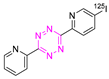
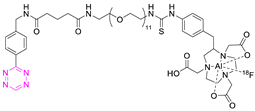
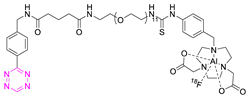
Recent Advances in the Development of Tetrazine Ligation Tools for Pretargeted Nuclear Imaging
Abstract
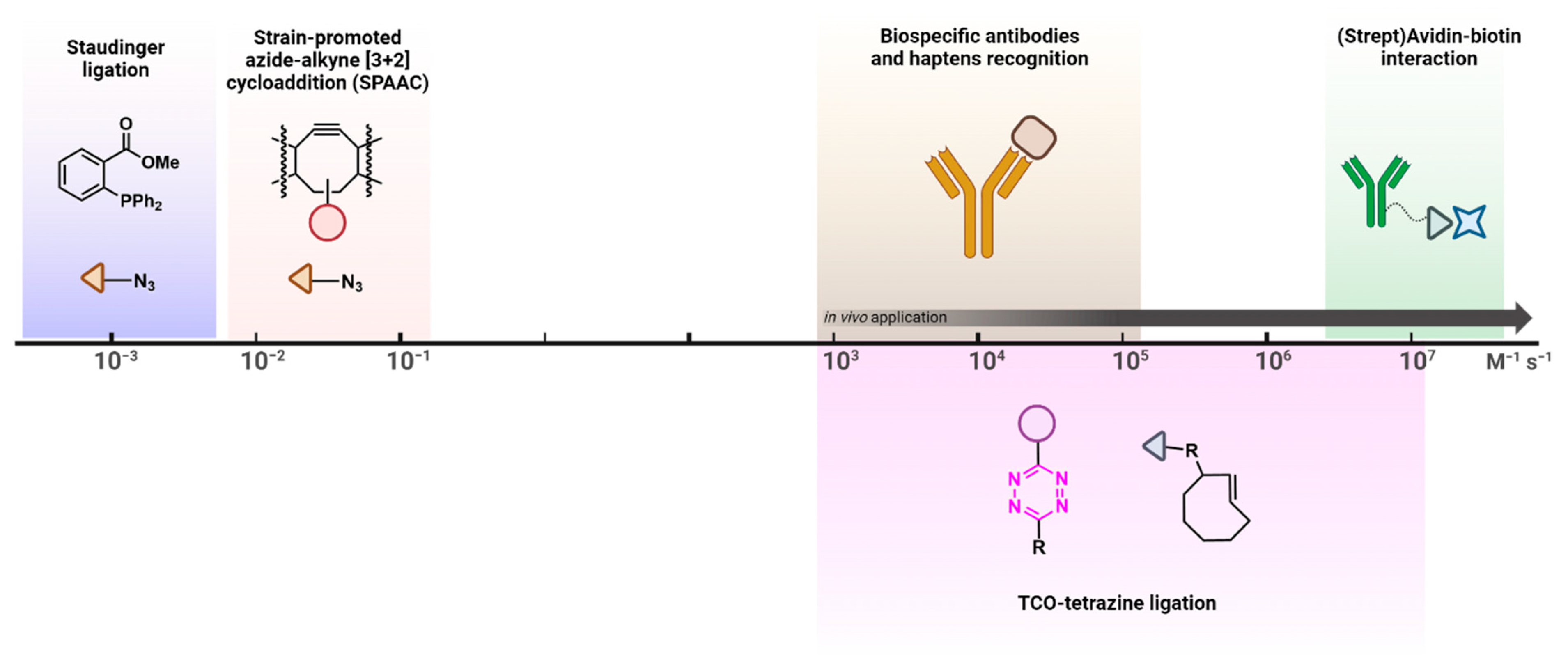
1. Introduction
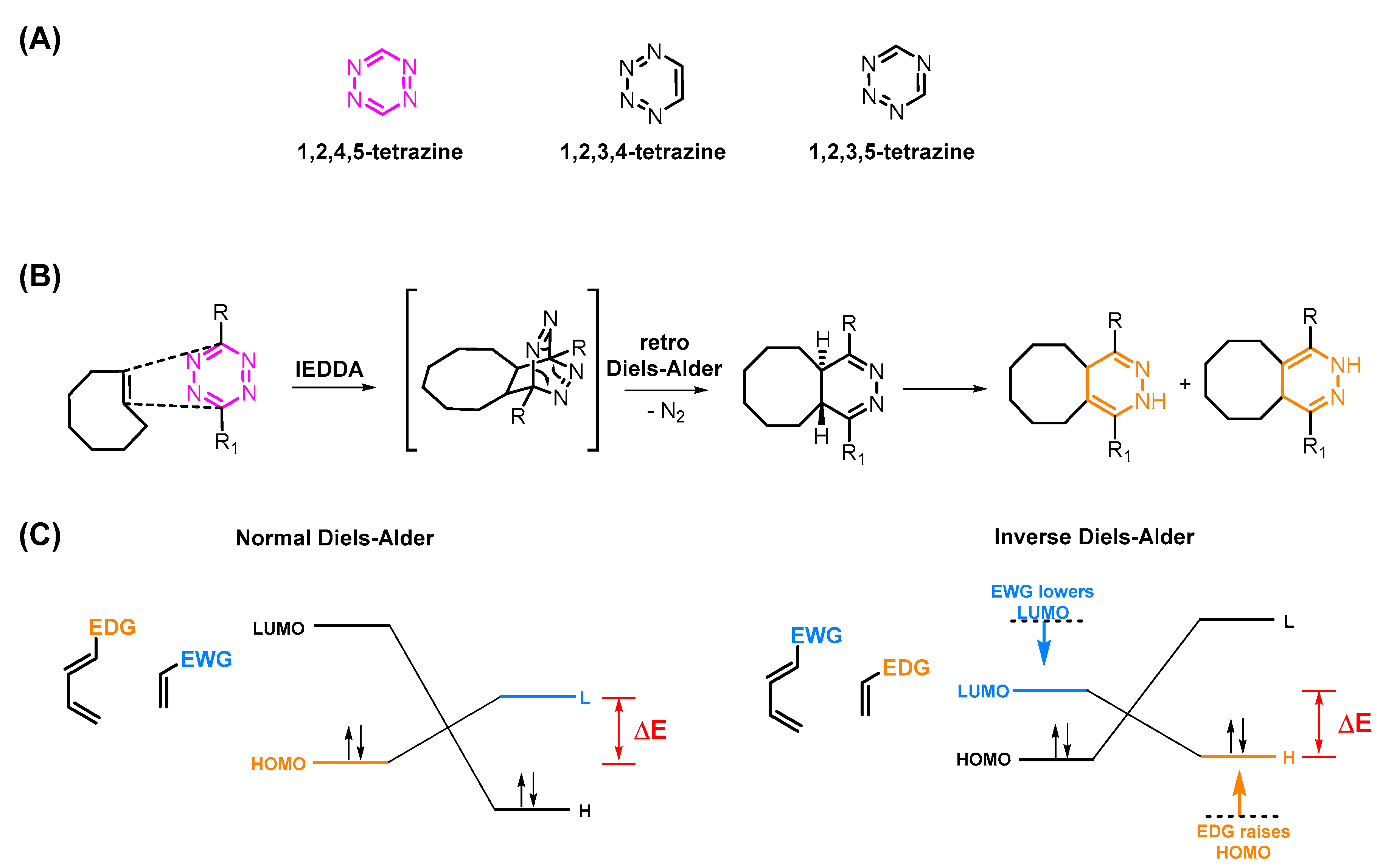
2. The Tetrazine–TCO Ligation
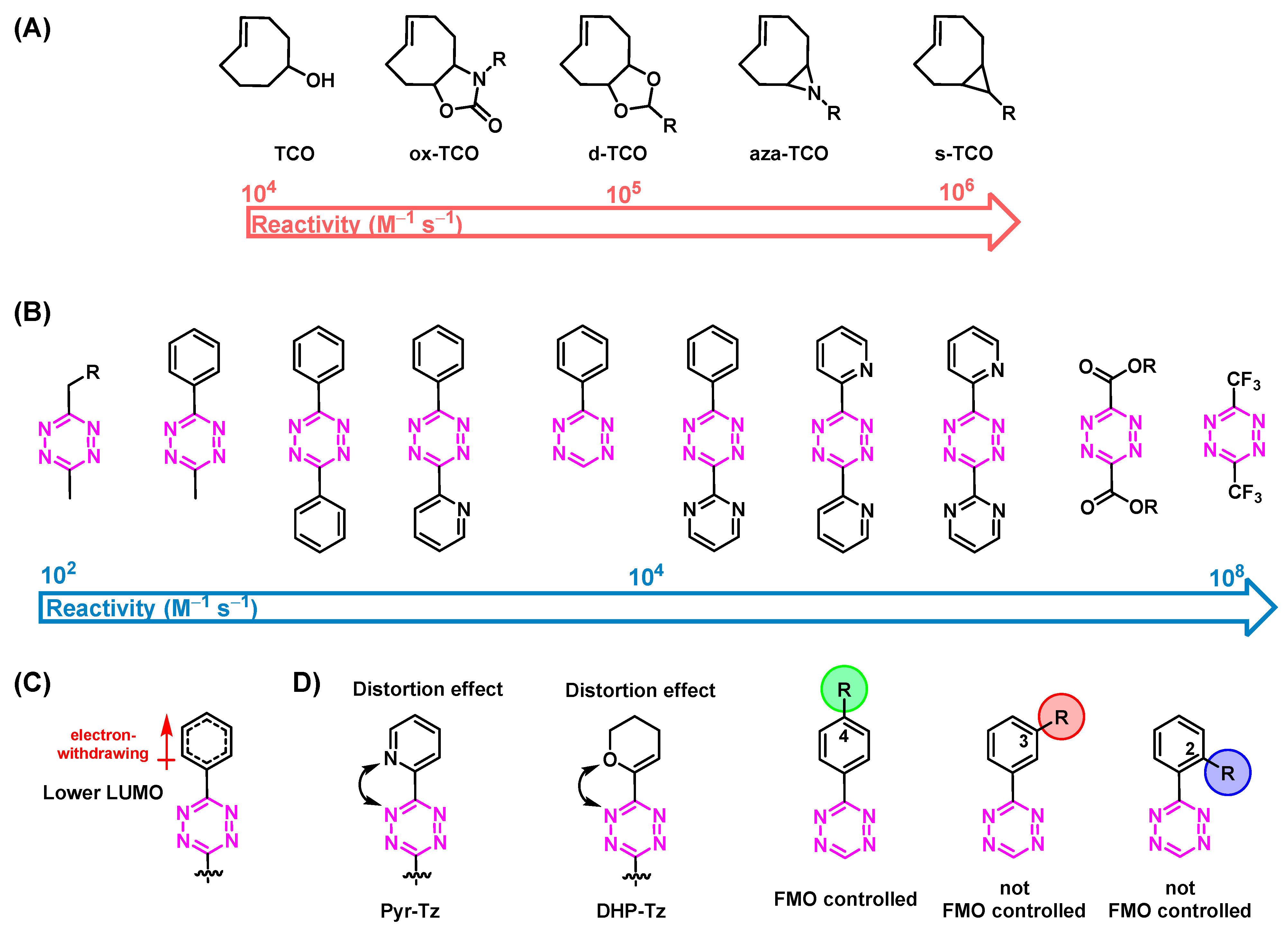
3. Influencing the Reaction Kinetics of the Tetrazine Ligation
3.1. Reactivity of TCOs
3.2. Reactivity of Tzs
4. The Use of Nanomedicines for Molecular Imaging
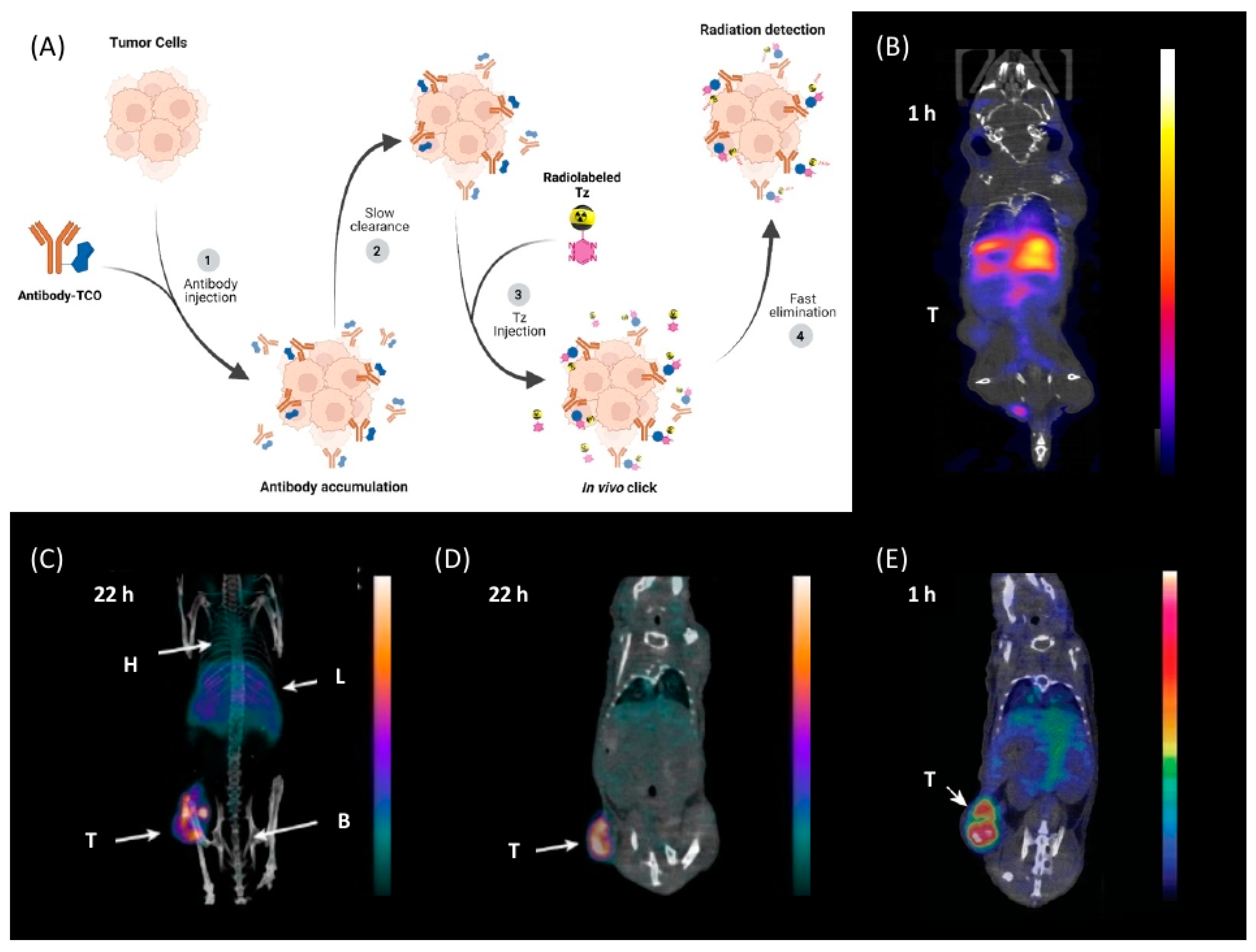
5. Pretargeted Nuclear Imaging
6. Tetrazine Labeling
7. Radiometals
| NO. | Chemical Structure | Refs. | NO. | Chemical Structure | Refs. |
|---|---|---|---|---|---|
| 1 |  | [6,75] | 7 |  | [78] |
| 2 | 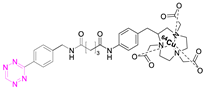 | [79,80] | 8 | 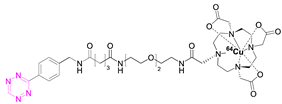 | [81] |
| 3 | 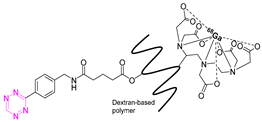 | [82] | 9 |  | [45] |
| 4 | 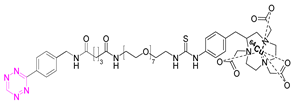 | [75,80] | 10 | 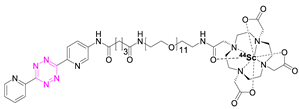 | [83] |
| 5 | 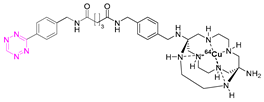 | [80] | 11 | 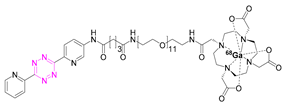 | [84] |
| 6 | 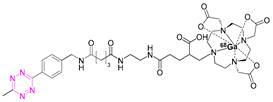 | [85] |
7.1. Indium-111
7.2. Copper-64
7.3. Gallium-68
7.4. Scandium-44
8. Non-Radiometal Radionuclides
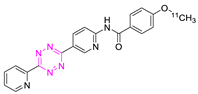
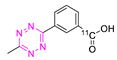
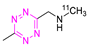
8.1. Carbon-11
8.2. Iodine-125
8.3. Fluorine-18
8.4. Indirect Labeling Strategies
9. Direct 18F-Labeling Approaches
| NO. | Chemical Structure | 18F-Labeling Method | Refs. |
|---|---|---|---|
| 16 | 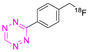 | Direct aliphatic nucleophilic substitution (SN2) | [113] |
| 17 | 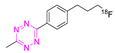 | Direct aliphatic nucleophilic substitution (SN2) | [100] |
| 18 | 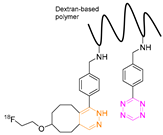 | Indirect aliphatic nucleophilic substitution (SN2) | [119] |
| 19 | 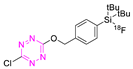 | Indirect fluoride anion exchange | [120] |
| 20 | 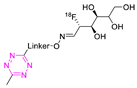 | Indirect aliphatic nucleophilic substitution (SN2) | [121] |
| 21 | 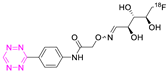 | Indirect aliphatic nucleophilic substitution (SN2) | [122,123,125] |
| 22 | 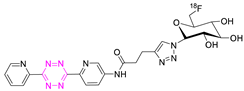 | Indirect aliphatic nucleophilic substitution (SN2) | [86] |
| 23 | 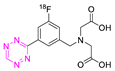 | Direct aromatic labeling (oxidative fluorination) | [126] |
| 24 |  | Aliphatic labeling (SN2) | [127] |
| 25 |  | Aliphatic labeling (SN2) | [76] |
| 26 |  | Fluoride anion exchange | [129] |
| 27 | 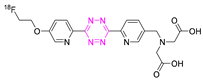 | Aliphatic labeling (SN2) | [130] |
10. Miscellaneous
11. Influence of Physicochemical Properties of Tz Derivatives on the Pharmacokinetic Profile
12. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devaraj, N.K.; Weissleder, R. Biomedical applications of tetrazine cycloadditions. Acc. Chem. Res. 2011, 44, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Cornelissen, B. Bioorthogonal chemistry: Implications for pretargeted nuclear (PET/SPECT) imaging and therapy. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 96–113. [Google Scholar] [PubMed]
- Carroll, L.; Evans, H.L.; Aboagye, E.O.; Spivey, A.C. Bioorthogonal chemistry for pre-targeted molecular imaging–progress and prospects. Organic Biomol. Chem. 2013, 11, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Zarschler, K.; Pietzsch, H.J.; Stephan, H.; Gasser, G. New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev. 2016, 45, 6415–6431. [Google Scholar] [CrossRef]
- Rossin, R.; Renart Verkerk, P.; van den Bosch, S.M.; Vulders, R.C.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. 2010, 122, 3447–3450. [Google Scholar] [CrossRef]
- Goodwin, D.; Mears, C.; McTigue, M.; David, G. Monoclonal antibody hapten radiopharmaceutical delivery. Nucl. Med. Commun. 1986, 7, 569–580. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Rossin, R.; ten Hoeve, W.; Janssen, H.M.; Robillard, M.S. Click to release: Instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. Engl. 2013, 52, 14112–14116. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Ten Hoeve, W.; Rossin, R.; de Geus, M.A.; Janssen, H.M.; Robillard, M.S. Click-to-Release from trans-Cyclooctenes: Mechanistic Insights and Expansion of Scope from Established Carbamate to Remarkable Ether Cleavage. Angew. Chem. Int. Ed. Engl. 2018, 57, 10494–10499. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M.; Paganelli, G.; Barbet, J.; Chatal, J.-F. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol. 2006, 24, 816. [Google Scholar] [CrossRef]
- Devaraj, N.K. The Future of Bioorthogonal Chemistry. ACS Cent. Sci. 2018, 4, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Pretze, M.; Pietzsch, D.; Mamat, C. Recent trends in bioorthogonal click-radiolabeling reactions using fluorine-18. Molecules 2013, 18, 8618–8665. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Saxon, E.; Armstrong, J.I.; Bertozzi, C.R. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org. Lett. 2000, 2, 2141–2143. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Stéen, E.J.L.; Edem, P.E.; Nørregaard, K.; Jørgensen, J.T.; Shalgunov, V.; Kjaer, A.; Herth, M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials 2018, 179, 209–245. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Wyffels, L.; Thomae, D.; Waldron, A.M.; Fissers, J.; Dedeurwaerdere, S.; Van der Veken, P.; Joossens, J.; Stroobants, S.; Augustyns, K.; Staelens, S. In vivo evaluation of (18)F-labeled TCO for pre-targeted PET imaging in the brain. Nucl. Med. Biol. 2014, 41, 513–523. [Google Scholar] [CrossRef]
- Ravasco, J.M.; Coelho, J.A. Predictive multivariate models for bioorthogonal inverse-electron demand Diels–Alder reactions. J. Am. Chem. Soc. 2020, 142, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Battisti, U.M.; Garcia-Vazquez, R.; Svatunek, D.; Herrmann, B.; Loffler, A.; Mikula, H.; Herth, M.M. Synergistic Experimental and Computational Investigation of the Bioorthogonal Reactivity of Substituted Aryltetrazines. Bioconjug. Chem. 2022, 33, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Yee, N.A.; Wu, K.; Zakharian, M.; Mahmoodi, A.; Royzen, M.; Mejía Oneto, J.M. SQ3370 Activates Cytotoxic Drug via Click Chemistry at Tumor and Elicits Sustained Responses in Injected and Non-Injected Lesions. Adv. Ther. 2021, 4, 2000243. [Google Scholar] [CrossRef] [PubMed]
- Trials, C. Phase 1 Study of SQ3370 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04106492 (accessed on 4 April 2022).
- Staudt, M.; Herth, M.M.; Poulie, C.B. Pretargeted Theranostics. In Theranostics-An Old Concept in New Clothing, IntechOpen: London, UK, 2021.
- Carlson, J.C.; Mikula, H.; Weissleder, R. Unraveling tetrazine-triggered bioorthogonal elimination enables chemical tools for ultrafast release and universal cleavage. J. Am. Chem. Soc. 2018, 140, 3603–3612. [Google Scholar] [CrossRef]
- Saracoglu, N. Recent advances and applications in 1,2,4,5-tetrazine chemistry. Tetrahedron 2007, 63, 4199–4236. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Liang, Y.; Liu, F.; Houk, K.N. Diels–Alder Reactivities of Benzene, Pyridine, and Di-, Tri-, and Tetrazines: The Roles of Geometrical Distortions and Orbital Interactions. J. Am. Chem. Soc. 2016, 138, 1660–1667. [Google Scholar] [CrossRef]
- Sauer, J.; Heldmann, D.K.; Hetzenegger, J.; Krauthan, J.; Sichert, H.; Schuster, J. 1,2,4,5-Tetrazine: Synthesis and Reactivity in [4 + 2] Cycloadditions. Eur. J. Org. Chem. 1998, 1998, 2885–2896. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Der Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Liu, F.; Liang, Y.; Houk, K. Theoretical elucidation of the origins of substituent and strain effects on the rates of Diels–Alder reactions of 1,2,4,5-tetrazines. J. Am. Chem. Soc. 2014, 136, 11483–11493. [Google Scholar] [CrossRef]
- Kronister, S.; Svatunek, D.; Denk, C.; Mikula, H. Acylation-Mediated ‘Kinetic Turn-On’of 3-Amino-1,2,4,5-tetrazines. Synlett 2018, 29, 1297–1302. [Google Scholar]
- Mayer, S.; Lang, K. Tetrazines in inverse-electron-demand Diels–Alder cycloadditions and their use in biology. Synthesis 2017, 49, 830–848. [Google Scholar]
- Selvaraj, R.; Fox, J.M. Trans-Cyclooctene—A stable, voracious dienophile for bioorthogonal labeling. Curr. Opin. Chem. Biol. 2013, 17, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Darko, A.; Wallace, S.; Dmitrenko, O.; Machovina, M.M.; Mehl, R.A.; Chin, J.W.; Fox, J.M. Conformationally strained trans-cyclooctene with improved stability and excellent reactivity in tetrazine ligation. Chem. Sci. 2014, 5, 3770–3776. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.T.; Blackman, M.L.; Dmitrenko, O.; Fox, J.M. Design and Synthesis of Highly Reactive Dienophiles for the Tetrazine–trans-Cyclooctene Ligation. J. Am. Chem. Soc. 2011, 133, 9646–9649. [Google Scholar] [CrossRef]
- Thalhammer, F.; Wallfahrer, U.; Sauer, J. Reaktivität einfacher offenkettiger und cyclischer dienophile bei Diels-Alder-reaktionen mit inversem elektronenbedarf. Tetrahedron Lett. 1990, 31, 6851–6854. [Google Scholar] [CrossRef]
- Lang, K.; Davis, L.; Wallace, S.; Mahesh, M.; Cox, D.J.; Blackman, M.L.; Fox, J.M.; Chin, J.W. Genetic Encoding of Bicyclononynes and trans-Cyclooctenes for Site-Specific Protein Labeling in Vitro and in Live Mammalian Cells via Rapid Fluorogenic Diels–Alder Reactions. J. Am. Chem. Soc. 2012, 134, 10317–10320. [Google Scholar] [CrossRef]
- Rossin, R.; Läppchen, T.; van den Bosch, S.M.; Laforest, R.; Robillard, M.S. Diels–Alder Reaction for Tumor Pretargeting: In Vivo Chemistry Can Boost Tumor Radiation Dose Compared with Directly Labeled Antibody. J. Nucl. Med. 2013, 54, 1989–1995. [Google Scholar] [CrossRef]
- Sauer, J.; Sustmann, R. Mechanistic Aspects of Diels-Alder Reactions: A Critical Survey. Angew. Chem. Int. Ed. Eng. 1980, 19, 779–807. [Google Scholar] [CrossRef]
- Boger, D.L.; Schaum, R.P.; Garbaccio, R.M. Regioselective Inverse Electron Demand Diels−Alder Reactions of N-Acyl 6-Amino-3-(methylthio)-1,2,4,5-tetrazines. J. Org. Chem. 1998, 63, 6329–6337. [Google Scholar] [CrossRef]
- Svatunek, D.; Denk, C.; Mikula, H. A computational model to predict the Diels–Alder reactivity of aryl/alkyl-substituted tetrazines. Mon. Chem. 2018, 149, 833–837. [Google Scholar] [CrossRef]
- Liang, Y.; Mackey, J.L.; Lopez, S.A.; Liu, F.; Houk, K.N. Control and Design of Mutual Orthogonality in Bioorthogonal Cycloadditions. J. Am. Chem. Soc. 2012, 134, 17904–17907. [Google Scholar] [CrossRef] [PubMed]
- Svatunek, D.; Wilkovitsch, M.; Hartmann, L.; Houk, K.; Mikula, H. Uncovering the Key Role of Distortion in Bioorthogonal Tetrazine Tools That Defy the Reactivity/Stability Trade-Off. J. Am. Chem. Soc. 2022, 144, 8171–8177. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.P.; Kozlowski, P.; Jackson, J.; Cunanan, K.M.; Adumeau, P.; Dilling, T.R.; Zeglis, B.M.; Lewis, J.S. Exploring Structural Parameters for Pretargeting Radioligand Optimization. J. Med. Chem. 2017, 60, 8201–8217. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Sauer, J. Donor-akzeptor substituierte dienophile bei diels-alder-reaktionen mit inversem elektronenbedarf. Tetrahedron Lett. 1990, 31, 6855–6858. [Google Scholar] [CrossRef]
- Haberkorn, U.; Eisenhut, M. Molecular imaging and therapy—A programme based on the development of new biomolecules. Eur. J. Nucl. Med. Mol. Imaging. 2005, 32, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Karmani, L.; Leveque, P.; Bouzin, C.; Bol, A.; Dieu, M.; Walrand, S.; Vander Borght, T.; Feron, O.; Gregoire, V.; Bonifazi, D.; et al. Biodistribution of (125)I-labeled anti-endoglin antibody using SPECT/CT imaging: Impact of in vivo deiodination on tumor accumulation in mice. Nucl. Med. Biol. 2016, 43, 415–423. [Google Scholar] [CrossRef]
- Kristensen, J.L.; Herth, M.M. Textbook of Drug Design and Discovery, 5th ed.; CRC Press: London, UK; New York, NY, USA, 2017. [Google Scholar]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Herth, M.M.; Barz, M.; Moderegger, D.; Allmeroth, M.; Jahn, M.; Thews, O.; Zentel, R.; Rosch, F. Radioactive labeling of defined HPMA-based polymeric structures using [18F]FETos for in vivo imaging by positron emission tomography. Biomacromolecules 2009, 10, 1697–1703. [Google Scholar] [CrossRef]
- Delso, G.; Ziegler, S. PET/MRI system design. Eur. J. Nucl. Med. Mol. Imaging 2009, 36 (Suppl. 1), S86–S92. [Google Scholar] [CrossRef]
- Piel, M.; Vernaleken, I.; Rosch, F. Positron emission tomography in CNS drug discovery and drug monitoring. J. Med. Chem. 2014, 57, 9232–9258. [Google Scholar] [CrossRef]
- Deng, X.; Rong, J.; Wang, L.; Vasdev, N.; Zhang, L.; Josephson, L.; Liang, S.H. Chemistry for positron emission tomography: Recent advances in 11C-, 18F-, 13N-, and 15O-labeling reactions. Angew. Chem. Int. Ed. 2019, 58, 2580–2605. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Ma, M.T. Site-specific chelator-antibody conjugation for PET and SPECT imaging with radiometals. Drug Discov. Today Technol. 2018, 30, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kwee, T.C.; Surti, S.; Akin, E.A.; Yoo, D.; Alavi, A. Fundamentals of PET and PET/CT imaging. Ann. N. Y. Acad. Sci. 2011, 1228, 1–18. [Google Scholar] [CrossRef]
- Stockhofe, K.; Postema, J.M.; Schieferstein, H.; Ross, T.L. Radiolabeling of Nanoparticles and Polymers for PET Imaging. Pharmaceuticals 2014, 7, 392–418. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Windhorst, A.D.; Zeglis, B.M. Radiopharmaceutical Chemistry; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Physics. 2016, 3, 8. [Google Scholar] [CrossRef]
- Barbet, J.; Bardiès, M.; Bourgeois, M.; Chatal, J.-F.; Chérel, M.; Davodeau, F.; Faivre-Chauvet, A.; Gestin, J.-F.; Kraeber-Bodéré, F. Radiolabeled antibodies for cancer imaging and therapy. Antib. Eng. 2012, 907, 681–697. [Google Scholar]
- Ross, T.; Wester, H. 18F: Labeling Chemistry and Labeled Compounds. In Handbook of nuclear chemistry; Springer: Berlin/Heidelberg, Germany, 2011; pp. 2021–2071. [Google Scholar]
- Rosar, F.; Buchholz, H.-G.; Michels, S.; Hoffmann, M.A.; Piel, M.; Waldmann, C.M.; Rösch, F.; Reuss, S.; Schreckenberger, M. Image quality analysis of 44Sc on two preclinical PET scanners: A comparison to 68Ga. EJNMMI Phys. 2020, 7, 16. [Google Scholar] [CrossRef]
- Edem, P.E.; Stéen, E.J.L. Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates, 1st ed.; Elsevier: Copenhagen, Denmark, 2018. [Google Scholar]
- Boerman, O.C.; Oyen, W.J. Immuno-PET of cancer: A revival of antibody imaging. J. Nucl. Med. 2011, 52, 1171–1172. [Google Scholar] [CrossRef]
- Boros, E.; Holland, J.P. Chemical aspects of metal ion chelation in the synthesis and application antibody-based radiotracers. J. Label. Comp. Radiopharm. 2018, 61, 652–671. [Google Scholar] [CrossRef]
- Sehlin, D.; Fang, X.T.; Cato, L.; Antoni, G.; Lannfelt, L.; Syvanen, S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat. Commun. 2016, 7, 10759. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, G.A.; Visser, G.W.; Lub-de Hooge, M.N.; de Vries, E.G.; Perk, L.R. Immuno-PET: A navigator in monoclonal antibody development and applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Eichenberger, L.S.; Fischer, G.; Holland, J.P. Photochemical Conjugation and One-Pot Radiolabelling of Antibodies for Immuno-PET. Angew. Chem. Int. Ed. Engl. 2019, 58, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Psimadas, D.; Valotassiou, V.; Alexiou, S.; Tsougos, I.; Georgoulias, P. Radiolabeled mAbs as Molecular Imaging and/or Therapy Agents Targeting PSMA. Cancer. Invest. 2018, 36, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef]
- Rossin, R.; Robillard, M.S. Pretargeted imaging using bioorthogonal chemistry in mice. Curr. Opin. Chem. Biol. 2014, 21, 161–169. [Google Scholar] [CrossRef]
- Lewis, M.R.; Wang, M.; Axworthy, D.B.; Theodore, L.J.; Mallet, R.W.; Fritzberg, A.R.; Welch, M.J.; Anderson, C.J. In vivo evaluation of pretargeted 64Cu for tumor imaging and therapy. J. Nucl. Med. 2003, 44, 1284–1292. [Google Scholar]
- Shi, X.; Gao, K.; Huang, H.; Gao, R. Pretargeted Immuno-PET Based on Bioorthogonal Chemistry for Imaging EGFR Positive Colorectal Cancer. Bioconjug. Chem. 2018, 29, 250–254. [Google Scholar] [CrossRef]
- Poulie, C.B.M.; Jørgensen, J.T.; Shalgunov, V.; Kougioumtzoglou, G.; Jeppesen, T.E.; Kjaer, A.; Herth, M.M. Evaluation of [(64)Cu]Cu-NOTA-PEG(7)-H-Tz for Pretargeted Imaging in LS174T Xenografts-Comparison to [(111)In]In-DOTA-PEG(11)-BisPy-Tz. Molecules 2021, 26, 544. [Google Scholar] [CrossRef]
- Battisti, U.M.; Bratteby, K.; Jorgensen, J.T.; Hvass, L.; Shalgunov, V.; Mikula, H.; Kjaer, A.; Herth, M.M. Development of the First Aliphatic (18)F-Labeled Tetrazine Suitable for Pretargeted PET Imaging-Expanding the Bioorthogonal Tool Box. J. Med. Chem. 2021, 64, 15297–15312. [Google Scholar] [CrossRef]
- Herth, M.M.; Ametamey, S.; Antuganov, D.; Bauman, A.; Berndt, M.; Brooks, A.F.; Bormans, G.; Choe, Y.S.; Gillings, N.; Häfeli, U.O.; et al. On the consensus nomenclature rules for radiopharmaceutical chemistry—Reconsideration of radiochemical conversion. Nucl. Med. Biol. 2021, 93, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Mohindra, P.; Weissmann, G.I.; Divilov, V.; Hilderbrand, S.A.; Weissleder, R.; Lewis, J.S. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjug. Chem. 2011, 22, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Sevak, K.K.; Reiner, T.; Mohindra, P.; Carlin, S.D.; Zanzonico, P.; Weissleder, R.; Lewis, J.S. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J. Nucl. Med. 2013, 54, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Brand, C.; Abdel-Atti, D.; Carnazza, K.E.; Cook, B.E.; Carlin, S.; Reiner, T.; Lewis, J.S. Optimization of a Pretargeted Strategy for the PET Imaging of Colorectal Carcinoma via the Modulation of Radioligand Pharmacokinetics. Mol. Pharm. 2015, 12, 3575–3587. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Choi, J.-s.; Garcia, M.A.; Xing, Y.; Chen, K.-J.; Chen, Y.-M.; Jiang, Z.K.; Ro, T.; Wu, L.; Stout, D.B.; et al. Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in Vivo Bioorthogonal Chemistry. ACS Nano 2016, 10, 1417–1424. [Google Scholar] [CrossRef]
- Nichols, B.; Qin, Z.; Yang, J.; Vera, D.R.; Devaraj, N.K. 68Ga chelating bioorthogonal tetrazine polymers for the multistep labeling of cancer biomarkers. Chem. Commun. 2014, 50, 5215–5217. [Google Scholar] [CrossRef]
- Edem, P.E.; Sinnes, J.P.; Pektor, S.; Bausbacher, N.; Rossin, R.; Yazdani, A.; Miederer, M.; Kjær, A.; Valliant, J.F.; Robillard, M.S.; et al. Evaluation of the inverse electron demand Diels-Alder reaction in rats using a scandium-44-labelled tetrazine for pretargeted PET imaging. EJNMMI Res. 2019, 9, 49. [Google Scholar] [CrossRef]
- Edem, P.E.; Jørgensen, J.T.; Nørregaard, K.; Rossin, R.; Yazdani, A.; Valliant, J.F.; Robillard, M.; Herth, M.M.; Kjaer, A. Evaluation of a (68)Ga-Labeled DOTA-Tetrazine as a PET Alternative to (111)In-SPECT Pretargeted Imaging. Molecules 2020, 25, 463. [Google Scholar] [CrossRef]
- Evans, H.L.; Nguyen, Q.D.; Carroll, L.S.; Kaliszczak, M.; Twyman, F.J.; Spivey, A.C.; Aboagye, E.O. A bioorthogonal (68)Ga-labelling strategy for rapid in vivo imaging. Chem. Commun. 2014, 50, 9557–9560. [Google Scholar] [CrossRef]
- Steen, E.J.L.; Jorgensen, J.T.; Denk, C.; Battisti, U.M.; Norregaard, K.; Edem, P.E.; Bratteby, K.; Shalgunov, V.; Wilkovitsch, M.; Svatunek, D.; et al. Lipophilicity and Click Reactivity Determine the Performance of Bioorthogonal Tetrazine Tools in Pretargeted In Vivo Chemistry. ACS Pharmacol. Transl. Sci. 2021, 4, 824–833. [Google Scholar] [CrossRef]
- Price, T.W.; Greenman, J.; Stasiuk, G.J. Current advances in ligand design for inorganic positron emission tomography tracers (68)Ga, (64)Cu, (89)Zr and (44)Sc. Dalton. Trans. 2016, 45, 15702–15724. [Google Scholar] [CrossRef] [PubMed]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with (8)(9)Zr: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J. Dextran conjugates in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Stroup, S.P.; Kane, C.J.; Farchshchi-Heydari, S.; James, C.M.; Davis, C.H.; Wallace, A.M.; Hoh, C.K.; Vera, D.R. Preoperative sentinel lymph node mapping of the prostate using PET/CT fusion imaging and Ga-68-labeled tilmanocept in an animal model. Clin. Exp. Metastasis 2012, 29, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.K.; Limmer, K.K.; Hall, D.J.; Han, S.-H.; Eckelman, W.C.; Kane, C.J.; Wallace, A.M.; Vera, D.R. A receptor-targeted fluorescent radiopharmaceutical for multireporter sentinel lymph node imaging. Radiology 2012, 265, 186–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallace, A.M.; Hoh, C.K.; Ellner, S.J.; Darrah, D.D.; Schulteis, G.; Vera, D.R. Lymphoseek: A molecular imaging agent for melanoma sentinel lymph node mapping. Ann. Surg. Oncol. 2007, 14, 913–921. [Google Scholar] [CrossRef]
- Vera, D.R.; Wallace, A.M.; Hoh, C.K.; Mattrey, R.F. A synthetic macromolecule for sentinel node detection: 99mTc-DTPA-mannosyl-dextran. J. Nucl. Med. 2001, 42, 951–959. [Google Scholar]
- Barendswaard, E.C.; Humm, J.L.; O’donoghue, J.A.; Sgouros, G.; Finn, R.D.; Scott, A.M.; Larson, S.M.; Welt, S. Relative therapeutic efficacy of 125I-and 131I-labeled monoclonal antibody A33 in a human colon cancer xenograft. J. Nucl. Med. 2001, 42, 1251–1256. [Google Scholar]
- Lee, F.T.; Hall, C.; Rigopoulos, A.; Zweit, J.; Pathmaraj, K.; O’Keefe, G.J.; Smyth, F.E.; Welt, S.; Old, L.J.; Scott, A.M. Immuno-PET of human colon xenograft–bearing BALB/c nude mice using 124I-CDR–grafted humanized A33 monoclonal antibody. J. Nucl.Med. 2001, 42, 764–769. [Google Scholar]
- Ackerman, M.E.; Chalouni, C.; Schmidt, M.M.; Raman, V.V.; Ritter, G.; Old, L.J.; Mellman, I.; Wittrup, K.D. A33 antigen displays persistent surface expression. Cancer Immunol. Immunother. 2008, 57, 1017–1027. [Google Scholar] [CrossRef]
- Humblet, Y. Cetuximab: An IgG1 monoclonal antibody for the treatment of epidermal growth factor receptor-expressing tumours. Expert Opin. Pharmacother. 2004, 5, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ujula, T.; Salomäki, S.; Autio, A.; Luoto, P.; Tolvanen, T.; Lehikoinen, P.; Viljanen, T.; Sipilä, H.; Härkönen, P.; Roivainen, A. 68Ga-chloride PET reveals human pancreatic adenocarcinoma xenografts in rats—Comparison with FDG. Mol. Imaging Biol. 2010, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Denk, C.; Svatunek, D.; Filip, T.; Wanek, T.; Lumpi, D.; Fröhlich, J.; Kuntner, C.; Mikula, H. Development of a (18) F-labeled tetrazine with favorable pharmacokinetics for bioorthogonal PET imaging. Angew. Chem. Int. Ed. Engl. 2014, 53, 9655–9659. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Stumbo, L.; Spoto, C.; D’Onofrio, L.; Pantano, F.; Iuliani, M.; Zoccoli, A.; Ribelli, G.; Virzì, V.; Vincenzi, B. Bisphosphonates as anticancer agents in early breast cancer: Preclinical and clinical evidence. Breast Cancer Res. 2015, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haim, S.; Israel, O. Breast Cancer: Role of SPECT and PET in Imaging Bone Metastases; Seminars in nuclear medicine; Elsevier: Amsterdam, The Netherlands, 2009; pp. 408–415. [Google Scholar]
- Liu, S.; Pietryka, J.; Ellars, C.E.; Edwards, D.S. Comparison of Yttrium and Indium Complexes of DOTA-BA and DOTA-MBA: Models for 90Y-and 111In-Labeled DOTA− Biomolecule Conjugates. Bioconjug. Chem. 2002, 13, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.; Bilton, H.; Vito, A.; Genady, A.R.; Rathmann, S.M.; Ahmad, Z.; Janzen, N.; Czorny, S.; Zeglis, B.M.; Francesconi, L.C. A bone-seeking trans-cyclooctene for pretargeting and bioorthogonal chemistry: A proof of concept study using 99mTc-and 177Lu-labeled tetrazines. J. Med. Chem. 2016, 59, 9381–9389. [Google Scholar] [CrossRef]
- Herth, M.M.; Andersen, V.L.; Lehel, S.; Madsen, J.; Knudsen, G.M.; Kristensen, J.L. Development of a (11)C-labeled tetrazine for rapid tetrazine-trans-cyclooctene ligation. Chem. Commun. 2013, 49, 3805–3807. [Google Scholar] [CrossRef]
- Steen, E.J.L.; Jorgensen, J.T.; Petersen, I.N.; Norregaard, K.; Lehel, S.; Shalgunov, V.; Birke, A.; Edem, P.E.; L’Estrade, E.T.; Hansen, H.D.; et al. Improved radiosynthesis and preliminary in vivo evaluation of the (11)C-labeled tetrazine [(11)C]AE-1 for pretargeted PET imaging. Bioorg. Med. Chem. Lett. 2019, 29, 986–990. [Google Scholar] [CrossRef]
- Denk, C.; Svatunek, D.; Mairinger, S.; Stanek, J.; Filip, T.; Matscheko, D.; Kuntner, C.; Wanek, T.; Mikula, H. Design, Synthesis, and Evaluation of a Low-Molecular-Weight (11)C-Labeled Tetrazine for Pretargeted PET Imaging Applying Bioorthogonal in Vivo Click Chemistry. Bioconjug. Chem. 2016, 27, 1707–1712. [Google Scholar] [CrossRef]
- Garcia-Vazquez, R.; Battisti, U.M.; Shalgunov, V.; Schafer, G.; Barz, M.; Herth, M.M. [11C]Carboxylated Tetrazines for Facile Labeling of Trans-Cyclooctene-Functionalized PeptoBrushes. Macromol. Rapid. Commun. 2021, e2100655. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.A.; Al-Karmi, S.A.; Vito, A.; Dzandzi, J.P.; Zlitni, A.; Beckford-Vera, D.; Blacker, M.; Janzen, N.; Patel, R.M.; Capretta, A.; et al. (125)I-Tetrazines and Inverse-Electron-Demand Diels-Alder Chemistry: A Convenient Radioiodination Strategy for Biomolecule Labeling, Screening, and Biodistribution Studies. Bioconjug. Chem. 2016, 27, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D. Fluorine-18 and medical imaging: Radiopharmaceuticals for positron emission tomography. J. Fluor. Chem. 2006, 127, 1488–1493. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. Engl. 2008, 47, 8998–9033. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Hassink, M.; Blackman, M.L.; Brown, R.C.; Conti, P.S.; Fox, J.M. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem. Commun. 2010, 46, 8043–8045. [Google Scholar] [CrossRef]
- Reiner, T.; Zeglis, B.M. The inverse electron demand Diels–Alder click reaction in radiochemistry. J. Label. Compd. Radiopharm. 2014, 57, 285–290. [Google Scholar] [CrossRef]
- van der Born, D.; Pees, A.; Poot, A.J.; Orru, R.V.A.; Windhorst, A.D.; Vugts, D.J. Fluorine-18 labelled building blocks for PET tracer synthesis. Chem. Soc. Rev. 2017, 46, 4709–4773. [Google Scholar] [CrossRef]
- Niemi, T.T.; Miyashita, R.; Yamakage, M. Colloid solutions: A clinical update. J. Anesth. 2010, 24, 913–925. [Google Scholar] [CrossRef]
- Bisht, S.; Maitra, A. Dextran–doxorubicin/chitosan nanoparticles for solid tumor therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2009, 1, 415–425. [Google Scholar] [CrossRef]
- Wingårdh, K.; Strand, S.-E. Evaluation in vitro and in vivo of two labelling techniques of different 99mTc-dextrans for lymphoscintigraphy. Eur. J. Nucl. Med. 1989, 15, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Thurber, G.M.; Keliher, E.J.; Marinelli, B.; Weissleder, R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. USA 2012, 109, 4762–4767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, S.; Wängler, C.; Wängler, B.; Lennox, R.B.; Schirrmacher, R. Synthesis of 3-chloro-6-((4-(di-tert-butyl[(18)F]fluorosilyl)-benzyl)oxy)-1,2,4,5-tetrazine ([(18)F]SiFA-OTz) for rapid tetrazine-based (18)F-radiolabeling. Chem. Commun. (Camb) 2015, 51, 12415–12418. [Google Scholar] [CrossRef]
- Rashidian, M.; Keliher, E.; Dougan, M.; Juras, P.K.; Cavallari, M.; Wojtkiewicz, G.R.; Jacobsen, J.; Edens, J.G.; Tas, J.M.; Victora, G.; et al. The use of (18)F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer. ACS Cent. Sci. 2015, 1, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, O.; Li, X.G.; Chenna, N.K.; Lumen, D.; Ott, J.; Molthoff, C.F.; Sarparanta, M.; Helariutta, K.; Vuorinen, T.; Windhorst, A.D.; et al. A New Highly Reactive and Low Lipophilicity Fluorine-18 Labeled Tetrazine Derivative for Pretargeted PET Imaging. ACS Med. Chem. Lett. 2016, 7, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Keinaänen, O.; Mäkilä, E.M.; Lindgren, R.; Virtanen, H.; Liljenbäck, H.; Oikonen, V.; Sarparanta, M.; Molthoff, C.; Windhorst, A.D.; Roivainen, A. Pretargeted PET imaging of trans-cyclooctene-modified porous silicon nanoparticles. ACS Omega 2017, 2, 62–69. [Google Scholar] [CrossRef]
- Bimbo, L.M.; Sarparanta, M.; Santos, H.A.; Airaksinen, A.J.; Makila, E.; Laaksonen, T.; Peltonen, L.; Lehto, V.-P.; Hirvonen, J.; Salonen, J. Biocompatibility of thermally hydrocarbonized porous silicon nanoparticles and their biodistribution in rats. ACS Nano 2010, 4, 3023–3032. [Google Scholar] [CrossRef]
- Keinänen, O.; Fung, K.; Pourat, J.; Jallinoja, V.; Vivier, D.; Pillarsetty, N.K.; Airaksinen, A.J.; Lewis, J.S.; Zeglis, B.M.; Sarparanta, M. Pretargeting of internalizing trastuzumab and cetuximab with a 18 F-tetrazine tracer in xenograft models. EJNMMI Res. 2017, 7, 95. [Google Scholar] [CrossRef]
- Garcia-Vazquez, R.; Battisti, U.M.; Jorgensen, J.T.; Shalgunov, V.; Hvass, L.; Stares, D.L.; Petersen, I.N.; Crestey, F.; Loffler, A.; Svatunek, D.; et al. Direct Cu-mediated aromatic (18)F-labeling of highly reactive tetrazines for pretargeted bioorthogonal PET imaging. Chem. Sci. 2021, 12, 11668–11675. [Google Scholar] [CrossRef]
- Bratteby, K.; Shalgunov, V.; Battisti, U.M.; Petersen, I.N.; van den Broek, S.L.; Ohlsson, T.; Gillings, N.; Erlandsson, M.; Herth, M.M. Insights into Elution of Anion Exchange Cartridges: Opening the Path toward Aliphatic (18)F-Radiolabeling of Base-Sensitive Tracers. ACS Pharmacol. Transl. Sci. 2021, 4, 1556–1566. [Google Scholar] [CrossRef]
- Bratteby, K.; Shalgunov, V.; Herth, M.M. Aliphatic (18) F-Radiofluorination: Recent Advances in the Labeling of Base-Sensitive Substrates. Chem. Med. Chem. 2021, 16, 2612–2622. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xu, H.; Wang, H.; Du, W.H.; Wang, N.; Xiong, H.; Gu, Y.; Noodleman, L.; Sharpless, K.B.; Yang, G.; et al. Sulfur [(18)F]Fluoride Exchange Click Chemistry Enabled Ultrafast Late-Stage Radiosynthesis. J. Am. Chem. Soc. 2021, 143, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- García-Vázquez, R.; Jørgensen, J.T.; Bratteby, K.E.; Shalgunov, V.; Hvass, L.; Herth, M.M.; Kjær, A.; Battisti, U.M. Development of 18F-Labeled Bispyridyl Tetrazines for In Vivo Pretargeted PET Imaging. Pharmaceuticals 2022, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. (18)F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels-Alder Click Chemistry. Bioconjug. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef]
- Jeppesen, T.E.; Simón, M.; Torp, J.; Knudsen, L.B.S.; Leth, J.M.; Crestey, F.; Ploug, M.; Jørgensen, J.T.; Madsen, J.; Herth, M.M.; et al. Optimization and Evaluation of Al18F Labeling Using a NOTA—or RESCA1-Conjugated AE105 Peptide Antagonist of uPAR. Front. Nucl. Med. 2021, 1, 8. [Google Scholar] [CrossRef]
- McBride, W.J.; Sharkey, R.M.; Karacay, H.; D’Souza, C.A.; Rossi, E.A.; Laverman, P.; Chang, C.H.; Boerman, O.C.; Goldenberg, D.M. A novel method of 18F radiolabeling for PET. J. Nucl. Med. 2009, 50, 991–998. [Google Scholar] [CrossRef]
- Hoigebazar, L.; Jeong, J.M.; Lee, J.Y.; Shetty, D.; Yang, B.Y.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C. Syntheses of 2-nitroimidazole derivatives conjugated with 1,4,7-triazacyclononane-N,N′-diacetic acid labeled with F-18 using an aluminum complex method for hypoxia imaging. J. Med. Chem. 2012, 55, 3155–3162. [Google Scholar] [CrossRef]
- Keinänen, O.; Fung, K.; Brennan, J.M.; Zia, N.; Harris, M.; van Dam, E.; Biggin, C.; Hedt, A.; Stoner, J.; Donnelly, P.S. Harnessing 64Cu/67Cu for a theranostic approach to pretargeted radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 28316–28327. [Google Scholar] [CrossRef]
- Rondon, A.; Schmitt, S.; Briat, A.; Ty, N.; Maigne, L.; Quintana, M.; Membreno, R.; Zeglis, B.M.; Navarro-Teulon, I.; Pouget, J.P.; et al. Pretargeted radioimmunotherapy and SPECT imaging of peritoneal carcinomatosis using bioorthogonal click chemistry: Probe selection and first proof-of-concept. Theranostics 2019, 9, 6706–6718. [Google Scholar] [CrossRef]
- Denk, C.; Wilkovitsch, M.; Aneheim, E.; Herth, M.M.; Jensen, H.; Lindegren, S.; Mikula, H. Multifunctional Clickable Reagents for Rapid Bioorthogonal Astatination and Radio-Crosslinking. Chem. Plus. Chem. 2019, 84, 775–778. [Google Scholar]
- Shah, M.A.; Zhang, X.; Rossin, R.; Robillard, M.S.; Fisher, D.R.; Bueltmann, T.; Hoeben, F.J.; Quinn, T.P. Metal-free cycloaddition chemistry driven pretargeted radioimmunotherapy using α-particle radiation. Bioconjug. Chem. 2017, 28, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Theek, B.; Rizzo, L.Y.; Ehling, J.; Kiessling, F.; Lammers, T. The Theranostic Path to Personalized Nanomedicine. Clin. Transl. Imaging 2014, 2, 66–76. [Google Scholar] [CrossRef] [PubMed]
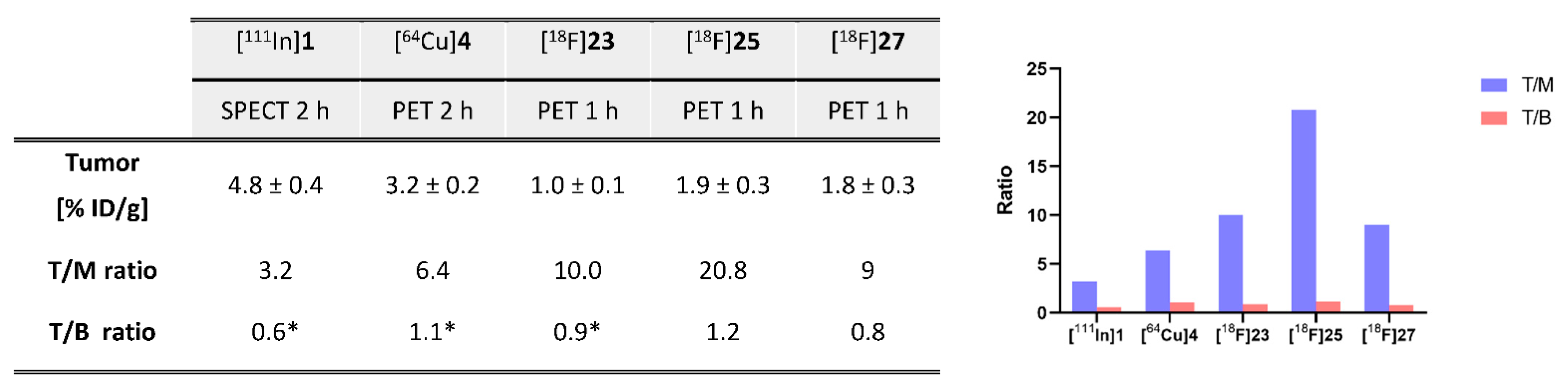

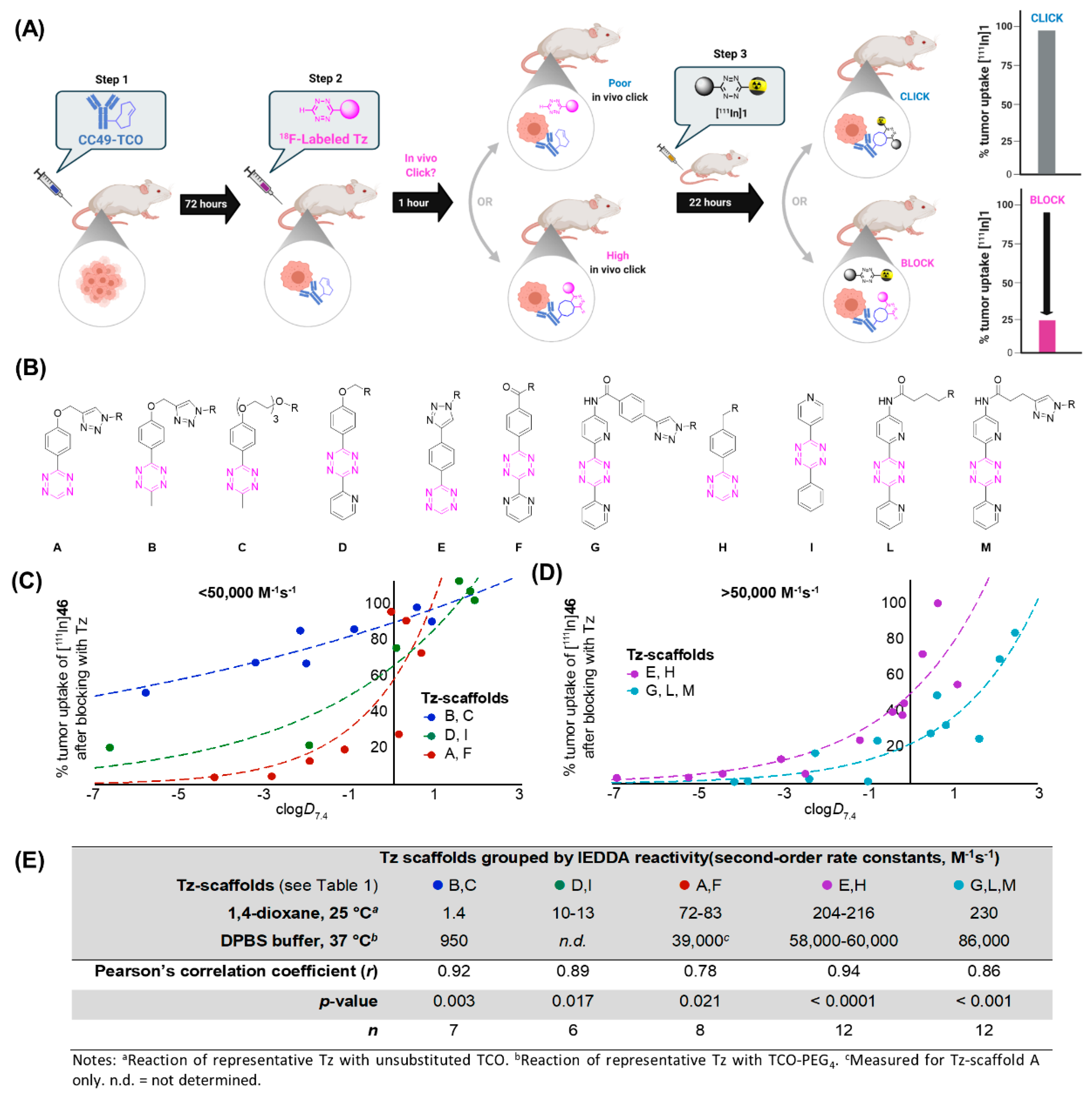
| Modality | Isotope | Half-Life | Branching Ratio(β+) (%) | Maximum Positron Range in Water (mm) | Gamma-Photon Energy (keV) |
|---|---|---|---|---|---|
| PET | Carbon-11 (11C) | 20.4 min | 99 | 4.5 | - |
| Gallium-68 (68Ga) | 68.4 min | 88 | 10.3 | - | |
| Fluorine-18 (18F) | 109.8 min | 97 | 2.3 | - | |
| Copper-64 (64Cu) | 12.7 h | 17.6 | 2.9 | - | |
| Arsenic-72 (72As) | 26.0 h | 88 | 18.2 | - | |
| Zirconium-89 (89Zr) | 78.4 h | 22.7 | 4.2 | - | |
| Scandium-44 (44Sc) | 4.04 h | 94.3 | 2.3 | ||
| Iodine-124 (124I) | 100.2 h | 22.8 | 11.7 | - | |
| SPECT | Iodine-123 (123I) | 13.3 h | - | - | 159 |
| Indium-111 (111In) | 67.3 h | - | - | 171 and 245 | |
| Technetium-99m (99mTc) | 6.01 h | - | - | 140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Vázquez, R.; Battisti, U.M.; Herth, M.M. Recent Advances in the Development of Tetrazine Ligation Tools for Pretargeted Nuclear Imaging. Pharmaceuticals 2022, 15, 685. https://doi.org/10.3390/ph15060685
García-Vázquez R, Battisti UM, Herth MM. Recent Advances in the Development of Tetrazine Ligation Tools for Pretargeted Nuclear Imaging. Pharmaceuticals. 2022; 15(6):685. https://doi.org/10.3390/ph15060685
Chicago/Turabian StyleGarcía-Vázquez, Rocío, Umberto Maria Battisti, and Matthias M. Herth. 2022. "Recent Advances in the Development of Tetrazine Ligation Tools for Pretargeted Nuclear Imaging" Pharmaceuticals 15, no. 6: 685. https://doi.org/10.3390/ph15060685
APA StyleGarcía-Vázquez, R., Battisti, U. M., & Herth, M. M. (2022). Recent Advances in the Development of Tetrazine Ligation Tools for Pretargeted Nuclear Imaging. Pharmaceuticals, 15(6), 685. https://doi.org/10.3390/ph15060685