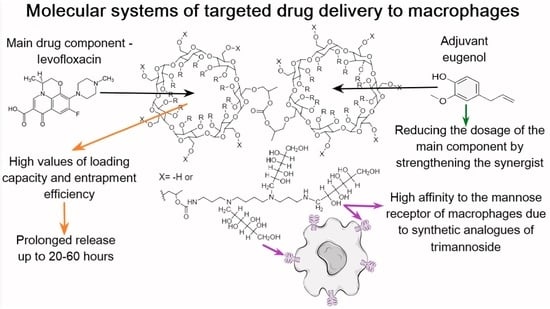
Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs
Abstract
1. Introduction
2. Results and Discussion
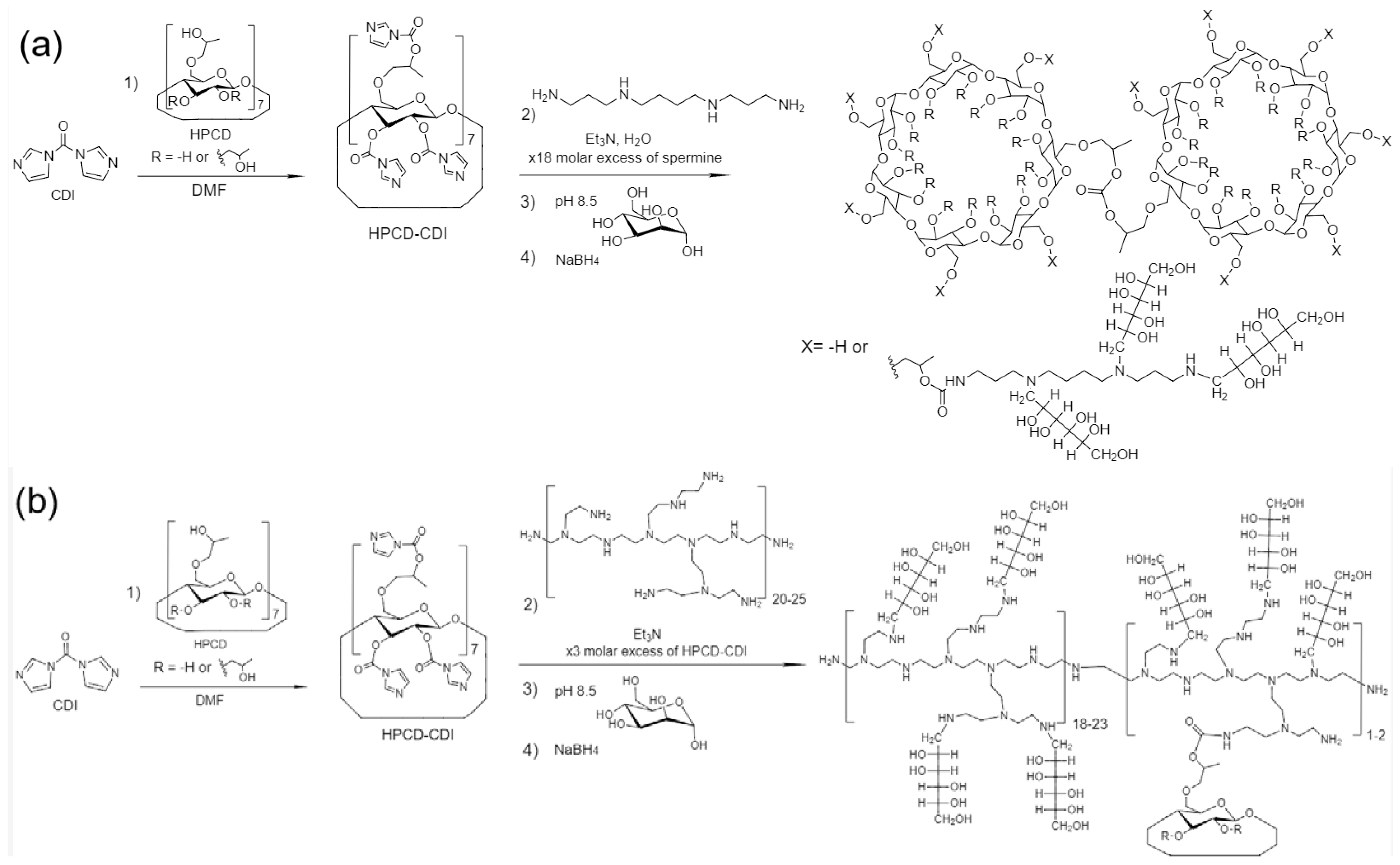
2.1. HPCD-PEI-Man & HPCD-Spermine-Man Conjugates Synthesis and Characterization
2.2. HPCD-PEI-Man & HPCD-Spermine-Man Conjugates Binding with a Model Mannose Receptor ConA
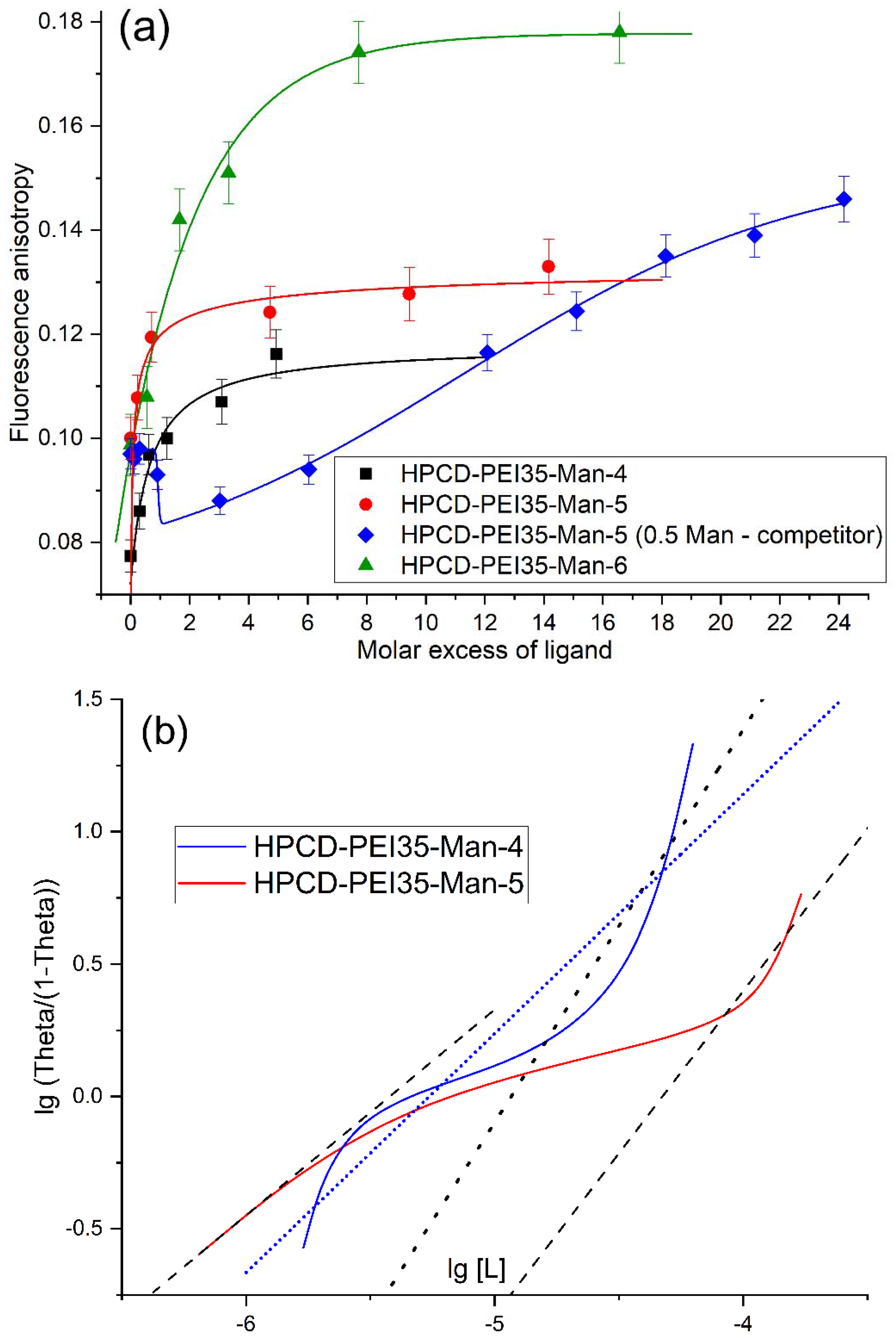
2.2.1. Fluorescence Quenching and Anisotropy
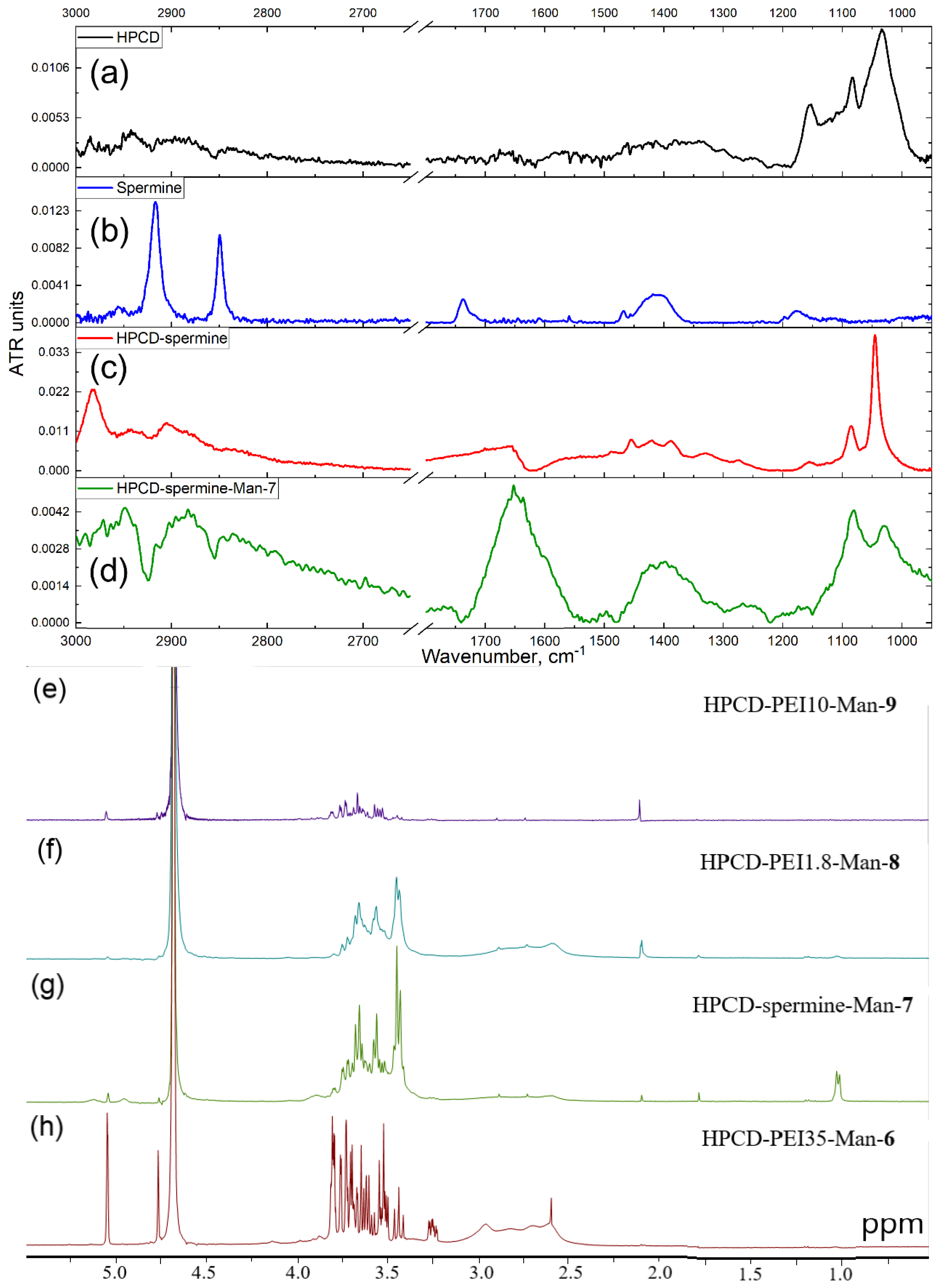
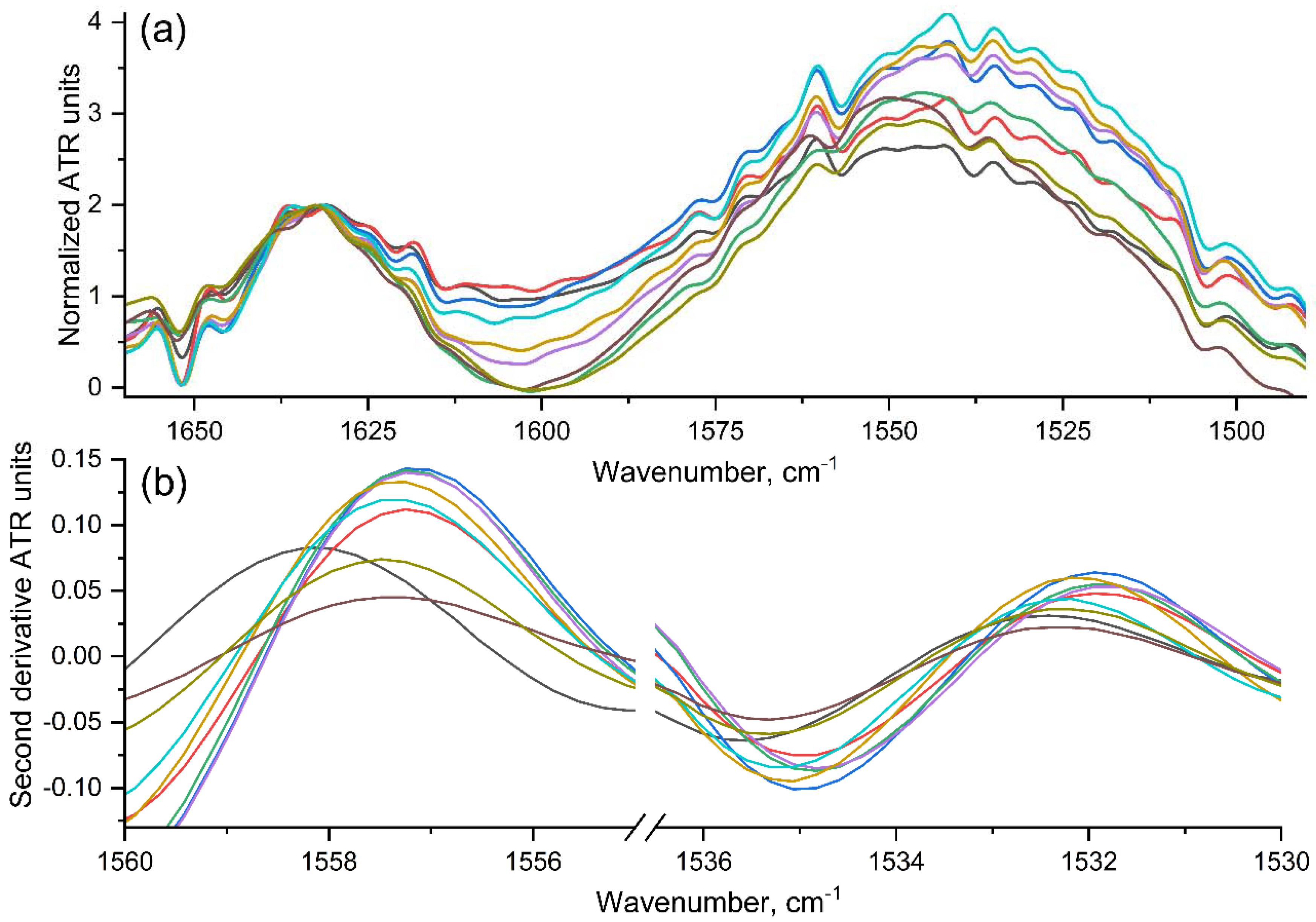
2.2.2. Fourier-Transform IR Spectroscopy (FTIR)
2.2.3. The Dependences of Conjugates’ Affinity to the Model ConA Receptor on the Parameters of the Molecular Containers
2.3. Levofloxacin and the Adjuvant Eugenol Interactions with HPCD-PEI-Man and HPCD-Spermine-Man Conjugates
2.3.1. Levofloxacin Fluorescence Quenching and Polarization
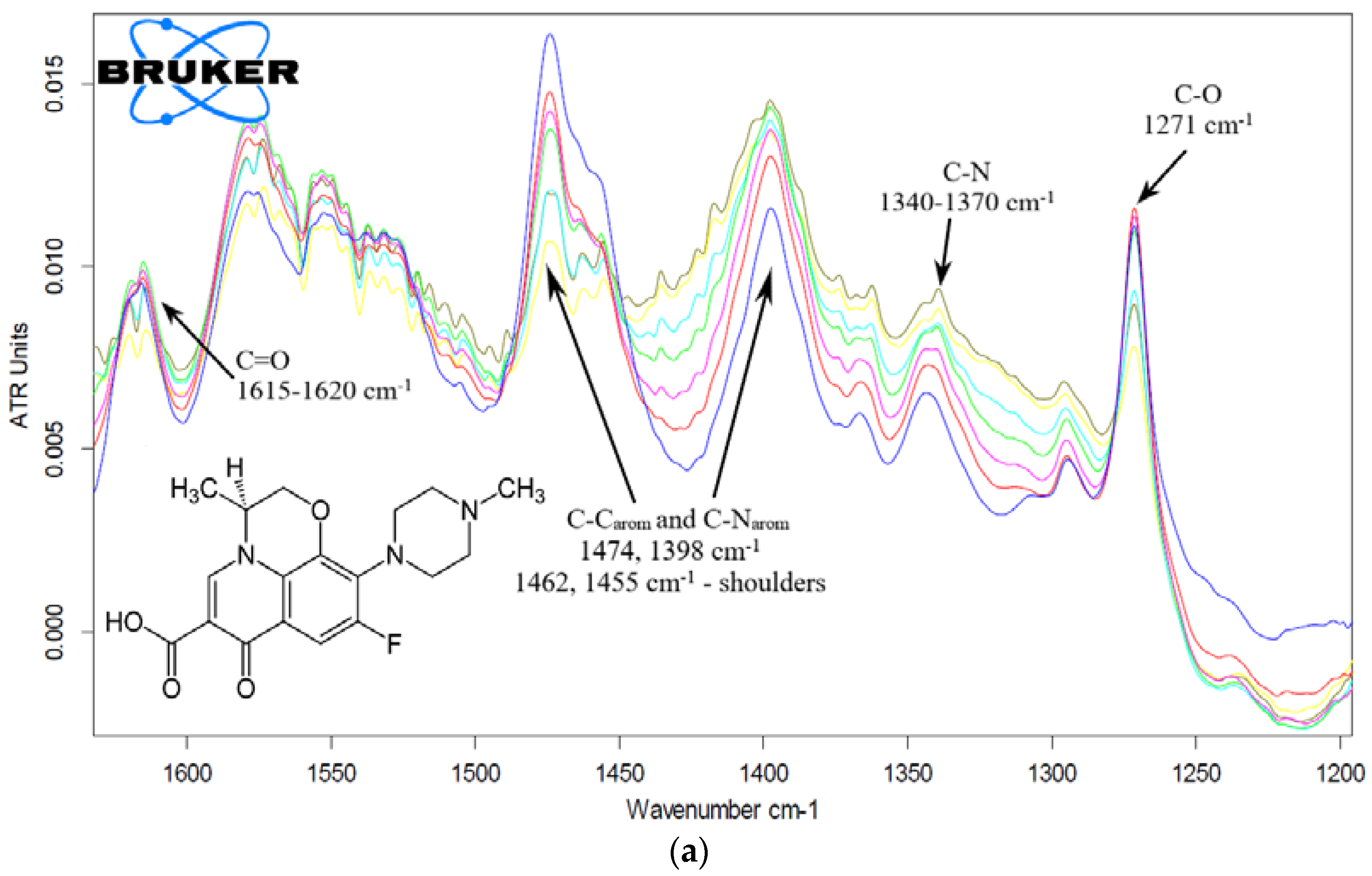
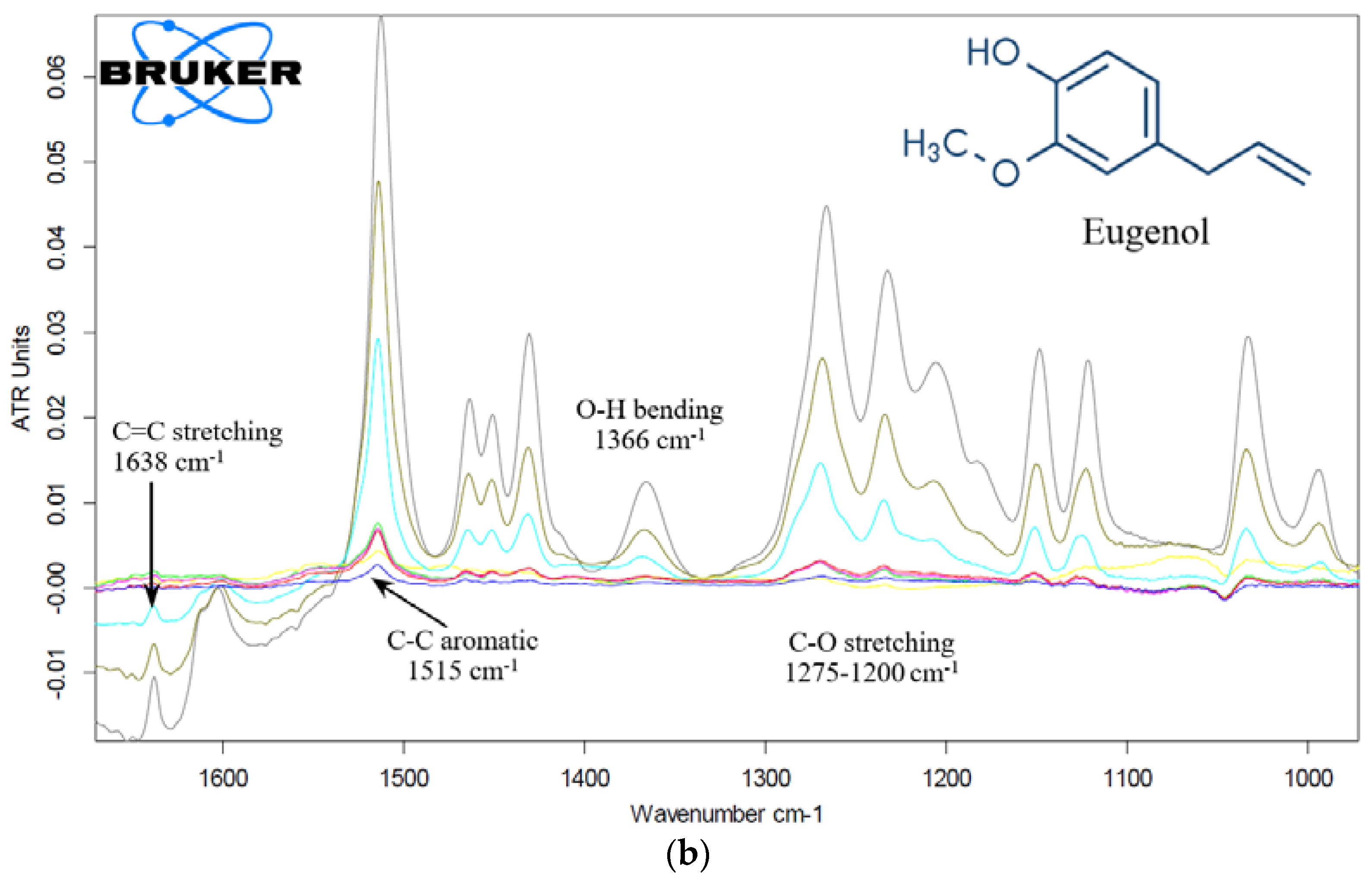
2.3.2. Eugenol and Levofloxacin: Fourier-Transform IR Spectroscopy Approach
Levofloxacin
Eugenol
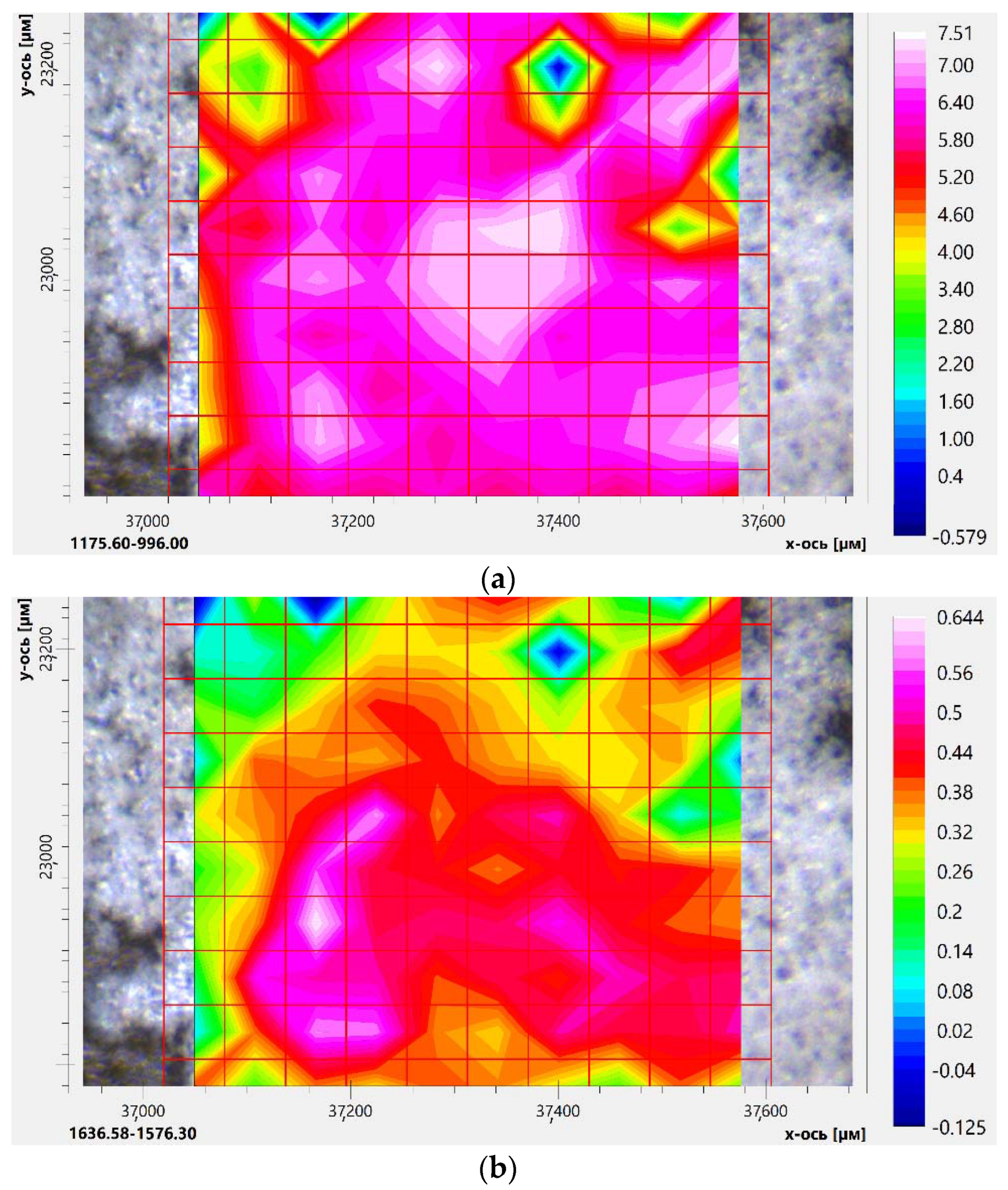
2.3.3. Eugenol and Levofloxacin Double Inclusion Complexes in Molecular Containers FTIR Microscopy Approach
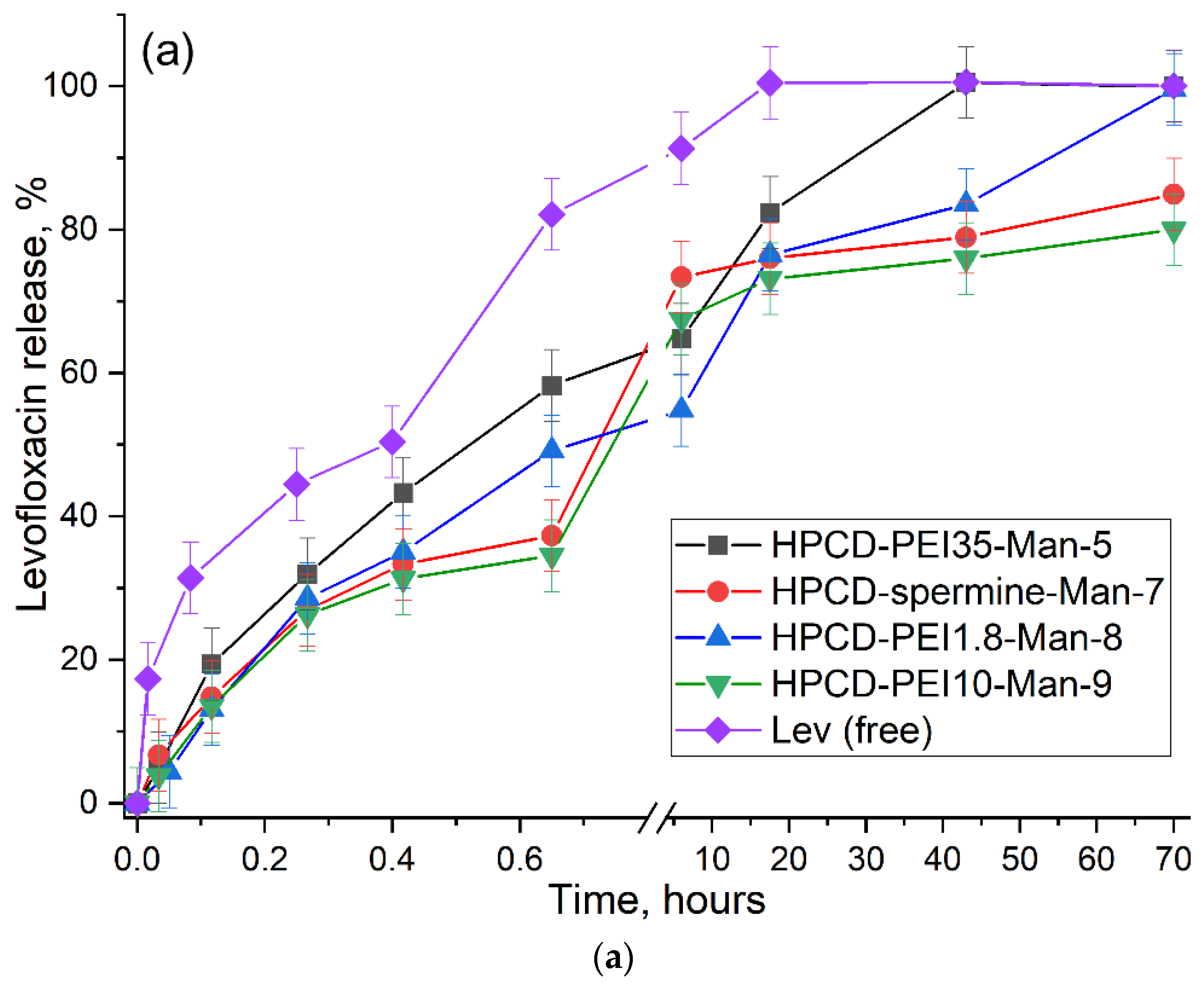
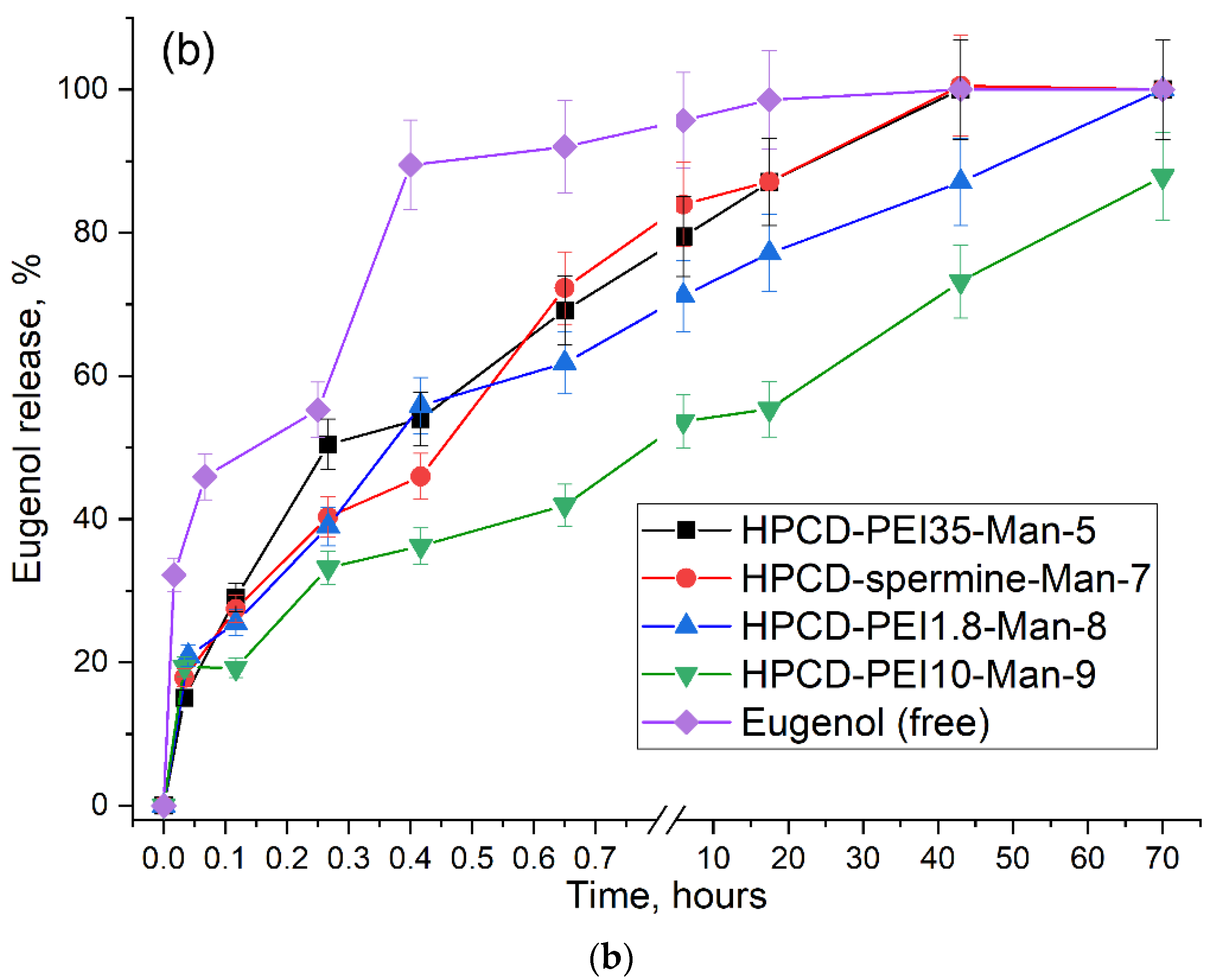
2.3.4. Kinetics of Drug Release from Molecular Containers
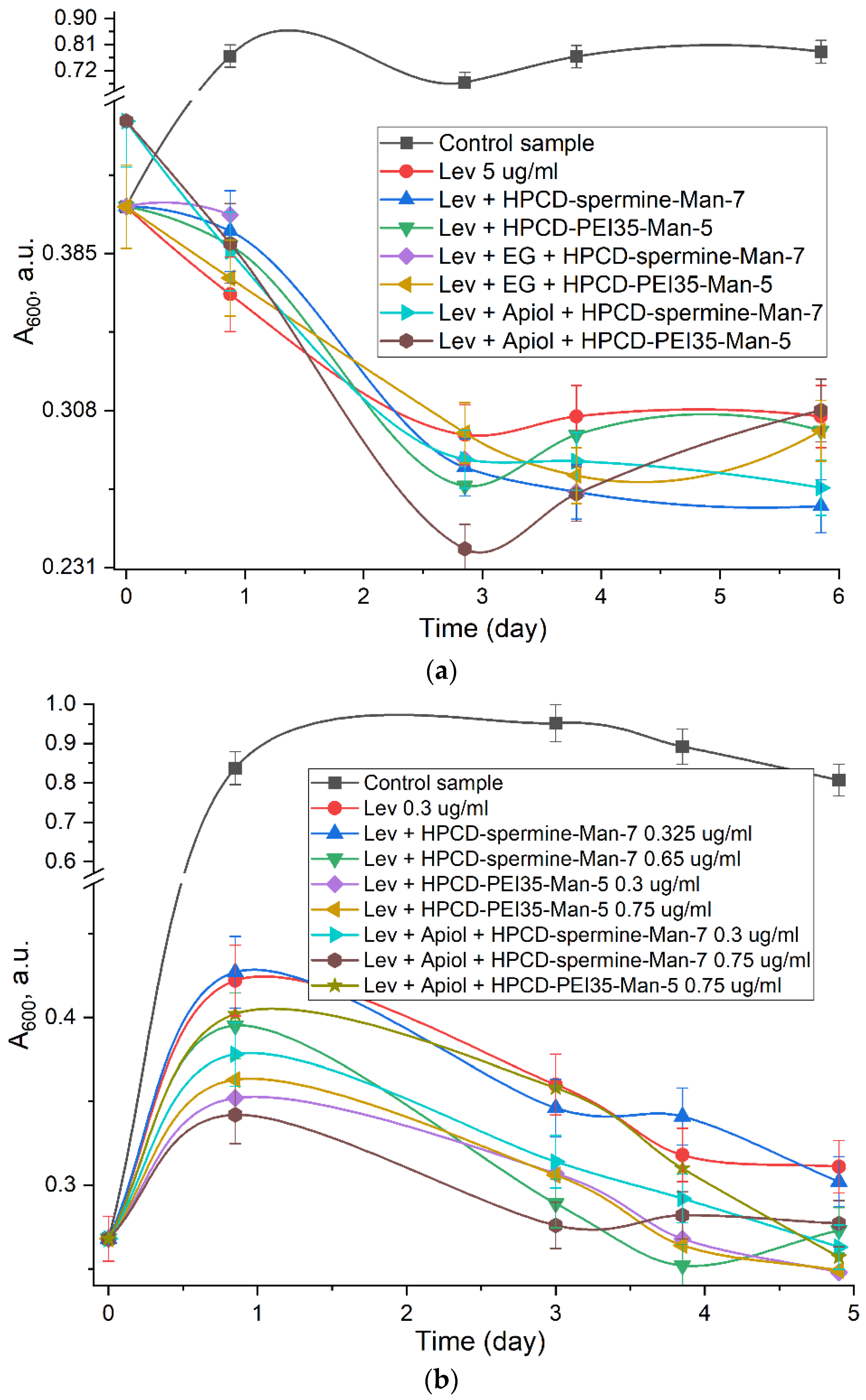
2.4. Antibacterial Activity of Levofloxacin and Adjuvants in Molecular Containers
3. Materials and Methods
3.1. Materials
3.2. Synthesis of HPCD Derivatives with PEI and Spermine
3.3. Mannosylation and Purification of HPCD-PEI and HPCD-Spermine Conjugates
3.4. FTIR-Spectroscopy
3.4.1. Complex Formation of ConA with Conjugates. FTIR
3.4.2. Complex Formation of Conjugates with Levofloxacin. FTIR
3.4.3. Complex Formation of Conjugates with Eugenol. FTIR
3.4.4. Formation of Double Drug Inclusion Complexes of Eugenol and Levofloxacin
3.5. Fluorescence Spectroscopy
3.5.1. Complex Formation of ConA with Conjugates. Fluorescence Methods
3.5.2. Complex Formation of ConA with FITC-Labeled Ligands. Fluorescence Methods
3.5.3. Complex Formation of Conjugates with Levofloxacin. Fluorescence Methods
3.6. Dynamic Light Scattering (DLS)
3.7. Levofloxacin and Eugenol Release by UV-Spectroscopy
3.8. NMR Spectroscopy
3.9. Antibacterial Activity of Levofloxacin and Adjuvants in Molecular Containers
3.10. Mathematical Processing of the Results
- (a)
- Calculation of dissociation constants of ConA-ligand complexes was carried out in 3 stages:
- (1)
- Fitting of the curves of change of the analytical signal ξ versus molar excess of the ligand x was carried out using the Hill and Boltzmann equations (Origin software):where start and end—horizontal asymptotes;Hill1: ξ = start + (end–start) · xn/(xn + kn)Boltzmann: ξ = (A1–A2)/(1 + exp((x−x0)/dx)) + A2
- (2)
- Calculation of the fraction of the bound receptor (α) through the values of the analyzed quantities (fluorescence intensity (F) or anisotropy (r), IR absorption (A)) for the receptor ξ0, the complex ξ∞ and the current value ξ, as described earlier [18]:
- (3)
- Calculation of the equilibrium concentrations of the receptor [R], ligand [L] and complex [R·L] and the Kd value by following equations by following equations [63,83]:material balance for receptor: C0(R) = [R] + [R·L]material balance for ligand: C0(ligand) = [L] + [R·L]complex: [R·L] = C0(R) ∙ αwhere θ = [R·L]/C0(R)—proportion of bound receptor, n—number of binding site.Hill’s linearization model: lg (θ/(1–θ)) = n · lg [L]—lg Kd,Scatchard’s linearization model: [R·L]/[L] = (C0(R)—[R·L])/Kd
- (b)
- Determination of drug loading parameters:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bongrand, P. Ligand-receptor interactions. Rep. Prog. Phys. 1999, 62, 921–968. [Google Scholar] [CrossRef]
- Rudd, P.M.; Wormald, M.R.; Dwek, R.A. Sugar-Mediated Ligand-Receptor Interactions in the Immune System. Trends Biotechnol. 2004, 22, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Managit, C.; Kawakami, S.; Yamashita, F.; Hashida, M. Effect of Galactose Density on Asialoglycoprotein Receptor-Mediated Uptake of Galactosylated Liposomes. J. Pharm. Sci. 2005, 94, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Kessner, S.; Krause, A.; Rothe, U.; Bendas, G. Investigation of the Cellular Uptake of E-Selectin-Targeted Immunoliposomes by Activated Human Endothelial Cells. Biochim. Biophys. Acta-Biomembr. 2001, 1514, 177–190. [Google Scholar] [CrossRef][Green Version]
- Bendas, G.; Krause, A.; Schmidt, R.; Vogel, J.; Rothe, U. Selectins as New Targets for Immunoliposome-Mediated Drug Delivery. A Potential Way of Anti-Inflammatory Therapy. Pharm. Acta Helv. 1998, 73, 19–26. [Google Scholar] [CrossRef]
- Brekke, O.H.; Sandlie, I. Therapeutic Antibodies for Human Diseases at the Dawn of the Twenty-First Century. Nat. Rev. Drug Discov. 2003, 2, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Filatova, L.Y.; Klyachko, N.L.; Kudryashova, E.V. Targeted Delivery of Anti-Tuberculosis Drugs to Macrophages: Targeting Mannose Receptors. Russ. Chem. Rev. 2018, 87, 374–391. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Kudryashova, E.V. Mannose Receptors of Alveolar Macrophages as a Target for the Addressed Delivery of Medicines to the Lungs. Russ. J. Bioorg. Chem. 2022, 48, 46–75. [Google Scholar] [CrossRef]
- Taylor, P.R.; Martinez-Pomares, L.; Stacey, M.; Lin, H.H.; Brown, G.D.; Gordon, S. Macrophage Receptors and Immune Recognition. Annu. Rev. Immunol. 2005, 23, 901–944. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The Mannose Receptor Family. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Stahl, P.D. The Macrophage Mannose Receptor: Current Status. Am. J. Respir. Cell Mol. Biol. 1990, 2, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Park-Snyder, S.; Kolatkar, A.R.; Heise, C.T.; Taylor, M.E.; Weis, W.I. Structure of a C-Type Carbohydrate Recognition Domain from the Macrophage Mannose Receptor. J. Biol. Chem. 2000, 275, 21539–21548. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Jégouzo, S.A.F.; Lasanajak, Y.; Smith, D.F.; Drickamer, K.; Weis, W.I.; Taylor, M.E. Structural Analysis of Carbohydrate Binding by the Macrophage Mannose Receptor CD206. J. Biol. Chem. 2021, 296, 100368. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Drickamer, K. Structural Requirements for High Affinity Binding of Complex Ligands by the Macrophage Mannose Receptor. J. Biol. Chem. 1993, 268, 399–404. [Google Scholar] [CrossRef]
- Hitchen, P.G.; Mullin, N.P.; Taylor, M.E. Orientation of Sugars Bound to the Principal C-Type Carbohydrate-Recognition Domain of the Macrophage Mannose Receptor. Biochem. J. 1998, 333, 601–608. [Google Scholar] [CrossRef]
- Mullin, N.P.; Hitchen, P.G.; Taylor, M.E. Mechanism of Ca2+ and Monosaccharide Binding to a C-Type Carbohydrate- Recognition Domain of the Macrophage Mannose Receptor. J. Biol. Chem. 1997, 272, 5668–5681. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Kudryashova, E.V. Computer Simulation of the Receptor–Ligand Interactions of Mannose Receptor CD206 in Comparison with the Lectin Concanavalin A Model. Biochem. 2022, 87, 54–69. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Vanichkin, D.A.; Kudryashova, E.V. Methods for Determining the Parameters of Receptor-Ligand Interactions on the Model of Concanavalin A and Mannosylated Chitosans Promising Carriers for Drug Delivery to Alveolar Macrophages. Biotekhnologiya 2021, 37, 28–40. [Google Scholar] [CrossRef]
- Loontiens, F.G.; Clegg, R.M.; Van Landschoot, A. Some Physicochemical Aspects of Oligosaccharide Binding to Concanavalin A and Wheat Germ Agglutinin. J. Biosci. 1983, 5, 105–120. [Google Scholar] [CrossRef]
- Sawyer, H.W.; Dabscheck, R.; Nott, P.R.; Selinger, B.K.; Kuntz, I.D. Hydrodynamic Changes Accompanying the Loss of Metal Ions from Concanavalin A. Biochem. J. 1975, 147, 613–615. [Google Scholar] [CrossRef]
- Mandal, D.K.; Kishore, N.; Brewer, C.F. Thermodynamics of Lectin-Carbohydrate Interactions. Titration Microcalorimetry Measurements of the Binding of N-Linked Carbohydrates and Ovalbumin to Concanavalin A. Biochemistry 1994, 33, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Drickamer, K. Structural Basis of Recognition Lectin-Carb Ohydrate. Anna Rev. Biochen. 19% 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Ghotbi, Z.; Haddadi, A.; Hamdy, S.; Hung, R.W.; Samuel, J.; Lavasanifar, A. Active Targeting of Dendritic Cells with Mannan-Decorated PLGA Nanoparticles. J. Drug Target. 2011, 19, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.X.; Chen, Y.Z.; Yao, X.L.; Du, A.; Tang, G.P.; Shen, Y.Q.; Tabata, Y.; Gao, J.Q. Macrophage Mannose Receptor-Specific Gene Delivery Vehicle for Macrophage Engineering. Acta Biomater. 2014, 10, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Nimje, N.; Agarwal, A.; Saraogi, G.K.; Lariya, N.; Rai, G.; Agrawal, H.; Agrawal, G.P. Mannosylated Nanoparticulate Carriers of Rifabutin for Alveolar Targeting. J. Drug Target. 2009, 17, 777–787. [Google Scholar] [CrossRef]
- Dam, T.K.; Roy, R.; Das, S.K.; Oscarson, S.; Brewer, C.F. Binding of Multivalent Carbohydrates to Concanavalin A and Dioclea Grandiflora Lectin. Thermodynamic Analysis of the ‘Multivalency Effect’. J. Biol. Chem. 2000, 275, 14223–14230. [Google Scholar] [CrossRef]
- VAN LANDSCHOOT, A.; LOONTIENS, F.G.; DE BRUYNE, C.K. Binding of Manno-oligosaccharides to Concanavalin A: Substitution Titration with a Fluorescent-Indicator Ligand. Eur. J. Biochem. 1980, 103, 307–312. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Mamaeva, P.V.; Skuredina, A.A.; Kudryashova, E.V. A Spectral Approach to Study Interaction between Chitosan Modified with Mannose and Concavalin A for the Creation of Address Delivery Systems of Antituberculosis Drugs. Moscow Univ. Chem. Bull. 2020, 75, 213–217. [Google Scholar] [CrossRef]
- So, L.L.; Goldstein, I.J. Protein-Carbohydrate Interaction. 13. The Interaction of Concanavalin A with Alpha-Mannans from a Variety of Microorganisms. J. Biol. Chem. 1968, 243, 2003–2007. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Ding, X.; Yang, Y. In Silico and Experimental Studies of Concanavalin a: Insights into Its Antiproliferative Activity and Apoptotic Mechanism. Appl. Biochem. Biotechnol. 2010, 162, 134–145. [Google Scholar] [CrossRef]
- Kaushik, S.; Mohanty, D.; Surolia, A. The Role of Metal Ions in Substrate Recognition and Stability of Concanavalin A: A Molecular Dynamics Study. Biophys. J. 2009, 96, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- SZEJTLI, J. ChemInform Abstract: Introduction and General Overview of Cyclodextrin Chemistry. ChemInform 2010, 29, no-no. [Google Scholar] [CrossRef]
- Connors, K.A. The Stability of Cyclodextrin Complexes in Solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef] [PubMed]
- Milcovich, G.; Antunes, F.E.; Grassi, M.; Asaro, F. Stabilization of Unilamellar Catanionic Vesicles Induced by β-Cyclodextrins: A Strategy for a Tunable Drug Delivery Depot. Int. J. Pharm. 2018, 548, 474–479. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-Based Pharmaceutics: Past, Present and Future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E.V. Regulation of Properties of Lipid Membranes by Interaction with 2-Hydroxypropyl β-Cyclodextrin: Molecular Details. Russ. J. Bioorganic Chem. 2020, 46, 692–701. [Google Scholar] [CrossRef]
- Lopukhov, A.V.; Yang, Z.; Haney, M.J.; Bronich, T.K.; Sokolsky-Papkov, M.; Batrakova, E.V.; Klyachko, N.L.; Kabanov, A.V. Mannosylated Cationic Copolymers for Gene Delivery to Macrophages. Macromol. Biosci. 2021, 21, 2000371. [Google Scholar] [CrossRef]
- Sato, A.; Kawakami, S.; Yamada, M.; Yamashita, F.; Hashida, M. Enhanced Gene Transfection in Macrophages Using Mannosylated Cationic Liposome-Polyethylenimine-Plasmid DNA Complexes. J. Drug Target. 2001, 9, 201–207. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Belogurova, N.G.; Kudryashova, E.V. The Formation of Quasi-Regular Polymeric Network of Cross-Linked Sulfobutyl Ether Derivative of β-Cyclodextrin Synthesized with Moxifloxacin as a Template. React. Funct. Polym. 2021, 159, 104811. [Google Scholar] [CrossRef]
- Zheng, H.; He, W.; Jiao, W.; Xia, H.; Sun, L.; Wang, S.; Xiao, J.; Ou, X.; Zhao, Y.; Shen, A. Molecular Characterization of Multidrug-Resistant Tuberculosis against Levofloxacin, Moxifloxacin, Bedaquiline, Linezolid, Clofazimine, and Delamanid in Southwest of China. BMC Infect. Dis. 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Qian, F.; Wang, Y.Y.; Liu, Y.; Sun, Y.; Zha, W.B.; Hao, K.; Zhou, F.; Wang, G.J.; Zhang, J.W. Ginsenoside 20(S)-Rh2 Promotes Cellular Pharmacokinetics and Intracellular Antibacterial Activity of Levofloxacin against Staphylococcus Aureus through Drug Efflux Inhibition and Subcellular Stabilization. Acta Pharmacol. Sin. 2021, 42, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Deygen, I.M.; Egorov, A.M.; Kudryashova, E.V. Structure and Stability of Fluoroquinolone-(2-Hydroxypropyl)-β-Cyclodextrin Complexes as Perspective Antituberculosis Drugs. Moscow Univ. Chem. Bull. 2016, 71, 387–392. [Google Scholar] [CrossRef]
- Jelić, R.; Tomović, M.; Stojanović, S.; Joksović, L.; Jakovljević, I.; Djurdjević, P. Study of Inclusion Complex of β-Cyclodextrin and Levofloxacin and Its Effect on the Solution Equilibria between Gadolinium(III) Ion and Levofloxacin. Monatshefte fur Chemie 2015, 146, 1621–1630. [Google Scholar] [CrossRef]
- Tychinina, A.S.; Skuredina, A.A.; Le-Deygen, I.M.; Kudryashova, E.V. Influence of Substituents in β-Cyclodextrin on the Interaction of Levofloxacin–β-Cyclodextrin Complexes with Liposomal Membrane. Colloid J. 2021, 83, 794–801. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium Aromaticum Essential Oil and Eugenol. Evidence-Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef]
- Gong, L.; Li, T.; Chen, F.; Duan, X.; Yuan, Y.; Zhang, D.; Jiang, Y. An Inclusion Complex of Eugenol into β-Cyclodextrin: Preparation, and Physicochemical and Antifungal Characterization. Food Chem. 2016, 196, 324–330. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Si, H.; Lin, L.; Chen, L. Release Characteristics and Antibacterial Activity of Solid State Eugenol/β-Cyclodextrin Inclusion Complex. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 207–213. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and Antioxidant Activities of Mexican Oregano Essential Oils (Lippia Graveolens H. B. K.) with Different Composition When Microencapsulated Inβ-Cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing Essential Oils (Trans-Cinnamaldehyde, Eugenol, Cinnamon Bark, and Clove Bud Extracts) for Antimicrobial Delivery Applications. LWT-Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Weisheimer, V.; Miron, D.; Silva, C.B.; Guterres, S.S.; Schapoval, E.E.S. Microparticles Containing Lemongrass Volatile Oil: Preparation, Characterization and Thermal Stability. Pharmazie 2010, 65, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Zhao, Q.Q.; Lv, T.F.; Shuai, W.P.; Zhou, J.; Tang, G.P.; Liang, W.Q.; Tabata, Y.; Hu, Y.L. Gene-Carried Chitosan-Linked-PEI Induced High Gene Transfection Efficiency with Low Toxicity and Significant Tumor-Suppressive Activity. Int. J. Pharm. 2010, 387, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Raviv, L.; Jaron-Mendelson, M.; David, A. Mannosylated Polyion Complexes for in Vivo Gene Delivery into CD11c+ Dendritic Cells. Mol. Pharm. 2015, 12, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, H.; Goh, S.H.; Li, J. Cationic Star Polymers Consisting of α-Cyclodextrin Core and Oligoethylenimine Arms as Nonviral Gene Delivery Vectors. Biomaterials 2007, 28, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ping, Y.; Xu, F.J.; Li, Z.H.; Wang, Q.Q.; Chen, J.H.; Yang, W.T.; Tang, G.P. Bifunctional Conjugates Comprising β-Cyclodextrin, Polyethylenimine, and 5-Fluoro-2′-Deoxyuridine for Drug Delivery and Gene Transfer. Bioconjug. Chem. 2010, 21, 1855–1863. [Google Scholar] [CrossRef]
- Tang, G.P.; Guo, H.Y.; Alexis, F.; Wang, X.; Zeng, S.; Lim, T.M.; Ding, J.; Yang, Y.Y.; Wang, S. Low Molecular Weight Polyethylenimines Linked by β-Cyclodextrin for Gene Transfer into the Nervous System. J. Gene Med. 2006, 8, 736–744. [Google Scholar] [CrossRef]
- Casillo, A.; Fabozzi, A.; Russo Krauss, I.; Parrilli, E.; Biggs, C.I.; Gibson, M.I.; Lanzetta, R.; Appavou, M.S.; Radulescu, A.; Tutino, M.L.; et al. Physicochemical Approach to Understanding the Structure, Conformation, and Activity of Mannan Polysaccharides. Biomacromolecules 2021, 22, 1445–1457. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Deme, R.; Mucsi, Z.; Rusu, A.; Mare, A.D.; Fiser, B.; Toma, F.; Sipos, E.; Tóth, G. Equilibrium, Structural and Antibacterial Characterization of Moxifloxacin-β-Cyclodextrin Complex. J. Mol. Struct. 2018, 1166, 228–236. [Google Scholar] [CrossRef]
- Cameron, K.S.; Fletcher, D.; Fielding, L. An NMR Study of Cyclodextrin Complexes of the Steroidal Neuromuscular Blocker Drug Rocuronium Bromide. Magn. Reson. Chem. 2002, 40, 251–260. [Google Scholar] [CrossRef]
- Rodríguez-Lavado, J.; De La Mata, M.; Jiménez-Blanco, J.L.; García-Moreno, M.I.; Benito, J.M.; Díaz-Quintana, A.; Sánchez-Alcázar, J.A.; Higaki, K.; Nanba, E.; Ohno, K.; et al. Targeted Delivery of Pharmacological Chaperones for Gaucher Disease to Macrophages by a Mannosylated Cyclodextrin Carrier. Org. Biomol. Chem. 2014, 12, 2289–2301. [Google Scholar] [CrossRef]
- Kanai, M.; Mortell, K.H.; Kiessling, L.L. Varying the Size of Multivalent Ligands: The Dependence of Concanavalin A Binding on Neoglycopolymer Length. J. Am. Chem. Soc. 1997, 119, 9931–9932. [Google Scholar] [CrossRef]
- Weatherman, R.V.; Kiessling, L.L. Fluorescence Anisotropy Assays Reveal Affinities of C- and O-Glycosides for Concanavalin A. J. Org. Chem. 1996, 61, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.I.; Le Novère, N. Cooperative Binding. PLoS Comput. Biol. 2013, 9, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bryce, R.A.; Hillier, I.H.; Naismith, J.H. Carbohydrate-Protein Recognition: Molecular Dynamics Simulations and Free Energy Analysis of Oligosaccharide Binding to Concanavalin A. Biophys. J. 2001, 81, 1373–1388. [Google Scholar] [CrossRef]
- Dani, M.; Manca, F.; Rialdi, G. Calorimetric study of concanavalin A binding to saccharides. Biochim. et Biophys. Acta BBA Protein Struct. 1981, 667, 108–117. [Google Scholar] [CrossRef]
- Mislovičová, D.; Masárová, J.; Švitel, J.; Mendichi, R.; Šoltés, L.; Gemeiner, P.; Danielsson, B. Neoglycoconjugates of Mannan with Bovine Serum Albumin and Their Interaction with Lectin Concanavalin A. Bioconjug. Chem. 2002, 13, 136–142. [Google Scholar] [CrossRef]
- López-López, M.; Fernández-Delgado, A.; Moyá, M.L.; Blanco-Arévalo, D.; Carrera, C.; de la Haba, R.R.; Ventosa, A.; Bernal, E.; López-Cornejo, P. Optimized Preparation of Levofloxacin Loaded Polymeric Nanoparticles. Pharmaceutics 2019, 11, 57. [Google Scholar] [CrossRef]
- Ameeduzzafar; Imam, S.S.; Bukhari, S.N.A.; Ahmad, J.; Ali, A. Formulation and Optimization of Levofloxacin Loaded Chitosan Nanoparticle for Ocular Delivery: In-Vitro Characterization, Ocular Tolerance and Antibacterial Activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Pirosa, A.; Sandreschi, S.; Chiellini, F. Levofloxacin-Loaded Star Poly(ε-Caprolactone) Scaffolds by Additive Manufacturing. J. Mater. Sci. Mater. Med. 2016, 27, 44. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I.V.; Kudryashova, E.V. Thermodynamics and Molecular Insight in Guest–Host Complexes of Fluoroquinolones with β-Cyclodextrin Derivatives, as Revealed by ATR-FTIR Spectroscopy and Molecular Modeling Experiments. Anal. Bioanal. Chem. 2017, 409, 6451–6462. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E.V. Effect of Cross-Linking on the Inclusion Complex Formation of Derivatized β-Cyclodextrins with Small-Molecule Drug Moxifloxacin. Carbohydr. Res. 2020, 498, 108183. [Google Scholar] [CrossRef] [PubMed]
- Sukhoverkov, K.V.; Le-Deygen, I.M.; Egorov, A.M.; Kudryashova, E.V. Physicochemical Properties of the Inclusion Complex of Moxifloxacin with Hydroxypropyl-β-Cyclodextrin Synthesized by RESS. Russ. J. Phys. Chem. B 2018, 12, 1193–1204. [Google Scholar] [CrossRef]
- Leite, A.M.; Lima, E.D.O.; De Souza, E.L.; Diniz, M.D.F.F.M.; Trajano, V.N.; De Medeiros, I.A. Inhibitory Effect of β-Pinene, α-Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Rev. Bras. Ciencias Farm. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic Interaction of Eugenol with Antibiotics against Gram Negative Bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Kopnova, T.Y.; Le-Deygen, I.M.; Kudryashova, E.V. Physical and Chemical Properties of the Guest–Host Inclusion Complexes of Cyprofloxacin with β-Cyclodextrin Derivatives. Moscow Univ. Chem. Bull. 2020, 75, 218–224. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial Activities of Eugenol and Cinnamaldehyde against the Human Gastric Pathogen Helicobacter Pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef]
- Lichtenstein, E.P.; Liang, T.T.; Schulz, K.R.; Schnoes, H.K.; Carter, G.T. Insecticidal and Synergistic Components Isolated from Dill Plants. J. Agric. Food Chem. 1974, 22, 658–664. [Google Scholar] [CrossRef]
- Samet, A.V.; Shevchenko, O.G.; Rusak, V.V.; Chartov, E.M.; Myshlyavtsev, A.B.; Rusanov, D.A.; Semenova, M.N.; Semenov, V.V. Antioxidant Activity of Natural Allylpolyalkoxybenzene Plant Essential Oil Constituents. J. Nat. Prod. 2019, 82, 1451–1458. [Google Scholar] [CrossRef]
- Pereira de Lira, M.H.; Fernandes Queiroga Moraes, G.; Macena Santos, G.; Patrício de Andrade Júnior, F.; De Oliveira Pereira, F.; Oliveira Lima, I. Synergistic Antibacterial Activity of Monoterpenes in Combination with Conventional Antimicrobials against Gram-Positive and Gram-Negative Bacteria. Rev. Ciências Médicas e Biológicas 2020, 19, 258. [Google Scholar] [CrossRef]
- Kudryashova, E.V.; Artemova, T.M.; Vinogradov, A.A.; Gladilin, A.K.; Mozhaev, V.V.; Levashov, A.V. Stabilization and Activation of α-Chymotrypsin in Water-Organic Solvent Systems by Complex Formation with Oligoamines. Protein Eng. 2003, 16, 303–309. [Google Scholar] [CrossRef]
- Beketov, V.I.; Voronina, R.D.; Zorov, N.B. Fluorimetric Determination of Amino Acids and Photochemical Stability of Their Reaction Products with Ortho-Phthalic Aldehyde under Irradiation by High-Power Pulsed Laser. Moscow Univ. Chem. Bull. 2012, 67, 149–153. [Google Scholar] [CrossRef]
- Deygen, I.M.; Kudryashova, E.V. New Versatile Approach for Analysis of PEG Content in Conjugates and Complexes with Biomacromolecules Based on FTIR Spectroscopy. Colloids Surfaces B Biointerfaces 2016, 141, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Endrenyi, L.; Kwong, F.H.F.; Fajszi, C. Evaluation of Hill Slopes and Hill Coefficients When the Saturation Binding or Velocity Is Not Known. Eur. J. Biochem. 1975, 51, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R. Eugenol-Loaded Chitosan Nanoparticles: I. Thermal Stability Improvement of Eugenol through Encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
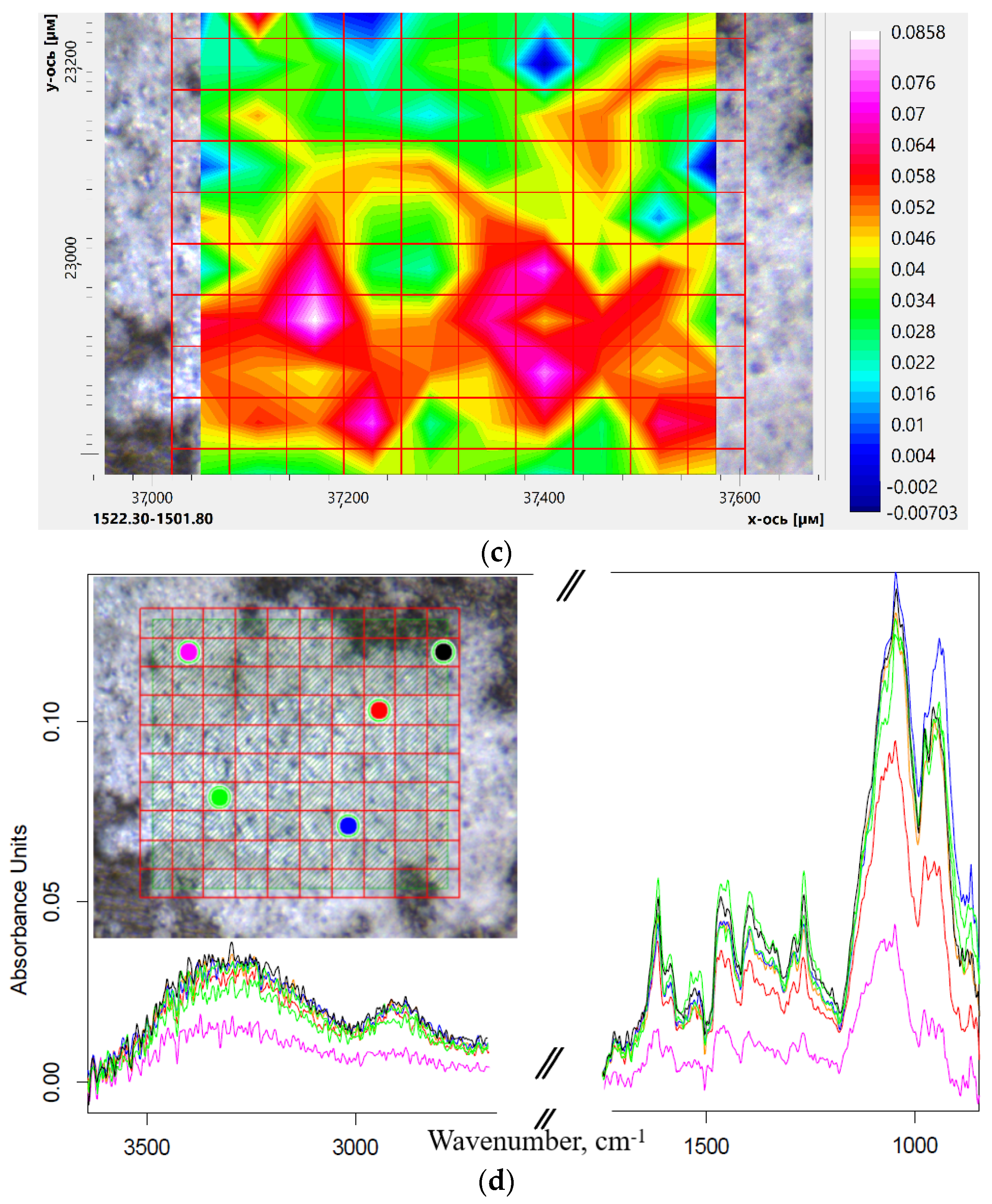
| Sample’s Designation * | Hydrodynamic Diameter (by DLS) **, nm | Mr, kDa | Molar Ratio HPCD: Spermine (or PEI): for both levofloxacin and eugenol.Man | Average Amount of Mannose Residues per Molecule | Degree of Mannosylation | ζ-Potential **, mV | References to Synthesis Schemes and FTIR/NMR Spectra |
|---|---|---|---|---|---|---|---|
| HPCD-spermine-Man-1 | 336 ± 68 | 6 | 7.4:1:6.2 | 6 | 95 ± 3 | −2.7 ± 0.8 | Supplement Figures S1, S7 and S8 |
| HPCD-spermine-Man-2 | 523 ± 106 | 9 | 5:1:6.9 | 7 | 98 ± 2 | −4.1 ± 1.9 | Supplement Figures S1 and S8 |
| HPCD-spermine-Man-3 | 781 ± 205 | 8.5 | 1.5:1:11 | 22 | 92 ± 3 | −4.9 ± 0.1 | Supplement Figures S2 and S8 |
| HPCD-PEI35-Man-4 | 52.8 ± 1.3 | 42 | 1:1:34.5 | 35 | 77 ± 3 | 5.1 ± 1.4 | Supplement Figures S3 and S9 |
| HPCD-PEI35-Man-5 | 62.3 ± 0.7 | 50 | 8.6:1:83 | 83 | 94 ± 4 | −9.4 ± 2.1 | Supplement Figures S4 and S9 |
| HPCD-PEI35-Man-6 | 59 ± 7 | 115 | 1:2:271 | 271 | 80 ± 4 | 19.0 ± 2.5 | Figure 2h, Figures S5 and S9 |
| HPCD-spermine-Man-7 | 152.2 ± 1.0 | 8 | 2:9:21 | 21 | 85 ± 5 | −6.0 ± 0.4 | Figure 1a and Figure 2 |
| HPCD-PEI1.8-Man-8 | 66.9 ± 0.3 | 20 | 1:6.1:45 | 45 | 83 ± 4 | −2.0 ± 0.1 | Figure 2f, Figures S6 and S10 |
| HPCD-PEI10-Man-9 | 373 ± 108 | 28 | 2:1:154 | 154 | 99 ± 1 | 4.8 ± 1.0 | Figure 1b, Figure 2e, Figure S10 |
| Ligand | Kd (ConA–Ligand), μM | |||
|---|---|---|---|---|
| Quenching of Trp Fluorescence | FTIR Spectroscopy | |||
| HPCD-spermine-Man-1 | 64 ± 11 | 50 ± 6 | ||
| HPCD-spermine-Man-2 | 69 ± 12 | 60 ± 11 | ||
| HPCD-spermine-Man-3 | 0.55 ± 0.19 | 0.49 ± 0.23 | ||
| HPCD-spermine-Man-7 | 0.24 ± 0.12 | 0.27 ± 0.08 | ||
| Ligand | Quenching of Trp fluorescence | Anisotropy of Trp fluorescence | FTIR spectroscopy | |
| HPCD-PEI35-Man-4 | 11 ± 6 | Kobs =17 ± 8 | 12 ± 3 | |
| HPCD-PEI35-Man-5 | 69 ± 21 | Kobs = 60 ± 19 Kmax = 5.0 ± 0.7 | 57 ± 17 | |
| HPCD-PEI35-Man-6 | Kobs = 31 ± 12 Kmax = 0.10 ± 0.03 | Kobs = 1.3 ± 0.4 Kmax = 0.97 ± 0.22 | 2.8 ± 0.7 | |
| HPCD-PEI1.8-Man-8 | 36 ± 13 | Kobs = 19 ± 6 Kmax = 2.9 ± 1.2 | 27 ± 10 | |
| HPCD-PEI10-Man-9 | 6.8 ± 0.4 | 8.1 ± 1.5 | 55 ± 14 * | |
| Mannan (control) | 1.9 ± 0.5 | 1.6 ± 0.9 | 1.7 ± 0.5 | |
| Levofloxacin | ||||||
|---|---|---|---|---|---|---|
| Conjugate | HPCD-spermine-Man-1 | HPCD-spermine-Man-2 | HPCD-spermine-Man-3 | HPCD-spermine-Man-7 | HPCD-PEI35-Man-5 | HPCD-PEI35-Man-6 |
| –lg Kd | 3.89 ± 0.24 | 3.75 ± 0.17 | 3.33 ± 0.19 | 4.46 ± 0.29 | 5.28 ± 0.41 | 3.15 ± 0.15 |
| N | 14 | 19 | 12 | 15 | 85 | 80 |
| EE, % | 97 ± 2 | 96 ± 3 | 82 ± 3 | 98 ± 2 | 98 ± 2 | 93 ± 3 |
| LC, % | 86 ± 5 | 78 ± 4 | 52 ± 4 | 69 ± 4 | 63 ± 5 | 26 ± 2 |
| Eugenol | ||||||
| Conjugate | HPCD-PEI35-Man-5 | HPCD-PEI35-Man-6 | HPCD-spermine-Man-7 | |||
| K * | 47 ± 4 | 7 ± 2 | 58 ± 5 | |||
| N | 6 ± 1 (mostly cyclodextrin inclusion) | 7 ± 2 (mostly polymer’s interaction) | 6 ± 1 | |||
| EE, % | 83 ± 5 | 62 ± 6 | 86 ± 4 | |||
| LC, % | 2.0 ± 0.1 | 1.0 ± 0.1 | 12 ± 1 | |||
| Conjugate (Sample) | Time of Semi-Release of Lev τ1/2, Min | Time of 80%–Release of Lev τ80%, Hours | Kinetic Constants, h−1 | The Proportion of Lev or EG Included in Cyclodextrin Tori and Retained by the Polymer Matrix, % | |
|---|---|---|---|---|---|
| The Total Process of Dissociation of the Complex and Release through the Membrane. ktot | Dissociation of the Complex. kdiss | ||||
| Levofloxacin free | 21 ± 3 | 0.62 ± 0.06 | 10.4 ± 1.3 | - | - |
| Lev in HPCD-PEI35-Man-5 | 32 ± 2 | 17 ± 1 | 1.5 ± 0.2 | 1.8 ± 0.2 | 40/60 |
| Lev in HPCD-spermine-Man-7 | 43 ± 3 | 66 ± 5 | 2.1 ± 0.3 | 2.6 ± 0.3 | 60/40 |
| Lev in HPCD-PEI1.8-Man-8 | 40 ± 2 | 30 ± 2 | 0.88 ± 0.07 | 0.96 ± 0.11 | 50/50 |
| Lev in HPCD-PEI10-Man-9 | 44 ± 2 | 70 ± 2 | 1.15 ± 0.12 | 1.3 ± 0.2 | 65/35 |
| Eugenol free | 9 ± 1 | 0.36 ± 0.04 | 19.3 ± 2.4 | - | - |
| EG in HPCD-PEI35-Man-5 | 16 ± 2 | 6.5 ± 0.5 | 4.6 ± 0.4 | 6.0 ± 0.5 | 30/70 |
| EG in HPCD-spermine-Man-7 | 27 ± 3 | 4.5 ± 0.6 | 5.4 ± 0.4 | 7.5 ± 0.6 | 25/35 |
| EG in HPCD-PEI1.8-Man-8 | 21 ± 3 | 25 ± 3 | 5.2 ± 0.3 | 7.1 ± 0.5 | 40/60 |
| EG in HPCD-PEI10-Man-9 | 300 ± 20 | 57 ± 4 | 2.6 ± 0.2 | 3.0 ± 0.3 | 55/45 |
| Sample | Inhibition Zone Diameters (mm, ±0.5 mm) and Corresponding Concentration of Drug | |||
|---|---|---|---|---|
| 0.5 μg/mL Lev | 1 μg/mL Lev | 2 μg/mL Lev | 5 μg/mL Lev | |
| Levofloxacin | 13 | 22.5 | 28 | 31 |
| HPCD-spermine-Man-7 + Lev (1:1) | 14 | 24 | 31 | 32 |
| HPCD-spermine-Man-7 + Lev/EG (1:5:5) | 15 | 24.5 | 30.5 | 33 |
| HPCD-PEI35-Man-5 + Lev (1:1) | 13 | 23 | 27 | 32 |
| HPCD-PEI35-Man-5 + Lev/EG (1:1:1) | 14.5 | 25 | 27.5 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zlotnikov, I.D.; Kudryashova, E.V. Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. Pharmaceuticals 2022, 15, 625. https://doi.org/10.3390/ph15050625
Zlotnikov ID, Kudryashova EV. Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. Pharmaceuticals. 2022; 15(5):625. https://doi.org/10.3390/ph15050625
Chicago/Turabian StyleZlotnikov, Igor D., and Elena V. Kudryashova. 2022. "Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs" Pharmaceuticals 15, no. 5: 625. https://doi.org/10.3390/ph15050625
APA StyleZlotnikov, I. D., & Kudryashova, E. V. (2022). Spectroscopy Approach for Highly-Efficient Screening of Lectin-Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. Pharmaceuticals, 15(5), 625. https://doi.org/10.3390/ph15050625