Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment
Abstract
1. Introduction
2. Results
2.1. Manufacture of 3D-Printed Minitablets
2.2. Solid State Characterization
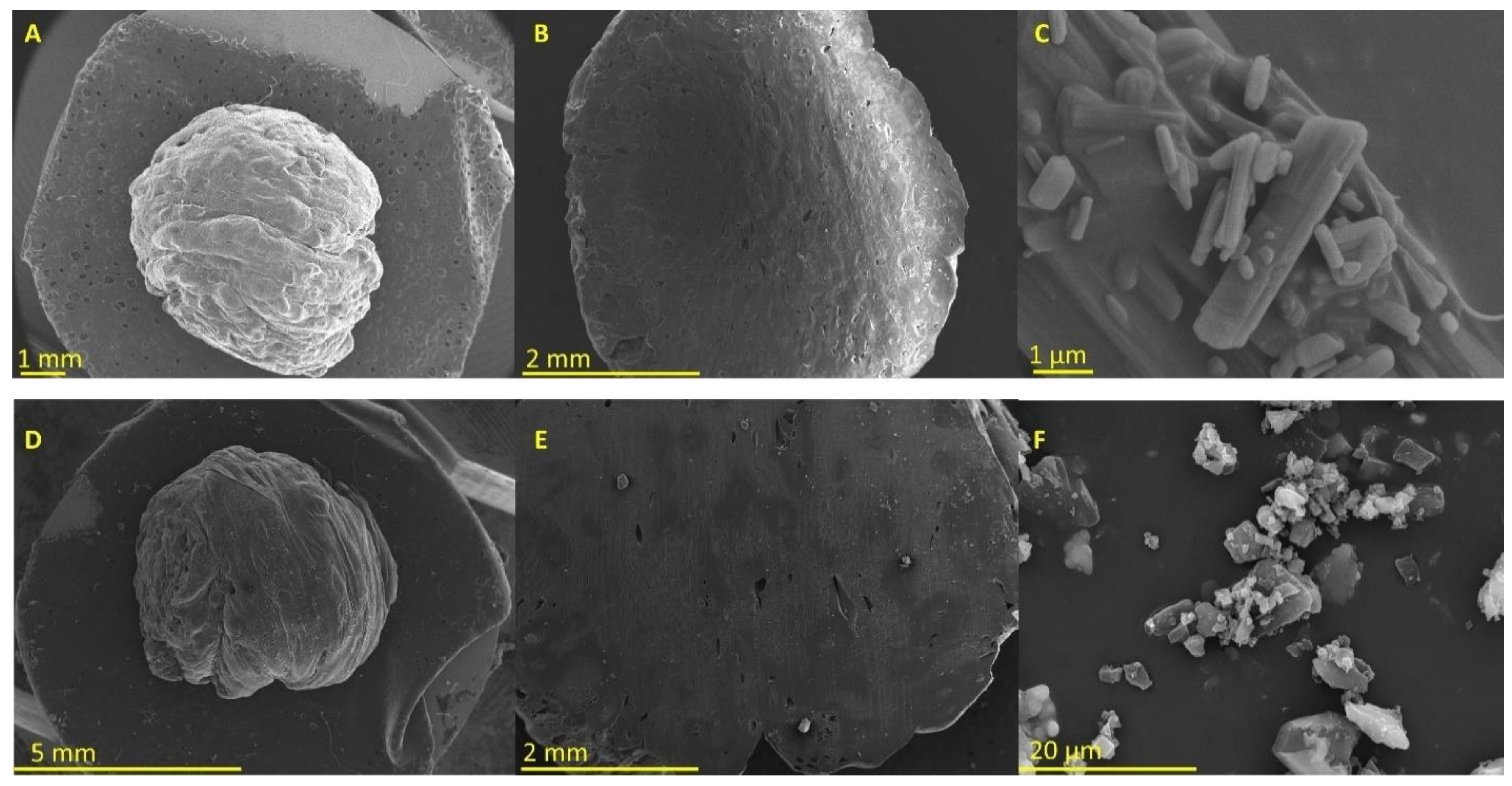
2.2.1. Morphology
2.2.2. Differential Scanning Calorimetry
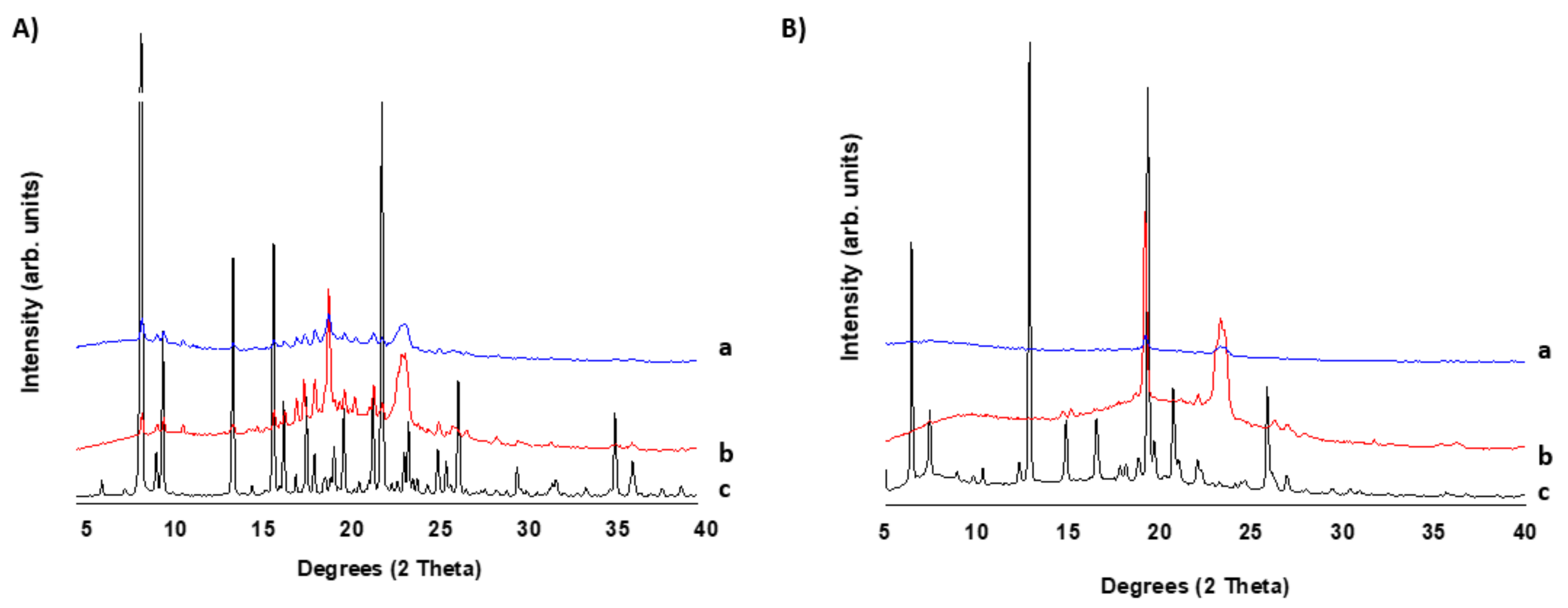
2.2.3. X-ray Powder Diffraction
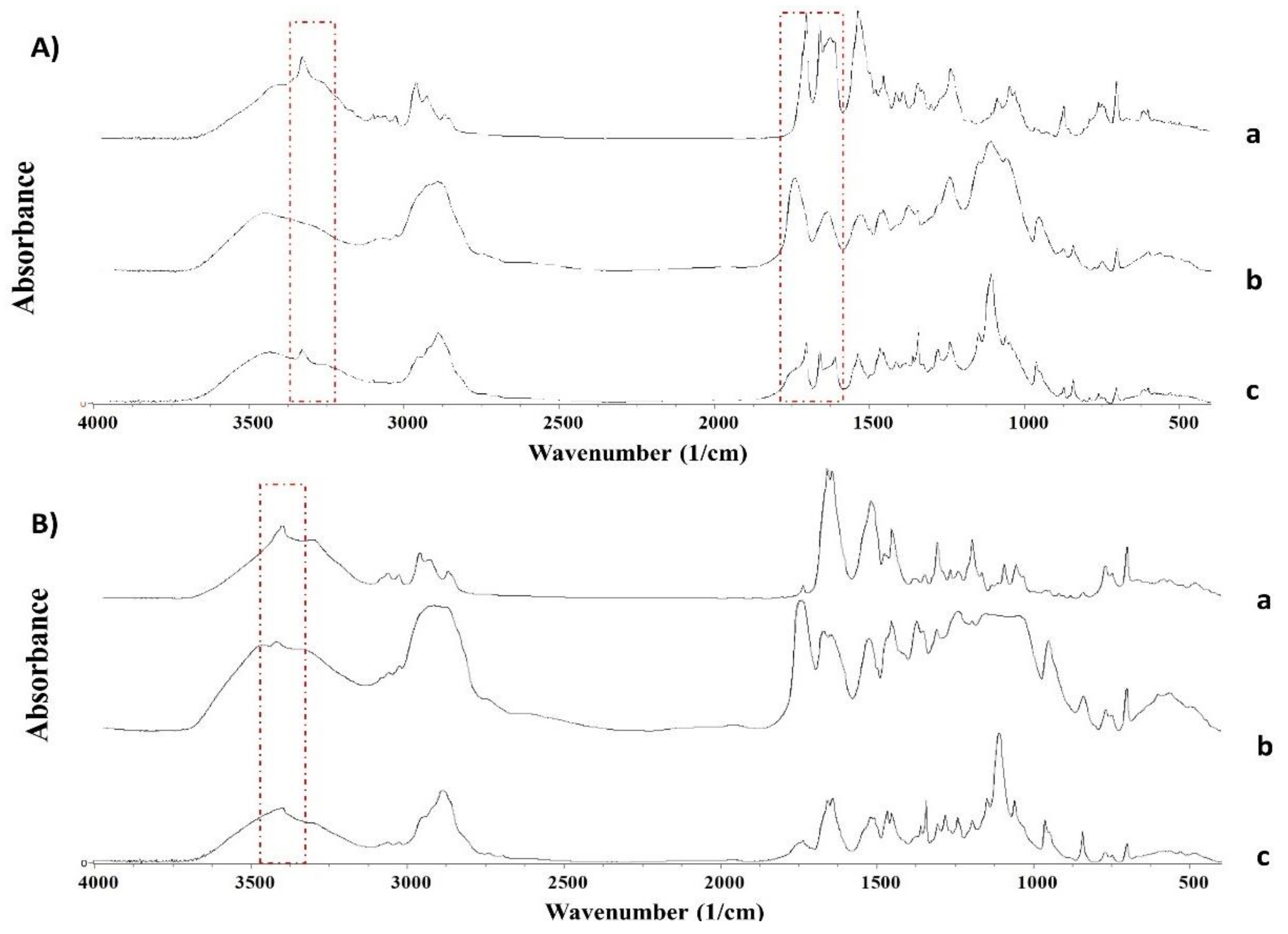
2.2.4. Fourier Transformed InfraRed Spectroscopy
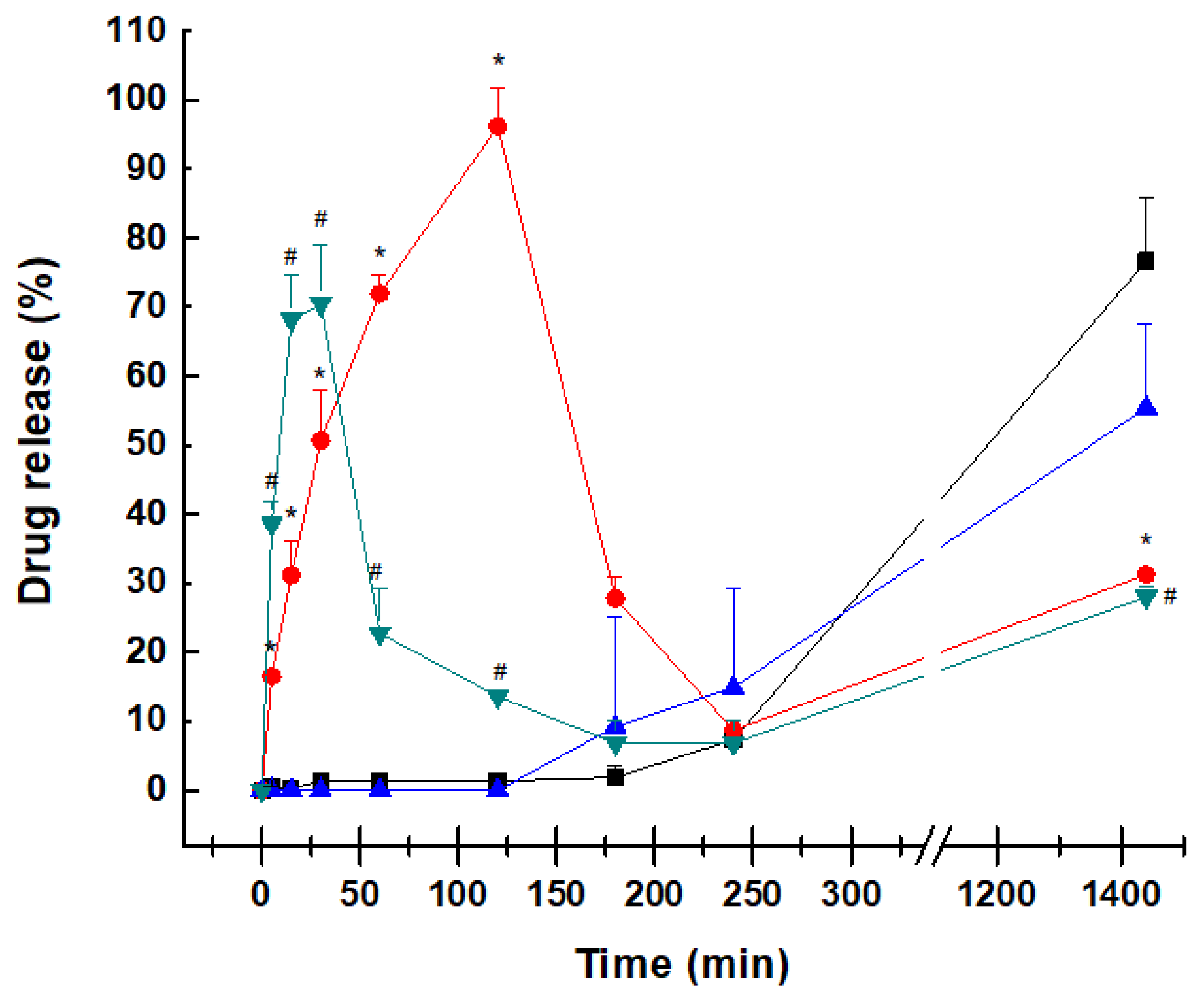
2.3. Dissolution Profile
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Geometrical Design
4.2.2. Formulation Development: DPE vs. FDM
4.2.3. DPE Printing Settings
4.2.4. HME Coupled with FDM
4.2.5. Mass and Content Uniformity
4.2.6. Scanning Electron Microscopy (SEM)
4.2.7. Solid-State Characterization
Fourier Transform Infrared (FTIR) Spectroscopy
X-ray Powder Diffraction (pXRD)
Differential Scanning Calorimetry (DSC)
4.2.8. Dissolution Studies
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; Garcia-Pina, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, R.; Prada, M.; Bolas-Fernandez, F.; Ballesteros, M.P.; Serrano, D.R. Oral Fixed-Dose Combination Pharmaceutical Products: Industrial Manufacturing Versus Personalized 3D Printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, E.; Brako, F.; Scarpa, M.; Lupo, M.; Bonifazi, D.; Pignataro, V.; Cavallo, M.; Cullufe, O.; Enache, C.; Nafria, B.; et al. Children’s Preferences for Oral Dosage Forms and Their Involvement in Formulation Research via EPTRI (European Paediatric Translational Research Infrastructure). Pharmaceutics 2021, 13, 730. [Google Scholar] [CrossRef]
- WHO. Draft Annex To 46th Report Of The Who Expert Committee On Specifications For Pharmacetical Preparations. Available online: https://www.who.int/childmedicines/partners/SabineKopp_Partners.pdf (accessed on 10 October 2021).
- World Health Organization. Treatment of Children Living with HIV. Available online: https://www.who.int/hiv/topics/paediatric/en/ (accessed on 23 May 2021).
- Penazzato, M.; Townsend, C.L.; Rakhmanina, N.; Cheng, Y.; Archary, M.; Cressey, T.R.; Kim, M.H.; Musiime, V.; Turkova, A.; Ruel, T.D.; et al. Prioritising the most needed paediatric antiretroviral formulations: The PADO4 list. Lancet HIV 2019, 6, 623–631. [Google Scholar] [CrossRef]
- Rautamo, M.; Kvarnstrom, K.; Siven, M.; Airaksinen, M.; Lahdenne, P.; Sandler, N. Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals. Pharmaceutics 2020, 12, 229. [Google Scholar] [CrossRef]
- Yuste, I.; Luciano, F.C.; Gonzalez-Burgos, E.; Lalatsa, A.; Serrano, D.R. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol. Res. 2021, 169, 105626. [Google Scholar] [CrossRef]
- Kara, A.; Vassiliadou, A.; Ongoren, B.; Keeble, W.; Hing, R.; Lalatsa, A.; Serrano, D.R. Engineering 3D Printed Microfluidic Chips for the Fabrication of Nanomedicines. Pharmaceutics 2021, 13, 2134. [Google Scholar] [CrossRef]
- Fernandez-Garcia, R.; Munoz-Garcia, J.C.; Wallace, M.; Fabian, L.; Gonzalez-Burgos, E.; Gomez-Serranillos, M.P.; Raposo, R.; Bolas-Fernandez, F.; Ballesteros, M.P.; Healy, A.M.; et al. Self-assembling, supramolecular chemistry and pharmacology of amphotericin B: Poly-aggregates, oligomers and monomers. J. Control. Release 2022, 341, 716–732. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An Overview of 3D Printing Technologies for Soft Materials and Potential Opportunities for Lipid-based Drug Delivery Systems. Pharm. Res. 2018, 36, 4. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernandez-Garcia, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef] [PubMed]
- Snachez-Guirales, S.; Jurado, N.; Kara, A.; Lalatsa, A.; Serrano, D.R. Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets. Pharmaceutics 2021, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Li, D.; Guo, S.; Penzak, S.; Dong, X. Development and in vivo evaluation of child-friendly lopinavir/ritonavir pediatric granules utilizing novel in situ self-assembly nanoparticles. J. Control. Release 2016, 226, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.; Lai, M.; Estrada, M.; Zhu, C. Developing a Flexible Pediatric Dosage Form for Antiretroviral Therapy: A Fast-Dissolving Tablet. J. Pharm. Sci. 2017, 106, 2173–2177. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Boniatti, J.; Januskaite, P.; Fonseca, L.B.D.; Vicosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Re, M.I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef]
- Mendibil, X.; Tena, G.; Duque, A.; Uranga, N.; Campanero, M.A.; Alonso, J. Direct Powder Extrusion of Paracetamol Loaded Mixtures for 3D Printed Pharmaceutics for Personalized Medicine via Low Temperature Thermal Processing. Pharmaceutics 2021, 13, 907. [Google Scholar] [CrossRef]
- Torrado-Salmeron, C.; Laguna, A.; Guillén, A.; Saro, M.G.; Matji, A.; Torrado, J.J.; Serrano, D.R. Tailoring Rational Manufacturing of Extemporaneous Compounding Oral Dosage Formulations with a Low Dose of Minoxidil. Pharmaceutics 2022, 14, 658. [Google Scholar] [CrossRef]
- United States Pharmacopeia and National Formulary (USP 38-NF 33). Reagents: Test Solutions; Rockville: Rockville, MD, USA, 2018. [Google Scholar]
- Morissette, S.L.; Soukasene, S.; Levinson, D.; Cima, M.J.; Almarsson, O. Elucidation of crystal form diversity of the HIV protease inhibitor ritonavir by high-throughput crystallization. Proc. Natl. Acad. Sci. USA 2003, 100, 2180–2184. [Google Scholar] [CrossRef]
- Henck, J.O.; Bernstein, J.; Ellern, A.; Boese, R. Disappearing and reappearing polymorphs. The benzocaine:picric acid system. J. Am. Chem. Soc. 2001, 123, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Chemburkar, S.R.; Bauer, J.; Deming, K.; Spiwek, H.; Patel, K.; Morris, J.; Henry, R.; Sapnton, S.; Dziki, W.; Quick, J.; et al. Dealing with the Impact of Ritonavir Polymorphs on the Late Stages of Bulk Drug Process Development. Org. Proc. Res. Dev. 2000, 4, 413–417. [Google Scholar] [CrossRef]
- Law, D.; Krill, S.L.; Schmitt, E.A.; Fort, J.J.; Qiu, Y.; Wang, W.; Porter, W.R. Physicochemical considerations in the preparation of amorphous ritonavir-poly(ethylene glycol) 8000 solid dispersions. J. Pharm. Sci. 2001, 90, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sunada, H. Comparison of nicotinamide, ethylurea and polyethylene glycol as carriers for nifedipine solid dispersion systems. Chem. Pharm. Bull. 1997, 45, 1688–1693. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lemmer, H.J.; Liebenberg, W. Preparation and evaluation of metastable solid-state forms of lopinavir. Pharmazie 2013, 68, 327–332. [Google Scholar]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An extraordinary example of conformational polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef]
- Spanish Agency of Medicines. Technical Data Sheet of Kaletra Tablets. Available online: https://cima.aemps.es/cima/dochtml/ft/01172008/FT_01172008.html#6-datos-farmac-uticos (accessed on 18 July 2021).
- Trasi, N.S.; Bhujbal, S.; Zhou, Q.T.; Taylor, L.S. Amorphous solid dispersion formation via solvent granulation—A case study with ritonavir and lopinavir. Int. J. Pharm. X 2019, 1, 100035. [Google Scholar] [CrossRef]
- Sakuma, S.; Matsumoto, S.; Ishizuka, N.; Mohri, K.; Fukushima, M.; Ohba, C.; Kawakami, K. Enhanced Boosting of Oral Absorption of Lopinavir Through Electrospray Coencapsulation with Ritonavir. J. Pharm. Sci. 2015, 104, 2977–2985. [Google Scholar] [CrossRef]
- Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available online: https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv/regimens-recommended-initial-therapy-antiretroviral-naive-children?view=full (accessed on 10 August 2021).
- Best, B.M.; Capparelli, E.V.; Diep, H.; Rossi, S.S.; Farrell, M.J.; Williams, E.; Lee, G.; van den Anker, J.N.; Rakhmanina, N. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. J. Acquir. Immune Defic. Syndr. 2011, 58, 385–391. [Google Scholar] [CrossRef]
- Hsu, A.; Granneman, G.R.; Bertz, R.J. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 1998, 35, 275–291. [Google Scholar] [CrossRef]
- Niu, W.J.; Sun, T.; Liu, L.; Liu, X.Q.; Zhang, R.F.; Yin, L.; Wang, J.R.; Jia, X.F.; Lu, H.Z.; Zhong, M.K.; et al. Population pharmacokinetics and dosing regimen optimisation of lopinavir in Chinese adults infected with HIV. Basic Clin. Pharmacol. Toxicol. 2019, 124, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zheng, Q.S.; Li, G.F. Similarities and differences in gastrointestinal physiology between neonates and adults: A physiologically based pharmacokinetic modeling perspective. AAPS J. 2014, 16, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Mohamed, E.M.; Rahman, Z.; Khan, M.A. 3D-printing of lopinavir printlets by selective laser sintering and quantification of crystalline fraction by XRPD-chemometric models. Int. J. Pharm. 2021, 592, 120059. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef]
- Lao, L.L.; Peppas, N.A.; Boey, F.Y.; Venkatraman, S.S. Modeling of drug release from bulk-degrading polymers. Int. J. Pharm. 2011, 418, 28–41. [Google Scholar] [CrossRef]
- Mamani, P.L.; Ruiz-Caro, R.; Veiga, M.D. Matrix tablets: The effect of hydroxypropyl methylcellulose/anhydrous dibasic calcium phosphate ratio on the release rate of a water-soluble drug through the gastrointestinal tract I. In vitro tests. AAPS PharmSciTech 2012, 13, 1073–1083. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]

| Drug | Tablet | Weight (mg) | Diameter (mm) | Density (g/cm3) | Drug Content (%) |
|---|---|---|---|---|---|
| Ritonavir | Mean | 149.3 | 6.3 | 1.13 | 95.8 |
| SD | 11.4 | 0.4 | 0.16 | 5.0 | |
| CV (%) | 7.7 | 5.8 | 14.11 | 5.2 | |
| Lopinavir | Mean | 158.3 | 6.6 | 1.06 | 97.7 |
| SD | 12.04 | 0.4 | 0.13 | 2.6 | |
| CV (%) | 7.6 | 6.8 | 12.49 | 2.3 |
| Parameter | Value |
|---|---|
| Temperature | 80 °C |
| Platform Temperature | 80 °C |
| Fan | 25% |
| Layer Height | 0.1 mm |
| Infill | 100% |
| Travel and Print Speed | 50 & 10 mm/s |
| Infill Type | Linear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malebari, A.M.; Kara, A.; Khayyat, A.N.; Mohammad, K.A.; Serrano, D.R. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals 2022, 15, 435. https://doi.org/10.3390/ph15040435
Malebari AM, Kara A, Khayyat AN, Mohammad KA, Serrano DR. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals. 2022; 15(4):435. https://doi.org/10.3390/ph15040435
Chicago/Turabian StyleMalebari, Azizah M., Aytug Kara, Ahdab N. Khayyat, Khadijah A. Mohammad, and Dolores R. Serrano. 2022. "Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment" Pharmaceuticals 15, no. 4: 435. https://doi.org/10.3390/ph15040435
APA StyleMalebari, A. M., Kara, A., Khayyat, A. N., Mohammad, K. A., & Serrano, D. R. (2022). Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals, 15(4), 435. https://doi.org/10.3390/ph15040435






