Disentangling a Thorny Issue: Myocarditis and Pericarditis Post COVID-19 and Following mRNA COVID-19 Vaccines
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Case Assessment
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency—COVID-19 Vaccines: Update on Ongoing Evaluation of Myocarditis and Pericarditis. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccines-update-ongoing-evaluation-myocarditis-pericarditis (accessed on 1 November 2021).
- Centers for Disease Control and Prevention (CDC)—Myocarditis and Pericarditis. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (accessed on 1 November 2021).
- Snyder, M.J.; Bepko, J.; White, M. Acute pericarditis: Diagnosis and management. Am. Fam. Physician 2014, 89, 553–560. [Google Scholar] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, M.; Schwartz, M.; Webber, E.; Shaffer, A.; Perry, T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Doctor, N.S.; Shah, A.B.; Coplan, N.; Kronzon, I. Acute Pericarditis. Prog. Cardiovasc. Dis. 2017, 59, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Fung, G.; Luo, H.; Qiu, Y.; Yang, D.; McManus, B. Myocarditis. Circ. Res. 2016, 118, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Mayosi, B.M.; Brucato, A.; Markel, G.; Trinchero, R.; Spodick, D.H.; Adler, Y. Triage and management of pericardial effusion. J. Cardiovasc. Med. 2010, 11, 928–935. [Google Scholar] [CrossRef]
- Dudley, M.Z.; Halsey, N.A.; Omer, S.B.; Orenstein, W.A.; O’Leary, S.T.; Limaye, R.J.; Salmon, D.A. The state of vaccine safety science: Systematic reviews of the evidence. Lancet Infect. Dis. 2020, 20, e80–e89. [Google Scholar] [CrossRef]
- Su, J.R.; McNeil, M.M.; Welsh, K.J.; Marquez, P.L.; Ng, C.; Yan, M.; Cano, M.V. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine 2021, 39, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Brighton Collaboration—Myocarditis/Pericarditis Case Definition. The Task Force for Global Health, 19 November 2021. Available online: https://brightoncollaboration.us/myocarditis-case-definition-update/ (accessed on 1 November 2021).
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 29 November–2 December 2021—PRAC Update on Risk of Myocarditis and Pericarditis with mRNA Vaccines. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-29-november-2-december-2021 (accessed on 15 December 2021).
- Montgomery, J.; Ryan, M.; Engler, R.; Hoffman, D.; McClenathan, B.; Collins, L.; Loran, D.; Hrncir, D.; Herring, K.; Platzer, M.; et al. Myocarditis Following Immunization with mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021, 6, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Hajjo, R.; Sabbah, D.A.; Bardaweel, S.K.; Tropsha, A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines 2021, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Viveiros, A.; Rasmuson, J.; Vu, J.; Mulvagh, S.L.; Yip, C.Y.Y.; Norris, C.M.; Oudit, G.Y. Sex differences in COVID-19: Candidate pathways, genetics of ACE2, and sex hormones. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H296–H304. [Google Scholar] [CrossRef] [PubMed]
- Keinath, K.; Church, T.; Kurth, B.; Hulten, E. Myocarditis secondary to smallpox vaccination. BMJ Case Rep. 2018, 22, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Cassimatis, D.C.; Atwood, J.E.; Engler, R.M.; Linz, P.E.; Grabenstein, J.D.; Vernalis, M.N. Smallpox vaccination and myopericarditis: A clinical review. J. Am. Coll. Cardiol. 2004, 43, 1503–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, R.J.; Nelson, M.R.; Collins, L.C.; Spooner, C.; Hemann, B.A.; Gibbs, B.T.; Atwood, J.E.; Howard, R.S.; Chang, A.S.; Cruser, D.L.; et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE 2015, 10, e0118283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). Vaccine Adverse Event Reporting System (VAERS). Available online: https://vaers.hhs.gov/index.html (accessed on 20 July 2021).
- Medical Dictionary for Regulatory Activities. Available online: meddra.org (accessed on 1 November 2021).
- Heymans, S.; Cooper, L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2022, 19, 75–77. [Google Scholar] [CrossRef] [PubMed]
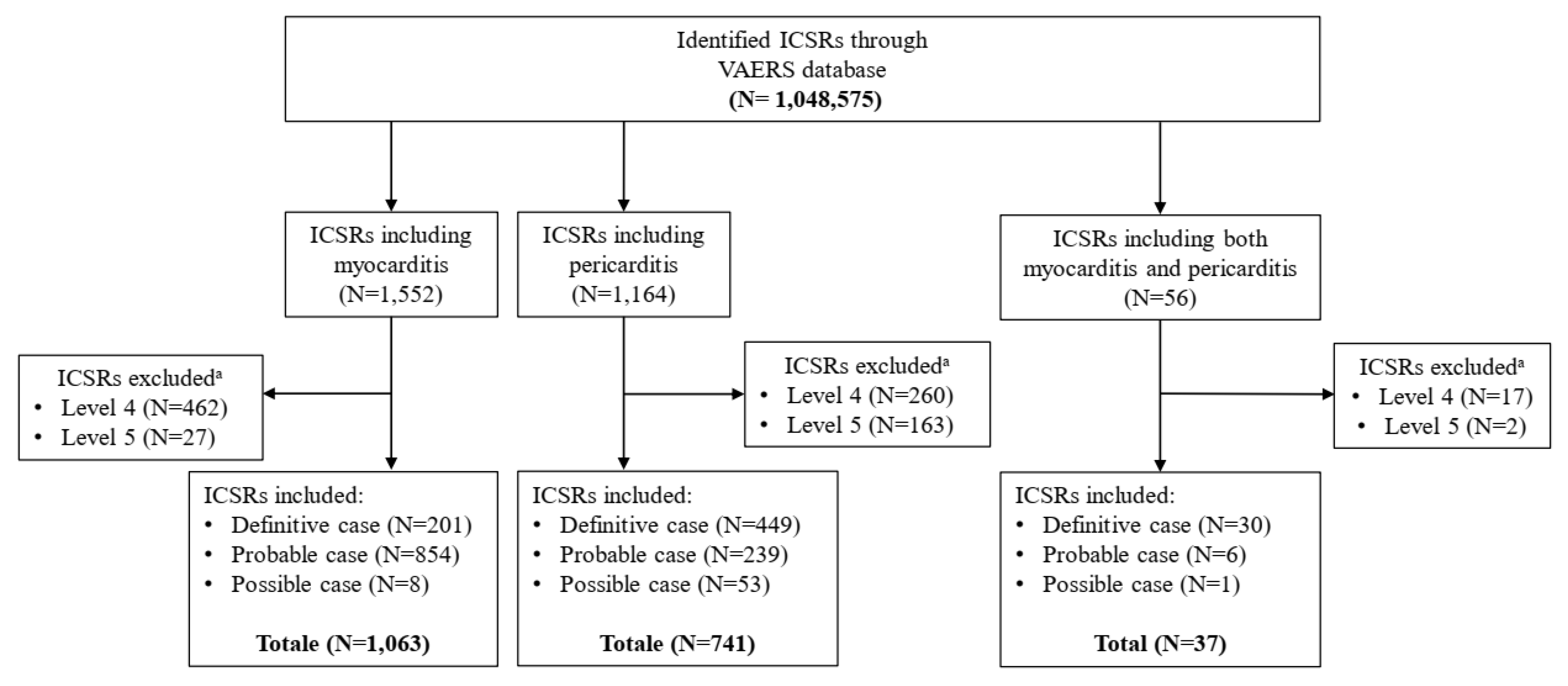


| Myocarditis N = 778 (%) | Pericarditis N = 472 (%) | |
|---|---|---|
| Gender | ||
| Male | 626 (80.5) | 336 (71.2) |
| Female | 145 (18.6) | 135 (28.6) |
| NA | 7 (0.9) | 1 (0.2) |
| Age group | ||
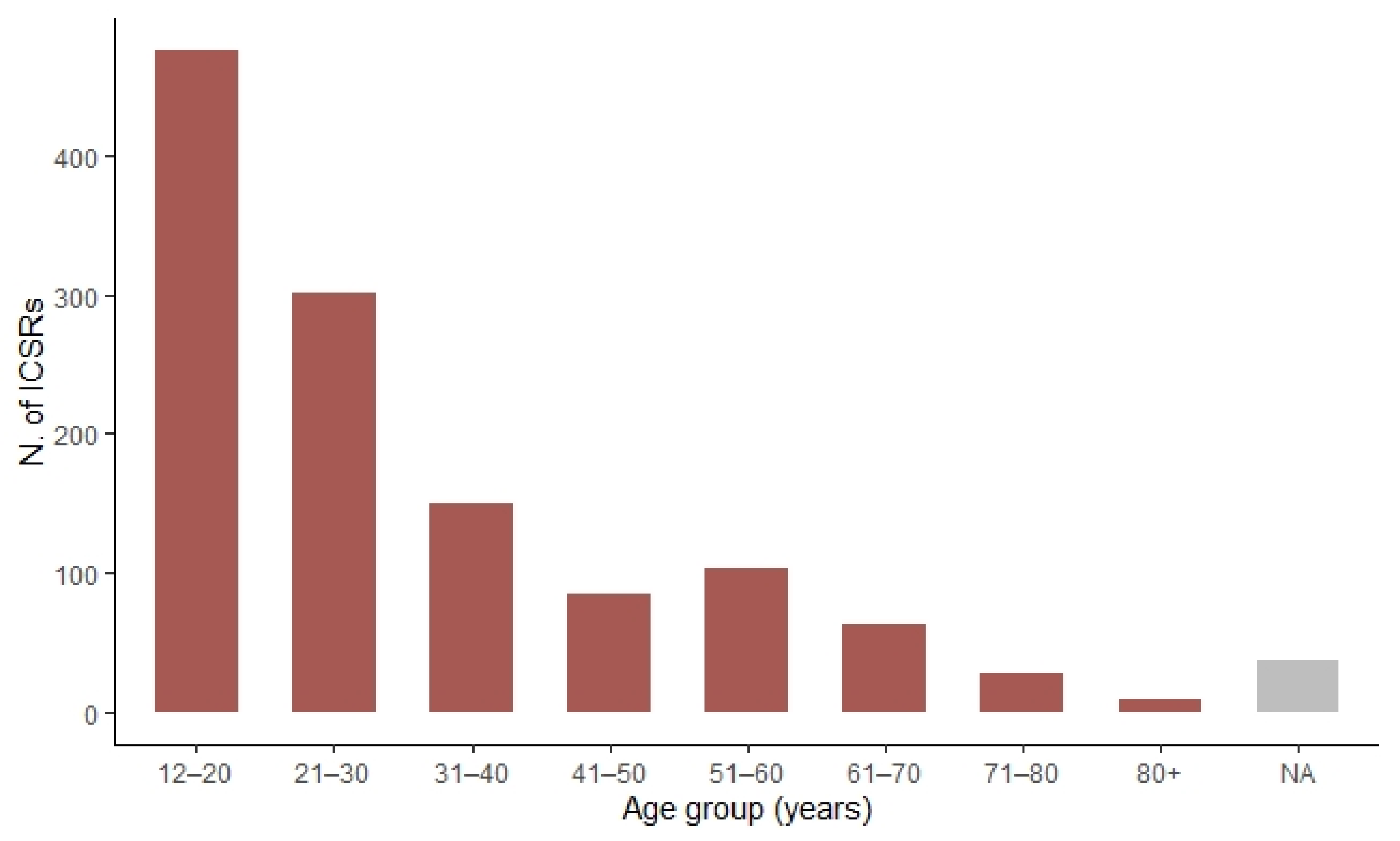
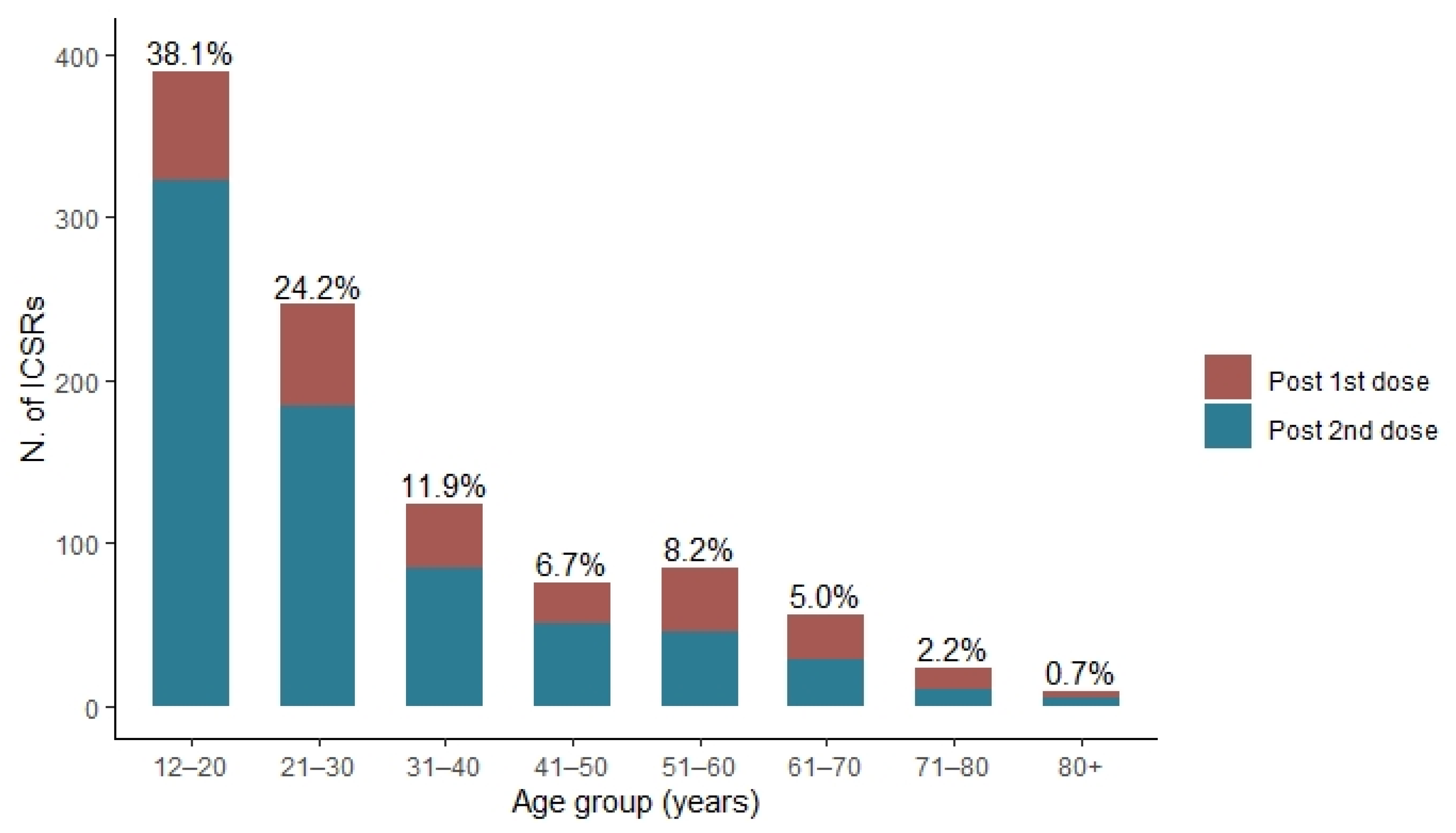
| 12–20 years | 360 (46.3) | 116 (24.6) |
| 21–30 years | 208 (26.7) | 94 (19.9) |
| 31–40 years | 82 (10.5) | 67 (14.2) |
| 41–50 years | 44 (5.7) | 40 (8.5) |
| 51–60 years | 34 (4.4) | 69 (14.6) |
| 61–70 years | 17 (2.2) | 46 (9.7) |
| 71–80 years | 5 (0.6) | 23 (4.9) |
| 80+ years | 1 (0.1) | 8 (1.7) |
| NA | 27 (3.5) | 9 (1.9) |
| Median age (IQR a) | 21 (16–30) | 34 (20–55) |
| Outcome | ||
| Fatal | 6 (0.8) | 1 (0.3) |
| Life threatening | 126 (16.0) | 67 (19.1) |
| Disability | 17 (2.1) | 16 (4.5) |
| Hospitalization | 640 (81.1) | 268 (76.1) |
| Mean hospitalization time in days (±SD b) | 3 (±1.7) | 3 (±2.1) |
| Dose | ||
| Post 1st dose | 152 (19.5) | 137 (29.0) |
| Post 2nd dose | 489 (62.9) | 262 (55.5) |
| NA | 137 (17.6) | 73 (15.5) |
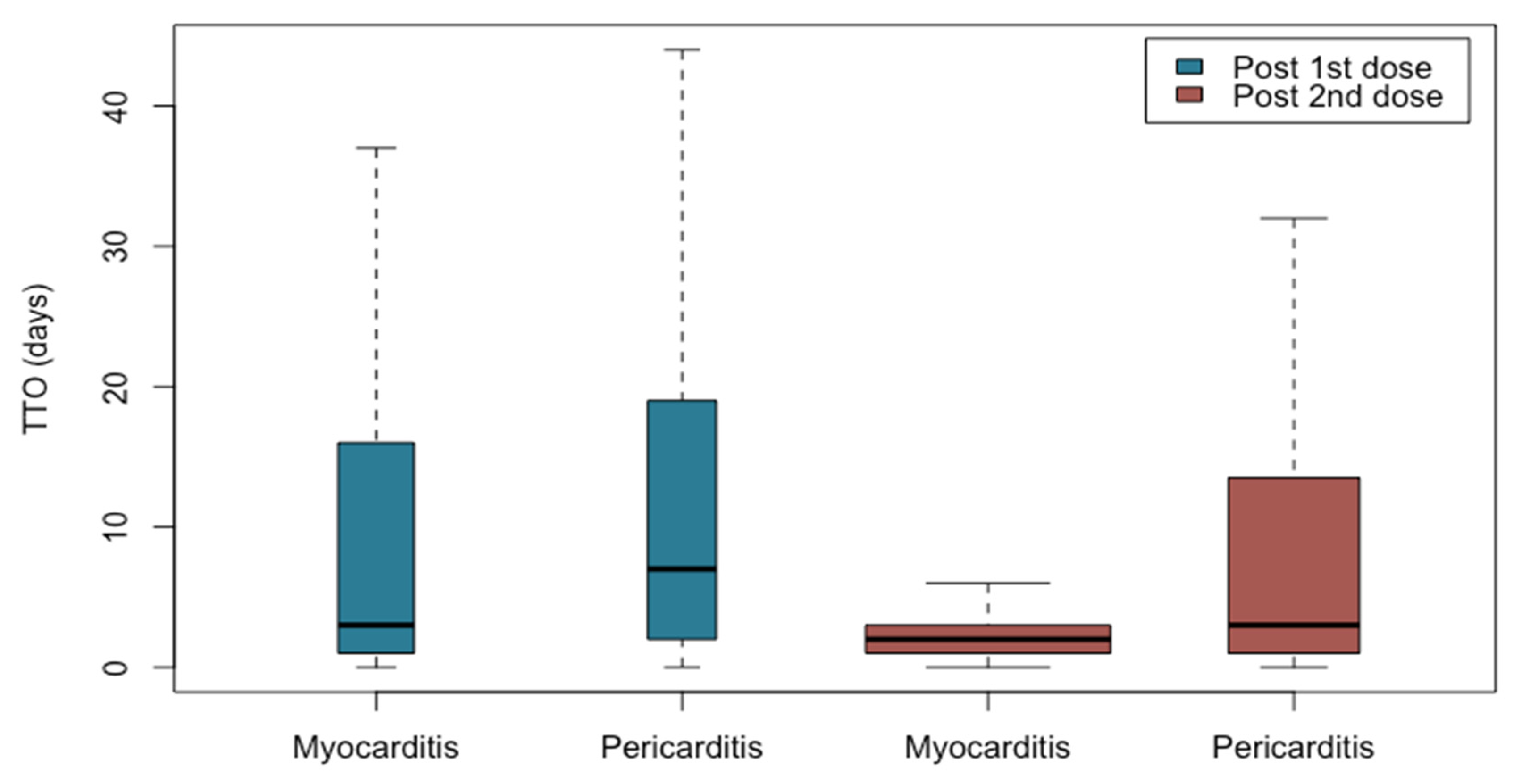
| Median TTO c post 1st dose (IQR) | 3 (1–16) | 7 (2–19) |
| Median TTO c post 2nd dose (IQR) | 2 (1–3) | 3 (1–13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafaniello, C.; Gaio, M.; Zinzi, A.; Sullo, M.G.; Liguori, V.; Ferraro, M.; Petronzelli, F.; Felicetti, P.; Marchione, P.; Marra, A.R.; et al. Disentangling a Thorny Issue: Myocarditis and Pericarditis Post COVID-19 and Following mRNA COVID-19 Vaccines. Pharmaceuticals 2022, 15, 525. https://doi.org/10.3390/ph15050525
Rafaniello C, Gaio M, Zinzi A, Sullo MG, Liguori V, Ferraro M, Petronzelli F, Felicetti P, Marchione P, Marra AR, et al. Disentangling a Thorny Issue: Myocarditis and Pericarditis Post COVID-19 and Following mRNA COVID-19 Vaccines. Pharmaceuticals. 2022; 15(5):525. https://doi.org/10.3390/ph15050525
Chicago/Turabian StyleRafaniello, Concetta, Mario Gaio, Alessia Zinzi, Maria Giuseppa Sullo, Valerio Liguori, Marialuisa Ferraro, Fiorella Petronzelli, Patrizia Felicetti, Pasquale Marchione, Anna Rosa Marra, and et al. 2022. "Disentangling a Thorny Issue: Myocarditis and Pericarditis Post COVID-19 and Following mRNA COVID-19 Vaccines" Pharmaceuticals 15, no. 5: 525. https://doi.org/10.3390/ph15050525
APA StyleRafaniello, C., Gaio, M., Zinzi, A., Sullo, M. G., Liguori, V., Ferraro, M., Petronzelli, F., Felicetti, P., Marchione, P., Marra, A. R., Rossi, F., De Angelis, A., & Capuano, A. (2022). Disentangling a Thorny Issue: Myocarditis and Pericarditis Post COVID-19 and Following mRNA COVID-19 Vaccines. Pharmaceuticals, 15(5), 525. https://doi.org/10.3390/ph15050525






