Exorbitant Drug Loading of Metformin and Sitagliptin in Mucoadhesive Buccal Tablet: In Vitro and In Vivo Characterization in Healthy Volunteers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solid-State Characterization
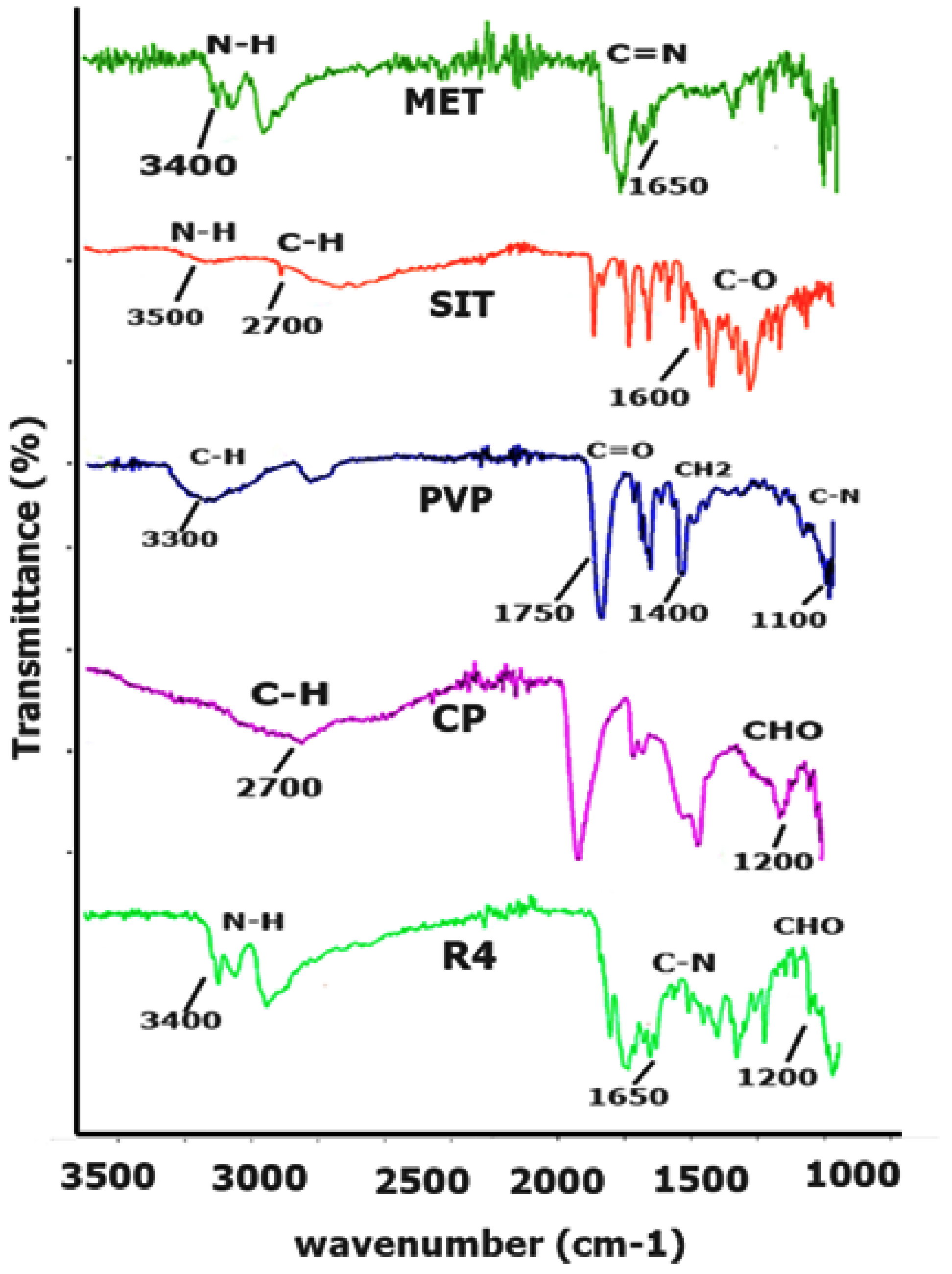
2.1.1. FTIR
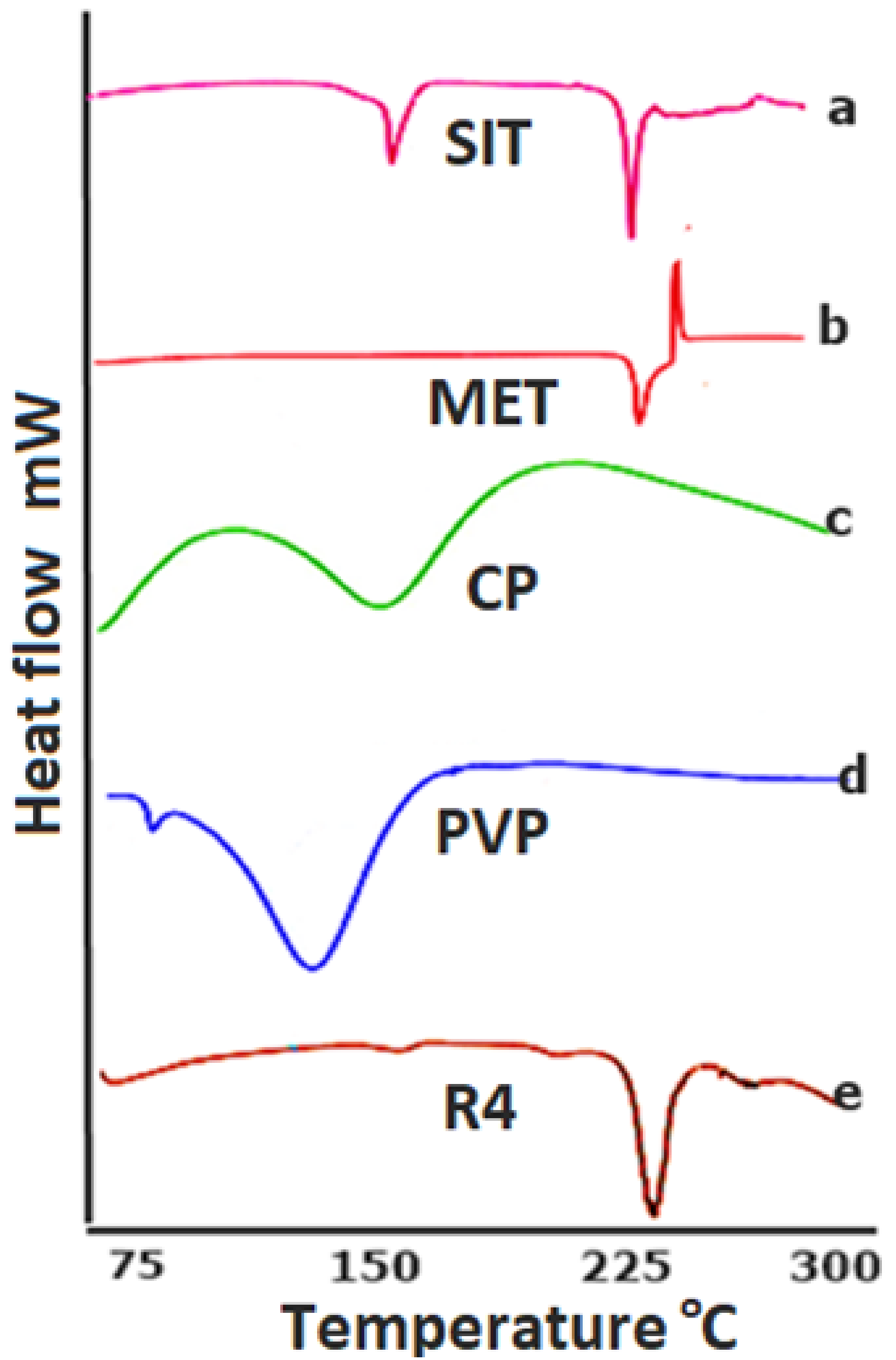
2.1.2. DSC
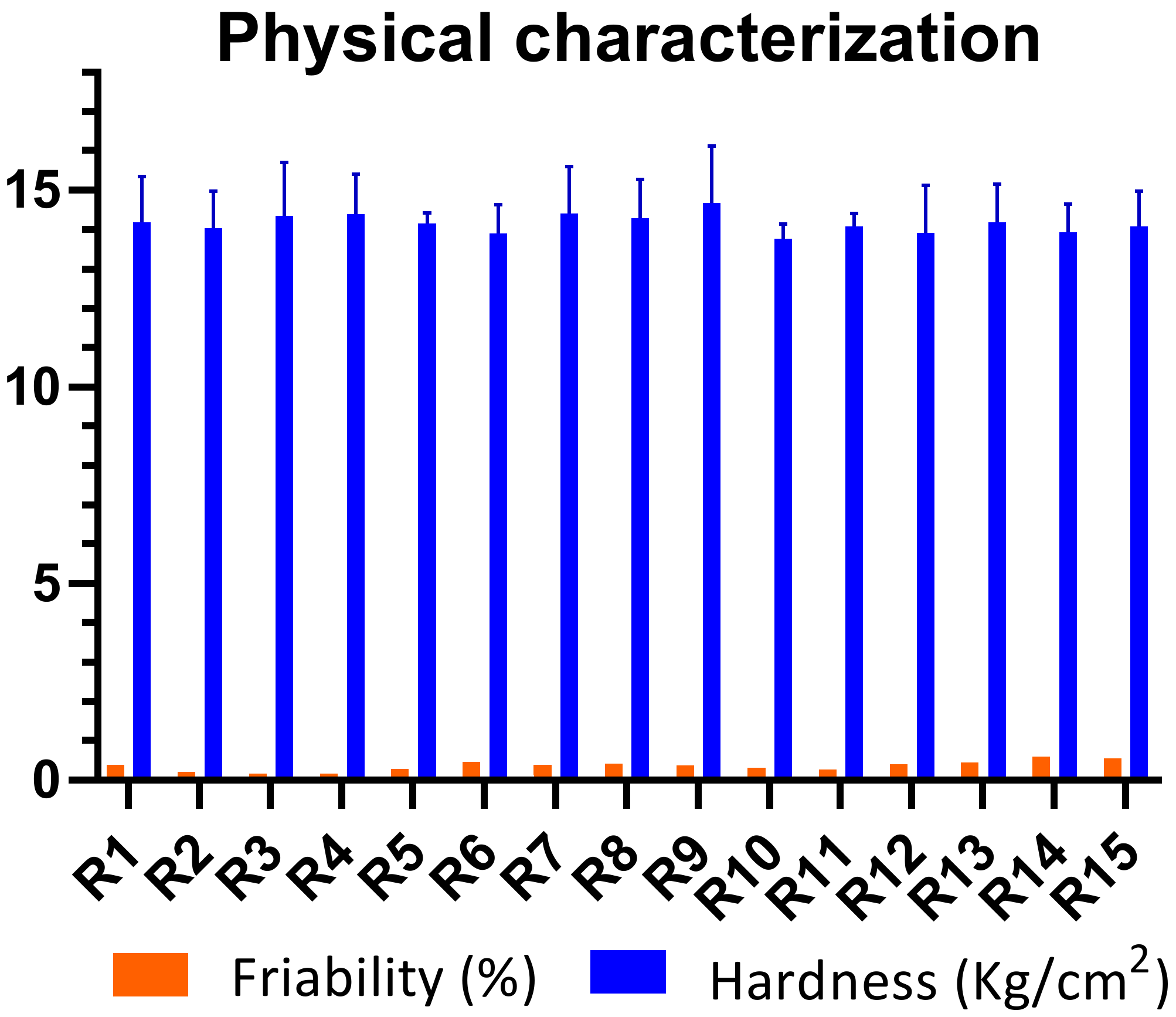
2.2. Physical Characterization of Mucoadhesive Buccal Tablets
2.3. Physicochemical Characterization
2.3.1. Content Uniformity
2.3.2. Surface pH
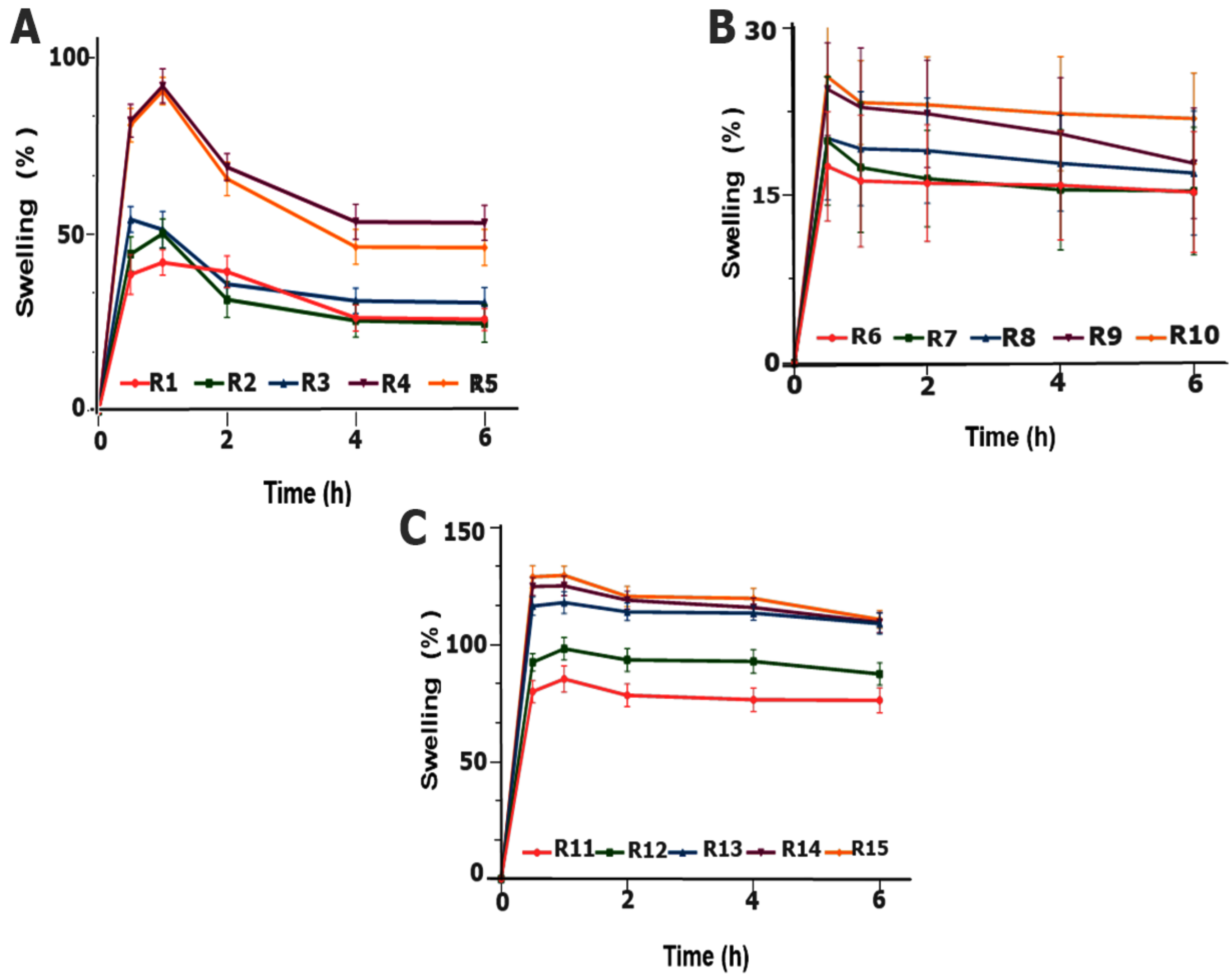
2.3.3. Swelling Index
2.3.4. Matrix Erosion
2.3.5. Ex Vivo Mucoadhesive Time (ET)
2.3.6. Mucoadhesive Strength (MS)
2.3.7. Mucoadhesive Study in Volunteers (MT)
2.3.8. In Vitro Hemolytic Analysis
2.3.9. In Vivo Histopathological Evaluation
2.3.10. In Vitro Cytotoxicity Analysis
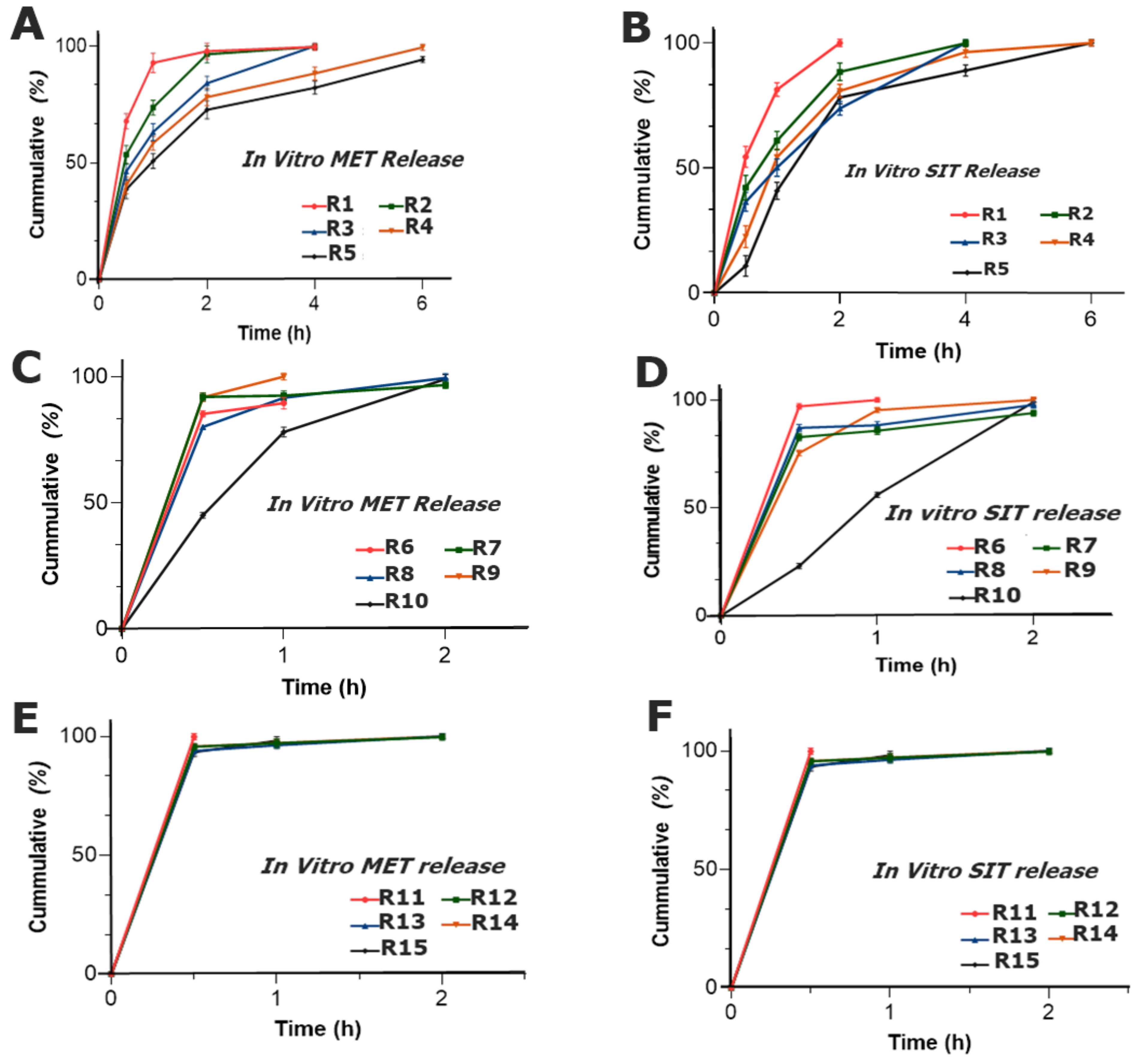
2.3.11. In Vitro Drug Release
Analytical Quantification of Metformin (MET) and Sitagliptin (SIT)
Dissolution Study
2.4. Optimization of Formulation
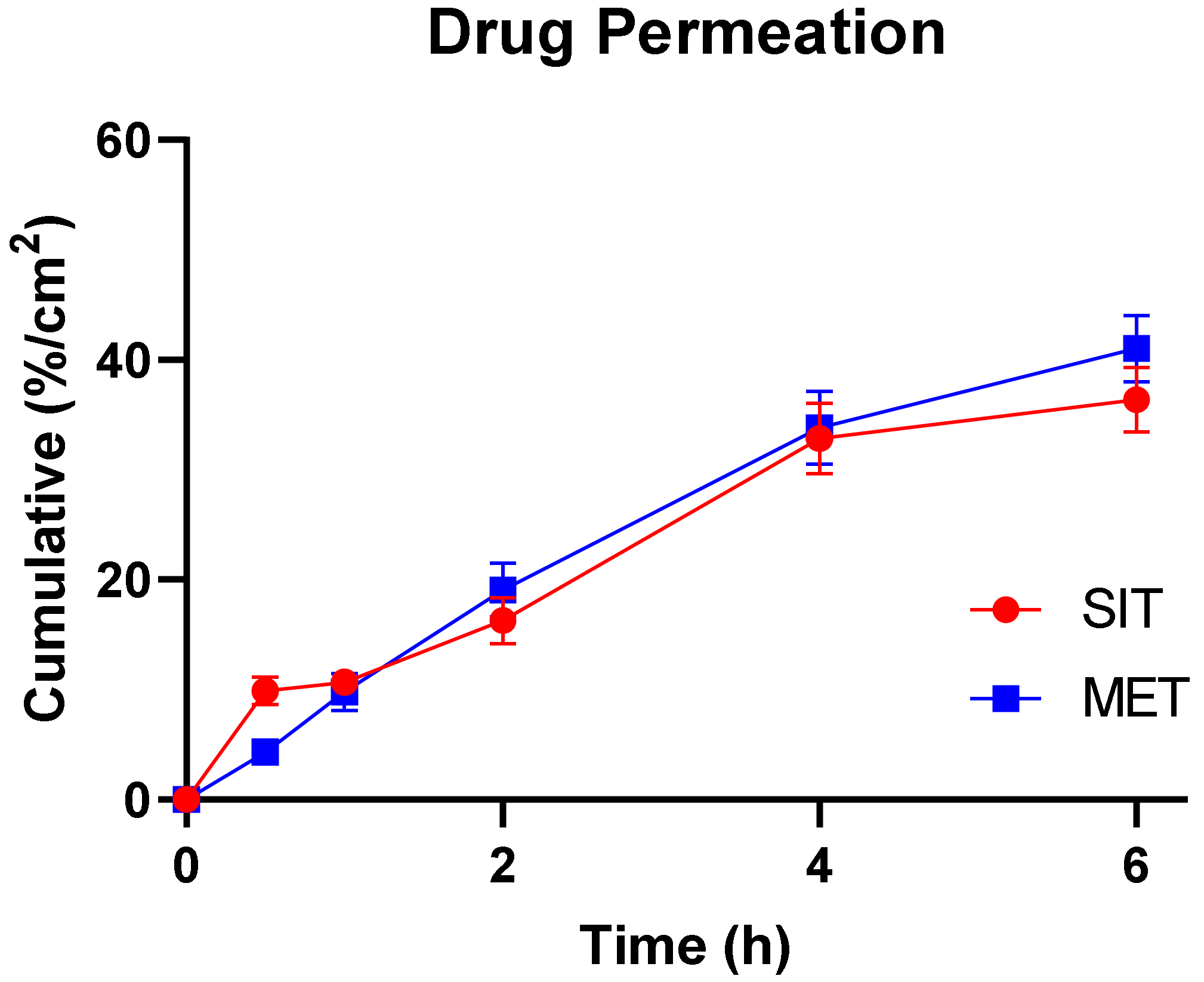
2.4.1. Ex Vivo Permeation Study
2.4.2. In Vitro Release Kinetics
2.4.3. Stability Study
2.4.4. Statistical Analysis
3. Materials and Methods
3.1. Materials
3.2. Formulation of Mucoadhesive Buccal Tablets
3.3. Dosage Form Design
3.4. Solid-State Characterization
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.4.2. Differential Scanning Calorimetry (DSC)
3.5. Physical Characterization of the Formualted Tablets
3.6. Physicochemical Characterization of Mucoadhesive Buccal Tablets
3.6.1. Surface pH
3.6.2. Content Uniformity
3.6.3. Swelling Index (SI)
3.6.4. Matrix Erosion
3.6.5. Ex Vivo Mucoadhesive Time (ET)
3.6.6. Ex Vivo Mucoadhesion Strength (MS)
3.6.7. Mucoadhesive Time in Volunteers (MT)
3.6.8. In Vitro Drug Release
HPLC Instrumental Conditions
Dissolution Study
3.6.9. In Vitro Drug Release Kinetics
3.6.10. Ex Vivo Permeation Study
3.6.11. In Vitro Hemolytic Analysis
3.6.12. In Vitro Cytotoxicity via MTT Assay
3.6.13. In Vivo Histopathological Analysis
3.6.14. Stability Study
3.6.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Targher, G.; Lonardo, A.; Byrne, C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.; Jha, J.C. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul. Surf. 2018, 16, 45–57. [Google Scholar] [CrossRef]
- Mansour, S.E.; Browning, D.J.; Wong, K.; Flynn, H.W., Jr.; Bhavsar, A.R. The evolving treatment of diabetic retinopathy. Clin. Ophthalmol. 2020, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, Y. Metformin and its benefits for various diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Shawky, L.M.; Morsi, A.A.; El Bana, E.; Hanafy, S.M. The biological impacts of sitagliptin on the pancreas of a rat model of type 2 diabetes mellitus: Drug interactions with metformin. Biology 2019, 9, 6. [Google Scholar] [CrossRef]
- Reifsnider, O.; Kansal, A.; Pimple, P.; Aponte-Ribero, V.; Brand, S.; Shetty, S. Cost-effectiveness analysis of empagliflozin versus sitagliptin as second-line therapy for treatment in patients with type 2 diabetes in the United States. Diabetes Obes. Metab. 2021, 23, 791–799. [Google Scholar] [CrossRef]
- Derosa, G.; Carbone, A.; Franzetti, I.; Querci, F.; Fogari, E.; Bianchi, L.; Bonaventura, A.; Romano, D.; Cicero, A.F.; Maffioli, P. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2012, 98, 51–60. [Google Scholar] [CrossRef]
- Haque, S.E.; Sheela, A. Development of polymer-bound fast-dissolving metformin buccal film with disintegrants. Int. J. Nanomed. 2015, 10, 199. [Google Scholar]
- Kanwal, A.; Iqbal, A.; Arshad, R.; Akhtar, S.; Razzaq, S.; Ahmad, N.M.; Naz, H.; Shahnaz, G. Formulation and Evaluation of Novel Thiolated Intra Pocket Periodontal Composite Membrane of Doxycycline. AAPS PharmSciTech 2019, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Minhas, M.U.; Irfan, M. Development and optimization of tibezonium iodide and lignocaine hydrochloride containing novel mucoadhesive buccal tablets: A pharmacokinetic investigation among healthy humans. Drug Dev. Ind. Pharm. 2021, 47, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; Irfan, N.; Danish, Z.; Hussain, N.; Ali, M.; Nasir, B.; Iqbal, J.; Saeed, H.; Ali, R.; Saleem, Z. Computer Aided Formulation and Characterization of Propranolol Hcl Buccal Tablet Using Polymeric Blend. Open Conf. Proc. J. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Razzaq, S.; Hanif, S.; Syed, M.A.; Iqbal, J.; Raza, S.A.; Riaz, H.; Abid, F. Development and evaluation of mucoadhesive buccal tablet containing metronidazole for the treatment of periodontitis and gingivitis. Pak. J. Pharm. Sci. 2018, 31, 1903–1910. [Google Scholar]
- Vijayakumar, T.M.; Jayram, J.; Meghana Cheekireddy, V.; Himaja, D.; Dharma Teja, Y.; Narayanasamy, D. Safety, Efficacy, and Bioavailability of Fixed-Dose Combinations in Type 2 Diabetes Mellitus: A Systematic Updated Review. Curr. Ther. Res. Clin. Exp. 2017, 84, 4–9. [Google Scholar] [CrossRef]
- Vaingankar, P.; Amin, P. Continuous melt granulation to develop high drug loaded sustained release tablet of Metformin HCl. Asian J. Pharm. Sci. 2017, 12, 37–50. [Google Scholar] [CrossRef]
- Batra, A.; Desai, D.; Serajuddin, A.T. Investigating the use of polymeric binders in twin screw melt granulation process for improving compactibility of drugs. J. Pharm. Sci. 2017, 106, 140–150. [Google Scholar] [CrossRef]
- Kelleher, J.F.; Madi, A.M.; Gilvary, G.C.; Tian, Y.; Li, S.; Almajaan, A.; Loys, Z.S.; Jones, D.S.; Andrews, G.P.; Healy, A.M. Metformin hydrochloride and sitagliptin phosphate fixed-dose combination product prepared using melt granulation continuous processing technology. Aaps PharmSciTech 2020, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal film preparations: Points to consider for patient centricity and manufacturing processes. Expert Opin. Drug Deliv. 2016, 13, 493–506. [Google Scholar] [CrossRef]
- Fahim, F.; Naseer, A.; Ahmed, S.; Sherazi, S.T.H.; Bhanger, M.I. A green approach for the determination of selected anti-diabetic drugs in pharmaceutical formulation by transmission FTIR spectroscopy. J. Braz. Chem. Soc. 2014, 25, 2032–2038. [Google Scholar] [CrossRef]
- Stofella, N.C.; Veiga, A.; Oliveira, L.J.; Montin, E.F.; Andreazza, I.F.; Carvalho Filho, M.A.; Bernardi, L.S.; Oliveira, P.R.; Murakami, F.S. Solid-state characterization of different crystalline forms of sitagliptin. Materials 2019, 12, 2351. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Khalid, I.; Minhas, M.U.; Barkat, K.; Khan, I.U.; Syed, H.K.; Umar, A. Preparation and in vitro evaluation of Chondroitin sulfate and carbopol based mucoadhesive controlled release polymeric composites of Loxoprofen using factorial design. Eur. Polym. J. 2019, 121, 109312. [Google Scholar] [CrossRef]
- Punčochová, K.; Ewing, A.V.; Gajdošová, M.; Sarvašová, N.; Kazarian, S.G.; Beránek, J.; Štěpánek, F. Identifying the mechanisms of drug release from amorphous solid dispersions using MRI and ATR-FTIR spectroscopic imaging. Int. J. Pharm. 2015, 483, 256–267. [Google Scholar] [CrossRef]
- Amal El Sayeh, F.; El Khatib, M.M. Formulation and evaluation of new long acting metoprolol tartrate ophthalmic gels. Saudi Pharm. J. 2014, 22, 555–563. [Google Scholar]
- Shavi, G.V.; Kumar, A.R.; Usha, Y.N.; Armugam, K.; Ranjan, O.P.; Ginjupalli, K.; Pandey, S.; Udupa, N. Enhanced dissolution and bioavailability of gliclazide using solid dispersion techniques. Int. J. Drug Deliv. 2010, 2, 49–57. [Google Scholar] [CrossRef]
- Gundogdu, N.; Cetin, M. Chitosan-poly (lactide-co-glycolide)(CS-PLGA) nanoparticles containing metformin HCl: Preparation and in vitro evaluation. Pak. J. Pharm. Sci. 2014, 27, 1923–1929. [Google Scholar]
- Hussain, A.; Syed, M.A.; Abbas, N.; Hanif, S.; Arshad, M.S.; Bukhari, N.I.; Hussain, K.; Akhlaq, M.; Ahmad, Z. Development of ann optimized mucoadhesive buccal tablet containing flurbiprofen and lidocaine for dental pain. Acta Pharm. 2016, 66, 245–256. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef]
- Çelik, B.; Özdemir, S.; Barla Demirkoz, A.; Üner, M. Optimization of piribedil mucoadhesive tablets for efficient therapy of Parkinson’s disease: Physical characterization and ex vivo drug permeation through buccal mucosa. Drug Dev. Ind. Pharm. 2017, 43, 1836–1845. [Google Scholar] [CrossRef]
- Muhammad, A.; Zahoor, A.F.; Iqbal, M.S.; Haroon, K.; Khan, I.U.; Shah, M.A.; Hanif, S.; Mohsin, N.A.; Islam, N.; Ikram, M.; et al. In vitro-Ex vivo Characterization of Agarose—Carbopol 934® Based Buccal Mucoadhesive Tablets Containing Benzocaine and Tibezonium Iodide as Model Drugs. Lat. Am. J. Pharm. 2022, 41, 1–10. [Google Scholar]
- Russo, E.; Selmin, F.; Baldassari, S.; Gennari, C.; Caviglioli, G.; Cilurzo, F.; Minghetti, P.; Parodi, B. A focus on mucoadhesive polymers and their application in buccal dosage forms. J. Drug Deliv. Sci. Technol. 2016, 32, 113–125. [Google Scholar] [CrossRef]
- Hamzavi, N.; Dewavrin, J.Y.; Drozdov, A.D.; Birgersson, E. Nonmonotonic swelling of agarose-carbopol hybrid hydrogel: Experimental and theoretical analysis. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 444–454. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Esim, O.; Savaser, A.; Ozkan, C.; Bayrak, Z.; Tas, C.; Ozkan, Y. Effect of polymer type on characteristics of buccal tablets using factorial design. Saudi Pharm. J. 2018, 26, 53–63. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K. Pharmaceutical Assessment of Polyvinylpyrrolidone (PVP): As Excipient from Conventional to Controlled Delivery Systems with a Spotlight on COVID-19 Inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Vasantha, P.V.; Puratchikody, A.; Mathew, S.T.; Balaraman, A.K. Development and characterization of Eudragit based mucoadhesive buccal patches of salbutamol sulfate. Saudi Pharm. J. 2011, 19, 207–214. [Google Scholar] [CrossRef]
- Srivalli, K.M.R.; Lakshmi, P.; Balasubramaniam, J. Design of a novel bilayered gastric mucoadhesive system for localized and unidirectional release of lamotrigine. Saudi Pharm. J. 2013, 21, 45–52. [Google Scholar] [CrossRef]
- Elbi, S.; Nimal, T.; Rajan, V.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of Salmonella. Colloids Surf. B 2017, 160, 40–47. [Google Scholar]
- Weber, D.; Torger, B.; Richter, K.; Nessling, M.; Momburg, F.; Woltmann, B.; Müller, M.; Schwartz-Albiez, R. Interaction of Poly(l-lysine)/Polysaccharide Complex Nanoparticles with Human Vascular Endothelial Cells. Nanomaterials 2018, 8, 358. [Google Scholar] [CrossRef]
- Javed, Q.U.A.; Syed, M.A.; Arshad, R.; Rahdar, A.; Irfan, M.; Raza, S.A.; Shahnaz, G.; Hanif, S.; Díez-Pascual, A.M.J.P. Evaluation and Optimization of Prolonged Release Mucoadhesive Tablets of Dexamethasone for Wound Healing: In Vitro–In Vivo Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 807. [Google Scholar] [CrossRef]
- Sakr, W.; Alanazi, F.; Sakr, A. Effect of Kollidon® SR on the release of Albuterol Sulphate from matrix tablets. Saudi Pharm. J. 2011, 19, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sheskey, P.J.; Cook, W.; Cable, C. Pharmaceutical Excipients. Med. Toxicol. Advers. Drug Exp. 2019, 3, 128–165. [Google Scholar]
- Chaudhary, J.P.; Kondaveeti, S.; Gupta, V.; Prasad, K.; Meena, R. Preparation and functional evaluation of agarose derivatives. J. Appl. Polym. Sci. 2014, 131, 40630. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Ali, S.; Iqbal, Z.; Shakir, R.; Iqbal, J. Formulation and Evaluation of Chitosan-Based Polymeric Biodegradable Mucoadhesive Buccal Delivery for Locally Acting Drugs: In Vitro, Ex Vivo and In Vivo Volunteers Characterization. Lat. Am. J. Pharm. 2021, 40, 670–681. [Google Scholar]
- Sander, C.; Nielsen, H.M.; Jacobsen, J. Buccal delivery of metformin: TR146 cell culture model evaluating the use of bioadhesive chitosan discs for drug permeability enhancement. Int. J. Pharm. 2013, 458, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Hussain, Z. Smart mucoadhesive buccal chitosan/HPMC scaffold for sore throat: In vitro, ex vivo and pharmacokinetic profiling in humans. J. Drug Deliv. Sci. Technol. 2022, 71, 103271. [Google Scholar] [CrossRef]
- Çelik, B. Risperidone mucoadhesive buccal tablets: Formulation design, optimization and evaluation. Drug Des. Dev. Ther. 2017, 11, 3355. [Google Scholar] [CrossRef]
- Syed, M.A.; Khan, I.U.; Iqbal, M.S.; Syed, H.K.; Irfan, M. Development of a Novel, Fast, Simple, Non-derived RP-HPLC Method for simultaneous Estimation of Benzocaine and Tibezonium Iodide from Mucoadhesive Dosage Form as well as Human Saliva and its Validation. Lat. Am. J. Pharm 2021, 40, 1281–1287. [Google Scholar]
- Nair, A.B.; Shah, J.; Jacob, S.; Al-Dhubiab, B.E.; Patel, V.; Sreeharsha, N.; Shinu, P. Development of Mucoadhesive Buccal Film for Rizatriptan: In Vitro and In Vivo Evaluation. Pharmaceutics 2021, 13, 728. [Google Scholar] [CrossRef]
- Hussain, A.; Shakeel, F.; Singh, S.K.; Alsarra, I.A.; Faruk, A.; Alanazi, F.K.; Christoper, G.P. Solidified SNEDDS for the oral delivery of rifampicin: Evaluation, proof of concept, in vivo kinetics, and in silico GastroPlusTM simulation. Int. J. Pharm. 2019, 566, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Mudakavi, R.J.; Vanamali, S.; Chakravortty, D.; Raichur, A.M. Development of arginine based nanocarriers for targeting and treatment of intracellular Salmonella. RSC Adv. 2017, 7, 7022–7032. [Google Scholar] [CrossRef]
- Elsharawy, A.M.; Shukr, M.H.; Elshafeey, A.H. Optimization and in vivo evaluation of duloxetine hydrochloride buccoadhesive lyophilized tablets. J. Drug Deliv. Sci. Technol. 2019, 52, 282–291. [Google Scholar] [CrossRef]




| Code | Average Weight | Diameter | Thickness |
|---|---|---|---|
| mg ± SD | mm ± SD | mm ± SD | |
| R1 | 649.9 ± 2.59 | 12.12 ± 0.02 | 5.67 ± 0.05 |
| R2 | 652.4 ± 2.19 | 12.13 ± 0.005 | 5.66 ± 0.12 |
| R3 | 650.3 ± 2.61 | 12.11 ± 0.04 | 5.67 ± 0.15 |
| R4 | 650.8 ± 1.69 | 12.13 ± 0.02 | 5.63 ± 0.09 |
| R5 | 651.1± 2.33 | 12.13 ± 0.04 | 5.66 ± 0.05 |
| R6 | 649.1 ± 3.18 | 12.15 ± 0.01 | 5.69 ± 0.11 |
| R7 | 645.4 ± 2.34 | 12.12 ± 0.06 | 5.63 ± 0.20 |
| R8 | 651.0 ± 3.23 | 12.15 ± 0.01 | 5.67 ± 0.08 |
| R9 | 648.2 ± 2.18 | 12.13 ± 0.07 | 5.66 ± 0.03 |
| R10 | 653.1 ± 2.76 | 12.12 ± 0.01 | 5.66 ± 0.46 |
| R11 | 647.9 ± 3.15 | 12.12 ± 0.01 | 5.67 ± 0.12 |
| R12 | 650.0 ± 1.97 | 12.13 ± 0.01 | 5.65 ± 0.09 |
| R13 | 650.7 ± 2.50 | 12.11 ± 0.01 | 5.66 ± 0.04 |
| R14 | 650.1 ± 2.85 | 12.13 ± 0.01 | 5.67 ± 0.10 |
| R15 | 651.6 ± 2.13 | 12.12 ± 0.01 | 5.66 ± 0.15 |
| Code | Content Uniformity | pH | ME% | |
|---|---|---|---|---|
| MET% ± SD | SIT% ± SD | |||
| R1 | 98.09 ± 1.56 | 100.25 ± 1.10 | 7.11 | 73.08 |
| R2 | 100.02 ± 0.75 | 99.59 ± 1.17 | 6.80 | 19.44 |
| R3 | 102.40 ± 0.95 | 99.98 ± 1.53 | 6.38 | 39.63 |
| R4 | 101.29 ± 1.66 | 100.53 ± 0.32 | 6.20 | 18.87 |
| R5 | 99.53 ± 1.11 | 101.76 ± 0.95 | 5.43 | 24.5 |
| R6 | 99.11 ± 1.39 | 100.92 ± 1.23 | 5.94 | 62.31 |
| R7 | 99.74 ± 1.56 | 99.49 ± 1.77 | 6.37 | 70.22 |
| R8 | 100.07 ± 1.05 | 98.71 ± 1.20 | 6.31 | 39.54 |
| R9 | 99.20 ± 1.63 | 97.13 ± 0.78 | 5.91 | 90.02 |
| R10 | 100.36 ± 0.80 | 100.90 ± 0.75 | 5.27 | 40.22 |
| R11 | 98.77 ± 1.36 | 101.73 ± 1.36 | 6.19 | 20.64 |
| R12 | 99.70 ± 1.18 | 102.71 ± 01.42 | 5.83 | 13.69 |
| R13 | 97.08 ± 1.29 | 98.33 ± 1.22 | 5.09 | 18.36 |
| R14 | 97.37 ± 1.17 | 99.82 ± 1.41 | 4.89 | 13.87 |
| R15 | 98.85 ± 0.64 | 99.75 ± 1.08 | 4.74 | 34.15 |
| Parameters | MET | SIT |
|---|---|---|
| Jss (mg/cm2.h) | 7.359 | 13.853 |
| Kp (cm/h) | 1.47 × 10−5 | 2.77 × 10−4 |
| Model | MET r2 (n) | SIT r2 (n) |
|---|---|---|
| Zero-order model | 0.9269 | 0.128 |
| First-order model | 0.9471 | 0.9765 |
| Higuchi model | 0.7433 | 0.8570 |
| Korsmeyer–Peppas model | 0.9633 (0.321) | 0.8813 (0.419) |
| Hixson–Crowell model | 0.8582 | 0.9747 |
| Time | Appearance | ET | MS | Contents % ± SD | |
|---|---|---|---|---|---|
| (Months) | h ± SD | g ± SD | SIT | MET | |
| 1 | White to off-white | 7.46 ± 3.76 | 24.54 ± 2.08 | 99.25 ± 0.54 | 99.20 ± 1.44 |
| 3 | White to off-white | 7.11 ± 2.19 | 23.68 ± 2.76 | 99.37 ± 1.01 | 98.59 ± 1.52 |
| 6 | White to off-white | 7.78 ± 3.33 | 26.37 ± 1.32 | 98.04 ± 1.23 | 98.31 ± 1.11 |
| Parameter | MET | SIT |
|---|---|---|
| Similarity factor (f2) | 67.47 | 62.35 |
| Dissimilarity factor (f1) | 5.88 | 7.17 |
| Before–After Stability | Mean | Standard Deviation | Standard Error Mean | 95% Confidence Interval of the Difference | t-Value | df | Significance (2-Tailed) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| MET | 3.57 | 2.73 | 1.11 | 0.697 | 6.445 | 3.194 | 5 | 0.21 |
| SIT | 3.25 | 4.95 | 2.02 | −1.947 | 8.457 | 1.608 | 5 | 0.346 |
| Code | CP | PVP | AG |
|---|---|---|---|
| R1 | 2 | 12 | - |
| R2 | 4 | 10 | - |
| R3 | 7 | 7 | - |
| R4 | 10 | 4 | - |
| R5 | 12 | 2 | - |
| R6 | - | 12 | 2 |
| R7 | - | 10 | 4 |
| R8 | - | 7 | 7 |
| R9 | - | 4 | 10 |
| R10 | - | 2 | 12 |
| R11 | 2 | - | 12 |
| R12 | 4 | - | 10 |
| R13 | 7 | - | 7 |
| R14 | 10 | - | 4 |
| R15 | 12 | - | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakir, R.; Hanif, S.; Salawi, A.; Arshad, R.; Sarfraz, R.M.; Irfan, M.; Raza, S.A.; Barkat, K.; Sabei, F.Y.; Almoshari, Y.; et al. Exorbitant Drug Loading of Metformin and Sitagliptin in Mucoadhesive Buccal Tablet: In Vitro and In Vivo Characterization in Healthy Volunteers. Pharmaceuticals 2022, 15, 686. https://doi.org/10.3390/ph15060686
Shakir R, Hanif S, Salawi A, Arshad R, Sarfraz RM, Irfan M, Raza SA, Barkat K, Sabei FY, Almoshari Y, et al. Exorbitant Drug Loading of Metformin and Sitagliptin in Mucoadhesive Buccal Tablet: In Vitro and In Vivo Characterization in Healthy Volunteers. Pharmaceuticals. 2022; 15(6):686. https://doi.org/10.3390/ph15060686
Chicago/Turabian StyleShakir, Rouheena, Sana Hanif, Ahmad Salawi, Rabia Arshad, Rai Muhammad Sarfraz, Muhammad Irfan, Syed Atif Raza, Kashif Barkat, Fahad Y. Sabei, Yosif Almoshari, and et al. 2022. "Exorbitant Drug Loading of Metformin and Sitagliptin in Mucoadhesive Buccal Tablet: In Vitro and In Vivo Characterization in Healthy Volunteers" Pharmaceuticals 15, no. 6: 686. https://doi.org/10.3390/ph15060686
APA StyleShakir, R., Hanif, S., Salawi, A., Arshad, R., Sarfraz, R. M., Irfan, M., Raza, S. A., Barkat, K., Sabei, F. Y., Almoshari, Y., Alshamrani, M., & Syed, M. A. (2022). Exorbitant Drug Loading of Metformin and Sitagliptin in Mucoadhesive Buccal Tablet: In Vitro and In Vivo Characterization in Healthy Volunteers. Pharmaceuticals, 15(6), 686. https://doi.org/10.3390/ph15060686










