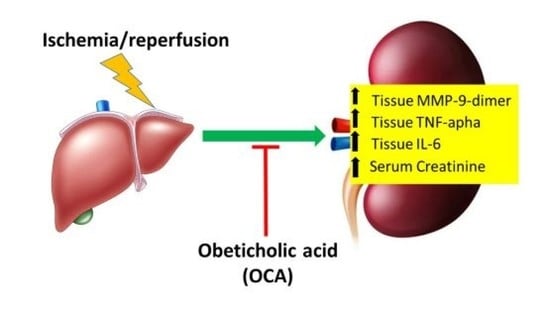
Obeticholic Acid Reduces Kidney Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats
Abstract
:1. Introduction
2. Results
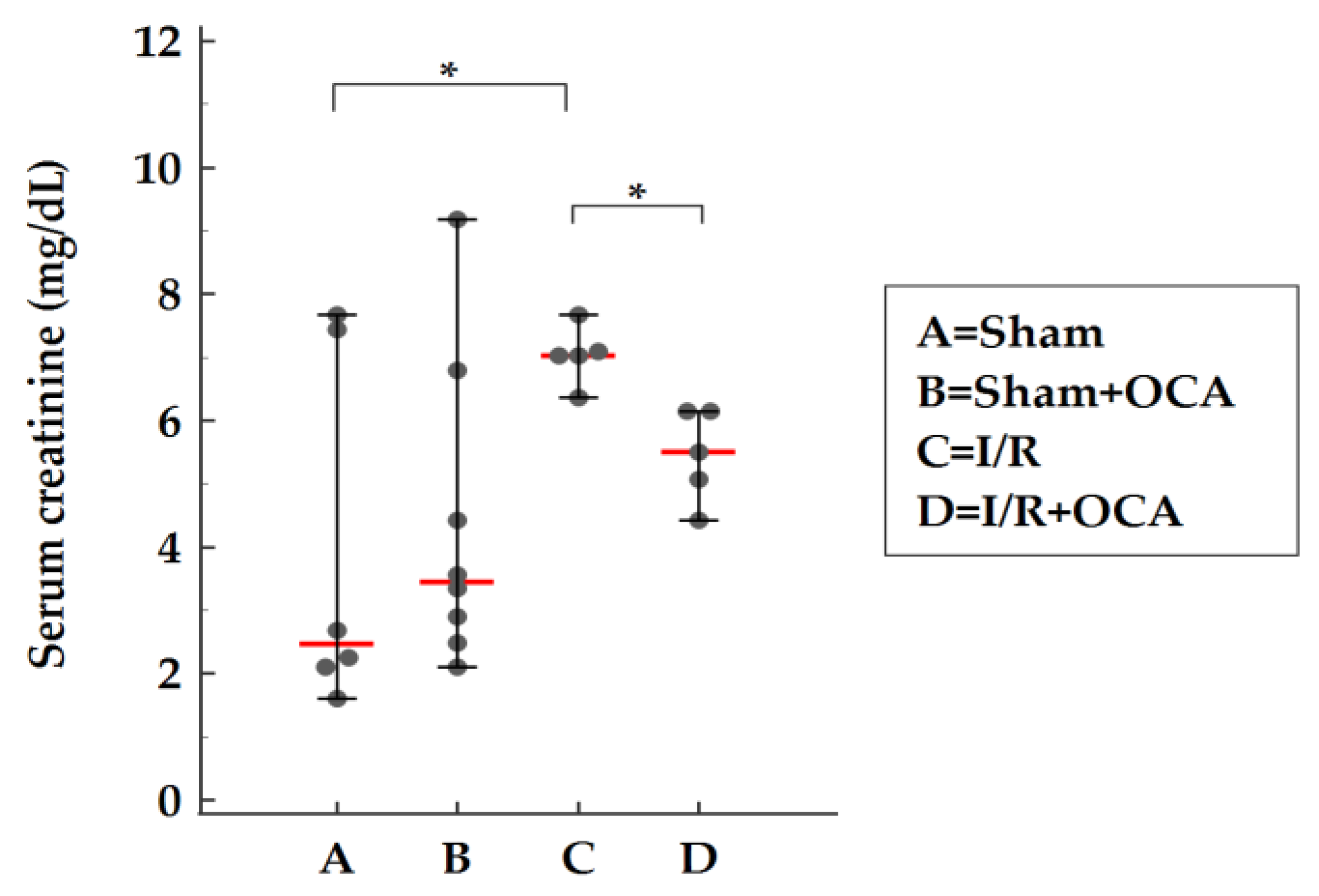
2.1. Hepatorenal Syndrome after Liver I/R
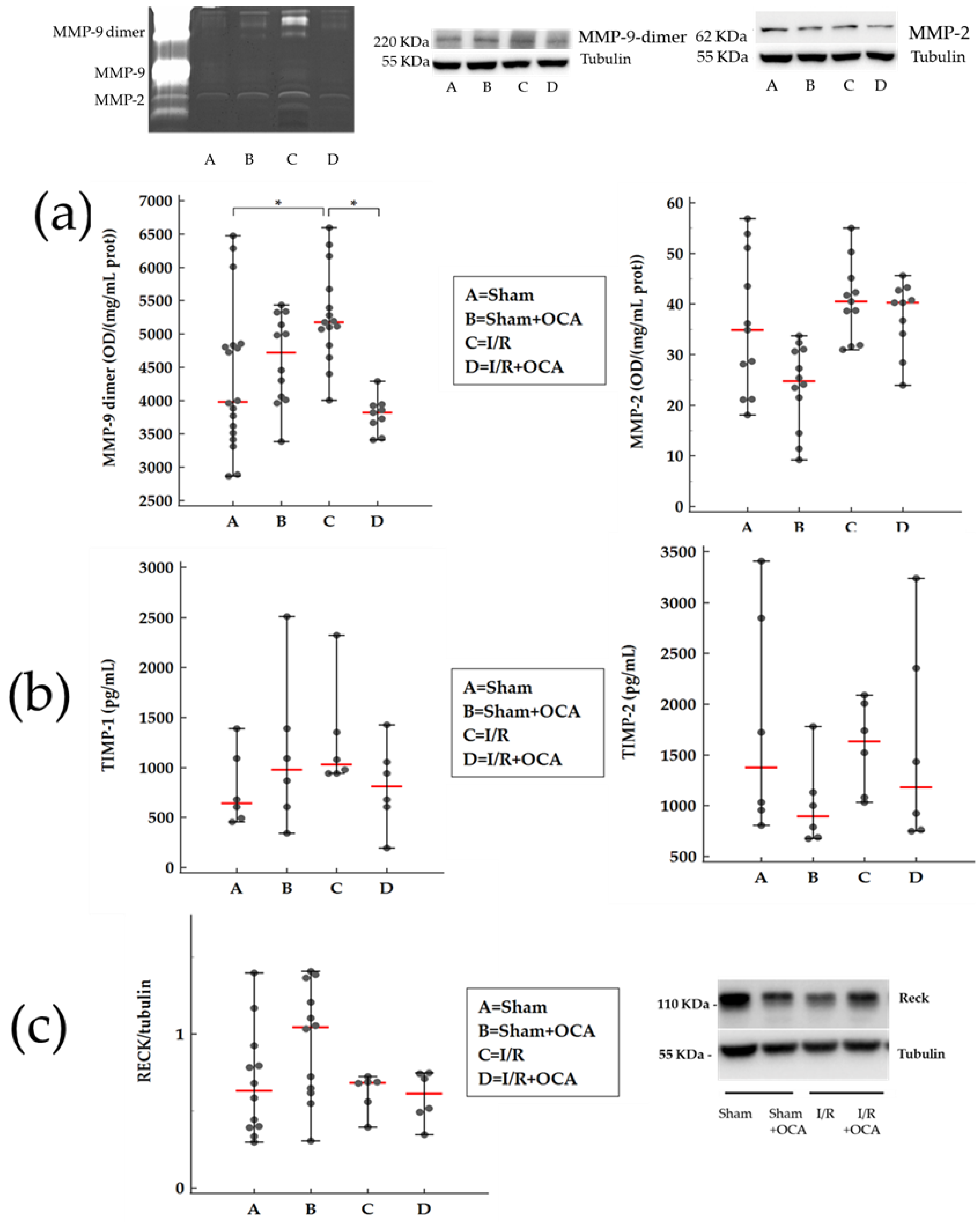
2.2. Changes in MMPs, TIMPs, and RECK in Kidney Cortex and Medulla after Liver I/R
2.3. OCA Treatment Reduces Serum Levels of Creatinine
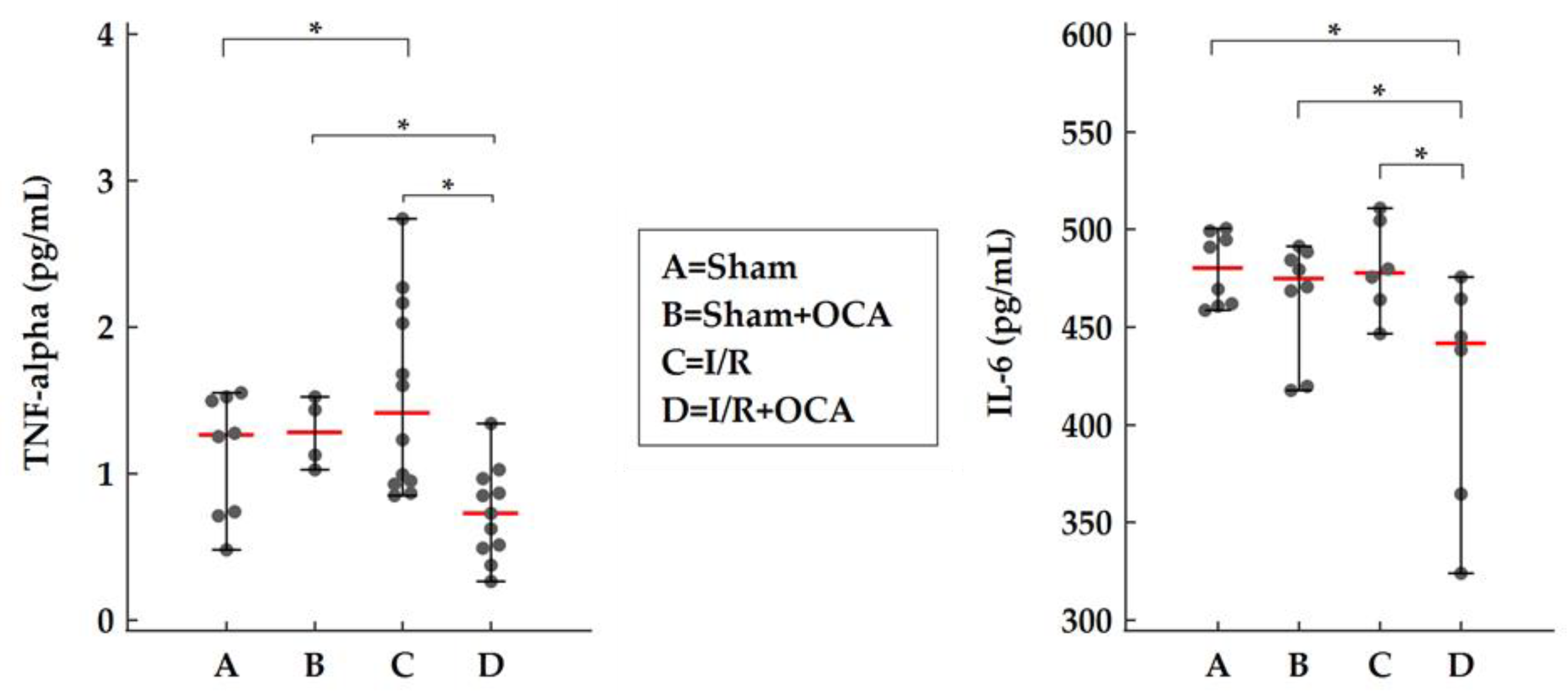
2.4. OCA Treatment Reduces Kidney Cortex Levels of TNF-Alpha and IL-6
2.5. FXR Expression in the Kidney
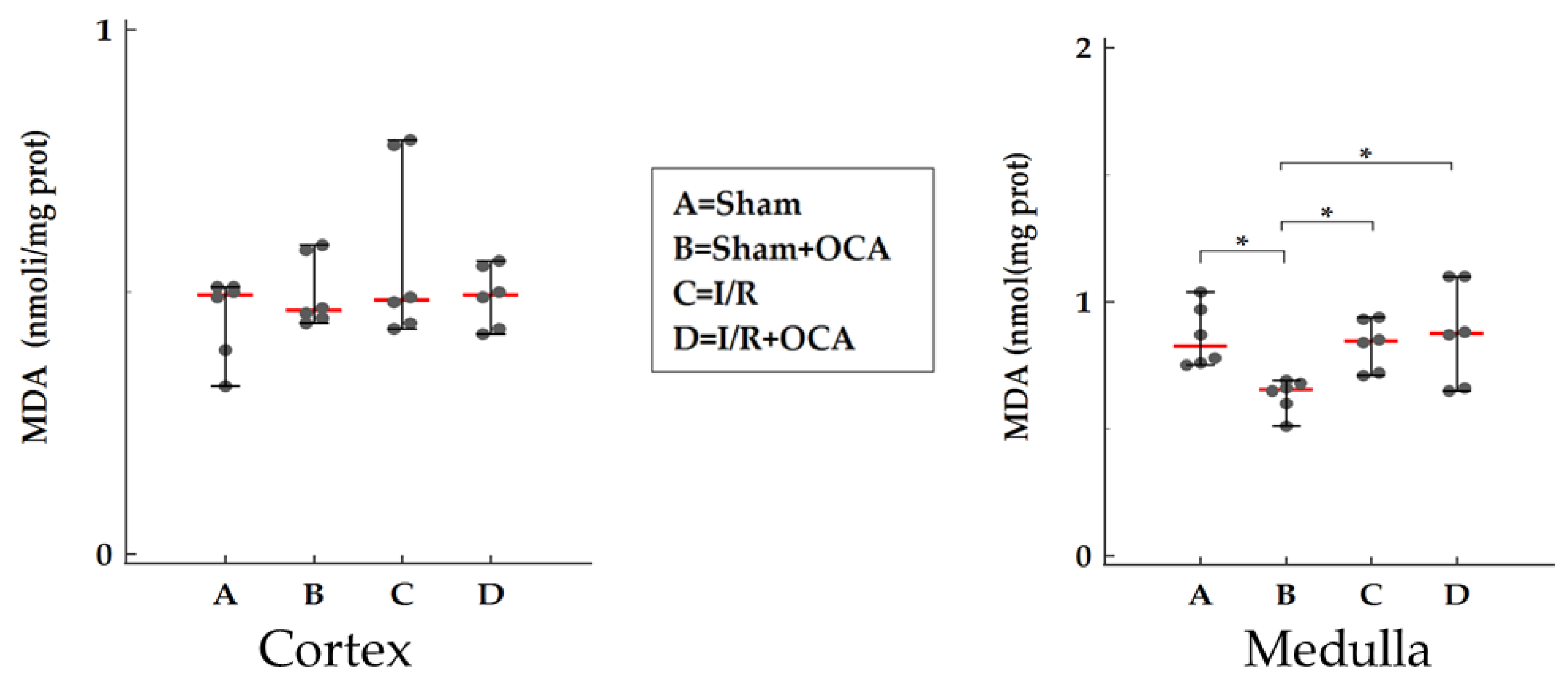
2.6. TBARS Formation in the Kidney
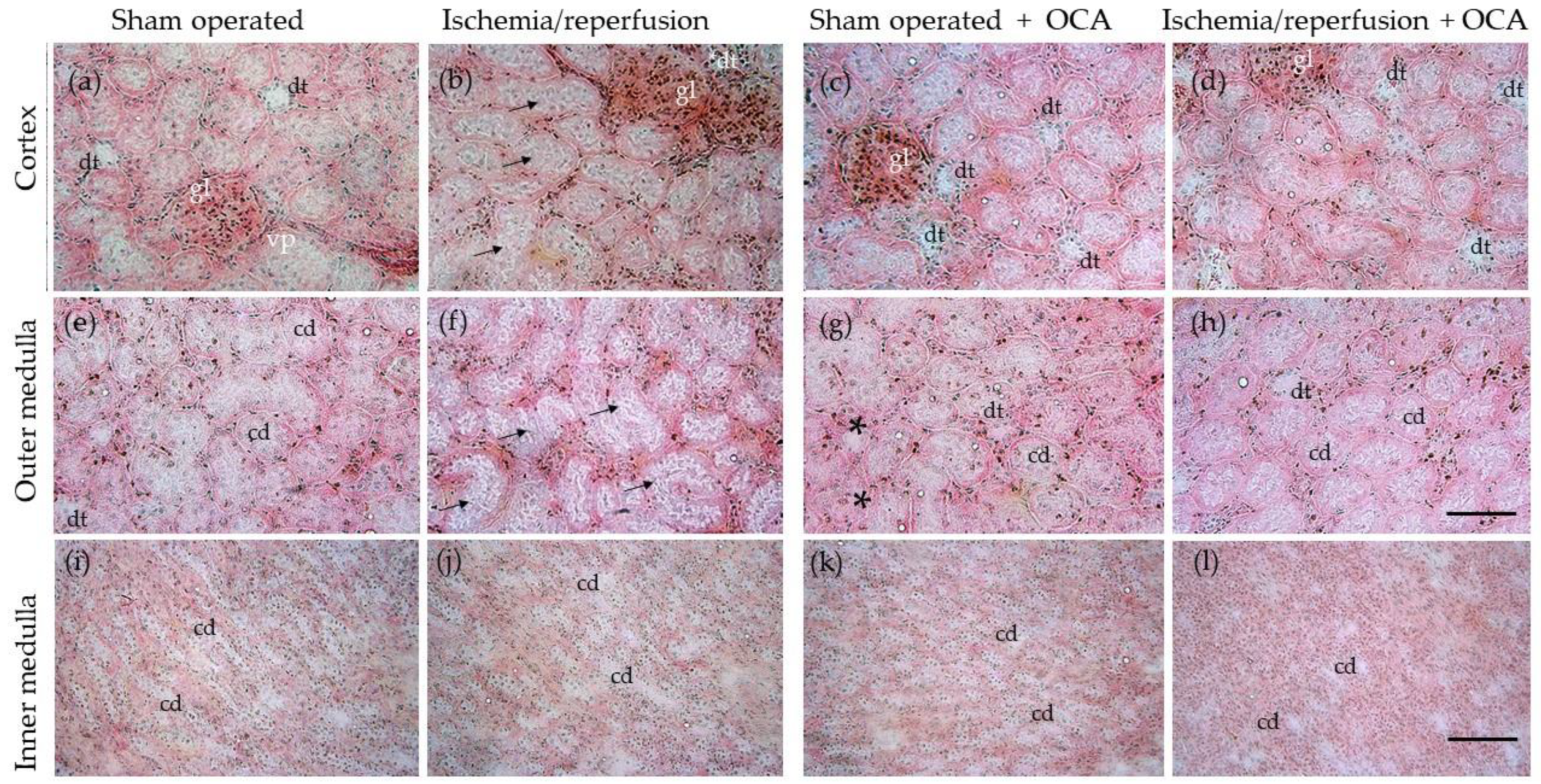
2.7. OCA Treatment and Histological Changes in the Kidney
3. Discussion
3.1. Hepatic Ischemia/Reperfusion and Renal Damage
3.2. Renal Damage Is Associated to MMPs Activation
3.3. OCA Treatment Reduces Kidney Gelatinases Activity following Partial Hepatic I/R Injury in Rats
4. Materials
4.1. Animals and Experimental Design
4.2. Biochemical Assays
4.3. TBARS Formation
4.4. Tissue Sources for MMPs Analysis
4.5. MMP Zymography
4.6. Western Blots
4.7. TIMP-1, TIMP-2, TNF-Alpha, and IL-6 Enzyme-Linked Immunosorbent Assay
4.8. FXR Expression
4.9. Tissue Morphology
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Makri, E.; Cholongitas, E.; Tziomalos, K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 9039–9043. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Nader, F.; Loomba, R.; Anstee, Q.M.; Ratziu, V.; Harrison, S.; Sanyal, A.J.; Schattenberg, J.M.; Barritt, A.S.; et al. Obeticholic Acid Impact on Quality of Life in Patients With Nonalcoholic Steatohepatitis: REGENERATE 18-Month Interim Analysis. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.M.; Chen, Y.S.; Harn, H.J. The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment. Molecules 2019, 24, 4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, S.; Hamada, T.; Kuriyama, N.; Busuttil, R.W.; Coito, A.J. TIMP-1 deficiency leads to lethal partial hepatic ischemia and reperfusion injury. Hepatology 2012, 56, 1074–1085. [Google Scholar] [CrossRef] [Green Version]
- Meng, N.; Li, Y.; Zhang, H.; Sun, X.F. RECK, a novel matrix metalloproteinase regulator. Histol. Histopathol. 2008, 23, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Ferrigno, A.; Palladini, G.; Di Pasqua, L.G.; Berardo, C.; Richelmi, P.; Cadamuro, M.; Fabris, L.; Perlini, S.; Adorini, L.; Vairetti, M. Obeticholic acid reduces biliary and hepatic matrix metalloproteinases activity in rat hepatic ischemia/reperfusion injury. PLoS ONE 2020, 15, e0238543. [Google Scholar] [CrossRef] [PubMed]
- Clichici, S.; David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Filip, M.; Nagy, A.; Luca, V.; Crivii, C.; Mircea, P.; et al. Hepatoprotective effects of silymarin coated gold nanoparticles in experimental cholestasis. Mater. Sci. Eng. C 2020, 115, 111117. [Google Scholar] [CrossRef]
- Nastos, C.; Kalimeris, K.; Papoutsidakis, N.; Tasoulis, M.K.; Lykoudis, P.M.; Theodoraki, K.; Nastou, D.; Smyrniotis, V.; Arkadopoulos, N. Global consequences of liver ischemia/reperfusion injury. Oxid. Med. Cell. Longev. 2014, 2014, 906965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palladini, G.; Ferrigno, A.; Rizzo, V.; Tarantola, E.; Bertone, V.; Freitas, I.; Perlini, S.; Richelmi, P.; Vairetti, M. Lung matrix metalloproteinase activation following partial hepatic ischemia/reperfusion injury in rats. Sci. World J. 2014, 2014, 867548. [Google Scholar] [CrossRef] [Green Version]
- Barri, Y.M.; Sanchez, E.Q.; Jennings, L.W.; Melton, L.B.; Hays, S.; Levy, M.F.; Klintmalm, G.B. Acute kidney injury following liver transplantation: Definition and outcome. Liver Transpl. 2009, 15, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, M.; Gonul, Y.; Bal, A.; Ozkececi, Z.T.; Celep, R.B.; Adali, F.; Hazman, O.; Koçak, A.; Tosun, M. Effect of IL-18 binding protein on hepatic ischemia-reperfusion injury induced by infrarenal aortic occlusion. Ann. Surg. Treat. Res. 2015, 88, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, V.; Ginès, P.; Gerbes, A.L.; Dudley, F.J.; Gentilini, P.; Laffi, G.; Reynolds, T.B.; Ring-Larsen, H.; Schölmerich, J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996, 23, 164–176. [Google Scholar] [CrossRef]
- Angeli, P.; Ginès, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.T.; Park, S.W.; Kim, M.; D’Agati, V.D. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab. Investig. 2009, 89, 196–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palladini, G.; Ferrigno, A.; Rizzo, V.; Boncompagni, E.; Richelmi, P.; Freitas, I.; Perlini, S.; Vairetti, M. Lobe-specific heterogeneity and matrix metalloproteinase activation after ischemia/reperfusion injury in rat livers. Toxicol. Pathol. 2012, 40, 722–730. [Google Scholar] [CrossRef] [Green Version]
- Teng, L.; Yu, M.; Li, J.M.; Tang, H.; Yu, J.; Mo, L.H.; Jin, J.; Liu, X.Z. Matrix metalloproteinase-9 as new biomarkers of severity in multiple organ dysfunction syndrome caused by trauma and infection. Mol. Cell. Biochem. 2012, 360, 271–277. [Google Scholar] [CrossRef]
- Davis, C.L.; Gonwa, T.A.; Wilkinson, A.H. Pathophysiology of renal disease associated with liver disorders: Implications for liver transplantation. Part I. Liver Transpl. 2002, 8, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Pose, E.; Solà, E.; Ginès, P. Hepatorenal syndrome in the era of acute kidney injury. Liver Int. 2018, 38, 1891–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manyalich, M.; Nelson, H.; Delmonico, F.L. The need and opportunity for donation after circulatory death worldwide. Curr. Opin. Organ Transplant. 2018, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Polat, C.; Tokyol, Ç.; Kahraman, A.; Sabuncuoǧlu, B.; Yìlmaz, S. The effects of desferrioxamine and quercetin on hepatic ischemia-reperfusion induced renal disturbance. Prostaglandins. Leukot. Essent. Fatty Acids 2006, 74, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef]
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal Physiol. 2012, 302, F1351–F1361. [Google Scholar] [CrossRef] [Green Version]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef] [PubMed]
- Bengatta, S.; Arnould, C.; Letavernier, E.; Monge, M.; De Préneuf, H.M.; Werb, Z.; Ronco, P.; Lelongt, B. MMP9 and SCF protect from apoptosis in acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Kunugi, S.; Shimizu, A.; Kuwahara, N.; Du, X.; Takahashi, M.; Terasaki, Y.; Fujita, E.; Mii, A.; Nagasaka, S.; Akimoto, T.; et al. Inhibition of matrix metalloproteinases reduces ischemia-reperfusion acute kidney injury. Lab. Investig. 2011, 91, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hörbelt, M.; Mang, H.E.; Knipe, N.L.; Bacallao, R.L.; Sado, Y.; Sutton, T.A. MMP-9 gene deletion mitigates microvascular loss in a model of ischemic acute kidney injury. Am. J. Physiol. Renal Physiol. 2011, 301, F101–F109. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Louis, G.; Loughlin, K.R.; Wiederschain, D.; Kilroy, S.M.; Lamb, C.C.; Zurakowski, D.; Moses, M.A. Tumor-specific urinary matrix metalloproteinase fingerprinting: Identification of high molecular weight urinary matrix metalloproteinase species. Clin. Cancer Res. 2008, 14, 6610–6617. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.W.; Bernardo, M.M.; Pietila, M.; Gervasi, D.C.; Toth, M.; Kotra, L.P.; Massova, I.; Mobashery, S.; Fridman, R. Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase-9. Differential rates for activation by stromelysin 1. J. Biol. Chem. 2000, 275, 2661–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrish, A.R. Matrix Metalloproteinases in Kidney Disease: Role in Pathogenesis and Potential as a Therapeutic Target. Prog. Mol. Biol. Transl. Sci. 2017, 148, 31–65. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.A.; Kelly, K.J.; Mang, H.E.; Plotkin, Z.; Sandoval, R.M.; Dagher, P.C. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am. J. Physiol. Renal Physiol. 2005, 288, F91–F97. [Google Scholar] [CrossRef] [PubMed]
- Ihtiyar, E.; Yaşar, N.F.; Erkasap, N.; Köken, T.; Tosun, M.; Öner, S.; Erkasap, S. Effects of doxycycline on renal ischemia reperfusion injury induced by abdominal compartment syndrome. J. Surg. Res. 2011, 167, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Chen, C.H.; Chen, Y.C.; Wu, Y.C.; Zhen, Y.Y.; Leu, S.; Tsai, T.H.; Ko, S.F.; Sung, P.H.; Yang, C.C.; et al. Sitagliptin protects rat kidneys from acute ischemia-reperfusion injury via upregulation of GLP-1 and GLP-1 receptors. Acta Pharmacol. Sin. 2015, 36, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anfuso, B.; Tiribelli, C.; Adorini, L.; Rosso, N. Obeticholic acid and INT-767 modulate collagen deposition in a NASH in vitro model. Sci. Rep. 2020, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Schrier, R.W. Renal failure in cirrhosis. N. Engl. J. Med. 2009, 361, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simbrunner, B.; Trauner, M.; Reiberger, T.; Mandorfer, M. Recent advances in the understanding and management of hepatorenal syndrome. Fac. Rev. 2021, 10. [Google Scholar] [CrossRef]
- Cai, D.; Duan, H.; Fu, Y.; Cheng, Z. Renal Tissue Damage Induced by Acute Kidney Injury in Sepsis Rat Model Is Inhibited by Cynaropicrin via IL-1β and TNF-α Down-Regulation. Dokl. Biochem. Biophys. 2021, 497, 151–157. [Google Scholar] [CrossRef]
- Ogura, J.; Terada, Y.; Tsujimoto, T.; Koizumi, T.; Kuwayama, K.; Maruyama, H.; Fujikawa, A.; Takaya, A.; Kobayashi, M.; Itagaki, S.; et al. The decrease in farnesoid X receptor, pregnane X receptor and constitutive androstane receptor in the liver after intestinal ischemia-reperfusion. J. Pharm. Pharm. Sci. 2012, 15, 616–631. [Google Scholar] [CrossRef] [Green Version]
- Wanner, G.A.; Ertel, W.; Müller, P.; Höfer, Y.; Leiderer, R.; Menger, M.D.; Messmer, K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock 1996, 5, 34–40. [Google Scholar] [CrossRef]
- Burdon, D.; Tiedje, T.; Pfeffer, K.; Vollmer, E.; Zabel, P. The role of tumor necrosis factor in the development of multiple organ failure in a murine model. Crit. Care Med. 2000, 28, 1962–1967. [Google Scholar] [CrossRef]
- Norman, J.T.; Lewis, M.P. Matrix metalloproteinases (MMPs) in renal fibrosis. Kidney Int. Suppl. 1996, 54, S61–S63. [Google Scholar]
- Kim, C.W.; Oh, E.T.; Kim, J.M.; Park, J.S.; Lee, D.H.; Lee, J.S.; Kim, K.K.; Park, H.J. Hypoxia-induced microRNA-590-5p promotes colorectal cancer progression by modulating matrix metalloproteinase activity. Cancer Lett. 2018, 416, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Di Pasqua, L.G.; Berardo, C.; Siciliano, V.; Richelmi, P.; Mannucci, B.; Croce, A.C.; Rizzo, V.; Perlini, S.; Vairetti, M.; et al. Fatty acid desaturase involvement in non-alcoholic fatty liver disease rat models: Oxidative stress versus metalloproteinases. Nutrients 2019, 11, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Mackey, K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal. Biochem. 1995, 225, 163–164. [Google Scholar] [CrossRef]
- Berardo, C.; Siciliano, V.; Di Pasqua, L.G.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Comparison between lipofectamine RNAiMAX and genmute transfection agents in two cellular models of human hepatoma. Eur. J. Histochem. 2019, 63, 189–194. [Google Scholar] [CrossRef]


| Sham | I/R | |
|---|---|---|
| AST (mU/mL) | 256 ± 33 | 9653 ± 956 * |
| ALT (mU/mL) | 64 ± 10 | 8635 ± 847 * |
| ALP (mU/mL) | 459 ± 50 | 803 ± 55 * |
| Total Bilirubin (mg/dL) | 0.15 ± 0.01 | 0.31 ± 0.02 * |
| Direct Bilitubin (mg/dL) | 0.05 ± 0.01 | 0.21 ± 0.01 * |
| Creatinine (mg/dL) | 3.96 ± 1.10 | 7.35 ± 0.40 * |
| r | p | |
| Creatinine/ALT | 0.94 | 0.002 |
| Gene | Sequence |
|---|---|
| rat FXR | Forward: CGCCTCATCGGCGGGAAGAA |
| Reverse: TCACGCAGTTGCCCCCGTTC | |
| rat GAPDH | Forward: AACCTGCCAAGTATGATGAC |
| Reverse: GGAGTTGCTGTTGAAGTCA | |
| rat UBQ | Forward: CACCAAGAAGGTCAAACAGGAA |
| Reverse: AAGACACCTCCCCATCAAACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palladini, G.; Cagna, M.; Di Pasqua, L.G.; Adorini, L.; Croce, A.C.; Perlini, S.; Ferrigno, A.; Berardo, C.; Vairetti, M. Obeticholic Acid Reduces Kidney Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats. Pharmaceuticals 2022, 15, 524. https://doi.org/10.3390/ph15050524
Palladini G, Cagna M, Di Pasqua LG, Adorini L, Croce AC, Perlini S, Ferrigno A, Berardo C, Vairetti M. Obeticholic Acid Reduces Kidney Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats. Pharmaceuticals. 2022; 15(5):524. https://doi.org/10.3390/ph15050524
Chicago/Turabian StylePalladini, Giuseppina, Marta Cagna, Laura Giuseppina Di Pasqua, Luciano Adorini, Anna Cleta Croce, Stefano Perlini, Andrea Ferrigno, Clarissa Berardo, and Mariapia Vairetti. 2022. "Obeticholic Acid Reduces Kidney Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats" Pharmaceuticals 15, no. 5: 524. https://doi.org/10.3390/ph15050524
APA StylePalladini, G., Cagna, M., Di Pasqua, L. G., Adorini, L., Croce, A. C., Perlini, S., Ferrigno, A., Berardo, C., & Vairetti, M. (2022). Obeticholic Acid Reduces Kidney Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats. Pharmaceuticals, 15(5), 524. https://doi.org/10.3390/ph15050524