitor of cyclic nucleotide phosphodiesterase type 3 and adenosine uptake. Cardiovasc. Drug Rev. 2001, 19, 369–386. [Google Scholar] [CrossRef]
- Ali, H.; Khan, F.; Musharraf, S.G. Cilostazol-mediated reversion of γ-globin silencing is associated with a high level of HbF production: A potential therapeutic candidate for β-globin disorders. Biomed. Pharmacother. 2021, 142, 112058. [Google Scholar] [CrossRef]
- Hahn, C.K.; Lowrey, C.H. Eukaryotic initiation factor 2alpha phosphorylation mediates fetal hemoglobin induction through a post-transcriptional mechanism. Blood 2013, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Perrine, S. Stressing HbF synthesis: Role of translation? Blood 2013, 122, 467–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xing, C.; Zhang, W.; Li, J.; Hu, T.; Li, L.; Li, H.; Cai, Y. Salubrinal, a novel inhibitor of eIF-2alpha dephosphorylation, promotes erythropoiesis at early stage targeted by ufmylation pathway. J. Cell Physiol. 2019, 234, 18560–18570. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.H.; Li, B.; Palani, C.; Siddaramappa, U.; Takezaki, M.; Xu, H.; Zhi, W.; Pace, B.S. Salubrinal induces fetal hemoglobin expression via the stress-signaling pathway in human sickle erythroid progenitors and sickle cell disease mice. PLoS ONE 2022, 17, e0261799. [Google Scholar] [CrossRef]
- Sun, Y.; Habara, A.; Le, C.Q.; Nguyen, N.; Chen, R.; Murphy, G.J.; Chui, D.H.K.; Steinberg, M.H.; Cui, S. Pharmacologic induction of PGC-1alpha stimulates fetal haemoglobin gene expression. Br. J. Haematol. 2022, 197, 97–109. [Google Scholar] [CrossRef]
| Agent | Mechanism of Action |
|---|---|
| Hydroxyurea |
|
| DNA methyltransferase inhibitors |
|
| HDAC inhibitors |
|
| Immunomodulators: Thalidomide and its derivatives |
|
| cGMP modulators: PDE-9 inhibitor |
|
| Agent | Mechanism of Action |
|---|---|
| EED inhibitors |
|
| LSD1 inhibitors |
|
| Modulators of redox-related transcriptional factors |
|
| Agents involved in the displacement/ suppression of γ-globin gene promoters |
|
| Modulators of cell signaling |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou-Fakhredin, R.; De Franceschi, L.; Motta, I.; Cappellini, M.D.; Taher, A.T. Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals 2022, 15, 753. https://doi.org/10.3390/ph15060753
Bou-Fakhredin R, De Franceschi L, Motta I, Cappellini MD, Taher AT. Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals. 2022; 15(6):753. https://doi.org/10.3390/ph15060753
Chicago/Turabian StyleBou-Fakhredin, Rayan, Lucia De Franceschi, Irene Motta, Maria Domenica Cappellini, and Ali T. Taher. 2022. "Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective" Pharmaceuticals 15, no. 6: 753. https://doi.org/10.3390/ph15060753
APA StyleBou-Fakhredin, R., De Franceschi, L., Motta, I., Cappellini, M. D., & Taher, A. T. (2022). Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals, 15(6), 753. https://doi.org/10.3390/ph15060753







