Significant Sex Differences in the Efficacy of the CSF1R Inhibitor-PLX5622 on Rat Brain Microglia Elimination
Abstract
:1. Introduction
2. Results
2.1. Steady-State Densities and Morphological Phenotypes of Microglia in the Brains of Female and Male Rat
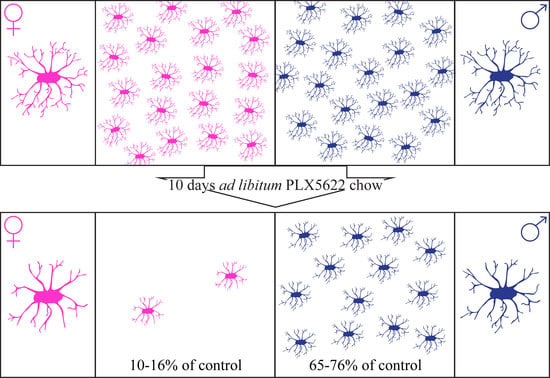
2.2. Sex-Related Microglia Elimination and Morphological Alterations of the Surviving Microglia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Processing for Immunohistology
4.3. Cryosectioning and Immunohistological Labeling
4.4. Microscopy
4.5. Image Processing, Analysis, and Statistics
4.6. Microglia’s Morphology Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, J.M.; Bilbo, S.D. Sex, glia, and development: Interactions in health and disease. Horm. Behav. 2012, 62, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017, 65, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; Garel, S. On place and time: Microglia in embryonic and perinatal brain development. Curr. Opin. Neurobiol. 2017, 47, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Cowan, M.; Petri, W.A., Jr. Microglia: Immune Regulators of Neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Della Torre, S.; Maggi, A. Sexual differentiation of microglia. Front. Neuroendocrinol. 2019, 52, 156–164. [Google Scholar] [CrossRef]
- Bordt, E.A.; Ceasrine, A.M.; Bilbo, S.D. Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors. Glia 2020, 68, 1085–1099. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Verdonk, F.; Roux, P.; Flamant, P.; Fiette, L.; Bozza, F.A.; Simard, S.; Lemaire, M.; Plaud, B.; Shorte, S.L.; Sharshar, T.; et al. Phenotypic clustering: A novel method for microglial morphology analysis. J. Neuroinflamm. 2016, 13, 153. [Google Scholar] [CrossRef] [Green Version]
- Fuger, P.; Hefendehl, J.K.; Veeraraghavalu, K.; Wendeln, A.C.; Schlosser, C.; Obermuller, U.; Wegenast-Braun, B.M.; Neher, J.J.; Martus, P.; Kohsaka, S.; et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 2017, 20, 1371–1376. [Google Scholar] [CrossRef]
- Sagar; Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernandez-Klett, F.; Lin, G.; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef]
- Zhan, L.; Krabbe, G.; Du, F.; Jones, I.; Reichert, M.C.; Telpoukhovskaia, M.; Kodama, L.; Wang, C.; Cho, S.H.; Sayed, F.; et al. Proximal recolonization by self-renewing microglia re-establishes microglial homeostasis in the adult mouse brain. PLoS Biol. 2019, 17, e3000134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hume, D.A.; Caruso, M.; Ferrari-Cestari, M.; Summers, K.M.; Pridans, C.; Irvine, K.M. Phenotypic impacts of CSF1R deficiencies in humans and model organisms. J. Leukoc. Biol. 2020, 107, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Green, K.N.; Crapser, J.D.; Hohs, L.A. To Kill a Microglia: A Case for CSF1R Inhibitors. Trends Immunol. 2020, 41, 771–784. [Google Scholar] [CrossRef]
- Eme-Scolan, E.; Dando, S.J. Tools and Approaches for Studying Microglia In vivo. Front. Immunol. 2020, 11, 583647. [Google Scholar] [CrossRef]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [Green Version]
- Elmore, M.R.; Lee, R.J.; West, B.L.; Green, K.N. Characterizing newly repopulated microglia in the adult mouse: Impacts on animal behavior, cell morphology, and neuroinflammation. PLoS ONE 2015, 10, e0122912. [Google Scholar] [CrossRef]
- Spangenberg, E.; Severson, P.L.; Hohsfield, L.A.; Crapser, J.; Zhang, J.; Burton, E.A.; Zhang, Y.; Spevak, W.; Lin, J.; Phan, N.Y.; et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019, 10, 3758. [Google Scholar] [CrossRef]
- Dagher, N.N.; Najafi, A.R.; Kayala, K.M.; Elmore, M.R.; White, T.E.; Medeiros, R.; West, B.L.; Green, K.N. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflamm. 2015, 12, 139. [Google Scholar] [CrossRef] [Green Version]
- Najafi, A.R.; Crapser, J.; Jiang, S.; Ng, W.; Mortazavi, A.; West, B.L.; Green, K.N. A limited capacity for microglial repopulation in the adult brain. Glia 2018, 66, 2385–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Given, K.S.; Dickson, E.L.; Owens, G.P.; Macklin, W.B.; Bennett, J.L. Concentration-dependent effects of CSF1R inhibitors on oligodendrocyte progenitor cells ex vivo and in vivo. Exp. Neurol. 2019, 318, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Easley-Neal, C.; Foreman, O.; Sharma, N.; Zarrin, A.A.; Weimer, R.M. CSF1R Ligands IL-34 and CSF1 Are Differentially Required for Microglia Development and Maintenance in White and Gray Matter Brain Regions. Front. Immunol. 2019, 10, 2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Models Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Homberg, J.R.; Wohr, M.; Alenina, N. Comeback of the Rat in Biomedical Research. ACS Chem. Neurosci. 2017, 8, 900–903. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Irvine, K.M.; Hume, D.A. Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Riquier, A.; Sollars, S.I. Astrocytic response to neural injury is larger during development than inadulthood and is not predicated upon the presence of microglia. Brain Behav. Immun. Health 2020, 1, 100010. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.L.; Yang, Q.; Zhou, X.; Tang, S.J. Microglial ablation does not affect opioid-induced hyperalgesia in rodents. Pain 2021, 163, 508–517. [Google Scholar] [CrossRef]
- Sharon, A.; Jankowski, M.M.; Shmoel, N.; Erez, H.; Spira, M.E. Inflammatory Foreign Body Response Induced by Neuro-Implants in Rat Cortices Depleted of Resident Microglia by a CSF1R Inhibitor and Its Implications. Front. Neurosci. 2021, 15, 646914. [Google Scholar] [CrossRef]
- Huang, S.H.; Shmoel, N.; Jankowski, M.M.; Erez, H.; Sharon, A.; Abu-Salah, W.; Nelken, I.; Weiss, A.; Spira, M.E. Immunohistological and Ultrastructural Study of the Inflammatory Response to Perforated Polyimide Cortical Implants: Mechanisms Underlying Deterioration of Electrophysiological Recording Quality. Front. Neurosci. 2020, 14, 926. [Google Scholar] [CrossRef]
- Tan, Y.L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Guneykaya, D.; Ivanov, A.; Hernandez, D.P.; Haage, V.; Wojtas, B.; Meyer, N.; Maricos, M.; Jordan, P.; Buonfiglioli, A.; Gielniewski, B.; et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018, 24, 2773–2783.e2776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Arjona, M.D.M.; Grondona, J.M.; Granados-Duran, P.; Fernandez-Llebrez, P.; Lopez-Avalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Biase, L.M.; Bonci, A. Region-Specific Phenotypes of Microglia: The Role of Local Regulatory Cues. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2019, 25, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Patkar, O.L.; Caruso, M.; Teakle, N.; Keshvari, S.; Bush, S.J.; Pridans, C.; Belmer, A.; Summers, K.M.; Irvine, K.M.; Hume, D.A. Analysis of homozygous and heterozygous Csf1r knockout in the rat as a model for understanding microglial function in brain development and the impacts of human CSF1R mutations. Neurobiol. Dis. 2021, 151. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Vallotton, P.; Lagerstrom, R.; Sun, C.; Buckley, M.; Wang, D.; De Silva, M.; Tan, S.S.; Gunnersen, J.M. Automated analysis of neurite branching in cultured cortical neurons using HCA-Vision. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lagerstrom, R.; Sun, C.; Bishof, L.; Valotton, P.; Gotte, M. HCA-vision: Automated neurite outgrowth analysis. J. Biomol. Screen. 2010, 15, 1165–1170. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharon, A.; Erez, H.; Spira, M.E. Significant Sex Differences in the Efficacy of the CSF1R Inhibitor-PLX5622 on Rat Brain Microglia Elimination. Pharmaceuticals 2022, 15, 569. https://doi.org/10.3390/ph15050569
Sharon A, Erez H, Spira ME. Significant Sex Differences in the Efficacy of the CSF1R Inhibitor-PLX5622 on Rat Brain Microglia Elimination. Pharmaceuticals. 2022; 15(5):569. https://doi.org/10.3390/ph15050569
Chicago/Turabian StyleSharon, Aviv, Hadas Erez, and Micha E. Spira. 2022. "Significant Sex Differences in the Efficacy of the CSF1R Inhibitor-PLX5622 on Rat Brain Microglia Elimination" Pharmaceuticals 15, no. 5: 569. https://doi.org/10.3390/ph15050569
APA StyleSharon, A., Erez, H., & Spira, M. E. (2022). Significant Sex Differences in the Efficacy of the CSF1R Inhibitor-PLX5622 on Rat Brain Microglia Elimination. Pharmaceuticals, 15(5), 569. https://doi.org/10.3390/ph15050569