Cobalt Bis-Dicarbollide Enhances Antibiotics Action towards Staphylococcus epidermidis Planktonic Growth Due to Cell Envelopes Disruption
Abstract
:1. Introduction
2. Results
2.1. Effect of Antibiotics or COSAN.Na on Planktonic Growth of S. epidermidis
2.2. Combined Effect of Antibiotics with COSAN.Na on Planktonic Growth of S. epidermidis
2.3. Cytotoxicity of Antibiotics and COSAN.Na Alone or in Their Combination
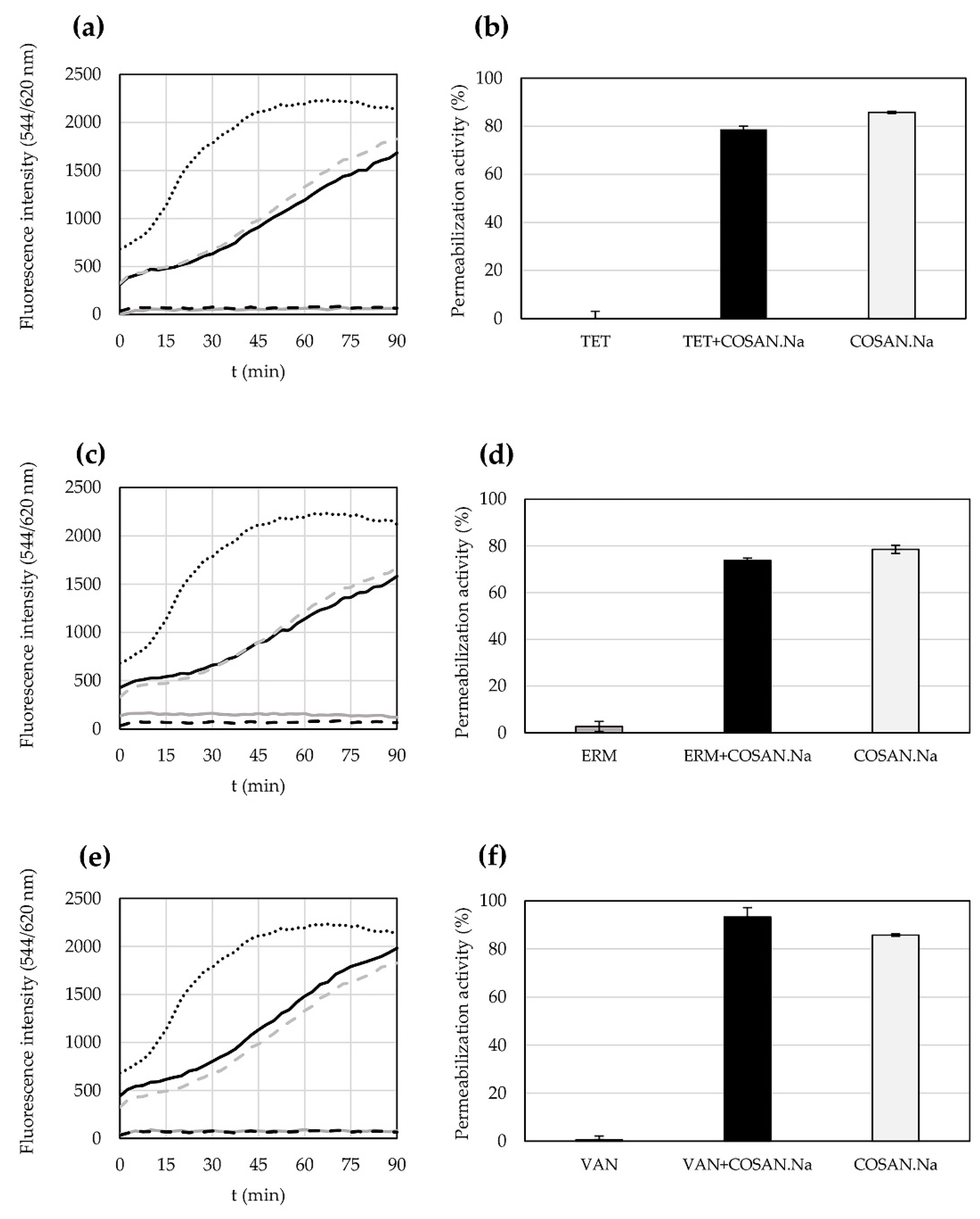
2.4. Permeabilization Activity of Antibiotics and COSAN.Na Alone or in Their Combination against S. epidermidis CNCTC 5671 Cells
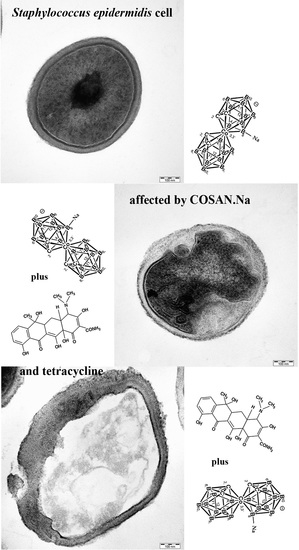
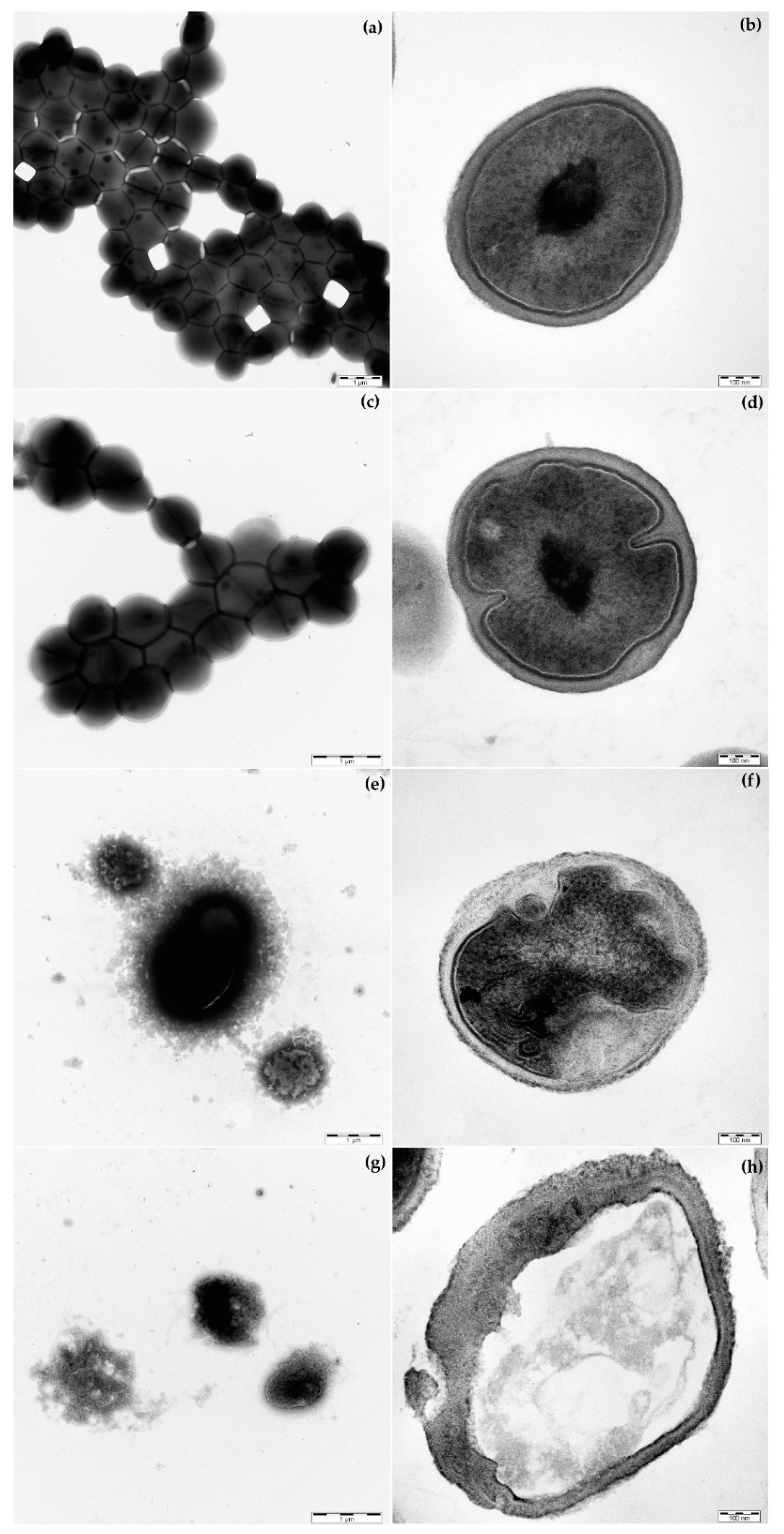
2.5. Morphology of S. epidermidis CNCTC 5671 Cells Affected by TET, COSAN.Na or Their Combination
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. Antimicrobial Agents
4.3. Antimicrobial Assay
4.4. Cytotoxicity Assay
4.5. Propidium Iodide Uptake Assay
4.6. Transmission Electron Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watnick, P.; Kolter, R. Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, M.; Padyana, S.; Dipin, K.; Kumar, S.; Nayak, B.; Varela, M. Antimicrobial compounds of plant origin as efflux pump inhibitors: New avenues for controlling multidrug resistant pathogens. J. Antimicrob. Agents 2018, 4, 1000159. [Google Scholar] [CrossRef]
- Yusuf, M. Natural antimicrobial agents for food biopreservation. In Food Packaging and Preservation; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 12; pp. 409–438. [Google Scholar] [CrossRef]
- Pizzolato-Cezar, L.R.; Okuda-Shinagawa, N.M.; Machini, M.T. Combinatory therapy antimicrobial peptide-antibiotic to minimize the ongoing rise of resistance. Front. Microbiol. 2019, 10, 1703. [Google Scholar] [CrossRef] [Green Version]
- Vaňková, E.; Kašparová, P.; Dulíčková, N.; Čeřovský, V. Combined effect of lasioglossin LL-III derivative with azoles against Candida albicans virulence factors: Biofilm formation, phospholipases, proteases and hemolytic activity. FEMS Yeast Res. 2020, 20, foaa020. [Google Scholar] [CrossRef]
- Kašparová, P.; Vaňková, E.; Brázdová, L.; Lokočová, K.; Maťátková, O.; Masák, J. Antibiofilm agent pterostilbene is able to enhance antibiotics action against Staphylococcus epidermidis. Microb. Pathog. 2021, 152, 104632. [Google Scholar] [CrossRef]
- Ishak, S.F.; Ghazali, A.R.; Zin, N.M.; Basri, D.F. Pterostilbene enhanced anti-methicillin resistant Staphylococcus aureus (MRSA) activity of oxacillin. Am. J. Infect. Dis. 2016, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Med. Ther. 2015, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Kašparová, P.; Zmuda, M.; Vaňková, E.; Maťátková, O.; Masák, J. Low-molecular weight chitosan enhances antibacterial effect of antibiotics and permeabilizes cytoplasmic membrane of Staphylococcus epidermidis biofilm cells. Folia Microbiol. 2021, 66, 983–996. [Google Scholar] [CrossRef]
- Lokočová, K.; Maťátková, O.; Vaňková, E.; Kolouchová, I.; Čejková, A.; Masák, J. Synergistic Inhibitory Effect of Chitosan and Amphotericin B on Planktonic and Biofilm Populations of C. albicans, C. parapsilosis and C. krusei. Microbiology 2021, 90, 370–382. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and synergistic effects of commercial piperine and piperlongumine in combination with conventional antimicrobials. Antibiotics 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-K.; Yusoff, K.; Mai, C.-W.; Lim, W.-M.; Yap, W.-S.; Lim, S.-H.E.; Lai, K.-S. Additivity vs. synergism: Investigation of the additive interaction of cinnamon bark oil and meropenem in combinatory therapy. Molecules 2017, 22, 1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.-Q.; Zhao, W.-H.; Yoda, Y.; Asano, N.; Hara, Y.; Shimamura, T. Additive, indifferent and antagonistic effects in combinations of epigallocatechin gallate with 12 non-β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2002, 50, 1051–1054. [Google Scholar] [CrossRef] [Green Version]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-801894-1. [Google Scholar]
- Lesnikowski, Z.J. Boron units as pharmacophores-new applications and opportunities of boron cluster chemistry. Collect. Czechoslov. Chem. Commun. 2007, 72, 1646–1658. [Google Scholar] [CrossRef]
- Plešek, J.; Baše, K.; Mareš, F.; Hanousek, F.; Štíbr, B.; Heřmánek, S. Potential uses of metallocarborane sandwich anions for analysis, characterization and isolation of various cations and organic bases. Collect. Czechoslov. Chem. Commun. 1984, 49, 2776–2789. [Google Scholar] [CrossRef]
- Matějíček, P.; Cígler, P.; Procházka, K.; Král, V. Molecular assembly of metallacarboranes in water: Light scattering and microscopy study. Langmuir 2006, 22, 575–581. [Google Scholar] [CrossRef]
- Tarrés, M.; Canetta, E.; Paul, E.; Forbes, J.; Azzouni, K.; Vinas, C.; Teixidor, F.; Harwood, A.J. Biological interaction of living cells with COSAN-based synthetic vesicles. Sci. Rep. 2015, 5, 7804. [Google Scholar] [CrossRef]
- Tarrés, M.; Canetta, E.; Vinas, C.; Teixidor, F.; Harwood, A.J. Imaging in living cells using ν B–H Raman spectroscopy: Monitoring COSAN uptake. Chem. Comm. 2014, 50, 3370–3372. [Google Scholar] [CrossRef] [Green Version]
- Verdiá-Báguena, C.; Alcaraz, A.; Aguilella, V.M.; Cioran, A.M.; Tachikawa, S.; Nakamura, H.; Teixidor, F.; Viñas, C. Amphiphilic COSAN and I2-COSAN crossing synthetic lipid membranes: Planar bilayers and liposomes. Chem. Comm. 2014, 50, 6700–6703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goszczyński, T.M.; Fink, K.; Boratyński, J. Icosahedral boron clusters as modifying entities for biomolecules. Expert Opin. Biol. Ther. 2018, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Goszczyński, T.M.; Fink, K.; Kowalski, K.; Leśnikowski, Z.J.; Boratyński, J. Interactions of boron clusters and their derivatives with serum albumin. Sci. Rep. 2017, 7, 9800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Coord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Volovetsky, A.B.; Sukhov, V.S.; Balalaeva, I.V.; Dudenkova, V.V.; Shilyagina, N.Y.; Feofanov, A.V.; Efremenko, A.V.; Grin, M.A.; Mironov, A.F.; Sivaev, I.B.; et al. Pharmacokinetics of chlorin e6-cobalt bis (dicarbollide) conjugate in Balb/c mice with engrafted carcinoma. Int. J. Mol. Sci. 2017, 18, 2556. [Google Scholar] [CrossRef] [Green Version]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef] [PubMed]
- Cígler, P.; Kožíšek, M.; Řezáčová, P.; Brynda, J.; Otwinowski, Z.; Pokorná, J.; Plešek, J.; Grüner, B.; Dolečková-Marešová, L.; Máša, M.; et al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. PNAS 2005, 102, 15394–15399. [Google Scholar] [CrossRef] [Green Version]
- Grüner, B.; Kugler, M.; El Anwar, S.; Holub, J.; Nekvinda, J.; Bavol, D.; Růžičková, Z.; Pospíšilová, K.; Fábry, M.; Král, V.; et al. Cobalt bis (dicarbollide) alkylsulfonamides: Potent and highly selective inhibitors of tumor specific carbonic anhydrase IX. ChemPlusChem. 2021, 86, 352–363. [Google Scholar] [CrossRef]
- Kvasničková, E.; Masák, J.; Čejka, J.; Maťátková, O.; Šícha, V. Preparation, characterization, and the selective antimicrobial activity of N-alkylammonium 8-diethyleneglycol cobalt bis-dicarbollide derivatives. J. Organomet. Chem. 2017, 827, 23–31. [Google Scholar] [CrossRef]
- Benkocká, M.; Kolářová, K.; Matoušek, J.; Semerádtová, A.; Šícha, V.; Kolská, Z. Nanocomposite of polystyrene foil grafted with metallaboranes for antimicrobial activity. Appl. Surf. Sci. 2018, 441, 120–129. [Google Scholar] [CrossRef]
- Popova, T.; Zaulet, A.; Teixidor, F.; Alexandrova, R.; Vinas, C. Investigations on antimicrobial activity of cobaltabisdicarbollides. J. Organomet. Chem. 2013, 747, 229–234. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhang, Y.; Liu, J.; van der Veen, S.; Duttwyler, S. The closo-Dodecaborate Dianion Fused with Oxazoles Provides 3D Diboraheterocycles with Selective Antimicrobial Activity. Chem. Eur. J. 2018, 24, 10364–10371. [Google Scholar] [CrossRef] [PubMed]
- Vaňková, E.; Lokočová, K.; Maťátková, O.; Křížová, I.; Masák, J.; Grüner, B.; Kaule, P.; Čermák, J.; Šícha, V. Cobalt bis-dicarbollide and its ammonium derivatives are effective antimicrobial and antibiofilm agents. J. Organomet. Chem. 2019, 899, 120891. [Google Scholar] [CrossRef]
- Nakagawa, T.; Watanabe, H.; Yoshizaki, T. Japan Patent [1,2-Dicarbaclovododecarboran(12)-1-yl] alkyl quaternary ammonium salts, 5031938 B4 19701015. Jpn. Kokai Tokkyo Koho 1970, 31, 940. [Google Scholar]
- Totani, T.; Aono, K.; Yamamoto, K.; Tawara, K. Synthesis and in vitro antimicrobial property of o-carborane derivatives. J. Med. Chem. 1981, 24, 1492–1499. [Google Scholar] [CrossRef]
- Adamska, A.; Rumijowska-Galewicz, A.; Ruszczynska, A.; Studzińska, M.; Jabłońska, A.; Paradowska, E.; Bulska, E.; Munier-Lehmann, H.; Dziadek, J.; Leśnikowski, Z.J.; et al. Anti-mycobacterial activity of thymine derivatives bearing boron clusters. Eur. J. Med. Chem. 2016, 121, 71–81. [Google Scholar] [CrossRef]
- Oros, G.; Ujvary, I.; Nachman, R. Antimicrobial activity of o-carboranylalanine. Amino Acids 1999, 17, 357–368. [Google Scholar] [CrossRef]
- Omarova, E.O.; Nazarov, P.A.; Firsov, A.M.; Strakhovskaya, M.G.; Arkhipova, A.Y.; Moisenovich, M.M.; Agapov, I.I.; Ol’shevskaya, V.A.; Zaitsev, A.V.; Kalinin, V.N.; et al. Carboranyl-chlorin e6 as a potent antimicrobial photosensitizer. PLoS ONE 2015, 10, e0141990. [Google Scholar] [CrossRef]
- Różycka, D.; Leśnikowski, Z.J.; Olejniczak, A.B. Synthesis of boron cluster analogs of penicillin and their antibacterial activity. J. Organomet. Chem. 2019, 881, 19–24. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Wei, Y.; Wu, C.; Gao, S.; Jiang, H.; Zhao, X.; Yan, H.; Wang, X. Antimicrobial activity of a ferrocene-substituted carborane derivative targeting multidrug-resistant infection. Biomaterials 2013, 34, 902–911. [Google Scholar] [CrossRef]
- Li, S.; Wu, C.; Tang, X.; Gao, S.; Zhao, X.; Yan, H.; Wang, X. New strategy for reversing biofilm-associated antibiotic resistance through ferrocene-substituted carborane ruthenium (II)-arene complex. Sci. China Chem. 2013, 56, 595–603. [Google Scholar] [CrossRef]
- Li, S.; Wu, C.; Zhao, X.; Jiang, H.; Yan, H.; Wang, X. Synergistic antibacterial activity of new isomeric carborane derivatives through combination with nanoscaled titania. J. Biomed. Nanotechnol. 2013, 9, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X. A highly potent antibacterial agent targeting methicillin-resistant Staphylococcus aureus based on cobalt bis (1, 2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Swietnicki, W.; Goldeman, W.; Psurski, M.; Nasulewicz-Goldeman, A.; Boguszewska-Czubara, A.; Drab, M.; Sycz, J.; Goszczyński, T.M. Metallacarborane derivatives effective against Pseudomonas aeruginosa and Yersinia enterocolitica. Int. J. Mol. Sci. 2021, 22, 6762. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Martinez-Medina, M.; Camprubí-Font, C.; Bennour, I.; Moreno, D.; Martínez, L.; Teixidor, F.; Fox, M.A.; Viñas, C. Metallacarborane Assemblies as Effective Antimicrobial Agents, Including a Highly Potent Anti-MRSA Agent. Organometallics 2020, 39, 4253–4264. [Google Scholar] [CrossRef]
- Semioshkin, A.; Ilinova, A.; Lobanova, I.; Bregadze, V.; Paradowska, E.; Studzińska, M.; Jabłońska, A.; Lesnikowski, Z.J. Synthesis of the first conjugates of 5-ethynyl-2′-deoxyuridine with closo-dodecaborate and cobalt-bis-dicarbollide boron clusters. Tetrahedron 2013, 69, 8034–8041. [Google Scholar] [CrossRef]
- Fuentes, I.; García-Mendiola, T.; Sato, S.; Pita, M.; Nakamura, H.; Lorenzo, E.; Teixidor, F.; Marques, F.; Viñas, C. Metallacarboranes on the road to anticancer therapies: Cellular uptake, DNA interaction, and biological evaluation of cobaltabisdicarbollide [COSAN]−. Chem. Eur. J. 2018, 24, 17239–17254. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Kosenko, I.D.; Sivaev, I.B.; Antonenko, Y.N.; Bregadze, V.I. Fast flip–flop of halogenated cobalt bis (dicarbollide) anion in a lipid bilayer membrane. Phys. Chem. Chem. Phys. 2017, 19, 25122–25128. [Google Scholar] [CrossRef] [Green Version]
- Assaf, K.I.; Begaj, B.; Frank, A.; Nilam, M.; Mougharbel, A.S.; Kortz, U.; Nekvinda, J.; Grüner, B.; Gabel, D.; Nau, W.M. High-affinity binding of metallacarborane cobalt bis (dicarbollide) anions to cyclodextrins and application to membrane translocation. J. Org. Chem. 2019, 84, 11790–11798. [Google Scholar] [CrossRef]
- Vaňková, E.; Paldrychová, M.; Kašparová, P.; Lokočová, K.; Kodeš, Z.; Maťátková, O.; Kolouchová, I.; Masák, J. Natural antioxidant pterostilbene as an effective antibiofilm agent, particularly for gram-positive cocci. World J. Microbiol. Biotechnol. 2020, 36, 101. [Google Scholar] [CrossRef]
- Ayala-Núñez, N.V.; Villegas, H.H.L.; Turrent, L.d.C.I.; Padilla, C.R. Silver nanoparticles toxicity and bactericidal effect against methicillin-resistant Staphylococcus aureus: Nanoscale does matter. Nanobiotechnology 2009, 5, 2–9. [Google Scholar] [CrossRef]
- Samadi, N.; Abadian, N.; Ahmadkhaniha, R.; Amini, F.; Dalili, D.; Rastkari, N.; Safaripour, E.; Mohseni, F.A. Structural characterization and surface activities of biogenic rhamnolipid surfactants from Pseudomonas aeruginosa isolate MN1 and synergistic effects against methicillin-resistant Staphylococcus aureus. Folia Microbiol. 2012, 57, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Maťátková, O.; Kolouchová, I.; Kvasničková, E.; Ježdík, R.; Masák, J.; Čejková, A. Synergistic action of amphotericin B and rhamnolipid in combination on Candida parapsilosis and Trichosporon cutaneum. Chem. Pap. 2017, 71, 1471–1480. [Google Scholar] [CrossRef]
- Guo, N.; Wu, X.; Yu, L.; Liu, J.; Meng, R.; Jin, J.; Lu, H.; Wang, X.; Yan, S.; Deng, X. In vitro and in vivo interactions between fluconazole and allicin against clinical isolates of fluconazole-resistant Candida albicans determined by alternative methods. FEMS Microbiol. Immunol. 2010, 58, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynek, J.; Koncošová, M.; Zelenka, J.; Křížová, I.; Ruml, T.; Kubát, P.; Demel, J.; Lang, K. Phosphinatophenylporphyrins tailored for high photodynamic efficacy. Org. Biomol. Chem. 2018, 16, 7274–7281. [Google Scholar] [CrossRef]
- Kočendová, J.; Vaňková, E.; Volejníková, A.; Nešuta, O.; Buděšínský, M.; Socha, O.; Hájek, M.; Hadravová, R.; Čeřovský, V. Antifungal activity of analogues of antimicrobial peptides isolated from bee venoms against vulvovaginal Candida spp. FEMS Yeast Res. 2019, 19, foz013. [Google Scholar] [CrossRef]
| MIC80 (µg/mL) | MBC99.9 (µg/mL) | MBC99.9 /MIC80 | Inhibitory Effect | ||||
|---|---|---|---|---|---|---|---|
| TET | ERM | VAN | COSAN.Na | COSAN.Na | COSAN.Na | COSAN.Na | |
| S. epidermidis DBM 3179 | 65 | 0.7 | 1.5 | 3.1 | 6.2 | 2 | bactericidal |
| S. epidermidis CNCTC 5671 | 45 | 0.75 | 2 | 2.8 | 14 | 5 | bacteriostatic |
| S. epidermidis CCM 2343 | 5 | 0.5 | 2.75 | 2 | 10 a | more than 5 | bacteriostatic |
| cATB; COSAN.Na (µg/mL) | FICi | Combined Effect | Reduction of cATB | ||||
|---|---|---|---|---|---|---|---|
| TET | ERM | VAN | COSAN.Na | ||||
| S. epidermidis DBM 3179 | 13 | 0.62 | 0.4 | synergistic | 5× | ||
| 6.5 | 1.24 | 0.5 | synergistic | 10× | |||
| 0.15 | 1.86 | 0.8 | additive | 5× | |||
| 0.075 | 2.48 | 0.9 | additive | 10× | |||
| 0.15 | 1.86 | 0.7 | additive | 10× a | |||
| S. epidermidis CNCTC 5671 | 27 | 0.28 | 0.7 | additive | 1.5× | ||
| 4.5 | 2.24 | 0.9 | additive | 10× | |||
| 0.45 | 0.28 | 0.7 | additive | 1.5× | |||
| 0.3 | 1.68 | 1.0 | additive | 2.5× | |||
| 0.15 | 2.24 | 0.9 | additive | 10× a | |||
| S. epidermidis CCM 2343 | 4 | 0.2 | 0.9 | additive | 1.25× | ||
| 1 | 1.6 | 1.0 | additive | 5× | |||
| 0.2 | 0.2 | 0.5 | synergistic | 2.5× | |||
| 0.1 | 1.6 | 1.0 | additive | 5× | |||
| 2.2 | 0.2 | 0.9 | additive | 1.25× | |||
| 1.65 | 0.8 | 1.0 | additive | 1.5× | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaňková, E.; Lokočová, K.; Kašparová, P.; Hadravová, R.; Křížová, I.; Maťátková, O.; Masák, J.; Šícha, V. Cobalt Bis-Dicarbollide Enhances Antibiotics Action towards Staphylococcus epidermidis Planktonic Growth Due to Cell Envelopes Disruption. Pharmaceuticals 2022, 15, 534. https://doi.org/10.3390/ph15050534
Vaňková E, Lokočová K, Kašparová P, Hadravová R, Křížová I, Maťátková O, Masák J, Šícha V. Cobalt Bis-Dicarbollide Enhances Antibiotics Action towards Staphylococcus epidermidis Planktonic Growth Due to Cell Envelopes Disruption. Pharmaceuticals. 2022; 15(5):534. https://doi.org/10.3390/ph15050534
Chicago/Turabian StyleVaňková, Eva, Kristýna Lokočová, Petra Kašparová, Romana Hadravová, Ivana Křížová, Olga Maťátková, Jan Masák, and Václav Šícha. 2022. "Cobalt Bis-Dicarbollide Enhances Antibiotics Action towards Staphylococcus epidermidis Planktonic Growth Due to Cell Envelopes Disruption" Pharmaceuticals 15, no. 5: 534. https://doi.org/10.3390/ph15050534
APA StyleVaňková, E., Lokočová, K., Kašparová, P., Hadravová, R., Křížová, I., Maťátková, O., Masák, J., & Šícha, V. (2022). Cobalt Bis-Dicarbollide Enhances Antibiotics Action towards Staphylococcus epidermidis Planktonic Growth Due to Cell Envelopes Disruption. Pharmaceuticals, 15(5), 534. https://doi.org/10.3390/ph15050534