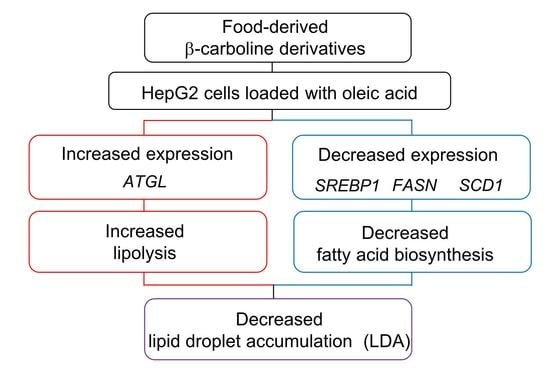
Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metabolite Fingerprinting of C. gigas and β-Carboline Alkaloid Identification
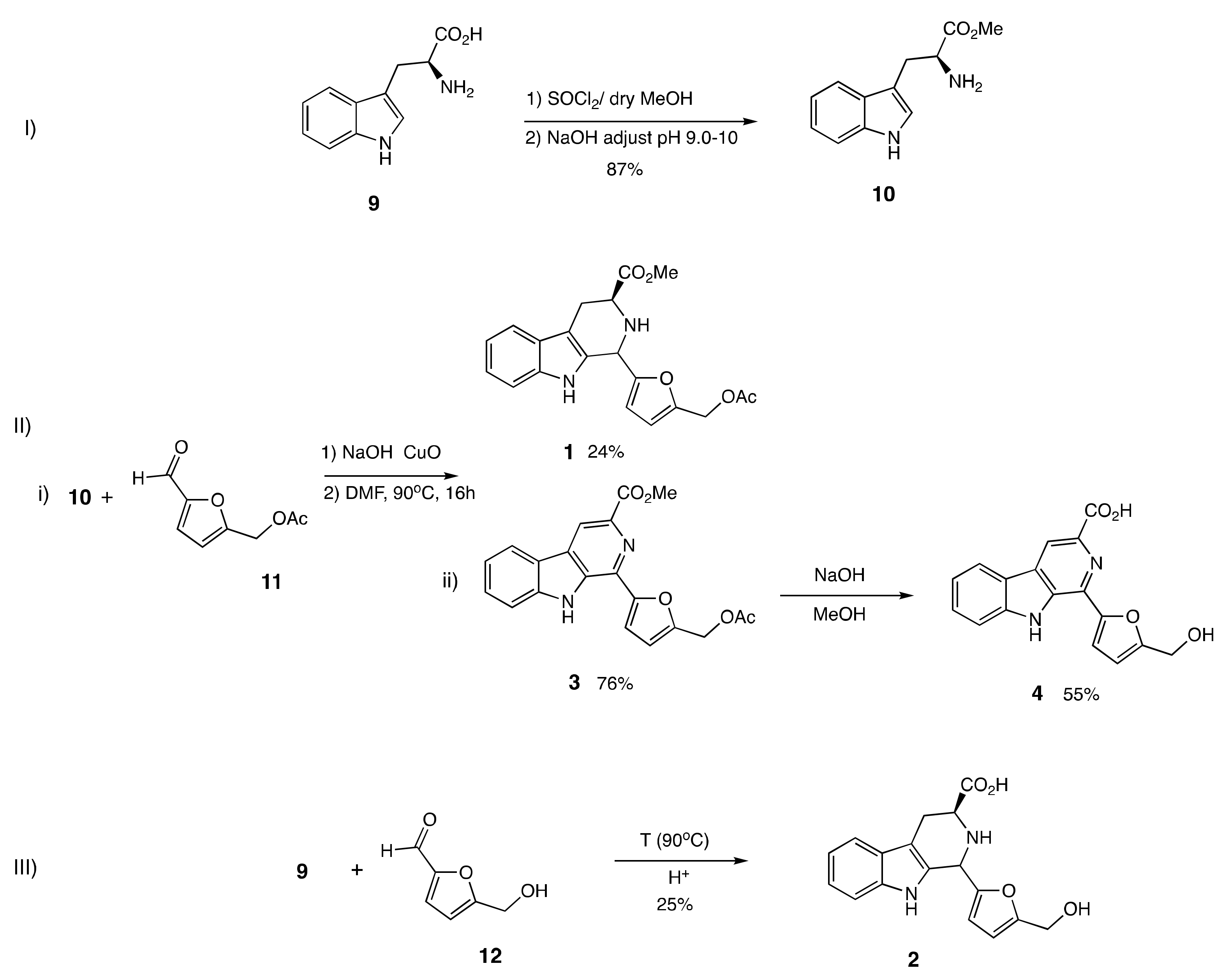
2.2. Design and Synthesis of β-Carboline Alkaloid Metabolite Derivatives (Pyridine and Piperidine Types)
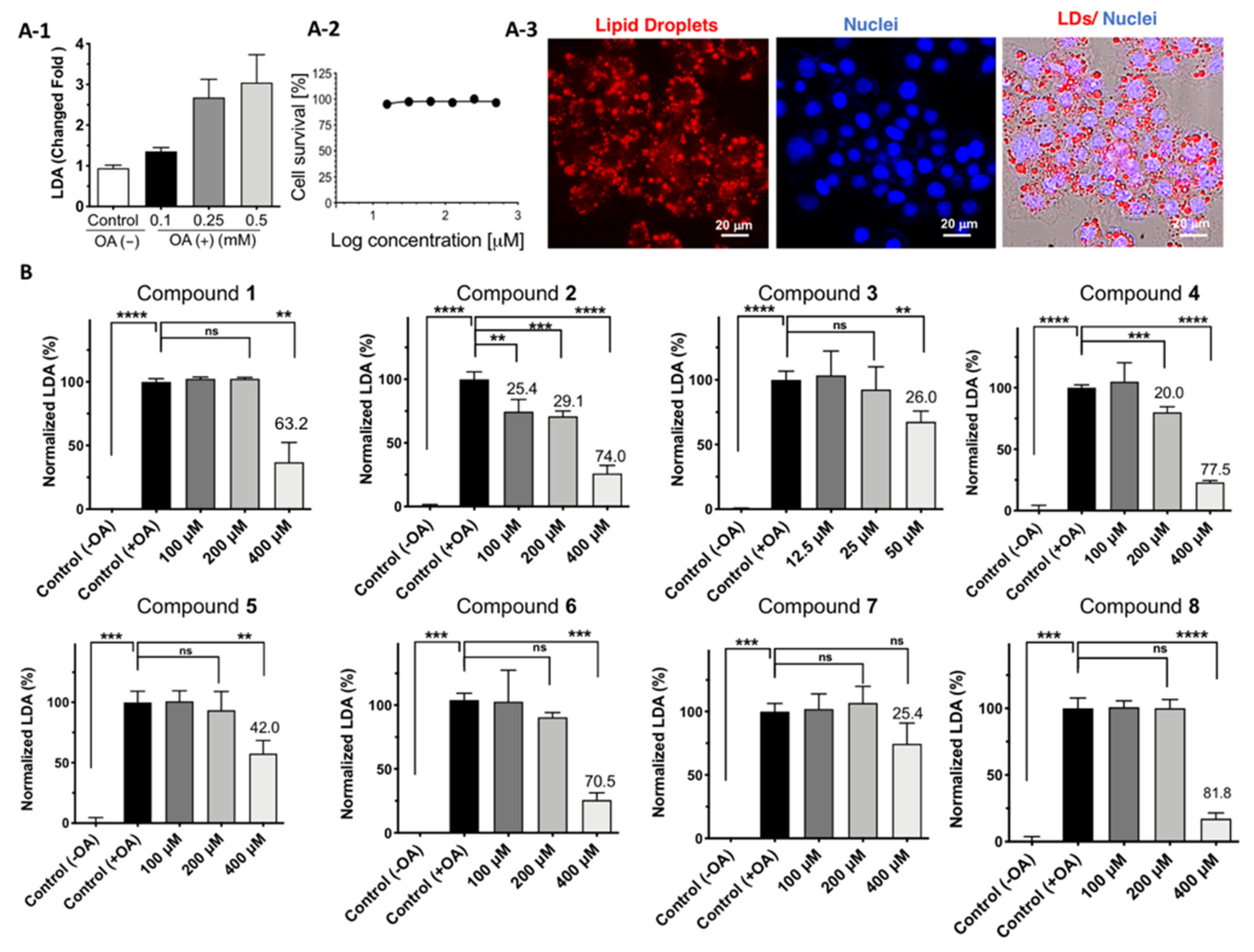
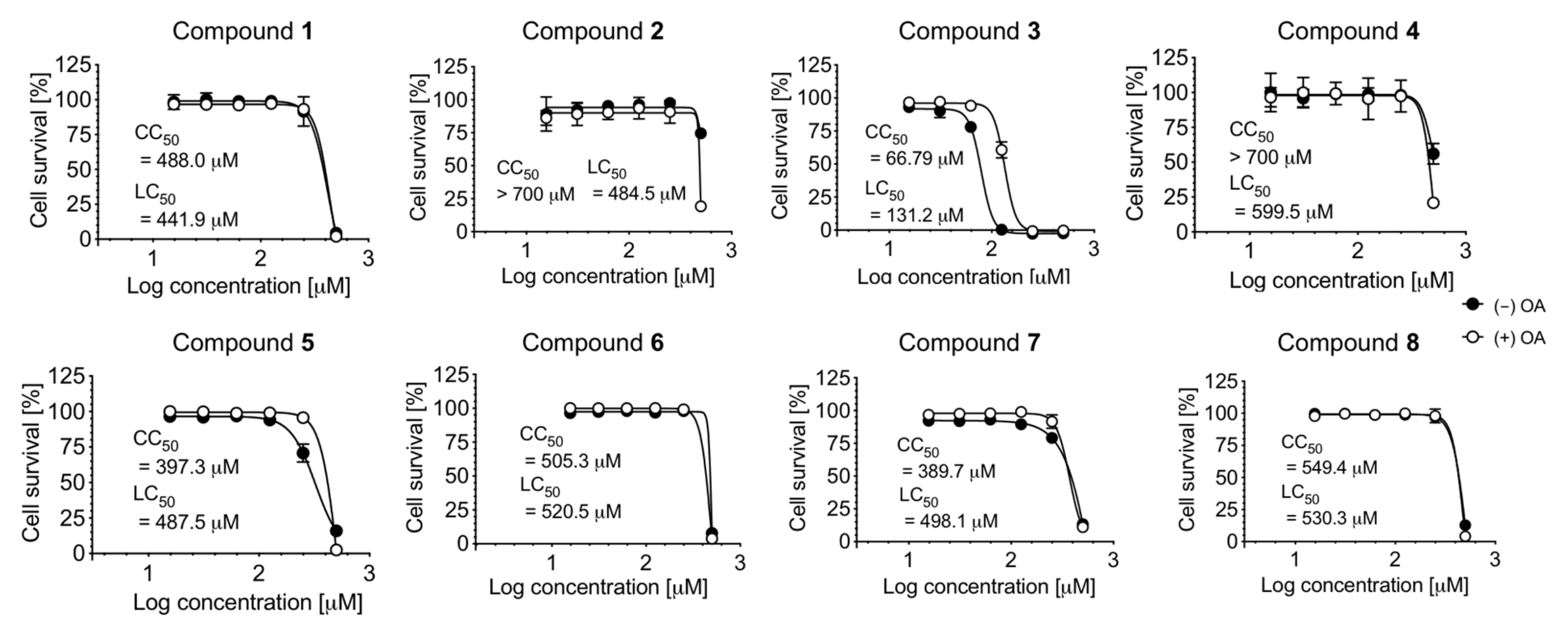
2.3. Cell Viability and LDAI Activity of β-Carboline Alkaloid Metabolite Derivatives
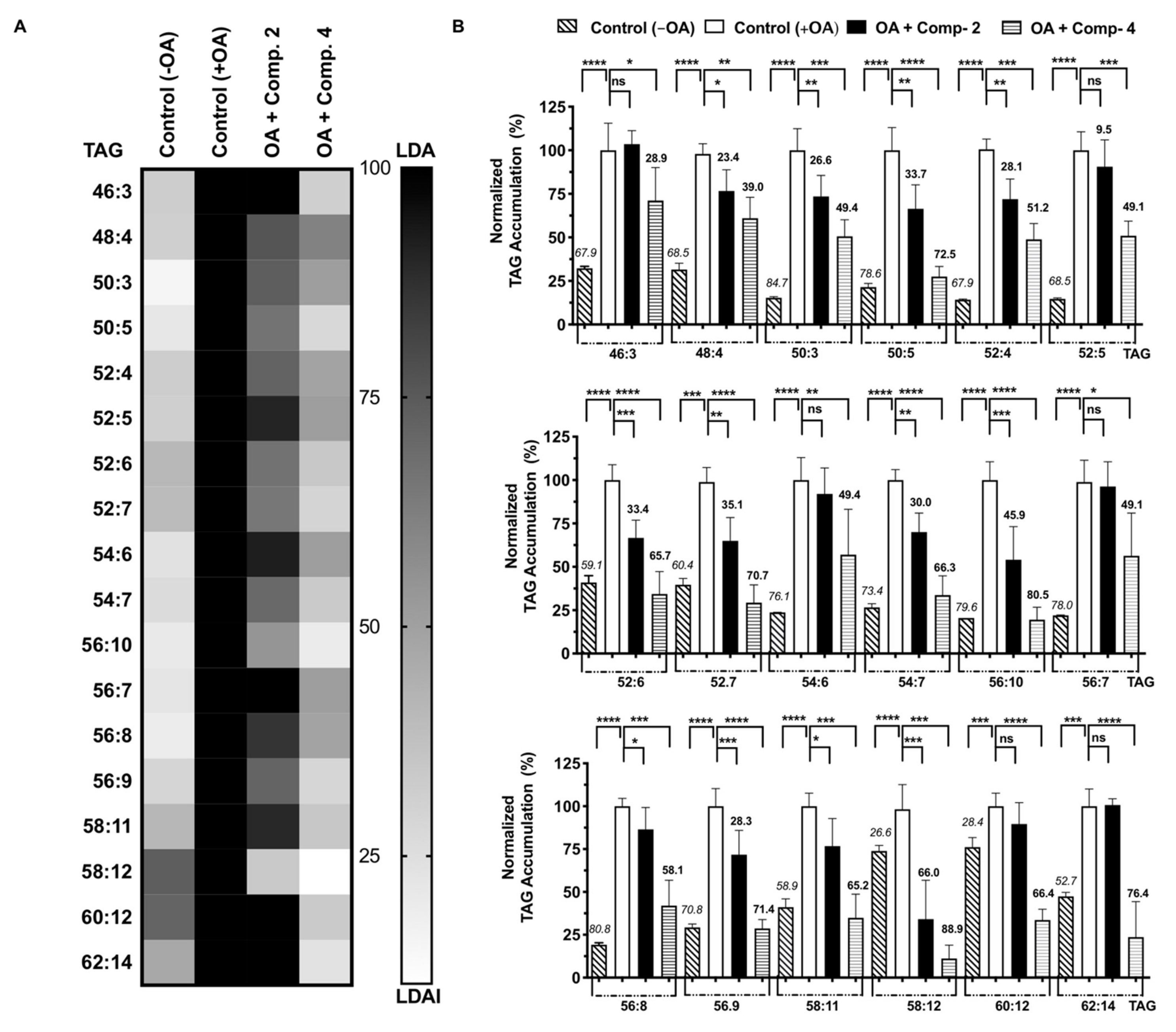
2.4. Quantification of Accumulated Triacylglycerol Species Inhibition
2.5. Gene Expression
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Metabolite Fingerprint of C. gigas and β-Carboline Alkaloid Dereplication
3.2.1. HPLC and LC/MS Profiling
3.2.2. Synthesis Procedure
3.3. Cell Culture and Cell Viability Assay
3.4. LDAI Assay, LD Fluorescence Staining Assay, and Real-Time LDAI Monitoring
3.5. Analysis of Accumulation on Triacylglycerols Species by LC-MS/MS
3.6. Lipid Metabolism–Related Gene Expression
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M. Non-Alcoholic Fatty Liver Disease—A Global Public Health Perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.R.; Kim, K.I. An overview of NAFLD/NASH in Japan. Yakugaku Zasshi 2016, 136, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, L.F.; Hernández, G.; Puerta, A.V.; Beltrán, Ó.; Botero, R.C.; Mejía, G. Non Alcoholic Fatty Liver Disease. The New Millennium Pandemia. Rev. Colomb. Gastroenterol. 2010, 25, 373–391. [Google Scholar]
- Torres, D.M.; Williams, C.D.; Harrison, S.A. Features, Diagnosis, and Treatment of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2012, 10, 837–858. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Neuschwander-Tetri, B.A. Nonalcoholic Fatty Liver Disease: Clinical Features and Pathogenesis. Gastroenterol. Hepatol. 2006, 2, 282–291. [Google Scholar]
- Ganguli, S.; DeLeeuw, P.; Satapathy, S.K. A Review of Current and Upcoming Treatment Modalities in Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Hepatic Med. Evid. Res. 2019, 11, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid Droplets and Liver Disease: From Basic Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Krahmer, N.; Farese, R.V.; Walther, T.C. Balancing the Fat: Lipid Droplets and Human Disease. EMBO Mol. Med. 2013, 5, 973–983. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and Future Pharmacological Therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef] [Green Version]
- Mashek, D.G. Hepatic Lipid Droplets: A Balancing Act Between Energy Storage and Metabolic Dysfunction in NAFLD. Mol. Metab. 2021, 50, 101115. [Google Scholar] [CrossRef]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. The Assembly of Lipid Droplets and Their Roles in Challenged Cells. EMBO J. 2018, 37, e98947. [Google Scholar] [CrossRef]
- Hernández-Pérez, E.; León García, P.E.; López-Díazguerrero, N.E.; Rivera-Cabrera, F.; del Ángel Benítez, E. Liver Steatosis and Nonalcoholic Steatohepatitis: From Pathogenesis to Therapy. Medwave 2016, 16, e6535. [Google Scholar] [CrossRef]
- Sumida, Y.; Okanoue, T.; Nakajima, A. Phase 3 Drug Pipelines in the Treatment of Non-Alcoholic Steatohepatitis. Hepatol. Res. 2019, 49, 1256–1262. [Google Scholar] [CrossRef]
- Pei, K.; Gui, T.; Kan, D.; Feng, H.; Jin, Y.; Yang, Y.; Zhang, Q.; Du, Z.; Gai, Z.; Wu, J.; et al. An Overview of Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Biomed Res. Int. 2020, 2020, 4020249. [Google Scholar] [CrossRef]
- Souza, M.R.d.A.; de Fátima Formiga de Melo Diniz, M.; de Medeiros-Filho, J.E.M.; de Araújo, M.S.T. Metabolic Syndrome and Risk Factors for Non-Alcoholic Fatty Liver Disease. Arq. Gastroenterol. 2012, 49, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Del Pilar López Panqueva, R. Pathological Aspects of Fatty Liver Disease. Rev. Colomb. Gastroenterol. 2014, 29, 82–88. [Google Scholar]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef]
- Khodair, R.; Da’aboul, I.; Barhoum, R. Synthesis of Novel Compounds from Harmine (β-Carboline Derivatives) Isolated from Syrian Peganum Harmala L. Seeds. Int. J. Acad. Sci. Res. 2017, 5, 57–76. [Google Scholar]
- Laine, A.E.; Lood, C.; Koskinen, A.M.P. Pharmacological Importance of Optically Active Tetrahydro-β-Carbolines and Synthetic Approaches to Create the C1 Stereocenter. Molecules 2014, 19, 1544–1567. [Google Scholar] [CrossRef] [Green Version]
- Fuda, H.; Watanabe, M.; Hui, S.P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Chiba, H. Anti-Apoptotic Effects of Novel Phenolic Antioxidant Isolated from the Pacific Oyster (Crassostrea gigas) on Cultured Human Hepatocytes under Oxidative Stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Hui, S.P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. A Phenolic Antioxidant from the Pacific Oyster (Crassostrea gigas) Inhibits Oxidation of Cultured Human Hepatocytes Mediated by Diphenyl-1-Pyrenylphosphine. Food Chem. 2012, 134, 2086–2089. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and Characterization of a Phenolic Antioxidant from the Pacific Oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fuda, H.; Okabe, H.; Joko, S.; Miura, Y.; Hui, S.P.; Yimin; Hamaoka, N.; Miki, E.; Chiba, H. Oyster Extracts Attenuate Pathological Changes in Non-Alcoholic Steatohepatitis (NASH) Mouse Model. J. Funct. Foods 2016, 20, 516–531. [Google Scholar] [CrossRef] [Green Version]
- Fuda, H.; Miyanaga, S.; Furukawa, T.; Umetsu, S.; Joko, S.; Roan, Y.; Suzuki, H.; Hui, S.P.; Watanabe, M.; Chiba, H. Flazin as a Promising Nrf2 Pathway Activator. J. Agric. Food Chem. 2019, 67, 12844–12853. [Google Scholar] [CrossRef]
- Baek, S.C.; Nam, K.H.; Yi, S.A.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Lee, J.; Kim, K.H. Anti-Adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum). Foods 2019, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-Carbolines in Food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.P.; Kaneda, T.; Morita, H. Plant Natural Products as an Anti-Lipid Droplets Accumulation Agent. J. Nat. Med. 2014, 68, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Shi, T.; Luo, L.; Liu, J.; Liu, R.; Liu, L.; Liang, M.; Ma, Y. One-Pot Synthesis of β-Carboline Derivatives Catalyzed by CuO Nanoparticles. Chem. J. Chin. Univ. 2018, 39, 2411–2418. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Cho, Y.Y.; Hsieh, S.-C.; Chiang, A.-N. Chinese olive extract ameliorates hepatic lipid accumulation in vitro and in vivo by regulating lipid metabolism. Sci. Rep. 2018, 8, 1057. [Google Scholar] [CrossRef]
- Kang, O.H.; Kim, S.B.; Mun, S.H.; Seo, Y.S.; Hwang, H.C.; Lee, Y.M.; Lee, H.S.; Kang, D.G.; Kwon, D.Y. Puerarin ameliorates hepatic steatosis by activating the PPARα and AMPK signaling pathways in hepatocytes. Int. J. Mol. Med. 2015, 35, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Vergas, P.M.; Rodriguez, A.S.; Echevarria, R.R.; Rosales, J.A.D.; Gracia, A.S.; Buranda, J.A. Delphinidin Ameliorates Hepatic Triglyceride Accumulation in Human HepG2 Cells, but Not in Diet-Induced Obese Mice. Nutrients 2018, 10, 1060. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.P.; Renaud, J.B.; Hoogstra, S.; McMullin, D.R.; Ibrahim, A.I. Diagnostic Fragmentation Filtering for the Discovery of New Chaetoglobosins and Cytochalasins. Rapid Commun. Mass Spectrom. 2019, 33, 133–139. [Google Scholar] [CrossRef]
- Miller Crotti, A.E.; Gates, P.J.; Luis Callegari Lopes, J.; Peporine Lopes, N. Electrospray MS-based characterization of β-carbolines- mutagenic constituents of thermally processes meat. Mol. Nutr. Food Res. 2010, 54, 433–439. [Google Scholar]
- Herraiz, T.; Galisteo, J.; Chamorro, C. L-Tryptophan reacts with naturally occurring and food-occurring phenolic aldehydes to give phenolic tetrahydro-β-carboline alkaloids: Activity as antioxidants and free radical scavengers. J. Agri. Food Chem. 2010, 51, 2168–2173. [Google Scholar] [CrossRef]
- Larghi, E.L.; Amongero, M.; Bracca, A.B.J.; Kaufman, T.S. The intermolecular Pictet-Spengler condensation with chiral carbonyl derivatives in the stereoselective syntheses of optically-active isoquinoline and indole alkaloids. Arkivoc 2005, 12, 98–153. [Google Scholar] [CrossRef] [Green Version]
- Goh, T.B.; Mordi, M.N.; Mansor, S.M. Mass Spectrometry (LC-MS-MS) as a Tool in the Maillard Reaction Optimisation and Characterisation of New 6-methoxy-tetrahydro-β-carboline Derivatives. Sains Malays. 2015, 44, 127–137. [Google Scholar] [CrossRef]
- Amaral, A.C.F.; Ramos, A.S.; Ferreira, J.L.P.; Santos, A.R.S.; Cruz, J.D.Z.; Luna, A.V.M.; Nery, V.V.C.; Lima, I.C.; Chaves, M.H.D.C.; Silva, J.R.D.A. LC-HRMS for the Identification of β-Carboline and Canthinone Alkaloids Isolated from Natural Sources. In Mass Spectrometry; Aliofkhazraei, M., Ed.; Intech: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Gao, P.; Zhang, M.; Liao, W.; Zhang, J. Synthesis and Biological Evaluation of a Novel Class of β-Carboline Derivatives. New J. Chem. 2014, 38, 4155–4166. [Google Scholar] [CrossRef]
- Tsukui, T.; Chen, Z.; Fuda, H.; Furukawa, T.; Oura, K.; Sakurai, T.; Hui, S.P.; Chiba, H. Novel Fluorescence-Based Method to Characterize the Antioxidative Effects of Food Metabolites on Lipid Droplets in Cultured Hepatocytes. J. Agric. Food Chem. 2019, 67, 9934–9941. [Google Scholar] [CrossRef]
- Seong, S.H.; Jung, H.A.; Choi, J.S. Discovery of Flazin, an Alkaloid Isolated from Cherry Tomato Juice, As a Novel Non-Enzymatic Protein Glycation Inhibitor via in Vitro and in Silico Studies. J. Agric. Food Chem. 2021, 69, 3647–3657. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Wang, Y.-M.; Yang, Y.-L.; Zeng, Y.; Wang, Q.-L.; Shao, Y.; Mei, L.-J.; Shi, Y.-P.; Tao, Y.-D. Isolation and identification of antioxidant and α-glucosidase inhibitory compounds from fruit juice of Nitraria tangutorum. Food Chem. 2017, 227, 93–101. [Google Scholar] [CrossRef]
- Mäkilä, L.; Laaksonen, O.; Alanne, A.-L.; Kortesniemi, M.; Kallio, H.; Yang, B. Stability of Hydroxycinnamic Acid Derivatives, Flavonol Glycosides, and Anthocyanins in Black Currant Juice. J. Agric. Food Chem. 2016, 64, 4584–4598. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Feng, B.; Goto, T.; Kihara, K. Structures of flazin and YS, highly fluorescent compounds isolated from japanese soy sauce. Tetrahedron Lett. 1986, 27, 3399–3402. [Google Scholar] [CrossRef]
- Gessner, W.P.; Brossi, A. β-Carbolines from Japanese Sake and Soy Sauce: Synthesis and Biological Activity of Flazin and Yellow Substance YS (Perlolyrine). Arch. Pharm. 1988, 321, 95–98. [Google Scholar] [CrossRef]
- Tang, J.G.; Wang, Y.H.; Wang, R.R.; Dong, J.Z.; Yang, L.M.; Zheng, Y.T.; Liu, J.K. Synthesis of Analogues of Flazin, in Particular, Flazinamide, as Promising Anti-HIV Agents. Chem. Biodivers. 1988, 5, 447–460. [Google Scholar] [CrossRef]
- Szabo, T.; Volk, B.; Milen, M. Recent Advances in the Synthesis of β-Carboline Alkaloids. Agents. Molecules 2021, 27, 663. [Google Scholar] [CrossRef]
- Rao, R.N.; Maiti, B.; Chanda, K. Application of Pictet–Spengler Reaction to Indole-Based Alkaloids Containing Tetrahydro-β-carboline Scaffold in Combinatorial Chemistry. ACS Comb. Sci. 2017, 19, 4–199. [Google Scholar] [CrossRef]
- Sakurai, T.; Chen, Z.; Yamahata, A.; Hayasaka, T.; Satoh, H.; Sekiguchi, H.; Chiba, H.; Hui, S.P. A Mouse Model of Short-Term, Diet-Induced Fatty Liver with Abnormal Cardiolipin Remodeling via Downregulated Tafazzin Gene Expression. J. Sci. Food Agric. 2021, 101, 4995–5001. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibwe, D.F.; Oba, S.; Takeishi, N.; Sakurai, T.; Tsukui, T.; Chiba, H.; Hui, S.-P. Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes. Pharmaceuticals 2022, 15, 578. https://doi.org/10.3390/ph15050578
Dibwe DF, Oba S, Takeishi N, Sakurai T, Tsukui T, Chiba H, Hui S-P. Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes. Pharmaceuticals. 2022; 15(5):578. https://doi.org/10.3390/ph15050578
Chicago/Turabian StyleDibwe, Dya Fita, Saki Oba, Nire Takeishi, Toshihiro Sakurai, Takayuki Tsukui, Hitoshi Chiba, and Shu-Ping Hui. 2022. "Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes" Pharmaceuticals 15, no. 5: 578. https://doi.org/10.3390/ph15050578
APA StyleDibwe, D. F., Oba, S., Takeishi, N., Sakurai, T., Tsukui, T., Chiba, H., & Hui, S.-P. (2022). Food-Derived β-Carboline Alkaloids Ameliorate Lipid Droplet Accumulation in Human Hepatocytes. Pharmaceuticals, 15(5), 578. https://doi.org/10.3390/ph15050578