Three-Dimensional (3D) Printing in Cancer Therapy and Diagnostics: Current Status and Future Perspectives
Abstract
:1. Introduction
2. 3D Printing Methods
2.1. Extrusion-Based Printing
2.2. Laser-Assisted 3D Printing System
2.3. SLA-Based Printing
2.4. Inkjet-Based Printing
3. Bio-Ink
3.1. Protein/Peptide Polymer-Based Bio-Ink
3.2. Carbohydrates-Based Bio-Ink
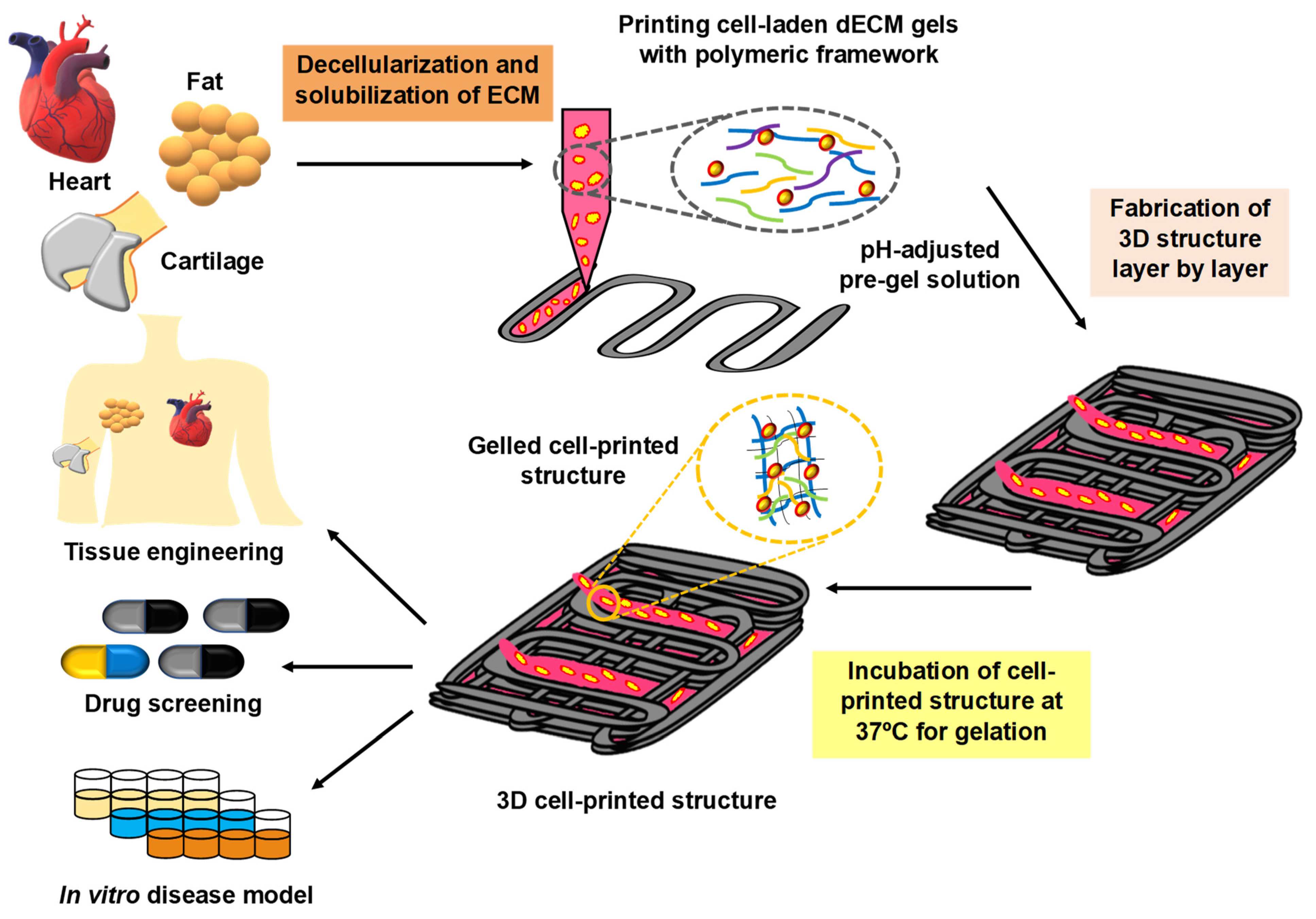
3.3. Decellularized Extracellular Based Bioink
3.4. Synthetic Polymer Bioink
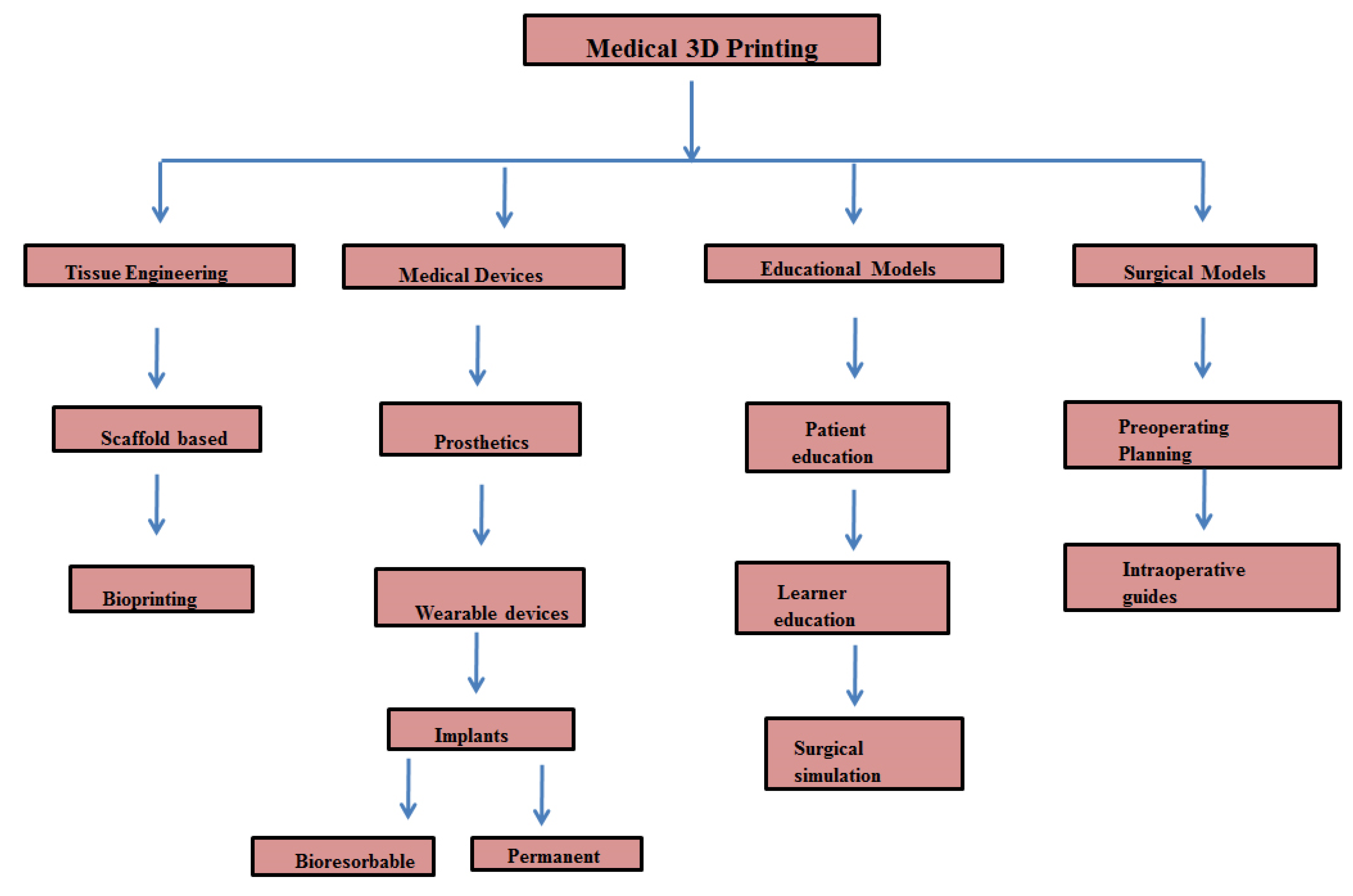
4. 3D Printing in Cancer Management
4.1. Cancer Surgery and 3D Printing: Clinical Studies
4.2. Tumor Microenvironment and 3D Printing
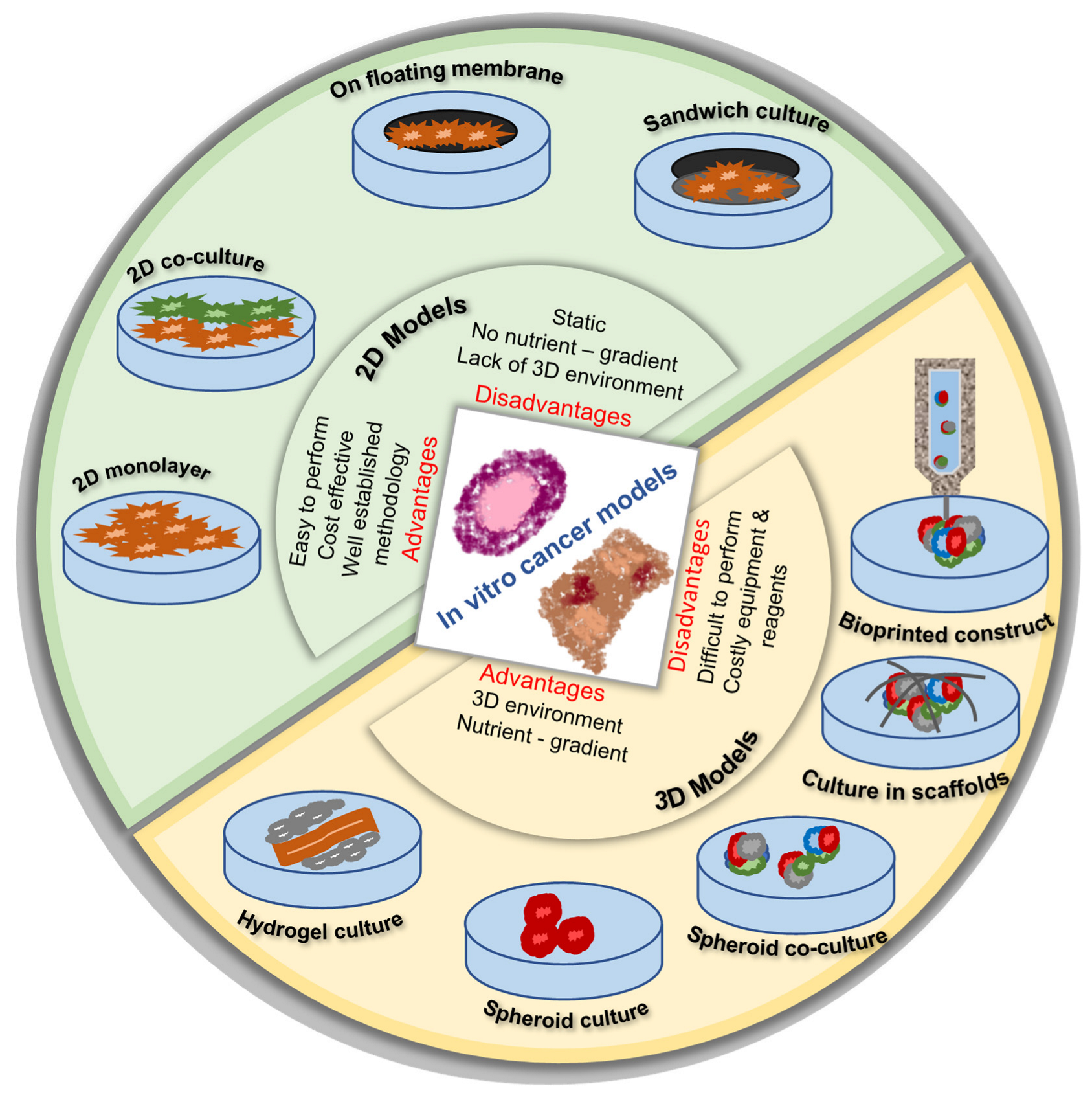
4.3. 3D Printing and In Vitro Cancer Models
4.4. Cancer Drug Delivery/Screening and 3D Printing
4.5. Drug-Eluting Implant and 3D Printing
4.6. Cancer Metastasis and 3D Printing
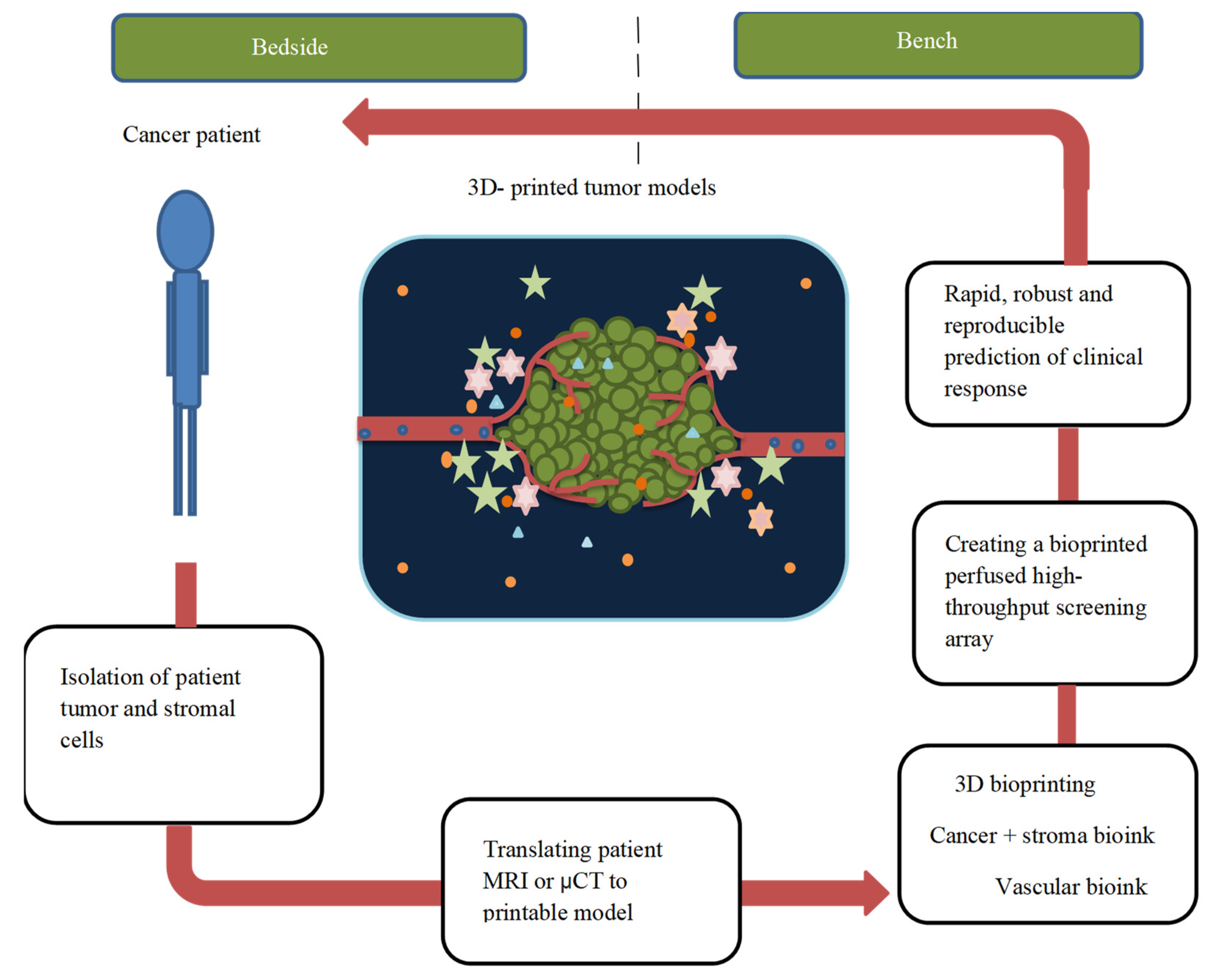
4.7. Cancer Diagnosis and 3D Printing
4.8. Cancer-On-A-Chip and 3D Printing
5. Nanomaterial, Cancer, and 3D Printing
6. Current Challenges and Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today (accessed on 24 February 2022).
- Globocan. 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 24 February 2022).
- Bhuskute, H.; Shende, P.; Prabhakar, B. 3D Printed Personalized Medicine for Cancer: Applications for Betterment of Diagnosis, Prognosis and Treatment. AAPS PharmSciTech 2021, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Almela, T.; Tayebi, L.; Moharamzadeh, K. 3D bioprinting for in vitro models of oral cancer: Toward development and validation. Bioprinting 2021, 22, e00132. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Su, Y.; Fang, D.; Qiao, S.; Ding, P.; Pan, W. Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development. Acta Pharm. Sin. B 2021, 11, 2488–2504. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D bioprinting for reconstituting the cancer microenvironment. npj Precis. Oncol. 2020, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Samitier, J.; Sanchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denizet, G.; Calame, P.; Lihoreau, T.; Kleinclauss, F.; Aubry, S. 3D multi-tissue printing for kidney transplantation. Quant. Imaging Med. Surg. 2018, 9, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.L.; Looi, T.; Lendvay, T.S.; Drake, J.M.; Farhat, W.A. Use of 3-Dimensional Printing Technology and Silicone Modeling in Surgical Simulation: Development and Face Validation in Pediatric Laparoscopic Pyeloplasty. J. Surg. Educ. 2014, 71, 762–767. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Yu, D.G.; Shen, X.X.; Branford-White, C.; Zhu, L.M.; White, K.; Yang, X.L. Novel oral fast-disintegrating drug delivery devices with predefined inner structure fabricated by three-dimensional printing. J. Pharm. Pharmacol. 2009, 61, 323–329. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Zhang, H.; Di, X.; Yu, H.; Wang, Z.; Zhang, L.; Zhao, J.; Liu, Z.; Sui, A.; Wang, J. Dosimetry study of three-dimensional print template-guided precision (125)I seed implantation. J. Cancer Res. Ther. 2016, 12, C159–C165. [Google Scholar]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-Specific Surgical Implants Made of 3D Printed PEEK: Material, Technology, and Scope of Surgical Application. BioMed Res. Int. 2018, 2018, 4520636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.; Meng, F.; Haag, R.; Zhong, Z. Actively targeted nanomedicines for precision cancer therapy: Concept, construction, challenges and clinical translation. J. Control. Release 2021, 329, 676–695. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Kim, B.S.; Jang, J.; Cho, D.-W. Recent Strategies in Extrusion-Based Three-Dimensional Cell Printing toward Organ Biofabrication. ACS Biomater. Sci. Eng. 2019, 5, 1150. [Google Scholar] [CrossRef]
- Lawlor, K.T.; Vanslambrouck, J.M.; Higgins, J.W.; Chambon, A.; Bishard, K.; Arndt, D.; Er, P.X.; Wilson, S.B.; Howden, S.E.; Tan, K.S.; et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 2020, 20, 260–271. [Google Scholar] [CrossRef]
- Gantumur, E.; Nakahata, M.; Kojima, M.; Sakai, S. Extrusion-Based Bioprinting through Glucose-Mediated Enzymatic Hydrogelation. Int. J. Bioprinting 2020, 6, 250. [Google Scholar] [CrossRef]
- Gospodinova, A.; Nankov, V.; Tomov, S.; Redzheb, M.; Petrov, P.D. Extrusion bioprinting of hydroxyethylcellulose-based bioink for cervical tumor model. Carbohydr. Polym. 2021, 260, 117793. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Ozbolat, V.; Datta, P.; Heo, D.N.; Ozbolat, I.T. Thermally-controlled extrusion-based bioprinting of collagen. J. Mater. Sci. Mater. Med. 2019, 30, 55. [Google Scholar] [CrossRef] [PubMed]
- Kuzucu, M.; Vera, G.; Beaumont, M.; Fischer, S.; Wei, P.; Shastri, V.P.; Forget, A. Extrusion-Based 3D Bioprinting of Gradients of Stiffness, Cell Density, and Immobilized Peptide Using Thermogelling Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Trucco, D.; Sharma, A.; Manferdini, C.; Gabusi, E.; Petretta, M.; Desando, G.; Ricotti, L.; Chakraborty, J.; Ghosh, S.; Lisignoli, G. Modeling and Fabrication of Silk Fibroin–Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater. Sci. Eng. 2021, 7, 3306–3320. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Azizi, L.; Turkki, P.; Janka, M.; Hytönen, V.P.; Tuukkanen, S. Extrusion-Based Bioprinting of Multilayered Nanocellulose Constructs for Cell Cultivation Using In Situ Freezing and Preprint CaCl2 Cross-Linking. ACS Omega 2020, 6, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, C.; Chen, T.; Qiu, R.; Liu, W. Photo-curing 3D printing of micro-scale bamboo fibers reinforced palm oil-based thermosets composites. Compos. Part A Appl. Sci. Manuf. 2021, 152, 106676. [Google Scholar] [CrossRef]
- Sakai, S.; Kotani, T.; Harada, R.; Goto, R.; Morita, T.; Bouissil, S.; Dubessay, P.; Pierre, G.; Michaud, P.; El Boutachfaiti, R.; et al. Development of phenol-grafted polyglucuronic acid and its application to extrusion-based bioprinting inks. Carbohydr. Polym. 2021, 277, 118820. [Google Scholar] [CrossRef]
- Neufeld, L.; Yeini, E.; Reisman, N.; Shtilerman, Y.; Ben-Shushan, D.; Pozzi, S.; Madi, A.; Tiram, G.; Eldar-Boock, A.; Ferber, S.; et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci. Adv. 2021, 7, eabi9119. [Google Scholar] [CrossRef]
- Koch, L.; Gruene, M.; Unger, C.; Chichkov, B. Laser assisted cell printing. Curr. Pharm. Biotechnol. 2013, 14, 91–97. [Google Scholar]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser Printing of Stem Cells for Biofabrication of Scaffold-Free Autologous Grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef]
- Hakobyan, D.; Médina, C.; Dusserre, N.; Stachowicz, M.-L.; Handschin, C.; Fricain, J.-C.; Guillermet-Guibert, J.; Oliveira, H. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 2020, 12, 035001. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Fricain, J.-C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Pflaum, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 2011, 3, 015005. [Google Scholar] [CrossRef]
- Nakielski, P.; Rinoldi, C.; Pruchniewski, M.; Pawłowska, S.; Gazińska, M.; Strojny, B.; Rybak, D.; Jezierska-Woźniak, K.; Urbanek, O.; Denis, P.; et al. Laser-Assisted Fabrication of Injectable Nanofibrous Cell Carriers. Small 2021, 18, 2104971. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Kang, H.W.; Cho, D.W. Development of an indirect stereolithography technology for scaffold fabrication with a wide range of biomaterial selectivity. Tissue Eng. Part C Methods 2012, 18, 719–729. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111773. [Google Scholar] [CrossRef]
- Burke, G.; Devine, D.M.; Major, I. Effect of Stereolithography 3D Printing on the Properties of PEGDMA Hydrogels. Polymers 2020, 12, 2015. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, A.; Lewin, S.; Eriksson, O.; Kreuger, J.; Persson, C. The Potential of Stereolithography for 3D Printing of Synthetic Trabecular Bone Structures. Materials 2021, 14, 3712. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, J.; Windmill, J.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, H.; Jiang, J.; Zhao, C.; Zhang, J.; Chen, P.; Lin, X.; Fan, S. Desktop-Stereolithography 3D Printing of a Polyporous Extracellular Matrix Bioink for Bone Defect Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 589094. [Google Scholar] [CrossRef]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Duffy, G.L.; Liang, H.; Williams, R.L.; Wellings, D.A.; Black, K. 3D reactive inkjet printing of poly-ε-lysine/gellan gum hydrogels for potential corneal constructs. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112476. [Google Scholar] [CrossRef]
- Lion, A.; Wildman, R.D.; Alexander, M.R.; Roberts, C.J. Customisable Tablet Printing: The Development of Multimaterial Hot Melt Inkjet 3D Printing to Produce Complex and Personalised Dosage Forms. Pharmaceutics 2021, 13, 1679. [Google Scholar] [CrossRef]
- Cader, H.K.; Rance, G.; Alexander, M.; Gonçalves, A.D.; Roberts, C.; Tuck, C.; Wildman, R. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Clark, E.A.; Alexander, M.; Irvine, D.J.; Roberts, C.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Hague, R.J.; Tuck, C.; Wildman, R.D. 3D printing of tablets using inkjet with UV photoinitiation. Int. J. Pharm. 2017, 529, 523–530. [Google Scholar] [CrossRef]
- Clark, E.A.; Alexander, M.R.; Irvine, D.J.; Roberts, C.; Wallace, M.J.; Yoo, J.; Wildman, R.D. Making tablets for delivery of poorly soluble drugs using photoinitiated 3D inkjet printing. Int. J. Pharm. 2020, 578, 118805. [Google Scholar] [CrossRef] [PubMed]
- Kyobula, M.; Adedeji, A.; Alexander, M.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Park, J.A.; Lee, H.-R.; Yoon, W.H.; Hwang, D.S.; Jung, S. Inkjet-Spray Hybrid Printing for 3D Freeform Fabrication of Multilayered Hydrogel Structures. Adv. Health Mater. 2018, 7, e1800050. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Park, J.A.; Kim, W.; Kim, S.; Lee, H.; Kim, W.; Yoo, J.; Jung, S. All-Inkjet-Printed 3D Alveolar Barrier Model with Physiologically Relevant Microarchitecture. Adv. Sci. 2021, 8, 2004990. [Google Scholar] [CrossRef] [PubMed]
- Dudman, J.P.R.; Ferreira, A.M.; Gentile, P.; Wang, X.; Ribeiro, R.D.C.; Benning, M.; Dalgarno, K.W. Reliable inkjet printing of chondrocytes and MSCs using reservoir agitation. Biofabrication 2020, 12, 045024. [Google Scholar] [CrossRef]
- Heid, S.; Boccaccini, A.R. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Thorat, N.D.; Pricl, S.; Patil, R.M.; Rohiwal, S.; Townley, H. Bioink: A 3D-bioprinting tool for anticancer drug discovery and cancer management. Drug Discov. Today 2021, 26, 1574–1590. [Google Scholar] [CrossRef]
- Nishiguchi, A.; Kapiti, G.; Höhner, J.R.; Singh, S.; Moeller, M. In Situ 3D-Printing using a Bio-ink of Protein–photosensitizer Conjugates for Single-cell Manipulation. ACS Appl. Bio Mater. 2020, 3, 2378–2384. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Hu, Q.; Shen, Z.; Rana, D.; Ramalingam, M. Designing vascular supportive albumen-rich composite bioink for organ 3D printing. J. Mech. Behav. Biomed. Mater. 2020, 104, 103642. [Google Scholar] [CrossRef]
- Kim, W.; Kim, G. Collagen/bioceramic-based composite bioink to fabricate a porous 3D hASCs-laden structure for bone tissue regeneration. Biofabrication 2019, 12, 015007. [Google Scholar] [CrossRef]
- Bakirci, E.; Toprakhisar, B.; Zeybek, M.C.; Ince, G.O.; Koc, B. Cell sheet based bioink for 3D bioprinting applications. Biofabrication 2017, 9, 024105. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cruz, M.R.; Postma, A.; Frith, J.E.; Meagher, L. Printability and bio-functionality of a shear thinning methacrylated xanthan–gelatin composite bioink. Biofabrication 2021, 13, 035023. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Wu, W.; Zhang, A.; Lu, B.; Zhang, T.; Kong, M. Dual cure (thermal/photo) composite hydrogel derived from chitosan/collagen for in situ 3D bioprinting. Int. J. Biol. Macromol. 2021, 182, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Wojciechowski, J.P.; Tang, J.; Guo, Y.; Stevens, M.M. Tunable Microgel-Templated Porogel (MTP) Bioink for 3D Bioprinting Applications. Adv. Health Mater. 2022, 11, 2200027. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, D.; Wang, L.; Hou, J.; Zhang, H.; Li, Y.; Zhong, S.; Wang, Y.; Wu, Y.; Huang, W. 3D bioprinted multiscale composite scaffolds based on gelatin methacryloyl (GelMA)/chitosan microspheres as a modular bioink for enhancing 3D neurite outgrowth and elongation. J. Colloid Interface Sci. 2020, 574, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mendes, B.B.; Gómez-Florit, M.; Hamilton, A.G.; Detamore, M.S.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Human platelet lysate-based nanocomposite bioink for bioprinting hierarchical fibrillar structures. Biofabrication 2019, 12, 015012. [Google Scholar] [CrossRef]
- Deng, F.; Dang, Y.; Tang, L.; Hu, T.; Ding, C.; Hu, X.; Wu, H.; Chen, L.; Huang, L.; Ni, Y.; et al. Tendon-inspired fibers from liquid crystalline collagen as the pre-oriented bioink. Int. J. Biol. Macromol. 2021, 185, 739–749. [Google Scholar] [CrossRef]
- Beketov, E.E.; Isaeva, E.V.; Yakovleva, N.D.; Demyashkin, G.A.; Arguchinskaya, N.V.; Kisel, A.A.; Lagoda, T.S.; Malakhov, E.P.; Kharlov, V.I.; Osidak, E.O.; et al. Bioprinting of Cartilage with Bioink Based on High-Concentration Collagen and Chondrocytes. Int. J. Mol. Sci. 2021, 22, 11351. [Google Scholar] [CrossRef]
- Aronsson, C.; Jury, M.; Naeimipour, S.; Boroojeni, F.R.; Christoffersson, J.; Lifwergren, P.; Mandenius, C.-F.; Selegård, R.; Aili, D. Dynamic peptide-folding mediated biofunctionalization and modulation of hydrogels for 4D bioprinting. Biofabrication 2020, 12, 035031. [Google Scholar] [CrossRef]
- Arab, W.; Rauf, S.; Al-Harbi, O.; Hauser, C.A.E. Novel ultrashort self-assembling peptide bioinks for 3D culture of muscle myoblast cells. Int. J. Bioprint. 2018, 4, 129. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; Panhuis, M.I.H.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.R.; Sharma, R.; Masri, N.Z.; Willerth, S.M. 3D Bioprinting Mesenchymal Stem Cell-Derived Neural Tissues Using a Fibrin-Based Bioink. Biomolecules 2021, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Smits, I.P.M.; De La Vega, L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Anil Kumar, S.; Alonzo, M.; Allen, S.C.; Abelseth, L.; Thakur, V.; Akimoto, J.; Ito, Y.; Willerth, S.M.; Suggs, L.; Chattopadhyay, M.; et al. A Visible Light-Cross-Linkable, Fibrin–Gelatin-Based Bioprinted Construct with Human Cardiomyocytes and Fibroblasts. ACS Biomater. Sci. Eng. 2019, 5, 4551–4563. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Enhe, J.; Yao, B.; Wang, Y.; Zhu, D.; Li, Z.; Song, W.; Duan, X.; Yuan, X.; et al. Bioactive nanoparticle reinforced alginate/gelatin bioink for the maintenance of stem cell stemness. Mater. Sci. Eng. C 2021, 126, 112193. [Google Scholar] [CrossRef]
- Sathish, P.B.; Gayathri, S.; Priyanka, J.; Shalini, M.; Narmadha, R.; Krishnakumar, G.S. Tricomposite gelatin-carboxymethylcellulose-alginate bioink for direct and indirect 3D printing of human knee meniscal scaffold. Int. J. Biol. Macromol. 2022, 195, 179–189. [Google Scholar] [CrossRef]
- Li, L.; Qin, S.; Peng, J.; Chen, A.; Nie, Y.; Liu, T.; Song, K. Engineering gelatin-based alginate/carbon nanotubes blend bioink for direct 3D printing of vessel constructs. Int. J. Biol. Macromol. 2020, 145, 262–271. [Google Scholar] [CrossRef]
- Amaral, A.J.R.; Gaspar, V.M.; Lavrador, P.; Mano, J.F. Double network laminarin-boronic/alginate dynamic bioink for 3D bioprinting cell-laden constructs. Biofabrication 2021, 13, 035045. [Google Scholar] [CrossRef]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef]
- Ruther, F.; Distler, T.; Boccaccini, A.R.; Detsch, R. Biofabrication of vessel-like structures with alginate di-aldehyde—gelatin (ADA-GEL) bioink. J. Mater. Sci. Mater. Med. 2018, 30, 1–14. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.C.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wenger, A.; Golzar, H.; Tang, X. 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sellaro, T.L.; Ranade, A.; Faulk, D.M.; McCabe, G.P.; Dorko, K.; Badylak, S.F.; Strom, S.C. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 2010, 16, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Luo, C.; Zhai, C.; Li, Z.; Zhang, Y.; Yuan, T.; Dong, S.; Zhang, J.; Fan, W. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C 2021, 118, 111388. [Google Scholar] [CrossRef]
- Sobreiro-Almeida, R.; Gómez-Florit, M.; Quinteira, R.; Reis, R.L.; Gomes, M.E.; Neves, N.M. Decellularized kidney extracellular matrix bioinks recapitulate renal 3D microenvironment in vitro. Biofabrication 2021, 13, 045006. [Google Scholar] [CrossRef]
- Hou, C.; Zheng, J.; Li, Z.; Qi, X.; Tian, Y.; Zhang, M.; Zhang, J.; Huang, X. Printing 3D vagina tissue analogues with vagina decellularized extracellular matrix bioink. Int. J. Biol. Macromol. 2021, 180, 177–186. [Google Scholar] [CrossRef]
- Jang, J.; Kim, T.G.; Kim, B.S.; Kim, S.-W.; Kwon, S.-M.; Cho, D.-W. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.-H.; Jang, J.; Kim, B.S.; Cho, D.-W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef]
- Visscher, D.O.; Lee, H.; van Zuijlen, P.P.; Helder, M.N.; Atala, A.; Yoo, J.J.; Lee, S.J. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2020, 121, 193–203. [Google Scholar] [CrossRef]
- Mao, Q.; Wang, Y.; Li, Y.; Juengpanich, S.; Li, W.; Chen, M.; Yin, J.; Fu, J.; Cai, X. Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater. Sci. Eng. C 2020, 109, 110625. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.H.; Leonard, A.; Camp, N.D.; Long, J.T.; Nawas, Z.Y.; Chavanachat, R.; Smith, A.S.; Choi, J.S.; Dong, Z.; Ahn, E.H.; et al. Tunable electroconductive decellularized extracellular matrix hydrogels for engineering human cardiac microphysiological systems. Biomaterials 2021, 272, 120764. [Google Scholar] [CrossRef] [PubMed]
- Kort-Mascort, J.; Bao, G.; Elkashty, O.; Flores-Torres, S.; Munguia-Lopez, J.G.; Jiang, T.; Ehrlicher, A.J.; Mongeau, L.; Tran, S.D.; Kinsella, J.M. Decellularized Extracellular Matrix Composite Hydrogel Bioinks for the Development of 3D Bioprinted Head and Neck in Vitro Tumor Models. ACS Biomater. Sci. Eng. 2021, 7, 5288–5300. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D bioprinting of photo-crosslinkable silk methacrylate (SilMA)-polyethylene glycol diacrylate (PEGDA) bioink for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2022, 110, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Li, M.; Zheng, S.; Qi, J. Adjusting the accuracy of PEGDA-GelMA vascular network by dark pigments via digital light processing printing. J. Biomater. Appl. 2021, 36, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ruan, C.; Ma, Y.; Cheng, D.; Wu, M.; Liu, W.; Zhao, X.; Pan, H.; Lu, W.W. 3D-Bioprinted Osteoblast-Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis both In Vitro and In Vivo. Adv. Sci. 2017, 5, 1700550. [Google Scholar] [CrossRef] [Green Version]
- VijayaVenkataRaman, S.; Vialli, N.; Fuh, J.Y.H.; Lu, W.F. Conductive Collagen/PPy-b-PCL hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprinting 2019, 5, 229. [Google Scholar] [CrossRef]
- Chae, S.; Lee, S.-S.; Choi, Y.-J.; Hong, D.H.; Gao, G.; Wang, J.H.; Cho, D.-W. 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 2021, 267, 120466. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Kade, J.C.; Jeiranikhameneh, A.; Ruberu, K.; Mukherjee, P.; Yue, Z.; Wallace, G.G. 3D hybrid printing platform for auricular cartilage reconstruction. Biomed. Phys. Eng. Express 2020, 6, 035003. [Google Scholar] [CrossRef]
- Firouzian, K.F.; Zhang, T.; Zhang, H.; Song, Y.; Su, X.; Lin, F. An Image-Guided Intrascaffold Cell Assembly Technique for Accurate Printing of Heterogeneous Tissue Constructs. ACS Biomater. Sci. Eng. 2019, 5, 3499–3510. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Salaoru, I.; Zhou, Z.; Morris, P.; Gibbons, G.J. Inkjet printing of polyvinyl alcohol multilayers for additive manufacturing applications. J. Appl. Polym. Sci. 2016, 133, 43572. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, S.; Anderson, G.D.; Robert Langer, R. Physical and mechanical properties of pla, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Galstyan, A.; Bunker, M.J.; Lobo, F.; Sims, R.; Inziello, J.; Stubbs, J.; Mukhtar, R.; Kelil, T. Applications of 3D printing in breast cancer management. 3D Print. Med. 2021, 7, 19. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Vaishya, R. 3D printing applications for the treatment of cancer. Clin. Epidemiology Glob. Health 2020, 8, 1072–1076. [Google Scholar] [CrossRef] [Green Version]
- Suvanasuthi, R.; Chimnaronk, S.; Promptmas, C. 3D printed hydrophobic barriers in a paper-based biosensor for point-of-care detection of dengue virus serotypes. Talanta 2021, 237, 122962. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Covalently modified enzymatic 3D-printed bioelectrode. Mikrochim. Acta 2021, 188, 374. [Google Scholar] [CrossRef]
- Marzo, A.M.L.; Mayorga-Martinez, C.C.; Pumera, M. 3D-printed graphene direct electron transfer enzyme biosensors. Biosens. Bioelectron. 2020, 151, 111980. [Google Scholar] [CrossRef]
- Cho, S.-J.; Byun, D.; Nam, T.-S.; Choi, S.-Y.; Lee, B.-G.; Kim, M.-K.; Kim, S. A 3D-Printed Sensor for Monitoring Biosignals in Small Animals. J. Health Eng. 2017, 2017, 9053764. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gao, W.; Ng, S.; Pumera, M. Chiral Protein–Covalent Organic Framework 3D-Printed Structures as Chiral Biosensors. Anal. Chem. 2021, 93, 5277–5283. [Google Scholar] [CrossRef] [PubMed]
- Kadimisetty, K.; Song, J.; Doto, A.M.; Hwang, Y.; Peng, J.; Mauk, M.G.; Bushman, F.D.; Gross, R.; Jarvis, J.N.; Liu, C. Fully 3D printed integrated reactor array for point-of-care molecular diagnostics. Biosens. Bioelectron. 2018, 109, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Malkoc, A.; La Belle, J.T. The Development of a Glucose Dehydrogenase 3D-Printed Glucose Sensor: A Proof-of-Concept Study. J. Diabetes Sci. Technol. 2017, 12, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, A.; Guardigli, M.; Calabria, D.; Calabretta, M.M.; Cevenini, L.; Michelini, E. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 2014, 139, 6494–6501. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, Y.; Kwon, E.Y.; Wang, L.; Cheng, N.; Niu, X.; Ding, S.; Van Wie, B.J.; Lin, Y.; Du, D. Nanomaterial-enhanced 3D-printed sensor platform for simultaneous detection of atrazine and acetochlor. Biosens. Bioelectron. 2021, 184, 113238. [Google Scholar] [CrossRef]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening. Ann. Biomed Eng. 2017, 45, 164–179. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bian, L.; Zhou, H.; Wu, D.; Xu, J.; Gu, C.; Fan, X.; Liu, Z.; Zou, J.; Xia, J.; et al. Usefulness of three-dimensional printing of superior mesenteric vessels in right hemicolon cancer surgery. Sci. Rep. 2020, 10, 11660. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, H.G.; Kim, J.H.; Kim, H.-S. The application of 3D-printing technology in pelvic bone tumor surgery. J. Orthop. Sci. 2021, 26, 276–283. [Google Scholar] [CrossRef]
- Zeng, N.; Yang, J.; Xiang, N.; Wen, S.; Zeng, S.; Qi, S.; Zhu, W.; Hu, H.; Fang, C. Application of 3D visualization and 3D printing in individualized precision surgery for Bismuth-Corlette type III and IV hilar cholangiocarcinoma. Nan Fang Yi Ke Da XueXue Bao 2020, 40, 1172–1177. (In Chinese) [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Wang, X.; Li, X.-D.; Cheng, K.; Zhou, Y.; Jiang, X.-B. A small 3D-printing model of macroadenomas for endoscopic endonasal surgery. Pituitary 2019, 22, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Emile, S.H.; Wexner, S.D. Systematic review of the applications of three-dimensional printing in colorectal surgery. Color. Dis. 2019, 21, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Lee, S.; Kim, T.; Baek, J.H.; Kim, W.W.; Chung, K.-W.; Kim, N.; Sung, T.-Y. Usefulness of a 3D-Printed Thyroid Cancer Phantom for Clinician to Patient Communication. World J. Surg. 2020, 44, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Burdall, O.C.; Makin, E.; Davenport, M.; Ade-Ajayi, N. 3D printing to simulate laparoscopic choledochal surgery. J. Pediatric Surg. 2016, 51, 828–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smelt, J.L.; Suri, T.; Valencia, O.; Jahangiri, M.; Rhode, K.; Nair, A.; Bille, A. Operative Planning in Thoracic Surgery: A Pilot Study Comparing Imaging Techniques and Three-Dimensional Printing. Ann. Thorac. Surg. 2019, 107, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Jiang, Y.; Ji, Z.; Guo, F.; Jiang, P.; Li, X.; Chen, Y.; Sun, H.; Fan, J.; et al. The efficacy and dosimetry analysis of CT-guided 125I seed implantation assisted with 3D-printing non-co-planar template in locally recurrent rectal cancer. Radiat. Oncol. 2020, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Park, S.; Kang, C.H.; Park, I.K.; Goo, J.M.; Kim, Y.T. Personalized 3D-Printed Model for Informed Consent for Stage I Lung Cancer: A Randomized Pilot Trial. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xie, Y.; Shang, X.; Xiong, G.; Chen, S.; Yao, Y.; Pan, Z.; Pan, H.; Dong, X.; Li, Y.; et al. The manufacturing procedure of 3D printed models for endoscopic endonasal transsphenoidal pituitary surgery. Technol. Health Care 2020, 28, 131–150. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Chen, Q.; Li, T.; Chen, K.; Yu, Q.; Lin, X. Three-dimensional printing technology for localised thoracoscopic segmental resection for lung cancer: A quasi-randomised clinical trial. World J. Surg. Oncol. 2020, 18, 223. [Google Scholar] [CrossRef]
- Lan, Q.; Zhu, Q.; Xu, L.; Xu, T. Application of 3D-Printed Craniocerebral Model in Simulated Surgery for Complex Intracranial Lesions. World Neurosurg. 2020, 134, e761–e770. [Google Scholar] [CrossRef]
- Anwar, S.; Singh, G.K.; Miller, J.; Sharma, M.; Manning, P.; Billadello, J.J.; Eghtesady, P.; Woodard, P.K. 3D Printing is a Transformative Technology in Congenital Heart Disease. JACC Basic Transl. Sci. 2018, 3, 294–312. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, X.; Ding, J.; Long, X.; Zhang, H.; Zhang, X.; Jiang, X.; Xu, T. 3D bioprinted glioma microenvironment for glioma vascularization. J. Biomed. Mater. Res. Part A 2021, 109, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, E.; Wei, X.; Xia, Y.; Wu, Z.; Gong, Z.; Shang, Z.; Guo, S. The acoustic droplet printing of functional tumor microenvironments. Lab Chip 2021, 21, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zheng, X.; Zhao, L.; Zhang, X. Recapitulating and Deciphering Tumor Microenvironment by Using 3D Printed Plastic Brick–Like Microfluidic Cell Patterning. Adv. Health Mater. 2020, 9, e1901713. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cao, Y.; Shen, Z.; Cheng, Y.; Ma, Z.; Wang, L.; Zhang, Y.; An, Y.; Sang, S. 3D Bioprinted GelMA/PEGDA Hybrid Scaffold for Establishing an In Vitro Model of Melanoma. J. Microbiol. Biotechnol. 2022, 32, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Kinsella, J.M. Bioprintable Alginate/Gelatin Hydrogel 3D In Vitro Model Systems Induce Cell Spheroid Formation. J. Vis. Exp. 2018, 137, e57826. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jang, J.; Cho, D.-W. Controlling Cancer Cell Behavior by Improving the Stiffness of Gastric Tissue-Decellularized ECM Bioink With Cellulose Nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 605819. [Google Scholar] [CrossRef]
- Aveic, S.; Janßen, S.; Nasehi, R.; Seidelmann, M.; Vogt, M.; Pantile, M.; Rütten, S.; Fischer, H. A 3D printed in vitro bone model for the assessment of molecular and cellular cues in metastatic neuroblastoma. Biomater. Sci. 2021, 9, 1716–1727. [Google Scholar] [CrossRef]
- Lv, K.; Zhu, J.; Zheng, S.; Jiao, Z.; Nie, Y.; Song, F.; Liu, T.; Song, K. Evaluation of inhibitory effects of geniposide on a tumor model of human breast cancer based on 3D printed Cs/Gel hybrid scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111509. [Google Scholar] [CrossRef]
- Li, R.; Ting, Y.-H.; Youssef, S.H.; Song, Y.; Garg, S. Three-Dimensional Printing for Cancer Applications: Research Landscape and Technologies. Pharmaceuticals 2021, 14, 787. [Google Scholar] [CrossRef]
- Zelis, J.M.; Meiburg, R.; Roijen, J.J.; Janssens, K.L.; Veer, M.V.; Pijls, N.H.; Johnson, N.P.; van de Vosse, F.N.; Tonino, P.A.; Rutten, M.C. 3D-printed stenotic aortic valve model to simulate physiology before, during, and after transcatheter aortic valve implantation. Int. J. Cardiol. 2020, 313, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Ayi, T.C.; Liu, Y.-C.; Sing, S.L.; Yeong, W.Y.; Tan, B.-H. Fabrication and Characterization of 3D Bioprinted Triple-layered Human Alveolar Lung Models. Int. J. Bioprinting 2021, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Sharma, P.K.; Murty, U.S.; Banerjee, S. Stereolithography-assisted fabrication of 3D printed polymeric film for topical berberine delivery: In-vitro, ex-vivo and in-vivo investigations. J. Pharm. Pharmacol. 2021, 158. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, A.; Surapaneni, S.K.; Huang, J.; Mondal, A.; Wang, V.Z.; Haruna, N.F.; Bagde, A.; Arthur, P.; Kutlehria, S.; Patel, N.; et al. Polysaccharide hydrogel based 3D printed tumor models for chemotherapeutic drug screening. Sci. Rep. 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Chung, J.; Cheng, K.; Gupta, R.; Haag, H.; Williams, Z.; Wallace, G. Invitro and Invivo Study of PCL-Hydrogel Scaffold to Advance Bioprinting Translation in Microtia Reconstruction. J. Craniofacial Surg. 2021, 32, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Won, J.-E.; Han, C.; Yin, X.Y.; Kim, H.K.; Nah, H.; Kwon, I.K.; Min, B.-H.; Kim, C.-H.; Shin, Y.S.; et al. Development of a three-dimensionally printed scaffold grafted with bone forming peptide-1 for enhanced bone regeneration with in vitro and in vivo evaluations. J. Colloid Interface Sci. 2019, 539, 468–480. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.; Elisseeff, J.H.; Dorafshar, A.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Mahmoudifar, N.; Doran, P.M. Tissue engineering of human cartilage and osteochondral composites using recirculation bioreactors. Biomaterials 2005, 26, 7012–7024. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.; González-Burgos, E.; Lalatsa, A.; Serrano, D. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Yi, H.-G.; Choi, Y.-J.; Kang, K.S.; Hong, J.M.; Pati, R.G.; Park, M.N.; Shim, I.K.; Lee, C.M.; Kim, S.C.; Cho, D.-W. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J. Control. Release 2016, 238, 231–241. [Google Scholar] [CrossRef]
- Germini, G.; Peltonen, L. 3D Printing of Drug Nanocrystals for Film Formulations. Molecules 2021, 26, 3941. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Yasa, I.C.; Yasa, O.; Tabak, A.F.; Giltinan, J.; Sitti, M. 3D-Printed Biodegradable Microswimmer for Theranostic Cargo Delivery and Release. ACS Nano 2019, 13, 3353–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, X.; Yang, Y.; Huang, R.; Shi, X.; Chen, H.; Wang, J.; Chen, Y.; Tan, Y.; Tan, Z. E-Jet 3D-Printed Scaffolds as Sustained Multi-Drug Delivery Vehicles in Breast Cancer Therapy. Pharm. Res. 2019, 36, 182. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R.; Achilefu, S. 3D Printing of Poloxamer 407 Nanogel Discs and Their Applications in Adjuvant Ovarian Cancer Therapy. Mol. Pharm. 2019, 16, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Do, A.-V.; Akkouch, A.; Green, B.; Ozbolat, I.; Debabneh, A.; Geary, S.; Salem, A.K. Controlled and Sequential Delivery of Fluorophores from 3D Printed Alginate-PLGA Tubes. Ann. Biomed. Eng. 2017, 45, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Fu, S.; Zuo, P.; Ye, B. A Novel Wick-Like Paper-Based Microfluidic Device for 3D Cell Culture and Anti-Cancer Drugs Screening. Biotechnol. J. 2021, 16, e2000126. [Google Scholar] [CrossRef]
- Kim, M.J.; Chi, B.H.; Yoo, J.J.; Ju, Y.M.; Whang, Y.M.; Chang, I.H. Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer. PLoS ONE 2019, 14, e0223689. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lo, T.-S.; Lin, Y.-T.; Chien, Y.-H.; Lu, C.-J.; Liu, S.-J. Fabrication of Drug-Eluting Polycaprolactone/poly(lactic-co-glycolic Acid) Prolapse Mats Using Solution-Extrusion 3D Printing and Coaxial Electrospinning Techniques. Polymers 2021, 13, 2295. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Chou, Y.-C.; Lai, Y.-H.; Lin, Y.-T.; Lu, C.-J.; Liu, S.-J. Fabrication of Drug-Eluting Nano-Hydroxylapatite Filled Polycaprolactone Nanocomposites Using Solution-Extrusion 3D Printing Technique. Polymers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.A.; Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Sumerel, J.; D’Agostino, H.B.; Mills, D.K.; Woodard, P.K. 3D Printed Antibiotic and Chemotherapeutic Eluting Catheters for Potential Use in Interventional Radiology: In Vitro Proof of Concept Study. Acad. Radiol. 2019, 26, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Farmer, Z.-L.; Utomo, E.; Domínguez-Robles, J.; Mancinelli, C.; Mathew, E.; Larrañeta, E.; Lamprou, D.A. 3D printed estradiol-eluting urogynecological mesh implants: Influence of material and mesh geometry on their mechanical properties. Int. J. Pharm. 2021, 593, 120145. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.-Y.; Lee, D.; Chen, S.-H.; Liao, C.-T.; Lo, L.-J.; Liu, S.-J. 3D-printed/electrospun bioresorbable nanofibrous drug-eluting cuboid frames for repair of alveolar bone defects. Int. J. Pharm. 2022, 615, 121497. [Google Scholar] [CrossRef]
- Lee, J.-H.; Baik, J.-M.; Yu, Y.-S.; Kim, J.-H.; Ahn, C.B.; Son, K.H.; Choi, E.S.; Lee, J.W. Development of a heat labile antibiotic eluting 3D printed scaffold for the treatment of osteomyelitis. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Misra, S.K.; Ostadhossein, F.; Babu, R.; Kus, J.; Tankasala, D.; Sutrisno, A.; Walsh, K.A.; Bromfield, C.R.; Pan, D. 3D-Printed Multidrug-Eluting Stent from Graphene-Nanoplatelet-Doped Biodegradable Polymer Composite. Adv. Health Mater. 2017, 6, 1700008. [Google Scholar] [CrossRef]
- Jang, J.; Kim, J.; Kim, Y.C.; Kim, S.; Chou, N.; Lee, S.; Choung, Y.; Kim, S.; Brugger, J.; Choi, H.; et al. A 3D Microscaffold Cochlear Electrode Array for Steroid Elution. Adv. Health Mater. 2019, 8, e1900379. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Y.; Wang, J.; Chen, H.; Wang, X.; Li, X.; Tan, W.; Tan, Z. 3D printed intelligent scaffold prevents recurrence and distal metastasis of breast cancer. Theranostics 2020, 10, 10652–10664. [Google Scholar] [CrossRef]
- Zhu, W.; Holmes, B.; Glazer, R.I.; Zhang, L.G. 3D printed nanocomposite matrix for the study of breast cancer bone metastasis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 69–79. [Google Scholar] [CrossRef]
- Han, W.; El Botty, R.; Montaudon, E.; Malaquin, L.; Deschaseaux, F.; Espagnolle, N.; Marangoni, E.; Cottu, P.; Zalcman, G.; Parrini, M.C.; et al. In vitro bone metastasis dwelling in a 3D bioengineered niche. Biomaterials 2021, 269, 120624. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Y.; Chen, J.; Huang, R.; Yang, Y.; Chen, H.; Huang, Y.; Tan, W.; Tan, Z. In Vitro Study of Colon Cancer Cell Migration Using E-Jet 3D Printed Cell Culture Platforms. Macromol. Biosci. 2018, 18, e1800205. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Akoury, E.; Luna, A.S.R.G.; Nour, A.; Weber, M.H.; Rosenzweig, D.H. Nanoporous 3D-Printed Scaffolds for Local Doxorubicin Delivery in Bone Metastases Secondary to Prostate Cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liang, Y.; Wang, Z.; Zhang, H.; Gao, Z.; Zhao, J.; Sui, A.; Zhao, J.; Liu, Z. Three-dimensional-printed individual template-guided 125I seed implantation for the cervical lymph node metastasis: A dosimetric and security study. J. Cancer Res. Ther. 2018, 14, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Mao, S.S.; Yao, R.; He, J.Y.; Zhou, Z.Z.; Feng, L.; Zhang, K.T.; Cheng, S.J.; Sun, W. TGF- β induced epithelial–mesenchymal transition in an advanced cervical tumor model by 3D printing. Biofabrication 2018, 10, 044102. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zheng, Z.; Zhu, L.; Pang, L.; Ma, J.; Zhu, S.; Du, L.; Jin, Y. 3D printing-based drug-loaded implanted prosthesis to prevent breast cancer recurrence post-conserving surgery. Asian J. Pharm. Sci. 2021, 16, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.S.; Pędziwiatr, M.; Major, P.; Budzyński, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [Green Version]
- Damiati, S.; Küpcü, S.; Peacock, M.; Eilenberger, C.; Zamzami, M.; Qadri, I.; Choudhry, H.; Sleytr, U.B.; Schuster, B. Acoustic and hybrid 3D-printed electrochemical biosensors for the real-time immunodetection of liver cancer cells (HepG2). Biosens. Bioelectron. 2017, 94, 500–506. [Google Scholar] [CrossRef]
- An, L.; Wang, G.; Han, Y.; Li, T.; Jin, P.; Liu, S. Electrochemical biosensor for cancer cell detection based on a surface 3D micro-array. Lab Chip 2018, 18, 335–342. [Google Scholar] [CrossRef]
- Tang, C.K.; Vaze, A.; Rusling, J.F. Automated 3D-printed unibody immunoarray for chemiluminescence detection of cancer biomarker proteins. Lab Chip 2017, 17, 484–489. [Google Scholar] [CrossRef]
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.-K. Electrochemiluminescence detection of human breast cancer cells using aptamer modified bipolar electrode mounted into 3D printed microchannel. Biosens. Bioelectron. 2018, 118, 217–223. [Google Scholar] [CrossRef]
- Park, C.; Abafogi, A.T.; Ponnuvelu, D.V.; Song, I.; Ko, K.; Park, S. Enhanced Luminescent Detection of Circulating Tumor Cells by a 3D Printed Immunomagnetic Concentrator. Biosensors 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Kadimisetty, K.; Malla, S.; Bhalerao, K.S.; Mosa, I.M.; Bhakta, S.; Lee, N.H.; Rusling, J.F. Automated 3D-Printed Microfluidic Array for Rapid Nanomaterial-Enhanced Detection of Multiple Proteins. Anal. Chem. 2018, 90, 7569–7577. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2019, 150, 111900. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, R.; Han, C.; Xu, S.; You, T.; Li, Y.; Xia, J.; Xu, X.; Wang, D.; et al. A Fully Automated and Integrated Microfluidic System for Efficient CTC Detection and Its Application in Hepatocellular Carcinoma Screening and Prognosis. ACS Appl. Mater. Interfaces 2021, 13, 30174–30186. [Google Scholar] [CrossRef] [PubMed]
- Kadimisetty, K.; Mosa, I.M.; Malla, S.; Satterwhite-Warden, J.E.; Kuhns, T.M.; Faria, R.C.; Lee, N.H.; Rusling, J.F. 3D-printed supercapacitor-powered electrochemiluminescent protein immunoarray. Biosens. Bioelectron. 2016, 77, 188–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heger, Z.; Žitka, J.; Cernei, N.; Krizkova, S.; Sztalmachova, M.; Kopel, P.; Masařík, M.; Hodek, P.; Zitka, O.; Adam, V.; et al. 3D-printed biosensor with poly(dimethylsiloxane) reservoir for magnetic separation and quantum dots-based immunolabeling of metallothionein. ELECTROPHORESIS 2015, 36, 1256–1264. [Google Scholar] [CrossRef]
- Damiati, S.; Peacock, M.; Leonhardt, S.; Damiati, L.; Baghdadi, M.A.; Becker, H.; Kodzius, R.; Schuster, B. Embedded Disposable Functionalized Electrochemical Biosensor with a 3D-Printed Flow Cell for Detection of Hepatic Oval Cells (HOCs). Genes 2018, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-de Santiago, G.T.; Flores-Garza, B.G.; Tavares-Negrete, J.A.; Lara-Mayorga, I.M.; González-Gamboa, I.; Zhang, Y.S.; Rojas-Martínez, A.; Ortiz-López, R.; Álvarez, M.M. The Tumor-on-Chip: Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials 2019, 12, 2945. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Bae, M.; Hwang, M.; Jang, J.; Cho, D.-W.; Yi, H.-G. 3D Cell-Printed Hypoxic Cancer-on-a-Chip for Recapitulating Pathologic Progression of Solid Cancer. J. Vis. Exp. 2021, 167, e61945. [Google Scholar] [CrossRef]
- Chiadò, A.; Palmara, G.; Chiappone, A.; Tanzanu, C.; Pirri, C.F.; Roppolo, I.; Frascella, F. A modular 3D printed lab-on-a-chip for early cancer detection. Lab Chip 2020, 20, 665–674. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Wu, G.; Wu, J.; Lu, S.; Lo, J.; He, Y.; Zhao, C.; Zhao, X.; Zhang, H.; et al. Metastasis-on-a-chip mimicking the progression of kidney cancer in the liver for predicting treatment efficacy. Theranostics 2020, 10, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, F.; Zhang, J.; Sun, X.; Yan, Y.; Wang, Y.; Ouyang, J.; Zhang, J.; Honore, T.; Ge, J.; et al. Study on Development of Composite Hydrogels With Tunable Structures and Properties for Tumor-on-a-Chip Research. Front. Bioeng. Biotechnol. 2020, 8, 611796. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Boerhan, R.; Liu, C.; Jiang, G. Nanoparticles Penetrate into the Multicellular Spheroid-on-Chip: Effect of Surface Charge, Protein Corona, and Exterior Flow. Mol. Pharm. 2017, 14, 4618–4627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, D.; Wang, Y.; Lin, S.; Jiang, Y. A novel 3D breast-cancer-on-chip platform for therapeutic evaluation of drug delivery systems. Anal. Chim. Acta 2018, 1036, 97–106. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, B.; Tian, Y.; Chen, H.; Liu, Y.; Fan, H.; Wang, K.; Zhang, C. Active fluidic chip produced using 3D-printing for combinatorial therapeutic screening on liver tumor spheroid. Biosens. Bioelectron. 2020, 151, 111966. [Google Scholar] [CrossRef]
- Lee, B.-E.; Kim, D.-K.; Lee, H.; Yoon, S.; Park, S.-H.; Lee, S.; Yoo, J. Recapitulation of First Pass Metabolism Using 3D Printed Microfluidic Chip and Organoid. Cells 2021, 10, 3301. [Google Scholar] [CrossRef]
- Dávila, S.; Cacheux, J.; Rodríguez, I. Microvessel-on-Chip Fabrication for the In Vitro Modeling of Nanomedicine Transport. ACS Omega 2021, 6, 25109–25115. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fernández-Garibay, X.; Castaño, A.G.; De Chiara, F.; Hernández-Albors, A.; Balaguer-Trias, J.; Ramón-Azcón, J. Muscle-on-a-chip with an on-site multiplexed biosensing system for in situ monitoring of secreted IL-6 and TNF-α. Lab Chip 2019, 19, 2568–2580. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Lindsay, C.D.; Roth, J.; LeSavage, B.L.; Seymour, A.J.; Krajina, B.A.; Ribeiro, R.; Costa, P.F.; Blaeser, A.; Heilshorn, S.C. Bioprinting Cell- and Spheroid-Laden Protein-Engineered Hydrogels as Tissue-on-Chip Platforms. Front. Bioeng. Biotechnol. 2020, 8, 374. [Google Scholar] [CrossRef]
- Chang, Y.; Jiang, J.; Chen, W.; Yang, W.; Chen, L.; Chen, P.; Shen, J.; Qian, S.; Zhou, T.; Wu, L.; et al. Biomimetic metal-organic nanoparticles prepared with a 3D-printed microfluidic device as a novel formulation for disulfiram-based therapy against breast cancer. Appl. Mater. Today 2020, 18, 100492. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, J.; Du, T.; Xu, X.; Deng, X.; Chen, S.; Liu, J.; Chen, Y.; Liu, X.; Xiong, M.; et al. Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 2019, 90, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shi, S.; Lan, X.; Quan, X.; Xu, Q.; Yao, G.; Liu, J.; Shuai, X.; Wang, C.; Li, X.; et al. Scaffold 3D-Printed from Metallic Nanoparticles-Containing Ink Simultaneously Eradicates Tumor and Repairs Tumor-Associated Bone Defects. Small Methods 2021, 5, 2100536. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Ma, B.; Li, B.; Huan, Z.; Ma, N.; Zhu, H.; Chang, J.; Xiao, Y.; Wu, C. 3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication 2020, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Luo, J.; Sun, Z.; Xia, L.; Shi, M.; Liu, M.; Chang, J.; Wu, C. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration. Biomaterials 2016, 111, 138–148. [Google Scholar] [CrossRef]
- Yang, C.; Ma, H.; Wang, Z.; Younis, M.R.; Liu, C.; Wu, C.; Luo, Y.; Huang, P. 3D Printed Wesselsite Nanosheets Functionalized Scaffold Facilitates NIR-II Photothermal Therapy and Vascularized Bone Regeneration. Adv. Sci. 2021, 8, 2100894. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Celada, L.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Chiara, M.-D. Three-dimensional bioprinted cancer models: A powerful platform for investigating tunneling nanotube-like cell structures in complex microenvironments. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112357. [Google Scholar] [CrossRef]
- Bordoni, M.; Karabulut, E.; Kuzmenko, V.; Fantini, V.; Pansarasa, O.; Cereda, C.; Gatenholm, P. 3D Printed Conductive Nanocellulose Scaffolds for the Differentiation of Human Neuroblastoma Cells. Cells 2020, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Babi, M.; Riesco, R.; Boyer, L.; Fatona, A.; Accardo, A.; Malaquin, L.; Moran-Mirabal, J. Tuning the Nanotopography and Chemical Functionality of 3D Printed Scaffolds through Cellulose Nanocrystal Coatings. ACS Appl. Bio Mater. 2021, 4, 8443–8455. [Google Scholar] [CrossRef]
- Hauser, D.; Estermann, M.; Milosevic, A.; Steinmetz, L.; Vanhecke, D.; Septiadi, D.; Drasler, B.; Petri-Fink, A.; Ball, V.; Rothen-Rutishauser, B. Polydopamine/Transferrin Hybrid Nanoparticles for Targeted Cell-Killing. Nanomaterials 2018, 8, 1065. [Google Scholar] [CrossRef] [Green Version]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Health Mater. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Kashte, S.; Jaiswal, A.K.; Kadam, S. Artificial Bone via Bone Tissue Engineering: Current Scenario and Challenges. Tissue Eng. Regen. Med. 2017, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.M.; Kolb, A.D.; Shupp, A.B.; Shine, K.M.; Bussard, K.M. Printing the Pathway Forward in Bone Metastatic Cancer Research: Applications of 3D Engineered Models and Bioprinted Scaffolds to Recapitulate the Bone–Tumor Niche. Cancers 2021, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Anjum, F.; Lienemann, P.S.; Metzger, S.; Biernaskie, J.; Kallos, M.S.; Ehrbar, M. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials 2016, 87, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Significant advancements of 4D printing in the field of orthopaedics. J. Clin. Orthop. Trauma 2020, 11, S485–S490. [Google Scholar] [CrossRef]

| S.No | Bioink Composition | Purpose | Mechanism | References |
|---|---|---|---|---|
| 1. | Protein-photosensitizer conjugates. | Regenerative medicine |
| [60] |
| 2. | Composite bioink comprises sodium alginate and egg white, often known as albumen. | Tissue and organ engineering |
| [61] |
| 3. | Composite bioink based on collagen/bioceramics. | Bone tissue regeneration |
| [62] |
| 4. | The production of bio-ink from cell sheets. | To aid in the creation of various 3D geometries via bioprinting |
| [63] |
| 5. | Bioink that self-assembles and thins under shear (Methacrylated xanthan gum with gelatin bioink). | Creating bio-functional bioink for 3D bioprinting application |
| [64] |
| 6. | Composite hydrogel bioink with dual-cure (thermal/photo). | In situ 3D bioprinting |
| [65] |
| 7. | Bioink with tunable Microgel-Templated Porogel (MTP). | To improve the use of 3D bioprinting. |
| [66] |
| 8. | Modular bioink: gelatin methacryloyl (GelMA)/chitosan microspheres | Nerve tissue engineering |
| [67] |
| 9. | Nanocomposite bioink | To produce tissue and organ surrogates for clinical use. |
| [68] |
| S.No | Biosensor | Application | Mechanism | Reference |
|---|---|---|---|---|
| 1. | Microfluidic paper-based analytical devices | Using tiny nucleotide sequence changes to distinguish dengue virus serotypes | 3D-printed barrier paper and a fluidic chip are combined. | [109] |
| 2. | 3D-printed nanocarbon electrode based on glucose oxidase | Detection of glucose in samples | To enable biosensing, a covalent linking approach was used to an enzyme on the surface of a 3D-printed electrode. | [110] |
| 3. | Enzyme biosensor | Detection of hydrogen peroxide | Direct electron transfer enzyme-based biosensors are built using 3D-printed graphene/polylactic electrodes and horseradish peroxidase immobilization. | [111] |
| 4. | Non-invasive 3Dprinted biosensor | Detect electrophysiological information | Sensor can measure electroencephalogram and electrocardiogram from zebrafish | [112] |
| 5. | 3D printed Chiral biosensor | Enantiomer recognition. | A 3D-printed electrochemical chiral sensor was functionalized with a magnetic covalent organic framework and BSA (chiral surface). | [113] |
| 6. | Microfluidic reactor array manufactured in 3D | Molecular diagnosis of infectious disease | Isothermal amplification by Loop mediation in 50 min. The exposure limits for Plasmodium falciparum were 100 FG and 50 CFU for Neisseria meningitidis per treatment. | [114] |
| 7. | Glucose dehydrogenase 3D printed glucose biosensor | To detect physiological glucose concentrations | As indicated by the slope and R2 correlation, a 3D-printed substance with a mylar substrate was immersed in an enzyme solution for 420 min. | [115] |
| 8. | 3D printed chemiluminiscencebiosensor | Lactate detection in oral fluid and sweat | 3D printing technology is utilized to create a disposable small cartridge that could be readily prototyped to turn any smartphone or tablet or into a portable luminometer capable of detecting chemiluminescence resulting from an enzyme-coupled reaction with detection limits of 0.5 mmol/L. | [116] |
| 9. | Nanomaterial enhanced 3D printed biosensor | Atrazine and acetochlor, two commonly used herbicides, were developed. | The catalyst of a mesoporous core-shell platium @palladium NPs on the redox reaction of thionin acetate and H2O2 produced an electrochemically driven signal that precisely showed the quantity of herbicide remains. | [117] |
| S.No | Nanomaterial | Disease | Mechanism | References |
|---|---|---|---|---|
| 1. | Ultrathin copper-tetrakis (4-carboxyphenyl) porphyrin (Cu-TCPP) nanosheets interface-beta structured -tricalcium phosphate (TCP) scaffold | Bone tumor and bone defect |
| [206] |
| 2. | Muscle-inspired nanostructure: 3D-printed bioceramics scaffolds with a Ca-P/polydopaminenanolayer surface that self-assembles consistently. | Bone Cancer therapy and bone regeneration |
| [207] |
| 3. | 3D Printed WesselsiteNanosheets (Wesselsite [SrCuSi4 O10] nanosheets, SC NSs) | Vascularized bone regeneration |
| [208] |
| 4. | Tunneling nanotube (TNT) -like functional cell projections | Renal tumor microenvironment |
| [209] |
| 5. | Cellulose nanofibrils (CNF), alginate, and SWCN are all examples of CNF-based materials. | Neuroblastoma |
| [210] |
| 6. | 3D printed materials containing cellulose nanocrystals (DS3000 and poly(ethylene glycol)diacrylate, PEG-DA) (CNCs). | Tumor microenvironment |
| [211] |
| 7. | Polydopamine/Transferrin Hybrid (PDA/Tf) NPs | Cell killing |
| [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safhi, A.Y. Three-Dimensional (3D) Printing in Cancer Therapy and Diagnostics: Current Status and Future Perspectives. Pharmaceuticals 2022, 15, 678. https://doi.org/10.3390/ph15060678
Safhi AY. Three-Dimensional (3D) Printing in Cancer Therapy and Diagnostics: Current Status and Future Perspectives. Pharmaceuticals. 2022; 15(6):678. https://doi.org/10.3390/ph15060678
Chicago/Turabian StyleSafhi, Awaji Y. 2022. "Three-Dimensional (3D) Printing in Cancer Therapy and Diagnostics: Current Status and Future Perspectives" Pharmaceuticals 15, no. 6: 678. https://doi.org/10.3390/ph15060678
APA StyleSafhi, A. Y. (2022). Three-Dimensional (3D) Printing in Cancer Therapy and Diagnostics: Current Status and Future Perspectives. Pharmaceuticals, 15(6), 678. https://doi.org/10.3390/ph15060678






