Effects of the Prior Use of Statins on Head and Neck Cancer Risk: A Hospital-Based Case–Control Study
Abstract
1. Introduction
2. Results
2.1. Characteristics of Cases and Controls and Factors Related to Head and Neck Cancer
2.2. Cancer History of Patients Diagnosed with Head and Neck Cancer
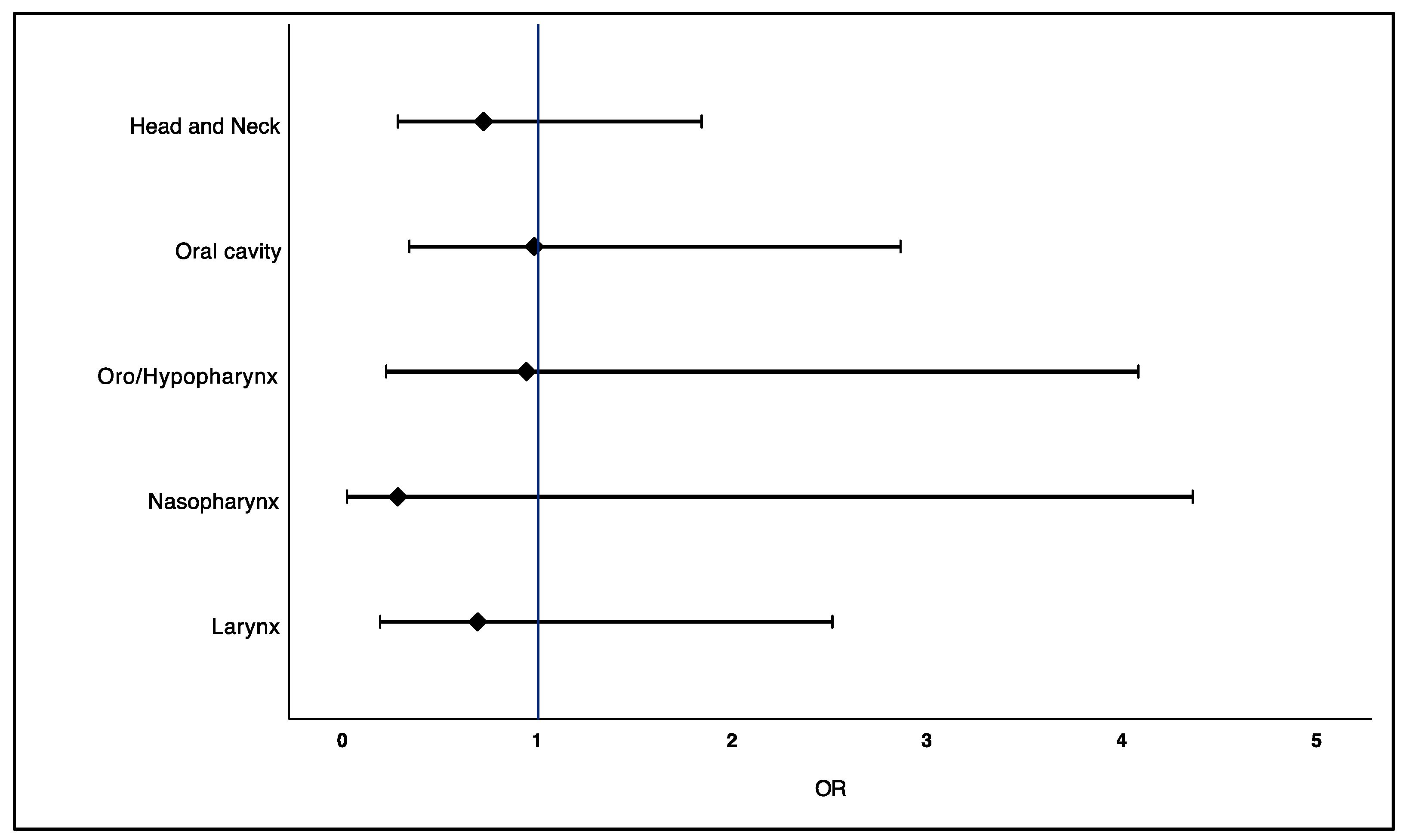
2.3. Association between Prior Use of Statins and Risk of Head and Neck Cancer
2.4. Association between Prior Use of Statins and Recurrence of Head and Neck Cancer
3. Discussion
4. Materials and Methods
4.1. Design and Study Population
4.2. Definition of Cases and Controls
4.3. Data Source, Identification of Cases and Controls and Recruitment
4.4. Sample Size
4.5. Data Collection
4.6. Exposure: Statins
4.7. Covariates
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehnavi, S.; Kiani, A.; Sadeghi, M.; Biregani, A.F.; Banach, M.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A. Targeting AMPK by Statins: A Potential Therapeutic Approach. Drugs 2021, 81, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Banno, K.; Kunitomi, H.; Nagai, S.; Takahashi, T.; Anko, M.; Iijima, M.; Takeda, T.; Matoba, Y.; Nakamura, K.; et al. Is antidyslipidemic statin use for cancer prevention a promising drug repositioning approach? Eur. J. Cancer Prev. 2019, 28, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Cuello, F.M.; Kato, C.S.; Díaz, S.D.; Owen, G. Efectos de las estatinas en cáncer: ¿potencial rol en terapéutica y prevención? Rev. Med. Chil. 2013, 141, 227–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zahra Bathaie, S.; Ashrafi, M.; Azizian, M.; Tamanoi, F. Mevalonate Pathway and Human Cancers. Curr. Mol. Pharmacol. 2017, 10, 77–85. [Google Scholar]
- Jakobisiak, M.; Golab, J. Potential antitumor effects of statins (Review). Int. J. Oncol. 2003, 23, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Loke, Y.; Beales, I.L.P. Systematic review and meta-analysis: Use of statins is associated with a reduced incidence of oesophageal adenocarcinoma. J. Gastrointest. Cancer 2018, 49, 442–454. [Google Scholar] [CrossRef]
- Nayan, M.; Punjani, N.; Juurlink, D.N.; Finelli, A.; Austin, P.C.; Kulkarni, G.S.; Uleryk, E.; Hamilton, R.J. Statin use and kidney cancer survival outcomes: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 52, 105–116. [Google Scholar] [CrossRef]
- Gray, R.T.; Coleman, H.G.; Hughes, C.; Murray, L.J.; Cardwell, C.R. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol. 2016, 45, 71–81. [Google Scholar] [CrossRef]
- Manthravadi, S.; Shrestha, A.; Madhusudhana, S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J. Cancer 2016, 139, 1281–1288. [Google Scholar] [CrossRef]
- Pavan, L.M.C.; Rêgo, D.F.; Elias, S.T.; De Luca Canto, G.; Guerra, E.N.S. In vitro anti-tumor effects of statins on head and neck squamous cell carcinoma: A systematic review. PLoS ONE 2015, 10, e0130476. [Google Scholar] [CrossRef]
- Ma, L.; Niknejad, N.; Gorn-Hondermann, I.; Dayekh, K.; Dimitroulakos, J. Lovastatin Induces Multiple Stress Pathways Including LKB1/AMPK Activation That Regulate Its Cytotoxic Effects in Squamous Cell Carcinoma Cells. PLoS ONE 2012, 7, e46055. [Google Scholar] [CrossRef] [PubMed]
- Takeda, I.; Maruya, S.I.; Shirasaki, T.; Mizukami, H.; Takahata, T.; Myers, J.N.; Kakehata, S.; Yagihashi, S.; Shinkawa, H. Simvastatin inactivates β1-integrin and extracellular signal-related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer Sci. 2007, 98, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, N.; Gorn-Hondermann, I.; Ma, L.; Zahr, S.; Johnson-Obeseki, S.; Corsten, M.; Dimitroulakos, J. Lovastatin-induced apoptosis is mediated by activating transcription factor 3 and enhanced in combination with salubrinal. Int. J. Cancer 2014, 134, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Mantha, A.J.; Hanson, J.E.L.; Goss, G.; Lagarde, A.E.; Lorimer, I.A.; Dimitroulakos, J. Targeting the mevalonate pathway inhibits the function of the epidermal growth factor receptor. Clin. Cancer Res. 2005, 11, 2398–2407. [Google Scholar] [CrossRef]
- Gabryś, D.; Dörfler, A.; Yaromina, A.; Hessel, F.; Krause, M.; Oertel, R.; Baumann, M. Effects of lovastatin alone or combined with irradiation on tumor cells in vitro and in vivo. Strahlenther. Onkol. 2008, 184, 48–53. [Google Scholar] [CrossRef]
- Saka Herrán, C.; Jané-Salas, E.; Estrugo Devesa, A.; López-López, J. Protective effects of metformin, statins and anti-inflammatory drugs on head and neck cancer: A systematic review. Oral Oncol. 2018, 85, 68–81. [Google Scholar] [CrossRef]
- Kao, L.T.; Hung, S.H.; Kao, P.F.; Liu, J.C.; Lin, H.C. Inverse association between statin use and head and neck cancer: Population-based case-control study in Han population. Head Neck 2019, 41, 1193–1198. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Wang, K.; Gerke, T.A.; Chen, X.; Prosperi, M. Association of statin use with risk of Gleason score-specific prostate cancer: A hospital-based cohort study. Cancer Med. 2019, 8, 7399–7407. [Google Scholar] [CrossRef]
- Chiu, H.F.; Ho, S.C.; Chang, C.C.; Wu, T.N.; Yang, C.Y. Statins are associated with a reduced risk of gastric cancer: A population-based case-control study. Am. J. Gastroenterol. 2011, 106, 2098–2103. [Google Scholar] [CrossRef]
- Kim, G.; Jang, S.Y.; Nam, C.M.; Kang, E.S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 2018, 68, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Cagan, A.; Cai, T.; Gainer, V.S.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; Liao, K.P.; Kohane, I. Statin use is associated with reduced risk of colorectal cancer in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2016, 14, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Baandrup, L.; Dehlendorff, C.; Friis, S.; Olsen, J.H.; Kjær, S.K. Statin use and risk for ovarian cancer: A Danish nationwide case-control study. Br. J. Cancer 2015, 112, 157–161. [Google Scholar] [CrossRef]
- Sperling, C.D.; Verdoodt, F.; Friis, S.; Dehlendorff, C.; Kjaer, S.K. Statin use and risk of endometrial cancer: A nationwide registry-based case–control study. Acta Obstet. Gynecol. Scand. 2017, 96, 144–149. [Google Scholar] [CrossRef]
- Dale, K.M.; Coleman, C.I.; Henyan, N.N.; Kluger, J.; White, M. Statins and cancer risk: A meta-analysis. JAMA 2006, 295, 74–80. [Google Scholar] [CrossRef] [PubMed]
- CTT Collaboration Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012, 7, e29849. [CrossRef]
- Farooqi, M.A.M.; Malhotra, N.; Mukherjee, S.D.; Sanger, S.; Dhesy-Thind, S.K.; Ellis, P.; Leong, D.P. Statin therapy in the treatment of active cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0209486. [Google Scholar] [CrossRef]
- Dwojak, S.; Bhattacharyya, N. Racial disparities in preventable risk factors for head and neck cancer. Laryngoscope 2017, 127, 1068–1072. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 491. [Google Scholar] [CrossRef]
- Tan, P.; Zhang, C.; Wei, S.Y.; Tang, Z.; Gao, L.; Yang, L.; Wei, Q. Effect of statins type on incident prostate cancer risk: A meta-analysis and systematic review. Asian J. Androl. 2017, 19, 666–671. [Google Scholar] [CrossRef]
- Gormley, M.; Yarmolinsky, J.; Dudding, T.; Burrows, K.; Martin, R.M.; Thomas, S.; Tyrrell, J.; Brennan, P.; Pring, M.; Boccia, S.; et al. Using genetic variants to evaluate the causal effect of cholesterol lowering on head and neck cancer risk: A Mendelian randomization study. PLoS Genet. 2021, 17, e1009525. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.; Garcia-Albeniz, X.; Logan, R.; Denaxas, S.; Hernan, M. Avoidable flaws in observational analyses: An application to statins and cancer. Nat. Med. 2019, 25, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Stokes, W.; Eguchi, M.; Hararah, M.; Amini, A.; Mueller, A.; Morgan, R.; Bradley, C.; Raben, D.; McDermott, J.D.; et al. Statin use associated with improved overall and cancer specific survival in patients with head and neck cancer. Oral Oncol. 2019, 90, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Lebo, N.L.; Griffiths, R.; Hall, S.; Dimitroulakos, J.; Johnson-Obaseki, S. Effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head Neck 2018, 40, 1697–1706. [Google Scholar] [CrossRef]
- Bravi, F.; Lee, Y.C.A.; Hashibe, M.; Boffetta, P.; Conway, D.I.; Ferraroni, M.; La Vecchia, C.; Edefonti, V.; Agudo, A.; Ahrens, W.; et al. Lessons learned from the INHANCE consortium: An overview of recent results on head and neck cancer. Oral Dis. 2021, 27, 73–93. [Google Scholar] [CrossRef]
- Brennan, S.; Baird, A.M.; O’Regan, E.; Sheils, O. The Role of Human Papilloma Virus in Dictating Outcomes in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2021, 8, 468. [Google Scholar] [CrossRef]
- Getz, K.R.; Bellile, E.; Zarins, K.R.; Rullman, C.; Chinn, S.B.; Taylor, J.M.G.; Rozek, L.S.; Wolf, G.T.; Mondul, A.M. Statin use and head and neck squamous cell carcinoma outcomes. Int. J. Cancer 2021, 148, 2440–2448. [Google Scholar] [CrossRef]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated Head and Neck Cancer: A Virus-related Cancer Epidemic—A Review of Epidemiology, Biology, Virus Detection and Issues in Management. Lancet Oncol. 2010, 176, 139–148. [Google Scholar] [CrossRef]
- Lewis, J.S.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human papillomavirus testing in head and neck carcinomas guideline from the college of American pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef]
- Ceacareanu, A.; Jolly, S.; Nimako, G.; Wintrob, Z.P. Statin type and cancer outcomes in patients with diabetes type 2 and solid tumors. J. Res. Pharm. Pract. 2021, 10, 50. [Google Scholar] [CrossRef]
- Beckwitt, C.H.; Shiraha, K.; Wells, A. Lipophilic statins limit cancer cell growth and survival, via involvement of Akt signaling. PLoS ONE 2018, 13, e0197422. [Google Scholar] [CrossRef] [PubMed]
- de Moura, A.C.; Assad, D.X.; Amorim dos Santos, J.; Porto de Toledo, I.; Barra, G.B.; Castilho, R.M.; Squarize, C.H.; Guerra, E.N.S. Worldwide prevalence of PI3K-AKT-mTOR pathway mutations in head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103284. [Google Scholar] [CrossRef] [PubMed]
- Warita, K.; Warita, T.; Beckwitt, C.H.; Schurdak, M.E.; Vazquez, A.; Wells, A.; Oltvai, Z.N. Statin-induced mevalonate pathway inhibition attenuates the growth of mesenchymal-like cancer cells that lack functional E-cadherin mediated cell cohesion. Sci. Rep. 2014, 4, 7593. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, nutrition, physical activity and cancers of the mouth, pharynx and larynx. In Continuous Update Project Report; Continuous Update Project: London, UK, 2018; pp. 1–67. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/mouth-pharynx-larynx-cancer-report.pdf (accessed on 3 April 2022).
- de Oliveira Figueiredo, R.A.; Weiderpass, E.; Tajara, E.H.; Ström, P.; Carvalho, A.L.; de Carvalho, M.B.; Kanda, J.L.; Moyses, R.A.; Wünsch-Filho, V. Diabetes mellitus, metformin and head and neck cancer. Oral Oncol. 2016, 61, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, F.; Ros, E.; Solá, R.; Godás, G.; Pérez-Heras, A.; Serra, M.; Mostaza, J.; Pintó, X. Consejos para ayudar a controlar el colesterol con una alimentación saludable. Clín. Investig. Arterioscler. 2006, 18, 104–110. [Google Scholar] [CrossRef]
| Characteristics | Total Sample (n = 202) n (%) | Cases (n = 101) n (%) | Controls (n = 101) n (%) | Adjusted OR † (95% CI) | p Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 138 (68.3) | 69 (68.3) | 69 (68.3) | ||
| Female | 64 (31.7) | 32 (31.7) | 32 (31.7) | ||
| Age (years) | 66 ± 10.2 * | 65.5 ± 9.8 * | 0.73 | ||
| 44–54 years | 30 (14.9) | 15 (14.9) | 15 (14.9) | ||
| 55–64 years | 58 (28.7) | 29 (28.7) | 29 (28.7) | ||
| ≥65 years | 114 (56.4) | 57 (56.4) | 57 (56.4) | ||
| Education | |||||
| Without education | 11 (5.6) | 9 (9.4) | 2 (2) | 2.58 (0.35–18.7) | 0.61 |
| Primary education | 101 (51.3) | 52 (54.2) | 49 (48.5) | 0.62 (0.22–1.78) | 0.39 |
| Secondary education | 43 (21.8) | 23 (24) | 20 (19.8) | 1.57 (0.52–4.74) | 0.98 |
| Higher education | 42 (21.3) | 12 (12.5) | 30 (29.7) | Reference | |
| Monthly income | |||||
| Low income | 71 (39) | 48 (59.3) | 23 (22.8) | 39.4 (4.08–381.3) | 0.03 |
| Middle income | 93 (51.1) | 32 (39.5) | 61 (60.4) | 8.86 (1.01–78.0) | 0.04 |
| High income | 18 (9.9) | 1 (1.2) | 17 (16.8) | Reference | |
| Smoking status | |||||
| Current smoker | 28 (13.9) | 12 (11.9) | 16 (15.8) | 0.76 (0.23–2.56) | 0.44 |
| Occasional smoker (<1 cigarette/day) | 4 (2) | 1 (1) | 3 (3) | 1.98 (0.16–23.9) | 0.58 |
| Ex-smoker | 91 (45) | 59 (58.4) | 32 (31.7) | 2.36 (1.01–5.57) | 0.03 |
| Never smoked | 79 (39.1) | 29 (28.7) | 50 (49.5) | Reference | |
| Cigarettes/day in smokers and ex-smokers | |||||
| >20 cigarettes/day | 47 (54) | 40 (69) | 7 (24.1) | 8.66 (1.38–54.2) | 0.02 |
| 10–20 cigarettes/day | 15 (17.2) | 6 (10.3) | 9 (31) | 0.38 (0.04–3.26) | 0.16 |
| 5–10 cigarettes/day | 11 (12.6) | 6 (10.3) | 5 (17.2) | 1.92 (0.22–16.4) | 0.82 |
| <5 cigarettes/day | 14 (16.1) | 6 (10.3) | 8 (27.6) | Reference | |
| Alcohol consumption | |||||
| Excessive alcohol consumption | 28 (13.9) | 24 (23.8) | 4 (4) | 10.1 (2.10–49.3) | 0.01 |
| Moderate alcohol consumption | 111 (55.2) | 40 (39.6) | 71 (71) | 1.04 (0.36–2.98) | 0.82 |
| Ex-consumer of alcoholic beverages | 16 (8) | 13 (12.9) | 3 (3) | 3.62 (0.69–18.9) | 0.28 |
| Alcohol never consumed | 46 (22.9) | 24 (23.8) | 22 (22) | Reference | |
| Comorbidities | |||||
| Diabetes Mellitus 2 | 36 (17.8) | 12 (11.9) | 24 (23.8) | 0.69 (0.26–1.82) | 0.62 |
| High blood pressure | 88 (43.6) | 39 (38.6) | 49 (48.5) | 0.90 (0.19–4.36) | 0.87 |
| Cardiovascular disease | 32 (15.8) | 21 (20.8) | 11 (10.9) | 1.66 (0.23–12.2) | 0.61 |
| Physical activity | |||||
| ≥3 times per week | 64 (34.2) | 26 (30.2) | 38 (37.6) | 0.42 (0.16–1.11) | 0.16 |
| 1–2 times per week | 55 (29.4) | 19 (22.1) | 36 (35.6) | 0.46 (0.18–1.21) | 0.14 |
| No practice of physical activity | 68 (36.4) | 41 (47.7) | 27 (26.7) | Reference | |
| Diet | |||||
| Healthy diet | 49 (27.2) | 10 (12.7) | 39 (38.6) | 0.29 (0.10–0.84) | 0.005 |
| Regular diet | 72 (40) | 32 (40.5) | 40 (39.6) | 0.77 (0.32–1.86) | 0.38 |
| Unhealthy diet | 59 (32.8) | 37 (46.8) | 22 (21.8) | Reference |
| Oncological History | Cases (n = 101) |
|---|---|
| Topographic location | |
| Oral cavity | 49.5% |
| Oro/Hypopharynx | 18.8% |
| Nasopharynx | 5% |
| Larynx | 26.7% |
| TNM staging | |
| I | 9.9% |
| II | 10.9% |
| III | 22.8% |
| IVA | 33.7% |
| IVB | 5.9% |
| HPV status | |
| HPV (+) | 3% |
| HPV (−) | 3% |
| Unknown | 94.1% |
| Cancer treatment | |
| Surgery | 15.8% |
| Surgery + radiotherapy | 20.8% |
| Induction chemotherapy + surgery + radiotherapy | 17.8% |
| Induction chemotherapy + radiotherapy | 37.6% |
| Radiotherapy + biological therapy (cetuximab) | 5.9% |
| Other (brachytherapy) | 2.0% |
| Recurrence rate | 13.9% |
| Local recurrence | 8.9% |
| Metastasis | 3.9% |
| Topographic Location | ||||
|---|---|---|---|---|
| TNM Staging | Oral Cavity (n = 50) n (%) | Oro/Hypopharynx (n = 19) n (%) | Nasopharynx (n = 5) n (%) | Larynx (n = 27) n (%) |
| I or II | 14 (33.3) | 5 (33.3) | 0 (0) | 2 (9.1) |
| III or IV | 28 (66.7) | 10 (66.7) | 5 (100) | 20 (90.9) |
| Statin Exposure | Total Sample (n = 202) n (%) | Cases (n = 101) n (%) | Controls (n = 101) n (%) | Crude OR (95% CI) n (%) | Adjusted OR † (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Overall | ||||||
| Non-use of statins | 145 (71.8) | 75 (74.3) | 70 (69.3) | Reference | Reference | |
| Regular use of statins | 57 (28.2) | 26 (25.7) | 31 (30.7) | 0.78 (0.42–1.45) | 0.72 (0.28–1.84) | 0.49 |
| Type of statins | ||||||
| Lipophilic | 51 (25.2) | 22 (21.8) | 29 (28.7) | 0.71 (0.37–1.34) | 0.71 (0.27–1.86) | 0.49 |
| Hydrophilic | 6 (3) | 4 (4) | 2 (2) | 1.86 (0.33–10.5) | 0.82 (0.06–10.9) | 0.88 |
| Daily dose | ||||||
| ≤10 mg/day | 12 (5.9) | 3 (3) | 9 (8.9) | 0.31 (0.09–1.19) | 0.39 (0.06–2.67) | 0.34 |
| 20 mg/day | 23 (11.4) | 9 (8.9) | 14 (13.9) | 0.60 (0.24–1.47) | 0.62 (0.16–2.37) | 0.48 |
| ≥40 mg/day | 22 (10.9) | 14 (13.9) | 8 (7.9) | 1.63 (0.65–4.13) | 1.13 (0.30–4.22) | 0.85 |
| Time (years) | 11.4 ± 8.3 * | 10 ± 9.1 * | 12.7 ± 7.5 * | 0.96 (0.89–1.03) | 0.85 (0.69–1.04) | 0.11 |
| Statin Exposure | Recurrence (n = 14) n (%) | No Recurrence (n = 79) n (%) | Crude OR (95% CI) | Adjusted OR † (95% CI) | p Value |
|---|---|---|---|---|---|
| Overall | |||||
| Non-use of statins | 10 (71.4) | 50 (74.7) | Reference | Reference | |
| Regular use of statins | 4 (28.6) | 20 (25.3) | 1.18 (0.33–4.18) | 0.80 (0.15–4.19) | 0.79 |
| Type of statin | |||||
| Lipophilic | 4 (28.6) | 17 (21.5) | 1.39 (0.39–4.98) | 0.91 (0.17–4.95) | 0.91 |
| Hydrophilic | 0 (0) | 3 (3.8) | Not estimable | Not estimable | |
| Daily dose | |||||
| ≤10 mg/day | 1 (7.1) | 1 (1.3) | 5.90 (0.34–102.1) | 4.43 (0.12–168.2) | 0.42 |
| 20 mg/day | 2 (14.3) | 6 (7.6) | 1.96 (0.35–11.1) | 1.98 (0.14–28.5) | 0.61 |
| ≥40 mg/day | 1 (7.1) | 13 (16.5) | 0.45 (0.05–3.86) | 0.32 (0.03–3.48) | 0.32 |
| Time (years) | 10 ± 8.4 | 10.2 ± 9.6 | 0.99 (0.85–1.17) | 0.99 (0.82–1.20) | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saka-Herrán, C.; Jané-Salas, E.; Mano-Azul, A.; Torrejón-Moya, A.; Estrugo-Devesa, A.; López-López, J. Effects of the Prior Use of Statins on Head and Neck Cancer Risk: A Hospital-Based Case–Control Study. Pharmaceuticals 2022, 15, 579. https://doi.org/10.3390/ph15050579
Saka-Herrán C, Jané-Salas E, Mano-Azul A, Torrejón-Moya A, Estrugo-Devesa A, López-López J. Effects of the Prior Use of Statins on Head and Neck Cancer Risk: A Hospital-Based Case–Control Study. Pharmaceuticals. 2022; 15(5):579. https://doi.org/10.3390/ph15050579
Chicago/Turabian StyleSaka-Herrán, Constanza, Enric Jané-Salas, Antonio Mano-Azul, Aina Torrejón-Moya, Albert Estrugo-Devesa, and José López-López. 2022. "Effects of the Prior Use of Statins on Head and Neck Cancer Risk: A Hospital-Based Case–Control Study" Pharmaceuticals 15, no. 5: 579. https://doi.org/10.3390/ph15050579
APA StyleSaka-Herrán, C., Jané-Salas, E., Mano-Azul, A., Torrejón-Moya, A., Estrugo-Devesa, A., & López-López, J. (2022). Effects of the Prior Use of Statins on Head and Neck Cancer Risk: A Hospital-Based Case–Control Study. Pharmaceuticals, 15(5), 579. https://doi.org/10.3390/ph15050579








