In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome
Abstract
1. Introduction
2. Results and Discussion
2.1. Headspace Composition
2.2. Composition of the Volatiles Obtained by Hydrodistillation
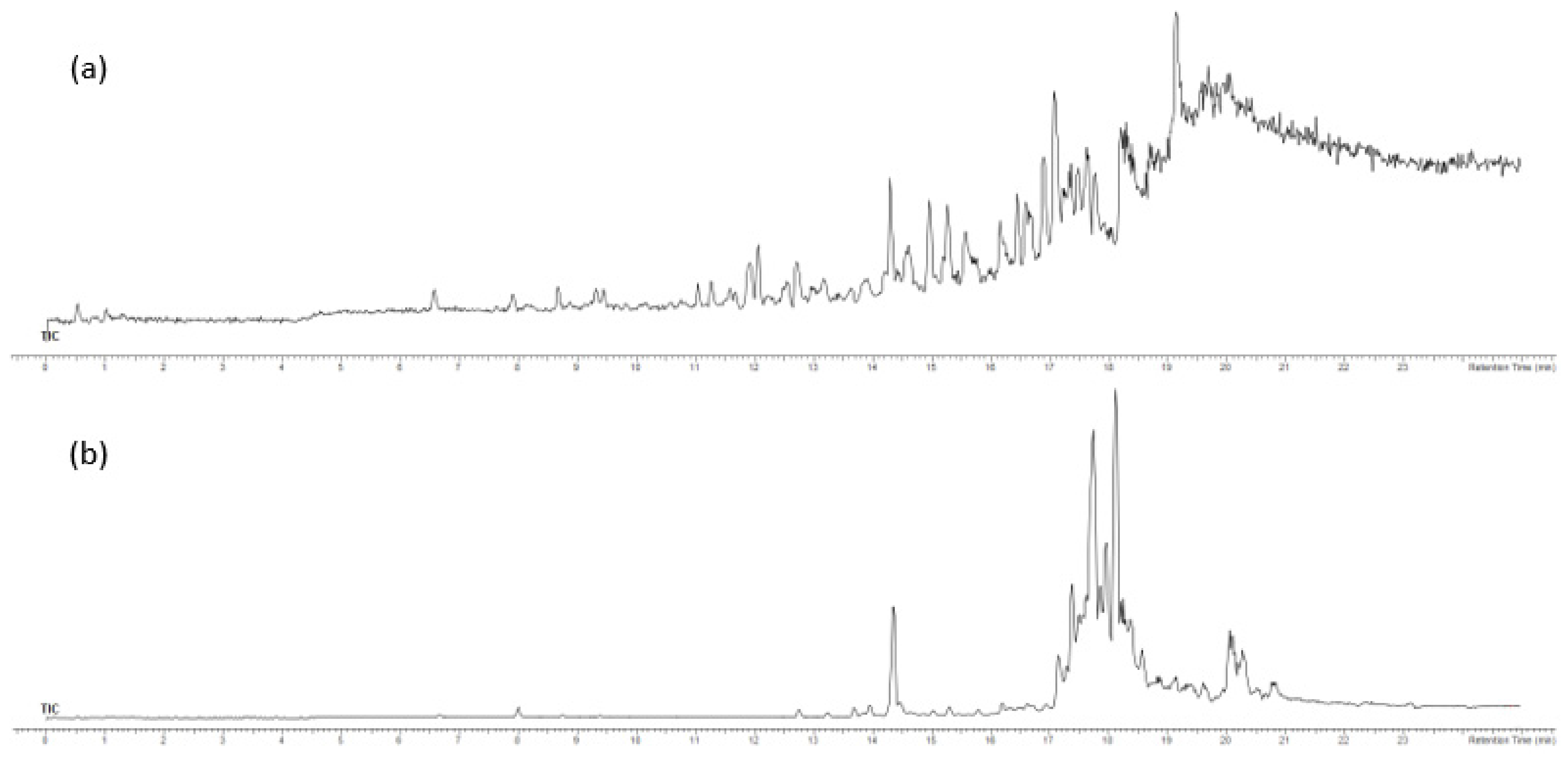
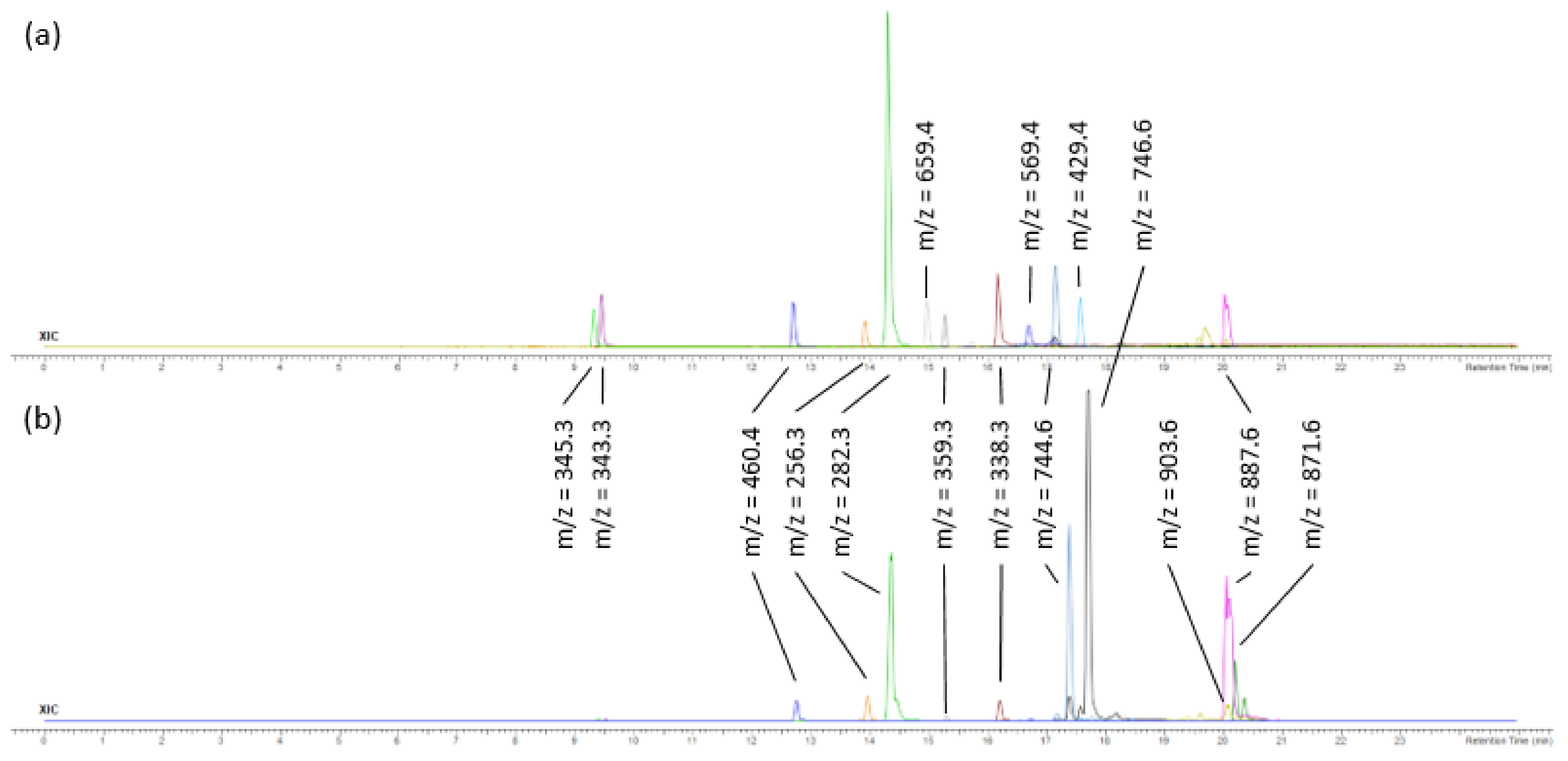
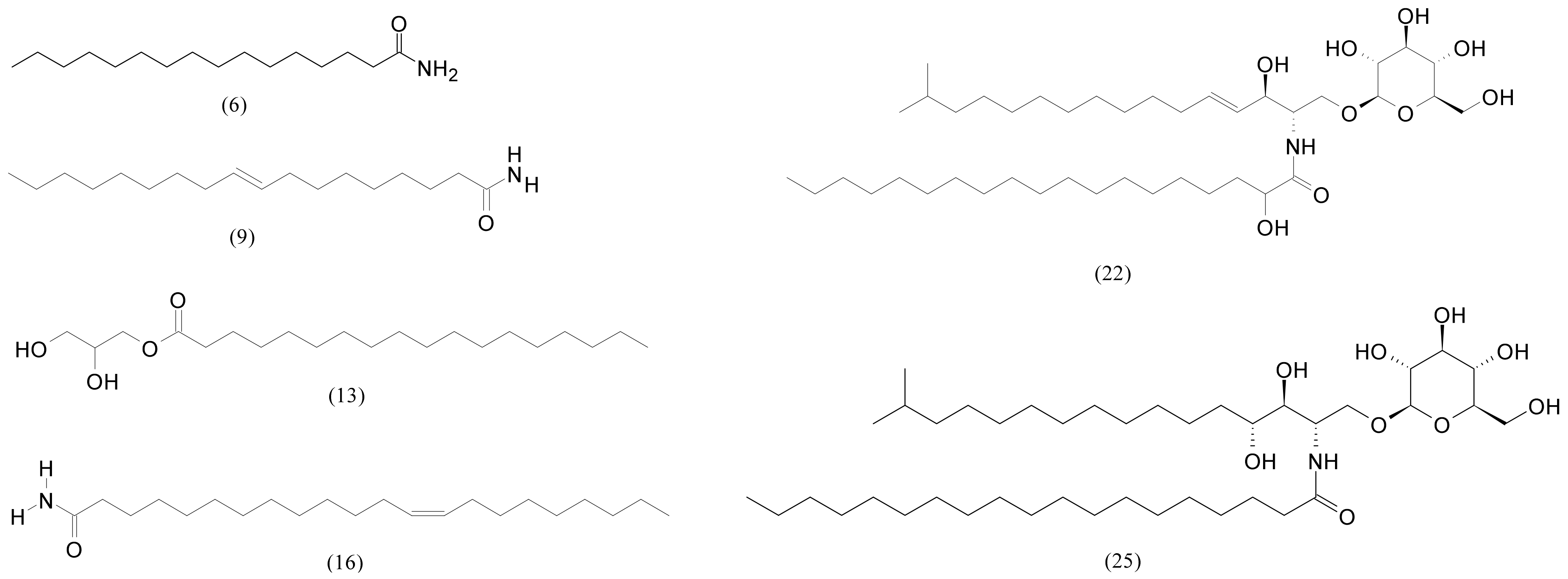
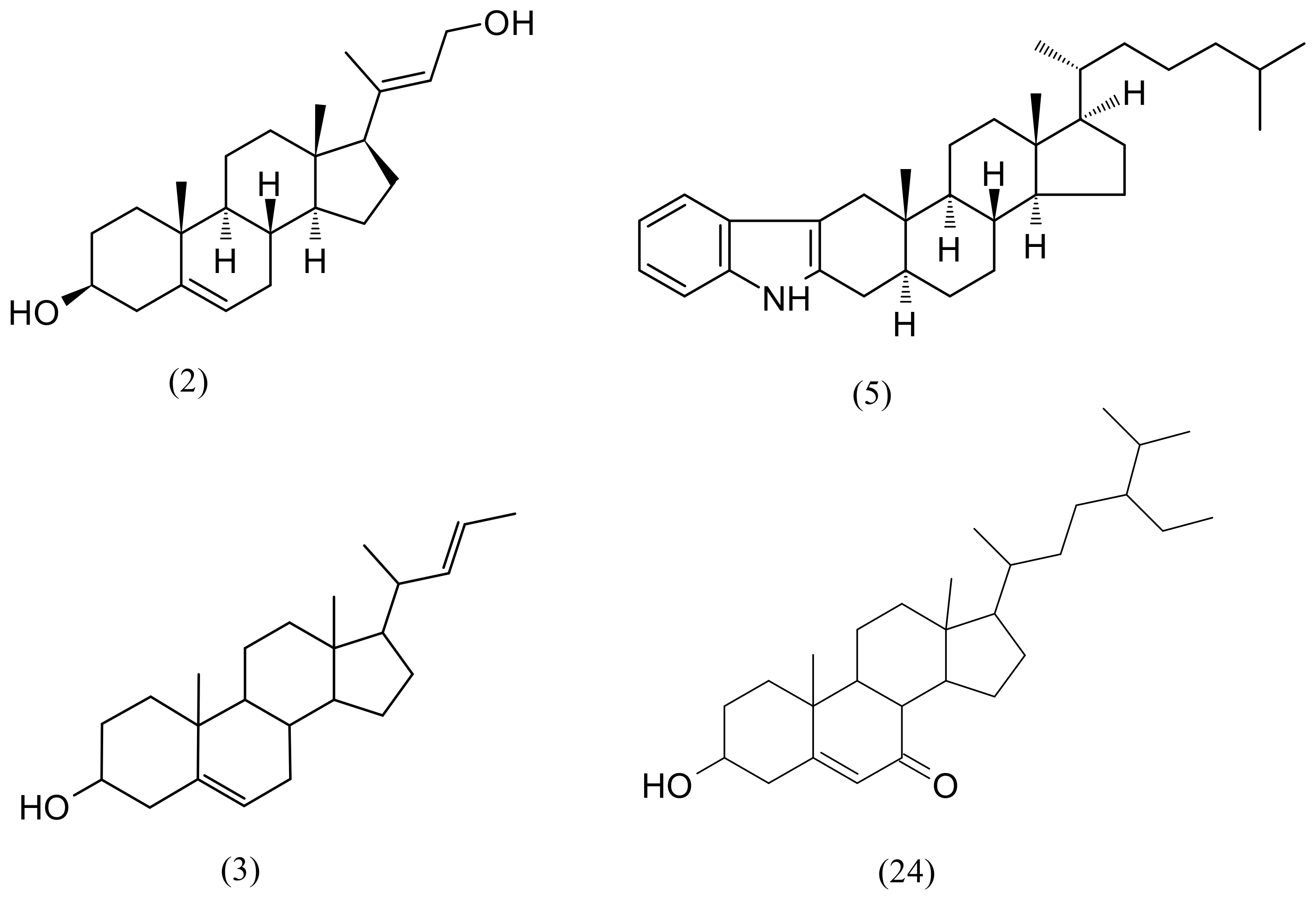
2.3. Analysis of F3 and F4 Fractions Containing Less Polar Nonvolatile Compounds
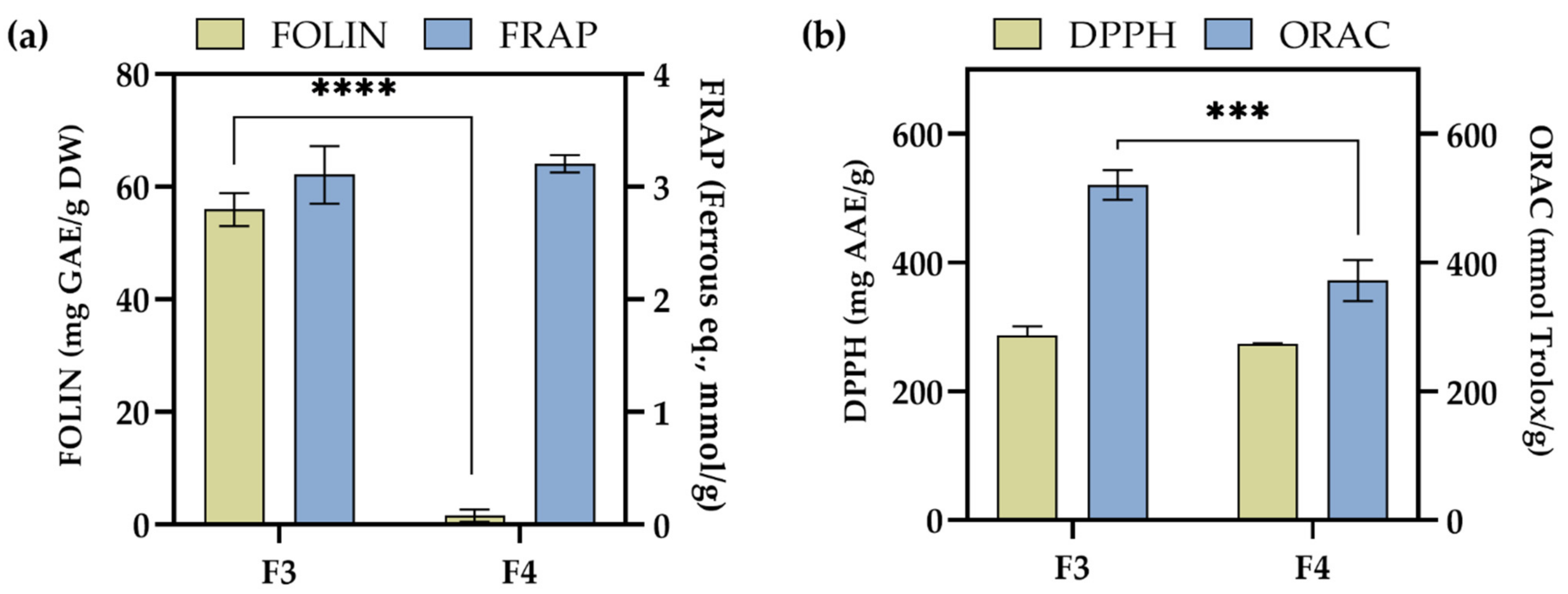
2.4. Antioxidant Activity of F3 and F4 Fractions In Vitro
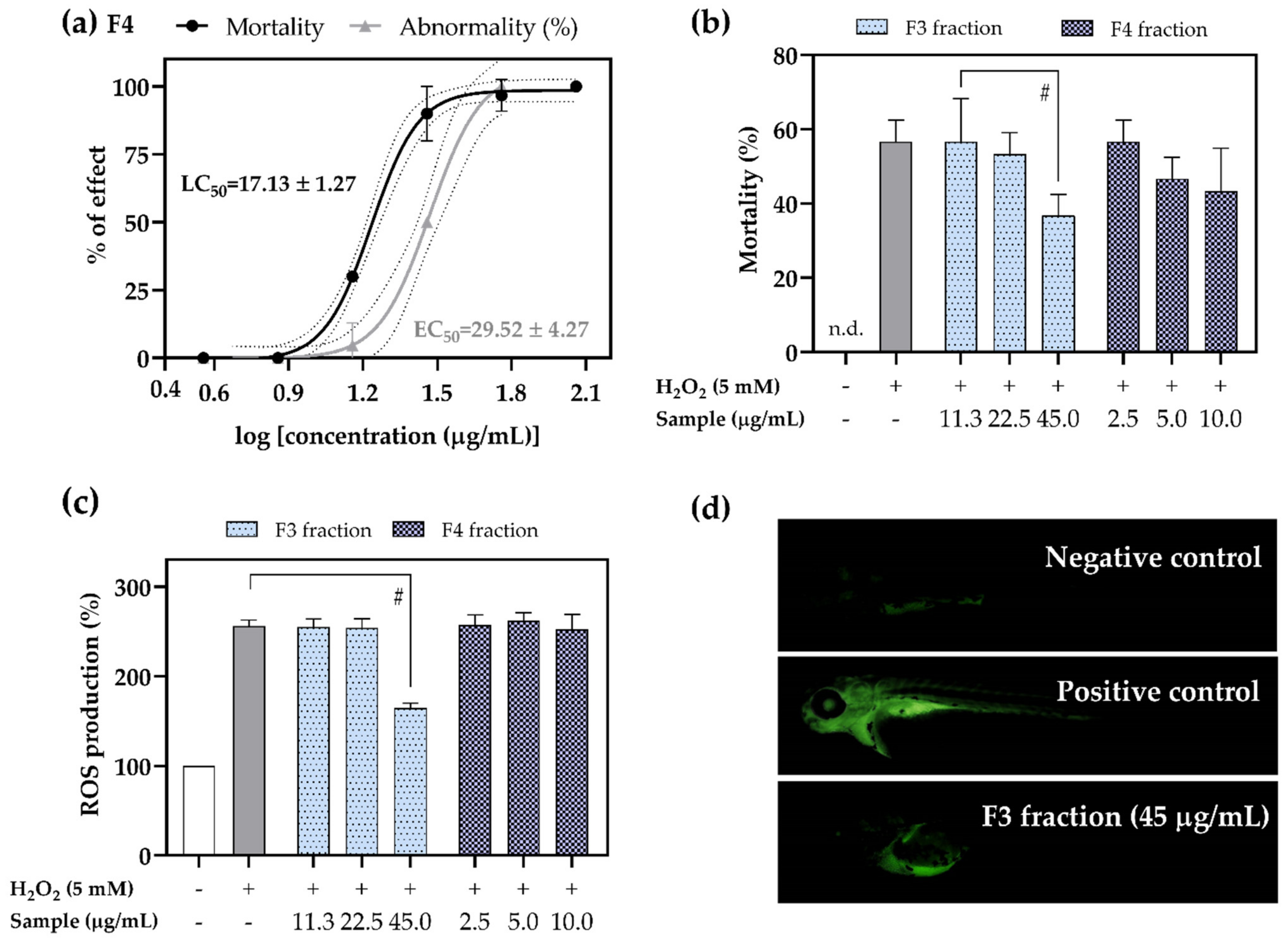
2.5. Antioxidant Activity of F3 and F4 Fractions In Vivo
3. Materials and Methods
3.1. Chemicals
3.2. Alga Sample
3.3. Headspace Solid-Phase Microextraction (HS-SPME)
3.4. Hydrodistillation (HD)
3.5. Gas Chromatography Mass Spectrometry Analysis of VOCs
3.6. Fractionation by Solid-Phase Extraction (SPE)
3.7. Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry (UHPLC-ESI–HRMS) of F3 and F4
3.8. Antioxidant Activity of Tested Fractions by In Vitro Assays
3.9. Embryotoxicity Potential
3.10. Antioxidant Potential In Vivo
3.11. Ethical Statement
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geraldes, V.; Pinto, E. Mycosporine-like amino acids (MAAs): Biology, chemistry and identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Tiwari, B.S. Cyanotherapeutics: An emerging field for future drug discovery. J. Appl. Phycol. 2020, 1, 44–57. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant potential of extracts obtained from macro-(Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Gopeechund, A.; Bhagooli, R.; Neergheen, V.S.; Bolton, J.J.; Bahorun, T. Anticancer activities of marine macroalgae: Status and future perspectives. In Biodiversity and Biomedicine; Academic Press: Cambridge, MA, USA, 2020; pp. 257–275. [Google Scholar]
- Liu, J.; Obaidi, I.; Nagar, S.; Scalabrino, G.; Sheridan, H. The antiviral potential of algal-derived Macromolecules. Curr. Res. Biotechnol. 2021, 3, 120–134. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and analytical characterization of novel sulfated coumarins in the marine green macroalga Dasycladus vermicularis (Scopoli) krasser. Molecules 2018, 23, 2735. [Google Scholar] [CrossRef]
- Grbec, B.; Matić, F.; Beg Paklar, G.; Morović, M.; Popović, R.; Vilibić, I. Long-term trends, variability and extremes of in situ sea surface temperature measured along the eastern Adriatic coast and its relationship to hemispheric processes. Pure Appl. Geophys. 2018, 175, 4031–4046. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.-M.; Flanjak, I.; Babić, S.; Čižmek, L.; Šubarić, D.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Less polar compounds and targeted antioxidant potential (in vitro and in vivo) of Codium adhaerens C. Agardh 1822. Pharmaceuticals 2021, 14, 944. [Google Scholar] [CrossRef]
- Radman, S.; Čižmek, L.; Babić, S.; Cikoš, A.-M.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. Bioprospecting of Less-Polar Fractions of Ericaria crinita and Ericaria amentacea: Developmental Toxicity and Antioxidant Activity. Mar. Drugs 2022, 20, 57. [Google Scholar] [CrossRef]
- Jerković, I.; Cikoš, A.-M.; Babić, S.; Čižmek, L.; Bojanić, K.; Aladić, K.; Ul’yanovskii, N.V.; Kosyakov, D.S.; Lebedev, A.T.; Čož-Rakovac, R.; et al. Bioprospecting of less-polar constituents from endemic brown macroalga Fucus virsoides J. Agardh from the Adriatic Sea and targeted antioxidant effects in vitro and in vivo (zebrafish model). Mar. Drugs 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal Variability of Volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef] [PubMed]
- Boland, W. The chemistry of gamete attraction: Chemical structures, biosynthesis, and (a)biotic degradation of algal pheromones. Proc. Natl. Acad. Sci. USA 1995, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Kawai, T.; Kajiwara, T.; Ishida, Y. Volatile components in protoplasts isolated from the marine brown alga Dictyopteris prolifera (Dictyotales). Plant Tissue Cult. Lett. 1994, 11, 34–39. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Kodama, K.; Ochi, S.; Fujimura, T. Dictyopterenes from three Japanese brown algae. Phytochemistry 1991, 30, 1805–1807. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Akakabe, Y.; Shimizu, H.; Kajiwara, T. Neodictyoprolenol and dictyoprolenol, the possible biosynthetic intermediates of dictyopterenes, in the Japanese brown algae Dictyopteris. J. Biosci. 2001, 56, 6–12. [Google Scholar] [CrossRef][Green Version]
- Endo, Y.; Usuki, R.; Kaneda, T. Antioxidant effects of chlorophyll and pheophytin on the autoxidation of oils in the dark. I. Comparison of the inhibitory effects. J. Am. Oil Chem. Soc. 1985, 62, 1375–1378. [Google Scholar] [CrossRef]
- Hiqashi-Okaj, K.; Otani, S.; Okai, Y. Potent suppressive effect of a Japanese edible seaweed, Enteromorpha prolifera (Sujiao-nori) on initiation and promotion phases of chemically induced mouse skin tumorigenesis. Cancer Lett. 1999, 104, 21–25. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S.G. Antioxidant properties of extract and fractions from. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Goss, R.; Latowski, D. Lipid Dependence of Xanthophyll Cycling in Higher Plants and Algae. Front. Plant Sci. 2020, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Liau, B.C.; Hong, S.E.; Chang, L.P.; Shen, C.T.; Li, Y.C.; Wu, Y.P.; Chang, C.-M.J. Separation of sight-protecting zeaxanthin from Nannochloropsis oculata by using supercritical fluids extraction coupled with elution chromatography. Sep. Purif. Technol. 2021, 78, 1–8. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Yanrong, L.; Yamin, L.; Tong, M.; Aiying, K. Sphingolipids in marine microalgae: Development and application of a mass spectrometric method for global structural characterization of ceramides and glycosphingolipids in three major phyla. Anal. Chim. Acta 2017, 986, 82–94. [Google Scholar]
- Nixon, G.F. Sphingolipids in inflammation: Pathological implications and potential therapeutic targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef]
- Bisogno, T.; Sepe, N.; De Petrocellis, L.; Mechoul, R.; Di Marzo, V. The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem. Biophys. Res. Commun. 1997, 239, 473–479. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.A.; Hong, J.H.; Kim, J.S.; Kim, D.K.; Kim, C.S. Oleamide suppresses inflammatory responses in LPS-induced RAW264.7 murine macrophages and alleviates paw edema in a carrageenan-induced inflammatory rat model. Int. Immunopharmacol. 2018, 56, 179–185. [Google Scholar] [CrossRef]
- Bertin, M.J.; Zimba, P.V.; Beauchesne, K.R.; Huncik, K.M.; Moeller, P.D. Identification of toxic fatty acid amides isolated from the harmful alga Prymnesium parvum carter. Harmful Algae 2012, 20, 111–116. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, S.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Chapman, M.J. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.L.; Burrin, D.G.; Guthrie, G.; Thijs, C. Plant-based sterols and stanols in health & disease: “Consequences of human development in a plant-based environment?”. Prog. Lipid Res. 2019, 74, 87–102. [Google Scholar]
- Catani, M.V.; Gasperi, V.; Bisogno, T.; Maccarrone, M. Essential Dietary Bioactive Lipids in Neuroinflammatory Diseases. Antioxid. Redox Signal. 2018, 29, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.W. The distribution of sterols in algae. Lipids 1971, 6, 120–127. [Google Scholar] [CrossRef]
- De Napoli, L.; Magno, S.; Mayol, L.; Novellino, E. Sterol composition of some Mediterranean green algae. Phytochemistry 1982, 21, 1993–1994. [Google Scholar] [CrossRef]
- El-Hamidi, M.; Zaher, F.A.; El-Shami, S.M. Interaction of oilseed pigments and phospholipids in the determination of total phenolic compounds using the Folin-Ciocalteu reagent. Int. J. Pharmtech. Res. 2016, 9, 207–214. [Google Scholar]
- Estevão, M.S.; Carvalho, L.C.; Ribeiro, D.; Couto, D.; Freitas, M.; Gomes, A.; Ferreira, L.M.; Fernandes, E.; Marques, M.B. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010, 45, 4869–4878. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chao, P.Y.; Hu, S.P.; Yang, C.M. The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Food Sci. Nutr. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Thomas, S.E.; Johnson, E.J. Xanthophylls. Adv. Nutr. 2018, 9, 160–162. [Google Scholar] [CrossRef]
- Alessenko, A.V.; Albi, E. Exploring sphingolipid implications in neurodegeneration. Front. Neurol. 2020, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Čižmek, L.; Maršavelski, A.; Malev, O.; Pflieger, M.; Strunjak-Perović, I.; Topić Popović, N.; Čož-Rakovac, R.; Trebše, P. Utilization of the zebrafish model to unravel the harmful effects of biomass burning during Amazonian wildfires. Sci. Rep. 2021, 11, 2527. [Google Scholar] [CrossRef] [PubMed]

| No. | Compounds | RI | Area (%) ± SD | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| 1 | Pent-1-en-3-ol | <900 | - | - | 1.34 ± 0.26 | - |
| 2 | Pentanal | <900 | - | - | - | 0.52 ± 0.10 |
| 3 | Pentan-1-ol | <900 | - | - | - | 0.72 ± 0.05 |
| 4 | (Z)-Pent-2-en-1-ol | <900 | - | - | - | 1.20 ± 0.10 |
| 5 | Hexanal | <900 | - | 3.40 ± 0.82 | - | 6.12 ± 0.29 |
| 6 | Nonane | 900 | - | - | 2.73 ± 0.15 | - |
| 7 | Heptanal | 907 | - | - | - | 2.35 ± 0.14 |
| 8 | Benzaldehyde | 970 | 27.79 ± 1.29 | 9.82 ± 0.06 | 27.34 ± 1.20 | 7.30 ± 0.26 |
| 9 | Phenol | 984 | 1.50 ± 0.01 | 6.64 ± 0.07 | 2.86 ± 0.08 | 7.02 ± 0.18 |
| 10 | Octan-3-one | 991 | 2.96 ± 0.09 | - | 1.96 ± 0.023 | - |
| 11 | 6-Methylhept-5-en-2-one | 995 | - | - | 2.13 ± 0.13 | - |
| 12 | 2-Pentylfuran | 996 | - | 2.18 ± 0.29 | - | 2.52 ± 0.05 |
| 13 | Octanal | 1007 | - | 2.54 ± 0.22 | - | 4.62 ± 0.15 |
| 14 | 2-Ethylhexan-1-ol | 1035 | - | 1.36 ± 0.00 | - | 0.83 ± 0.33 |
| 15 | Benzyl alcohol | 1041 | 2.07 ± 0.19 | 57.92 ± 0.84 | 2.59 ± 0.23 | 52.48 ± 0.44 |
| 16 | (E)-Oct-2-enal | 1064 | 1.73 ± 0.24 | 0.86 ± 0.30 | 1.15 ± 0.09 | |
| 17 | Acetophenone | 1073 | 1.41 ± 0.11 | 0.87 ± 0.08 | 1.23 ± 0.02 | - |
| 18 | (E,E)-Octa-3,5-dien-2-one | 1076 | 2.79 ± 0.17 | 1.41 ± 0.19 | 2.50 ± 0.16 | 1.46 ± 0.05 |
| 19 | Nonanal | 1108 | - | 4.43 ± 0.30 | - | 2.72 ± 0.47 |
| 20 | 2,6-Dimethylcyclohexan-1-ol | 1114 | - | - | - | 1.62 ± 0.16 |
| 21 | 6-[(1Z)-Butenyl]-1,4-cycloheptadiene] (Dictyopterene D′) | 1158 | 7.78 ± 0.48 | 1.81 ± 0.00 | 7.12 ± 0.20 | 1.41 ± 0.14 |
| 22 | (Z)-Non-2-enal | 1169 | 3.93 ± 0.24 | - | 4.45 ± 0.27 | - |
| 23 | [6-Butyl-1,4-cycloheptadiene] (Dictyopterene C′) | 1175 | 8.65 ± 0.78 | - | 9.34 ± 1.16 | - |
| 24 | Decanal | 1204 | 2.02 ± 0.20 | 1.46 ± 0.00 | 2.02 ± 0.18 | 1.03 ± 0.09 |
| 25 | 2-Phenylbut-2-enal (2-Phenylcrotonaldehyde) | 1278 | 24.20 ± 1.36 | - | 21.72 ± 0.69 | - |
| 26 | Heptadecane | 1703 | - | 3.25 ± 0.20 | - | 2.32 ± 0.10 |
| 27 | (E)-Nonadec-9-ene | 1878 | 5.89 ± 0.83 | 2.90 ± 0.08 | 1.54 ± 0.32 | 2.60 ± 0.31 |
| No. | Compound | RI | Area% ± SD | |
|---|---|---|---|---|
| V | VI | |||
| 1 | Nonane | 900 | 0.36 ± 0.15 | 0.06 ± 0.01 |
| 2 | Heptanal | 904 | - | 0.12 ± 0.03 |
| 3 | Benzaldehyde | 968 | 0.08 ± 0.00 | 0.33 ± 0.12 |
| 4 | Oct-1-en-3-ol | 984 | 0.24 ± 0.05 | 0.22 ± 0.02 |
| 5 | 2-Pentylfuran | 994 | 0.10 ± 0.01 | 0.22 ± 0.06 |
| 6 | Octanal | 1006 | - | 0.09 ± 0.03 |
| 7 | (E,E)-Hepta-2,4-dienal | 1015 | - | 0.06 ± 0.00 |
| 8 | Benzyl alcohol | 1040 | - | 0.15 ± 0.02 |
| 9 | Phenylacetaldehyde | 1051 | 0.15 ± 0.02 | 0.08 ± 0.00 |
| 10 | (E)-Oct-2-enal | 1064 | - | 0.08 ± 0.03 |
| 11 | (E)-Oct-2-en-1-ol | 1073 | 0.22 ± 0.07 | 0.24 ± 0.05 |
| 12 | (E,E)-Octa-3,5-dien-2-one | 1076 | 0.48 ± 0.23 | 0.16 ± 0.02 |
| 13 | Nonan-2-one | 1096 | 0.77 ± 0.17 | 0.03 ± 0.00 |
| 14 | Linalool | 1103 | 0.11 ± 0.00 | - |
| 15 | Nonanal | 1107 | - | 0.08 ± 0.01 |
| 16 | 2,6-Dimethylcyclohexan-1-ol | 1113 | - | 0.40 ± 0.13 |
| 17 | 6-[(1Z)-Butenyl]-cyclohepta-1,4-diene] (Dictyopterene D′) | 1158 | 0.17 ± 0.02 | - |
| 18 | (Z)-Non-2-enal | 1165 | - | 0.07 ± 0.02 |
| 19 | [6-Butylcyclohepta-1,4-diene] (Dictyopterene C′) | 1174 | 0.13 ± 0.03 | - |
| 20 | 2,4-Dimethylbenzaldehyde | 1177 | 0.15 ± 0.01 | - |
| 21 | Decan-2-one | 1192 | 0.26 ± 0.07 | - |
| 22 | Decanal | 1204 | 3.66 ± 0.25 | 0.07 ± 0.01 |
| 23 | (Z,E)-Nona-2,4-dienal | 1218 | - | 0.06 ± 0.00 |
| 24 | β-Cyclocitral | 1226 | - | 0.08 ± 0.03 |
| 25 | Decan-1-ol | 1277 | - | 0.14 ± 0.04 |
| 26 | 2-Phenylbut-2-enal | 1278 | 0.17 ± 0.06 | - |
| 27 | 2,6,11-Trimethyldodecane | 1283 | 0.25 ± 0.04 | - |
| 28 | Indole | 1296 | - | 0.28 ± 0.09 |
| 29 | (Z)-Tridec-3-ene | 1296 | 0.46 ± 0.11 | - |
| 30 | Undecanal | 1311 | - | 0.09 ± 0.01 |
| 31 | (E,E)-Deca-2,4-dienal | 1320 | - | 0.26 ± 0.10 |
| 32 | (E)-Undec-2-en-1-ol | 1347 | - | 0.12 ± 0.03 |
| 33 | β-Cubebene | 1394 | 0.15 ± 0.06 | |
| 34 | β-Elemene | 1395 | 0.54 ± 0.10 | 0.18 ± 0.06 |
| 35 | Dodecanal | 1413 | 0.08 ± 0.01 | 0.13 ± 0.03 |
| 36 | α-Ionone | 1433 | 0.10 ± 0.01 | 0.12 ± 0.01 |
| 37 | (Z)-Geranylacetone | 1458 | - | 0.18 ± 0.04 |
| 38 | (E)-Dodec-5-en-1-ol | 1465 | - | 0.18 ± 0.03 |
| 39 | Dodecan-1-ol | 1479 | 1.18 ± 0.21 | 0.51 ± 0.15 |
| 40 | Germacrene D | 1485 | 0.72 ± 0.17 | - |
| 41 | β-Ionone | 1490 | 0.27 ± 0.11 | 2.61 ± 0.30 |
| 42 | Pentadec-1-ene | 1495 | 1.04 ± 0.17 | 0.29 ± 0.02 |
| 43 | (E)-β-Guaiene | 1498 | 0.76 ± 0.30 | - |
| 44 | Tridecan-2-one | 1499 | - | 0.31 ± 0.10 |
| 45 | Pentadecane | 1500 | 0.75 ± 0.22 | 0.27 ± 0.09 |
| 46 | Germacrene A | 1509 | 2.31 ± 0.31 | 0.20 ± 0.05 |
| 47 | Tridecanal | 1514 | 1.01 ± 0.18 | 0.23 ± 0.03 |
| 48 | β-Cadinene | 1520 | 1.31 ± 0.12 | 0.26 ± 0.02 |
| 49 | Myristicine | 1527 | 2.03 ± 0.32 | - |
| 50 | Zonarene | 1530 | 0.27 ± 0.03 | - |
| 51 | (E)-Cadina-1,4-diene | 1537 | 0.31 ± 0.07 | - |
| 52 | Tridecan-1-ol | 1581 | 1.25 ± 0.20 | 0.50 ± 0.05 |
| 53 | Gleenol | 1590 | 0.11 ± 0.00 | - |
| 54 | Hexadecane | 1600 | 0.21 ± 0.03 | - |
| 55 | Tetradecanal | 1616 | 0.33 ± 0.02 | 0.37 ± 0.03 |
| 56 | Cubenol | 1648 | 3.46 ± 0.56 | 0.65 ± 0.10 |
| 57 | α-Cadinol | 1660 | 0.47 ± 0.19 | 0.22 ± 0.02 |
| 58 | Tetradecan-1-ol | 1682 | 1.95 ± 0.20 | 3.16 ± 0.33 |
| 59 | (E)-Heptadec-8-ene | 1697 | 1.39 ± 0.31 | 0.37 ± 0.12 |
| 60 | Heptadecane | 1700 | 1.41 ± 0.10 | 1.32 ± 0.20 |
| 61 | Pentadecanal | 1719 | 2.02 ± 0.08 | 0.74 ± 0.08 |
| 62 | Tetradecanoic acid | 1770 | 1.86 ± 0.33 | - |
| 63 | Octadec-1-ene | 1780 | 0.15 ± 0.06 | 0.20 ± 0.02 |
| 64 | Pentadecan-1-ol | 1784 | 0.54 ± 0.09 | 1.64 ± 0.34 |
| 65 | Octadecane | 1800 | - | 0.10 ± 0.00 |
| 66 | Hexadecanal | 1821 | 0.57 ± 0.11 | 1.25 ± 0.25 |
| 67 | 6,10,14-Trimethylpentadecan-2-one | 1850 | 0.56 ± 0.21 | 2.14 ± 0.36 |
| 68 | (Z)-Hexadeca-1,9-diene | 1865 | 0.42 ± 0.07 | 5.26 ± 0.44 |
| 69 | Diisobutyl phthalate | 1873 | 0.66 ± 0.08 | 1.66 ± 0.27 |
| 70 | (E)-Nonadec-9-ene | 1878 | 5.77 ± 0.61 | 12.79 ± 0.82 |
| 71 | Hexadecan-1-ol | 1885 | 1.42 ± 0.30 | 10.37 ± 0.36 |
| 72 | Nonadec-1-ene | 1897 | 0.36 ± 0.06 | 0.28 ± 0.07 |
| 73 | Nonadecane | 1900 | 0.85 ± 0.11 | 2.06 ± 0.34 |
| 74 | Heptadecan-2-one | 1911 | 0.19 ± 0.05 | 0.34 ± 0.10 |
| 75 | (E,E)-Farnesyl acetone | 1923 | - | 0.56 ± 0.11 |
| 76 | Isophytol | 1953 | - | 0.46 ± 0.10 |
| 77 | Dibutyl phtalate | 1967 | - | 0.57 ± 0.13 |
| 78 | Hexadecanoic acid | 1970 | 1.94 ± 0.26 | 0.76 ± 0.14 |
| 79 | (Z)-Octadec-9-enal | 1998 | 0.43 ± 0.10 | 0.28 ± 0.06 |
| 80 | Eicosane | 2000 | 0.09 ± 0.00 | 0.14 ± 0.04 |
| 81 | Octadecanal | 2024 | 0.59 ± 0.23 | 0.89 ± 0.24 |
| 82 | Geranyllinalool | 2033 | - | 0.16 ± 0.06 |
| 83 | (Z)-Falcarinol | 2045 | 2.52 ± 0.44 | 2.02 ± 0.59 |
| 84 | Methyl heptadeca-5-8-11-trienoate | 2049 | 0.91 ± 0.18 | 0.59 ± 0.18 |
| 85 | (Z,Z,Z)-Octadeca-9,12,15-trien-1-ol | 2056 | 0.36 ± 0.16 | 0.42 ± 0.08 |
| 86 | (Z)-Octadec-9-en-1-ol | 2061 | 0.20 ± 0.07 | 8.13 ± 0.96 |
| 87 | (Z,Z)-Octadeca-3,13-dien-1-ol | 2070 | 0.23 ± 0.03 | - |
| 88 | Heneicos-10-ene | 2075 | 0.83 ± 0.15 | 2.42 ± 0.42 |
| 89 | Octadecan-1-ol | 2088 | 0.78 ± 0.32 | 0.62 ± 0.11 |
| 90 | Heneicosane | 2100 | 0.36 ± 0.14 | 0.46 ± 0.02 |
| 91 | (Z,Z)-Octadeca-9,12-dienoic acid | 2110 | 1.34 ± 0.35 | 0.40 ± 0.15 |
| 92 | (E)-Phytol | 2116 | 16.69 ± 0.65 | 16.32 ± 1.01 |
| 93 | Docosane | 2200 | 7.69 ± 0.90 | 0.33 ± 0.14 |
| 94 | (E)-Geranylgeraniol | 2206 | 0.45 ± 0.12 | - |
| 95 | Cembra-4,7,11,15-tetraen-3-ol | 2231 | 4.30 ± 0.20 | 0.90 ± 0.20 |
| F3 | F4 | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | tR (min) | Name | Structure | Mono-Isotopic Mass | [M + H]+ | Mass Difference (ppm) | Area (Counts) | |
| Pigments | ||||||||
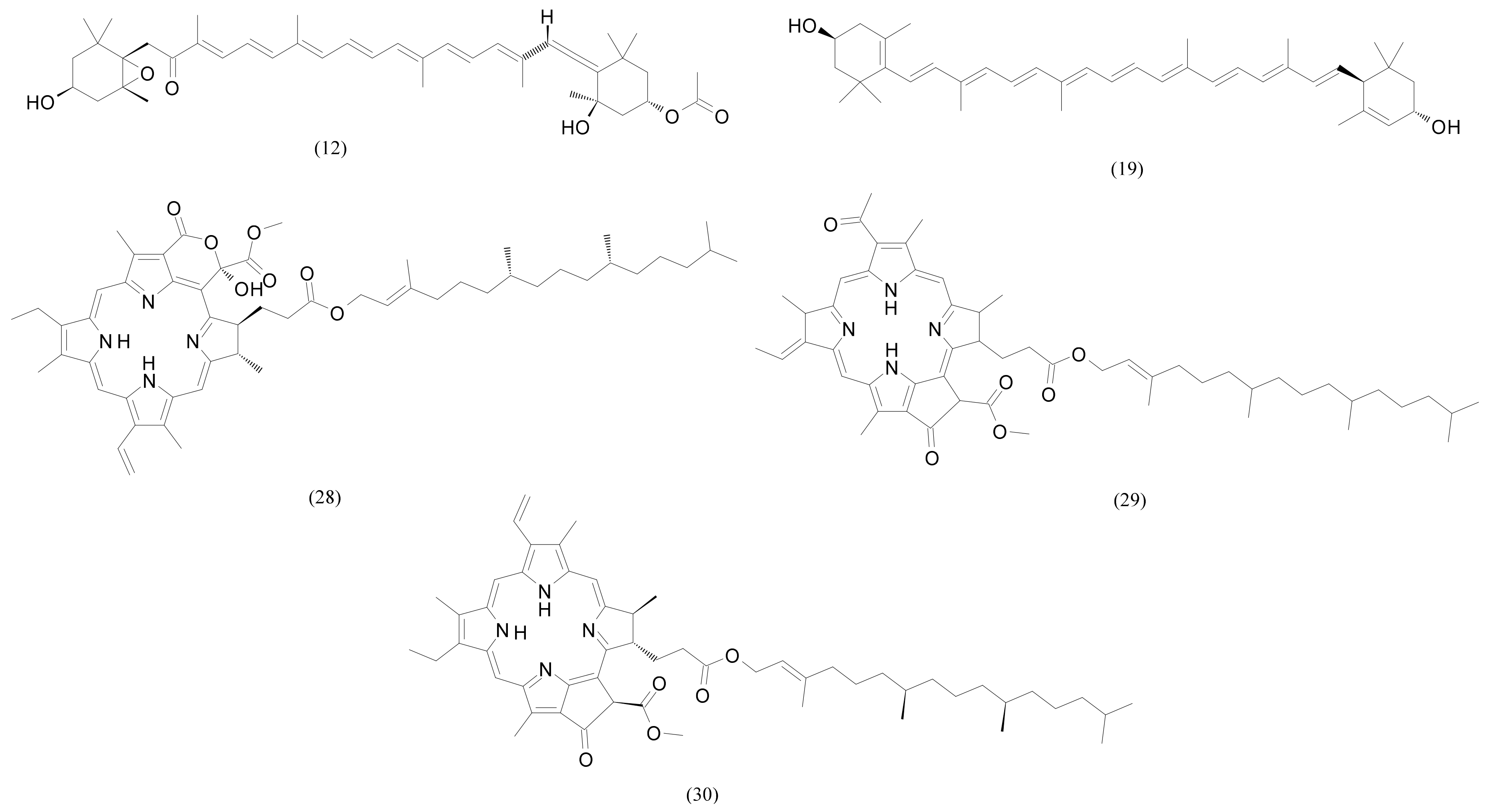
| 12 | 14.96 | Fucoxanthin | C42H58O6 | 658.423340 | 659.43062 | 3.5 | 388,447 | 11,204 |
| 15 | 15.57 | Pheophorbide a | C35H36N4O5 | 592.268570 | 593.27585 | 0.5 | 14,419 | 5581 |
| 19 | 16.68 | Zeaxanthin/Lutein | C40H56O2 | 568.428040 | 569.43531 | 6.5 | 463,265 | 309,962 |
| 23 | 17.52 | Siphonein | C52H76O5 | 780.56928 | 781.57655 | 5.2 | 97,166 | 131,660 |
| 28 | 20.04 | Methyl (3R,10Z,14Z,20Z,22S,23S)-12-ethyl-3-hydroxy-13,18,22,27-tetramethyl-5-oxo-23-(3-oxo-3-{[(2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-yl]oxy}propyl)-17-vinyl-4-oxa-8,24,25,26-tetraazahexacycl;o [19.2.1.16,9.111,14.116,19.02,7]heptacosa-1(24),2(7),6(27),8,10,12,14,16,18,20-decaene-3-carboxylate | C55H74N4O7 | 902.555725 | 903.56303 | 0.4 | 69,855 | 2,573,134 |
| 29 | 20.05 | 3-Phorbinepropanoic acid, 9-acetyl-14-ethylidene-13,14-dihydro-21-(methoxycarbonyl)-4,8,13,18-tetramethyl-20-oxo-, 3,7,11,15-tetramethyl-2-hexadecen-1-yl ester | C55H74N4O6 | 886.560852 | 887.56811 | 2.0 | 677,361 | 38,222,272 |
| 30 | 20.18 | Pheophytin a | C55H74N4O5 | 870.565918 | 871.5732 | 1.4 | 25,152 | 738,479 |
| Fatty Acid Derivatives | ||||||||
| 6 | 13.96 | Palmitamide | C16H33NO | 255.25621 | 256.26349 | −2.0 | 243,821 | 3,675,708 |
| 7 | 13.99 | 1,3-Dihydroxy-2-propanyl 5,8,11,14-icosatetraenoate | C23H38O4 | 378.277008 | 379.28429 | 3.0 | 16,874 | - |
| 8 | 14.23 | 2,3-Dihydroxypropyl palmitate | C19H38O4 | 330.277008 | 331.28429 | −5.2 | 110,342 | 253,278 |
| 9 | 14.35 | Oleamide | C18H35NO | 281.271851 | 282.27914 | 0.9 | 3,107,219 | 30,659,656 |
| 10 | 14.57 | 2,3-Dihydroxypropyl 9-octadecenoate | C21H40O4 | 356.292664 | 357.29994 | 2.0 | 19,415 | - |
| 13 | 15.30 | 2,3-Dihydroxypropyl stearate | C21H42O4 | 358.308319 | 359.31559 | 2.9 | 221,566 | 518,492 |
| 16 | 16.19 | Erucamide | C22H43NO | 337.334473 | 338.34174 | 2.4 | 670,677 | 2,888,407 |
| 17 | 16.38 | 2-Hydroxypropyl stearate | C21H42O3 | 342.313385 | 343.32067 | 2.1 | - | 188,262 |
| 18 | 16.48 | 3-{[6-O-(α-D-Galactopyranosyl)-β-D-galactopyranosyl]oxy}-2-[(Z)-9-hexadec-9-enoyloxy]propyl (Z,Z,Z)-9,12,15-octadecatrienoate | C49H84O15 | 912.580994 | 913.58830 | −0.3 | 67,693 | 16,012 |
| 20 | 16.94 | 1-Hexadecanoyl-2-(9Z,12Z,15Z-octadecatrienoyl)-3-O-(α-D-galactosyl-1-6-β-D-galactosyl)-sn-glycerol | C49H86O15 | 914.596672 | 915.60395 | 0.3 | 161,165 | 68,963 |
| 22 | 17.38 | N-(2-hydroxynonadecanoyl)-1-O-β-D-glucosyl-15-methylhexadecasphing-4-enine | C42H81NO9 | 743.59113 | 744.59841 | −2.0 | 1,591,978 | 59,570,336 |
| 25 | 17.70 | N-Nonadecanoyl-1-O-β-D-glucosyl-4-hydroxy-15-methylhexadecasphinganine | C42H83NO9 | 745.60678 | 746.61406 | 2.3 | 207,250 | 106,970,216 |
| 27 | 19.70 | 3-Hydroxy-1,2-propanediyl bis(9-octadecenoate) | C39H72O5 | 620.537964 | 621.54525 | 3.6 | 23,062 | - |
| Steroids and Terpenes | ||||||||
| 1 | 6.50 | Loliolide | C11H16O3 | 196.10994 | 197.11722 | 1.9 | 44,368 | 4657 |
| 2 | 9.30 | (3S,8S,9S,10R,13S,14S,17R)-17-[(2E)-4-Hydroxy-2-buten-2-yl]-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol | C23H36O2 | 344.271515 | 345.27881 | 4.9 | 575,025 | 283,200 |
| 3 | 9.44 | (22E)-Chola-5,22-dien-3-ol | C24H38O | 342.292267 | 343.29954 | 2.6 | 748,132 | 268,543 |
| 4 | 11.91 | Sargaquinoic acid | C27H36O4 | 424.261353 | 425.26864 | −3.5 | 5778 | - |
| 5 | 12.69 | 1′H-5α-Cholest-2-eno [3,2-b]indole | C33H49N | 459.38650 | 460.39378 | 3.1 | 714,421 | 5,802,712 |
| 11 | 14.94 | (3aR,4aR,6S,8aS)-1-Isopropyl-3a,8a-dimethyl-5-methylene-2,3,3a,4,4a,5,6,7,8,8a-decahydrobenzo[f]azulene-4a,6-diol | C20H30O2 | 302.22458 | 303.23186 | 5.6 | 146,533 | 3685 |
| 14 | 15.57 | (3aR,4aR,6S,8aR)-1-Isopropyl-3a,8a-dimethyl-5-methylene-2,3a,4,5,6,7,8,8a,9,10-decahydrobenzo[f]azulene-4a,6(3H)-diol (Isoamijiol) | C20H32O2 | 304.240234 | 305.24751 | 2.7 | 173,463 | 16,323 |
| 21 | 17.02 | 11-Hydroxy-3,20-dioxopregn-4-en-21-yl (9E)-9-octadecenoate | C39H62O5 | 610.459717 | 611.4670 | −2.4 | 129,163 | 22,853 |
| 24 | 17.57 | 6β-Hydroxystigmast-4-en-3-one | C29H48O2 | 428.36543 | 429.37271 | 1.5 | 504,060 | 230,167 |
| 26 | 18.44 | (3β)-3-Hydroxystigmast-5-en-7-one | C29H48O2 | 428.365431 | 429.37271 | −4.9 | - | 68,565 |
| Sample | IC50 Value (mg/mL) | Confidence Interval | Hillslope | R2 Value |
|---|---|---|---|---|
| F3 | 0.498 | 0.419–0.595 | 2.48 | 0.981 |
| F4 | 0.798 | 0.696–0.937 | 1.97 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radman, S.; Cikoš, A.-M.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome. Pharmaceuticals 2022, 15, 743. https://doi.org/10.3390/ph15060743
Radman S, Cikoš A-M, Babić S, Čižmek L, Čož-Rakovac R, Jokić S, Jerković I. In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome. Pharmaceuticals. 2022; 15(6):743. https://doi.org/10.3390/ph15060743
Chicago/Turabian StyleRadman, Sanja, Ana-Marija Cikoš, Sanja Babić, Lara Čižmek, Rozelindra Čož-Rakovac, Stela Jokić, and Igor Jerković. 2022. "In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome" Pharmaceuticals 15, no. 6: 743. https://doi.org/10.3390/ph15060743
APA StyleRadman, S., Cikoš, A.-M., Babić, S., Čižmek, L., Čož-Rakovac, R., Jokić, S., & Jerković, I. (2022). In Vivo and In Vitro Antioxidant Activity of Less Polar Fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the Chemical Composition of Fractions and Macroalga Volatilome. Pharmaceuticals, 15(6), 743. https://doi.org/10.3390/ph15060743