Abstract
We report efficient synthetic methodologies for the preparation of 3-amino and 3-hydroxy 3-pyrrolin-2-ones (unsaturated γ-lactams) through a multicomponent reaction of amines, aldehydes and acetylene or pyruvate derivatives. The densely substituted γ-lactam substrates show in vitro cytotoxicity, inhibiting the growth of the carcinoma human tumor cell lines RKO (human colon epithelial carcinoma), SKOV3 (human ovarian carcinoma) and A549 (carcinomic human alveolar basal epithelial cell). In view of the possibilities for the diversity of the substituents that offer a multicomponent, synthetic methodology, an extensive structure–activity profile is presented. In addition, the bioisosteric replacement of the flat ester group by a tetrahedral phosphonate or phosphine oxide moiety in γ-lactam substrates leads to increased growth inhibition activity. Cell morphology analysis and flow cytometry assays indicate that the main pathway by which our compounds induce cytotoxicity is based on the activation of the intracellular apoptotic mechanism.
1. Introduction
Due to a rise in life expectancy and/or decline in the fertility rate, virtually every country in the world is experiencing growth in the number and proportion of older persons in the population. According to data from the United Nations [1], in 2019, 9% of people in the world were over aged age 65. By 2050, it is expected that 16% of the world’s population will be aged over 65, and one in four persons living in Europe and Northern America will be aged 65 or over. Moreover, the number of persons aged 80 years or over is projected to triple, from 143 million in 2019 to 426 million in 2050. These longevity gains are one of the greatest accomplishments of humankind but, at the same time, one of the most formidable challenges for the future with implications for nearly all socioeconomic sectors [2] and an especially high impact in all health care systems worldwide. During the last century, the causes of mortality changed from infectious and parasitic to chronic and degenerative diseases, and accordingly, cancer has become one of the world’s greatest health problems [3]. The systemic treatment of cancer often involves the administration of chemotherapeutic agents, which possess the ability to travel throughout the body and destroy malignant cells [4]. The development of antineoplasic drugs has undergone exponential growth in the last decades, but there is still a serious need to search for newer, safer and more potent cytotoxic drugs, especially due to the known ability of cancer cells to develop resistance to traditional therapies [5,6]. Here, drug discovery plays a crucial role through, first, the identification of drug candidates; second, the synthesis and characterization of target molecules; and finally, the evaluation of their therapeutic efficacy prior to drug development and subsequent clinical trials.
Among the innumerable potential chemotherapeutic agents, the γ-lactam ring (Figure 1) is a key structural scaffold used in medicinal chemistry that is found in the structures of many natural and synthetic bioactive compounds [7]. In particular, 3-pyrrolin-2-ones (Figure 1) are unsaturated γ-lactam derivatives that present a conjugated ring system which possesses latent reactivity for further modifications [8,9,10,11]. Moreover, the structures of these unsaturated γ-lactam derivatives are essential parts of the skeletons of numerous relevant bioactive molecules that show a large variety of biological activities [12,13,14], such as the cytotoxic polyketides Myceliothermophins E, C and D [15], cytotoxic Pukeleumid E present in Lyngbya majuscule algae [16], the HIV-integrase inhibitor Oteromicyn [17,18] and the antibiotic Pyrrocidine A [19].
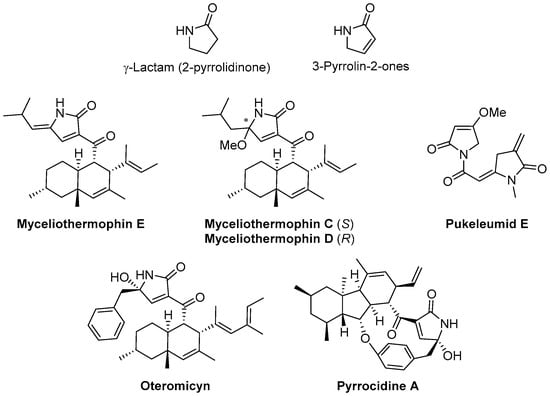
Figure 1.
Relevant γ-lactam-containing structures.
Interestingly, some unsaturated γ-lactam substrates have been identified as p53−MDM2 [20] and STAT3 [21] inhibitors that have strong antiproliferative activity. In addition, several 2-pyrrolidone derivatives have been described as antitumor agents [22,23,24,25,26,27,28,29].
As a part of our ongoing research on the multicomponent synthesis of γ-lactam derivatives, in 2006, we reported that a three-component reaction of amines, aldehydes and pyruvate derivatives in the presence of a Brønsted acid catalyst leads to the formation of 3-amino 3-pyrrolin-2-ones [30]. More recently, we developed an enantioselective version of this reaction [31] and extended this multicomponent protocol to the synthesis of phosphorus- and fluorine-containing isotetronic acid-based γ-lactams [32]. In addition, we were able to demonstrate that these substrates can be prepared through a similar multicomponent reaction using acetylene carboxylates instead of pyruvate derivatives [33]. These multicomponent protocols are considered essential tools in diversity-oriented synthesis [34,35] due to the high degree of molecular diversity achieved and, accordingly, they have become a preferential methodology in the field of medicinal chemistry [36,37]. 3-Amino 3-pyrrolin-2-ones can be seen as cyclic α-dehydro α-amino acids, and such skeletons are known to be present in many bioactive molecules, such as antimicrobials with anti-biofilm activity, caspase-3 inhibitors, analgesics, and antipyretics [38,39,40,41,42]. They also represent the basic structure of dithiopyrrolone antibiotics [43]. In addition, 3-hydroxy 3-pyrrolin-2-ones have been described as HIV integrase inhibitors [44,45], antibacterials [46,47,48], nootropics [49] and antivirals [50]. Additionally they show anticancer activity [26,27].
In this context, very recently, we conducted a study on the antiproliferative activity of 3-amino 3-pyrrolin-2-ones, showing evidence of the ability of these substrates to activate the intracellular apoptotic mechanism [29]. Taking into consideration the potential of five-membered heterocycles containing the 3-pyrrolin-2-one skeleton to act as anticancer agents, we believe that this report on the synthesis of unsaturated 3-hydroxy and 3-amino γ-lactam derivatives obtained by multicomponent methodologies and study of their applications as antiproliferative agents will be of great value to this field.
2. Results and Discussion
2.1. Chemistry
The multicomponent protocol for the synthesis of 3-amino 3-pyrrolin-2-ones 4 implies the reaction of aromatic amines 1, aldehydes 2 and acetylene carboxylate derivatives 3 in the presence of a catalytic amount of BINOL-derived phosphoric acid for several hours in refluxing toluene [32]. The presence of MgSO4 is necessary in order to remove the water generated in the process. The mechanism of the reaction comprises the initial formation of imine and enamine species 5 and 6 through the reaction of two equivalents of amine substrate 1 with aldehydes 2 and acetylene dicarboxylate derivatives 3. Next, an acid-promoted Mannich reaction leads to the formation of intermediate 7, which spontaneously evolves through intramolecular cyclization between the amine and the ester groups to yield γ-lactam substrates 4 (Scheme 1).
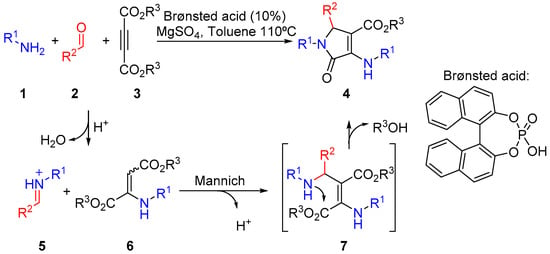
Scheme 1.
Multicomponent synthesis of 3-amino 3-pyrrolin-2-ones 4.
Following this approach, 13 densely functionalized substrates were synthesized in order to illustrate the synthetic potential of the reaction. First, the reaction was applied to the use of different amines 1 using benzaldehyde (2a, R2 = Ph) and diethyl acetylenedicarboxylate (3a, R3 = Et). The reaction with weakly activated p-toluidine (1a, R1 = p-CH3C6H4) as an amine substrate provided a very good yield of γ-lactam derivative 4a after 48 h. (Figure 2, 4a). However, the use of an electron-rich aniline or aliphatic amines, such as p-anisidine (1b, R1 = p-CH3OC6H4) or benzylamine (1c, R1 = Bn), led to a slight decrease in yield (Figure 2, 4b,c). Remarkably, when electron-deficient amines were used as substrates, the formation of imine and enamine intermediates was initially observed but, in this case, the reaction failed to provide the γ-lactam substrates. It should be noted that, in view of the mechanism proposed for the transformation (Scheme 1), the electronic character of the amine substrate might be a crucial factor in the reactivity of the key step of the multicomponent process. Accordingly, while the use of electron-rich amines may benefit the nucleophilic character of enamine species 6, this would result in a decrease in the electrophilic character of imine species 5. On the other hand, the use of deactivated amines would result in the activation of imine electrophile 5 and the collateral deactivation of enamine nucleophile 6. In addition, the slightly lower yield obtained using p-anisidine compared to p-toluidine can be also attributed to the formation of side products 8a,b, generated by a subsequent nucleophilic addition of an additional molecule of amine to the carboxylate group of lactam 4b. Amides 8a,b were isolated in 3% and 13% yield from the crude reaction (Figure 2).
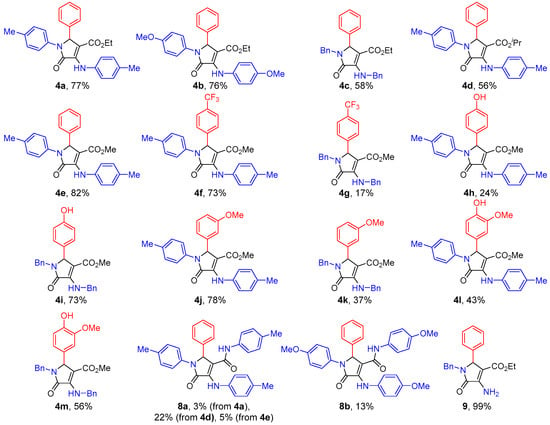
Figure 2.
3-Amino 3-pyrrolin-2-ones 4, 8 and 9 obtained.
The use of other acetylenedicarboxylates 3b,c (R3 = iPr or Me) as electrophile substrates in the multicomponent reaction with p-toluidine (1a, R1 = p-CH3C6H4) and benzaldehyde 2a (R2 = Ph) led to the formation of γ-lactams 4d,e in moderate to good yields (Figure 2). The bulkier di-iso-propyl acetylenedicarboxylate 3b (R3 = iPr) required a longer reaction time (72 h), which may have also facilitated the formation of the amide side product 8a as a result of the greater coexistence of p-toluidine (1a, R1 = p-CH3C6H4) and γ-lactam 4d. In the case of dimethyl acetylenedicarboxylate 3c (R3 = Me), amide 8a formed in equal proportion as with the use of diethyl acetylenedicarboxylate 3a, even though the reaction proceeded to full conversion with a shorter reaction time (24 h).
Next, the scope of the reaction was extended to the use of different aldehydes by utilizing dimethyl acetylenedicarboxylate 3c (R3 = Me) as the electrophile and p-toluidine (1a, R1 = p-CH3C6H4) and benzylamine (1c, R1 = Bn) as amine substrates. The use of electron-deficient p-trifluoromethyl benzaldehyde (2b, R2 = p-CF3C6H4) in the multicomponent reaction provided a more electrophilic imine species 5, favoring the reactivity of the Mannich intermediate process. Indeed, when p-toluidine (1a, R1 = p-CH3C6H4) was used as the reaction partner, a very good yield of lactam 4f was obtained with no presence of the amide side product, as expected due to the less nucleophilic character of the amine (Scheme 1). However, although the reaction with benzylamine (1c, R1 = Bn) led to the formation of a more nucleophilic enamine intermediate 6 for the Mannich reaction, for this particular case, a low yield of γ-lactam 4g was obtained, possibly due to the lower electrophilic character found for N-benzylimine intermediate 5 (Scheme 1).
On the contrary, it was expected that the intermediate Mannich reaction would be disfavored by the use of an aldehyde holding a strong electron-donating substituent, which would generate, in this case, a less electrophilic imine species 5. Accordingly, the use of p-hydroxybenzaldehyde (2c, R2 = p-HOC6H4) and p-toluidine (1a, R1 = p-CH3C6H4) in the multicomponent reaction led to the formation of a modest yield of γ-lactam 4h, possibly due to the poorer electrophilic character expected for N-arylimine intermediate 5 (Scheme 1). Nevertheless, the same reaction using benzylamine (1c, R1 = Bn) produced a good yield of γ-lactam derivative 4i, where an increase in the nucleophilic character of the enamine intermediate seems to drive the Mannich reaction and overcome the decreased electrophilicity expected for the N-benzylimine species 5 (Scheme 1). In accordance with the observed reactivity for the multicomponent reaction, m-anisaldehyde (2d, R2 = m-MeOC6H4) provided similar results as benzaldehyde (2a, R2 = Ph), with a very good yield for p-toluidine-derived γ-lactam 4j and a slightly lower yield for benzylamine-derived γ-lactam 4k (Figure 2). Finally, the scope of the reaction was completed using a disubstituted benzaldehyde. In this case, by following the same multicomponent protocol with dimethyl acetylenedicarboxylate 3c (R3 = Me) and vanillin (2e, R2 = 3-MeO-4-HO-C6H3), γ-lactams 4l–m were obtained with moderate to good yields (Figure 2). In addition, the benzyl group at the enamine moiety in 4c was selectively removed by hydrogenolysis through treatment with EtOH under an H2 atmosphere at 80 psi in the presence of 10% mol of palladium on carbon, leading to the production of lactam derivative 9 with a quantitative yield (Figure 2). Remarkably, under those conditions, only one of the benzyl groups was removed, and the carbon–carbon double bond remained intact.
Due to their chemical similitude to natural phosphate metabolites, phosphonate derivatives show multiple biological activities, and for this reason, they have numerous applications in medicine and agrochemistry [51,52,53,54,55,56]. Thus, in order to further broaden the scope of the Brønsted acid-catalyzed multicomponent reaction, we tried to extend the synthetic protocol to the use of activated-acetylene-bearing phosphorated groups (substitution of a carboxylate by a phosphonate group). However, the reaction using methyl 3-(diethoxyphosphinyl)-2-propynoate (MeO2C-C≡C-P(O)(OEt)2) instead of dialkyl acetylenedicarboxylates gave complex mixtures. Nevertheless, the use of phosphorus-substituted pyruvates as surrogates of acetylenedicarboxylates proved to be an excellent choice. To our delight, the Brønsted-acid-catalyzed multicomponent reaction of amines 1, aldehydes 2 and phosphorated pyruvates 10 in refluxing MTBE led to the formation of an excellent yield of phosphorus-substituted γ-lactam derivatives 12 after 48 h. (Figure 3). Similar to the multicomponent reaction using acetylene carboxylates (Scheme 1, vide supra), the enamine intermediate 6 was generated in this case through an amine-carbonyl condensation reaction between pyruvate derivative 10 and amine 1 using MgSO4 to remove the water released. Next, 3-amino 3-pyrrolin-2-ones 11 were formed in an identical Mannich reaction. Due to the high steric hindrance expected in the highly functionalized heterocycle, enamine derivatives 11 are not isolable, and 3-hydroxy 3-pyrrolin-2-ones 12 were obtained in this case after spontaneous hydrolysis of the enamine moiety (Figure 3).
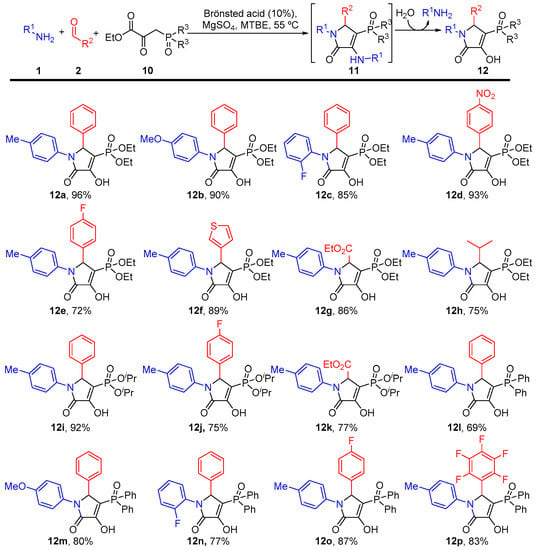
Figure 3.
3-Hydroxy 3-pyrrolin-2-ones 12 obtained.
First, the reaction was applied to different amine substrates 1 using benzaldehyde 2a (R2 = Ph) and diethylphosphoryl substituted pyruvate 10a (R3 = OEt), providing good to excellent yields of γ-lactam derivatives 12a–c (Figure 3). The scope of the reaction was extended to the use of different aldehydes using diethylphosphoryl-substituted pyruvate 10a (R1 = OEt) and p-toluidine (1a, R1 = p-CH3C6H4) as the reaction partners and providing good to excellent yields of γ-lactam derivatives 12d–h (Figure 3). In addition, the multicomponent reaction was applied to diverse phosphorus-substituted pyruvates to produce several different phosphonate and phosphine oxide-substituted lactams 12i–p (Figure 3).
Next, in order to extend the structural diversity obtained in this study, some additional 3-hydroxy 3-pyrrolin-2-ones 13 were prepared from alkyloxycarbonyl-substituted 3-amino 3-pyrrolin-2-ones 4 previously obtained from the multicomponent reaction using acetylenedicarboxylates. Upon treatment of γ-lactams 4 under acidic conditions, selective hydrolysis of the enamine moiety was observed, leading to the formation of enol-containing lactam substrates 13a–g. The reaction was applied successfully to ethyl ester-substituted γ-lactam substrates 13a–c derived from p-toluidine (1a, R1 = p-CH3C6H4), p-anisidine (1b, R1 = p-CH3OC6H4) or benzylamine (1c, R1 = Bn) and could be also extended to methyl esters derived from a variety of aldehydes for the preparation of γ-lactams 13d–g (Figure 4).
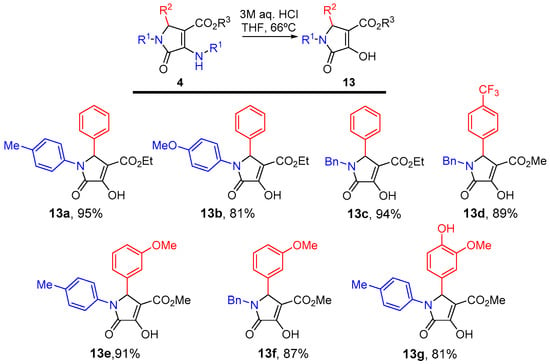
Figure 4.
3-Hydroxy 3-pyrrolin-2-ones 13 obtained.
With this collection of highly functionalized γ-lactam derivatives in our hands, we studied their biological activity. The antiproliferative activity of the substrates against several cancer cell lines was investigated.
2.2. Biological Results
The in vitro cytotoxicity of the γ-lactam derivatives was evaluated by testing their antiproliferative activity against several human cancer cell lines. The cell counting kit (CCK-8) assay was used for the evaluation of growth inhibition. Moreover, nonmalignant MRC5 lung fibroblasts were tested to study the selective toxicity [57], and chemotherapeutic doxorubicin was used as a reference.
In the first study, we tested the cytotoxicity of 3-amino 3-pyrrolin-2-ones 4, 8 and 9 obtained from SKOV3 (human ovarian carcinoma) and A549 (carcinomic human alveolar basal epithelial cell) cell lines. The cell proliferation inhibitory activity of the γ-lactams is shown as IC50 values (Table 1).

Table 1.
Antiproliferative activity of 3-amino γ-lactam derivatives 4, 8 and 9.
Accordingly, γ-lactam derivative 4a, derived from p-toluidine (1a), benzaldehyde (2a) and diethyl acetylenedicarboxylate (3a) showed a modest IC50 value of 11.70 ± 1.02 µM against the A549 cell line (Table 1, Entry 1). Analogous γ-lactams 4b,c, derived from p-anisidine or benzylamine presented IC50 values of 14.26 ± 1.80 and 2.42 ± 0.15 µM (Table 1, Entries 2–3). Remarkably, substrates 4a–c did not show significant cytotoxicity against SKOV3, while they displayed very good selectivity against nonmalignant cells with IC50 values higher than 50 µM (Table 1, Entries 1–3). Switching the ethyl ester with an iso-propylester group at the 5-membered ring resulted in an improved level of toxicity towards the A549 and SKOV3 cell lines with IC50 values of 3.34 ± 0.29 and 48.45 ± 2.90 µM, respectively, in substrate 4d while maintaining good selectivity with respect to the MRC5 cell line (Table 1, Entry 4). Remarkably, the presence of a methyl ester substituent in 4e provided a notable improvement in the inhibition of cell growth in the A549 cell line with a very good IC50 value of 1.67 ± 0.49 µM and very good selectivity against the SKOV3 and MRC5 cell lines (Table 1, Entry 5).
With the view that the best toxicity level was obtained for methyl ester derivative 4e, we next extended the structure–activity relationship study to an investigation of influence of the substituent at position 5 of the γ-lactam ring using 4e as the model. Although the effect of the introduction of fluorine atoms into the structure of organic compounds is rather difficult to predict, it very often leads to increased activity [58,59,60,61]. The key properties that make fluorine-containing compounds attractive in chemical biology include the small atomic radius and high electronegativity of the fluorine atom and the low polarizability of the C–F bond. In addition, the fact that the only natural isotope of 19F atom has a nuclear spin of ½ makes it ideal for monitoring studies by NMR. For this reason, we next tested the in vitro cytotoxicity of trifluoromethyl-containing γ-lactams 4f,g. The introduction of a para-trifluorophenyl substituent at the 5-membered ring did not result in improved activity, and IC50 values of 42.58 ± 2.55 and 7.64 ± 0.17 µM were obtained in A549 cell line for compounds 4f,g, respectively. However, compound 4f did show some toxicity against the SKOV3 cell line with a moderate IC50 value of 30.27 ± 1.03 µM. Remarkably, both compounds exhibited very high selectivity towards malignant cells with IC50 values higher than 50 µM in the MRC5 cell line. (Table 1, Entries 6–7).
The antioxidant properties of phenols are known to be associated with the antitumor activities of a plethora of compounds bearing this moiety [62]. Accordingly, the antiproliferative activity of phenol-substituted γ-lactams 4h,i, was tested. Indeed, an excellent IC50 value of 1.98 ± 0.18 µM was found for the A549 cell line for p-toluidine-derived γ-lactam 4h. Although compound 4h also showed some toxicity against nonmalignant cells, its selectivity was found to be 5 times higher compared with A549 cells. Likewise, in this case, the toxicity towards ovarian carcinoma was comparable to that observed in the MRC5 cell line (Table 1, Entry 8). In addition, benzylamine-derived γ-lactam 4i presented IC50 values of 10.71 ± 1.35 and 21.91 ± 1.53 µM in the A549 and SKOV3 cell lines, respectively, although a similar level of toxicity was found for nonmalignant cells (Table 1, Entry 9).
The methoxy group is a strong electron-donating substituent in aromatic rings that is known to be a widespread motif in drugs and natural products. The introduction of this moiety to potential anticancer agents very often leads to increased selective activity [63,64], which is attributed in part to its weak to medium antimitotic activity. Consequently, the cytotoxicity of m-methoxyphenyl-substituted γ-lactams 4j,k was next explored. For the particular case of p-toluidine-derived γ-lactam 4j, some cytotoxicity was observed against the A549 and SKOV3 cell lines with IC50 values of 13.03 ± 1.48 and 43.93 ± 1.66 µM, respectively, although not much selectivity was obtained compared with nonmalignant cells (Table 1, Entry 10). Moreover, switching p-toluidine with a benzylamine group in 4k did not have a positive effect on the cytotoxicity against the A549 cell line with an IC50 value of 11.39 ± 1.49 µM. However, compound 4k was found to be very selective in A549 cells compared with the SKOV3 or MRC5 cell lines (Table 1, Entry 11). To our surprise, the combination of phenol and methoxy moieties in γ-lactams 4l,m provided excellent IC50 values of 0.11 ± 0.016 and 6.02 ± 1.01 µM in the A549 cell line for p-toluidine- and benzylamine-derived substrates, respectively, with a high level of selectivity towards nonmalignant cells. Noticeably, p-toluidine derivative 4l delivered a very good IC50 value of 1.23 ± 0.31 µM against SKOV3 cells (Table 1, Entries 12–13).
In addition, the effect of the replacement of the methyl ester with an amide group had disparate effects on the antiproliferative activity of γ-lactam substrates. While a very good IC50 value of 2.97 ± 0.29 µM was obtained for the A549 cell line for p-toluidine derivative 8a, a modest IC50 value of 32.38 ± 1.58 µM was observed for p-anisidine derived substrate 8b. Further, compounds 8a,b also showed toxicity in the SKOV3 cell line with IC50 values of 6.95 ± 0.59 and 16.62 ± 0.19 µM, respectively. In addition, compound 8a was found to be very selective towards malignant cells, although substrate 8b presented significant toxicity in the MRC5 cell line (Table 1, Entries 14–15). Likewise, N-debenzylated substrate 9 gave slightly worse values compared with its precursor, γ-lactam 4c (Table 1, Entry 3 vs. Entry 16).
In order to further extend our structure–activity study, we next tested the antiproliferative activity of the 3-hydroxy γ-lactam derivatives 13 obtained from the hydrolysis of their parent enamine derivatives (see Figure 4). The replacement of enamine with an enol moiety in ethyl ester substituted structures 4a–c resulted in similar or slighter lower cytotoxic activity against the A549 cell line in compounds 13a–c (Table 2, Entries 1–3 vs. Table 1, Entries 1–3), showing IC50 values of 15.73 ± 1.27, 13.05 ± 0.56 and 4.50 ± 0.18 µM, respectively. Moreover, compounds 13a–c did not present significant toxicity in the SKOV3 or MRC5 cell lines. Similarly, methyl-ester-substituted 3-hydroxy γ-lactam 13d holding a p-trifluoromethyl substituent at the chiral carbon of the lactam ring presented lower toxicity against the A549 cell line compared with the parent enamine substrate 4g, with an IC50 value of 19.13 ± 3.00 µM and no toxicity towards the SKOV3 and MRC5 cell lines (Table 2, Entry 4 vs. Table 1, Entry 7). The same lowering of antiproliferative activity was observed in m-anisyl derivatives 13e,f and vanillin derivative 13g relative to their enamine precursors 4j–l, with IC50 values of 17.64 ± 3.76, 15.96 ± 1.97 and 13.30 ± 2.19 µM, respectively (Table 2, Entries 5–7 vs. Table 1, Entries 10–12). Compounds 13d,e showed no toxicity in the SKOV3 and MRC5 cell lines.

Table 2.
Antiproliferative activity of 3-hydroxy γ-lactam derivatives 13.
Bioisosterism represents an approach that is widely used for the rational modification of lead compounds into safer and more clinically effective agents [65]. Accordingly, it is well known that the substitution of a carboxylate with a phosphonate group in active substrates may result in new or increased activity [51,52,53,54,55,56]. For this reason, we next studied the antiproliferative activity of phosphorus-substituted γ-lactam derivatives 12 against the A549 and SKOV3 cell lines (Table 3). Indeed, the replacement of the ethyl carboxylate group with a diethyl phosphonate substituent in p-toluidine- and p-anisidine-derived lactams 13a,b, resulted in an increase in the cytotoxic activity towards the A549 cell line in 12a,b with IC50 values of 3.11 ± 0.31 and 4.56 ± 0,44 µM, respectively, and a high level of selectivity compared with the SKOV3 and MCR5 cell lines (Table 3, Entries 1–2 vs. Table 2, Entries 1–2). However, phosphorated γ-lactam 12c derived from o-fluoroaniline presented decreased activity in the A549 cell line with an IC50 value of 16.03 ± 1.49 µM.

Table 3.
Antiproliferative activity of 3-hydroxy γ-lactam derivatives 12.
With these results in hand, we next studied the effect of the substitution at the chiral carbon of the five membered ring 3-hydroxy 3-pyrrolin-2-ones 12d–h using the most active substrate 12a, derived from p-toluidine and diethyl phosphonate, as a model. The introduction of a strong electron-withdrawing p-nitrophenyl group at the stereogenic carbon of the γ-lactam ring had a very negative effect on the cytotoxicity, and IC50 values higher than 50 μM were found for compound 12d in both the A549 and SKOV3 cell lines (Table 3, Entry 4).
In spite of the benefits expected from the introduction of a fluorine atom into the heterocyclic structure [58,59,60,61], p-fluorophenyl-substituted lactam 12e provided a slightly worse IC50 value of 6.6 ± 0.58 μM in the A549 cell line relative to the parent compound 12a (Table 3, Entry 5 vs. Entry 1). The introduction of other heteroaromatic, ester or aliphatic substituents also had a negative effect on the toxicity of substrates 12. Accordingly, a drop in antiproliferative activity towards the A549 cell line was observed for 2-thienyl, ethoxycarbonyl and iso-propyl substituted γ-lactams 12f–h with IC50 values of 23.29 ± 2.4, 8.27 ± 0.91 and 24.20 ± 0.81 μM, respectively. However, compounds 12f–h presented high selectivity compared with the SKOV3 cell line and nonmalignant cells (Table 3, Entries 6–8).
Continuing with our interest on phosphorus-containing heterocycles, we next tested the cell growth inhibition activity of bulkier di-iso-propyl phosphonates 12i–k. Although slightly higher IC50 values of 5.36 ± 0.28 and 5.91 ± 0.69 μM were found in the A549 cell line for phenyl- and p-fluorophenyl-substituted lactams 12i,j with respect to their parent diethyl phosphonate derivatives 12a,e, those substrates presented significant toxicity in SKOV3 cells with IC50 values of 11.56 ± 3.36 and 15.55 ± 1.60 μM and high selectivity towards nonmalignant cells (Table 3, Entries 9, 10 vs. Entries 1, 5). Moreover, the presence of a carboxylate group in di-iso-propyl phosphonate substituted γ-lactam 12f led to a complete loss of cytotoxicity in all cancer cell lines (Table 3, Entry 11).
Although the appearance of phosphine oxides in drug discovery is rare compared with their counterparts phosphates, phosphonates or phosphoramidates, a few of these derivatives have been proven to be excellent drug candidates, such as the anticancer drugs ridaforolimus [66,67] or brigatinib [68,69]. For this reason, in order to further expand the study of the effect of the phosphorus substituent at the γ-lactam ring, we extended the structure–activity study to substrates 12l–p, holding a phosphine oxide substituent at C-4. Accordingly, first, the antiproliferative activity of 5-phenyl-substituted lactam substrates was studied, holding a diphenylphosphine oxide moiety at C-4. In this case, p-toluidine-derived substrate 12l presented lower cytotoxicity than its phosphonate analogs 12a,i with an IC50 value of 11.86 ± 1.35 μM in the A549 cell line (Table 3, Entry 12 vs. Entries 1, 9). However, the presence of a diphenylphosphine oxide moiety in p-anisidine and o-fluoroaniline derivatives 12m,n resulted in an improvement in cytotoxicity towards the A549 cell line compared with the parent diethyl phosphonate substrates 12b,c, showing IC50 values of 3.72 ± 0.32 and 5.5 ± 1.35 μM, respectively (Table 3, Entries 13, 14 vs. Entries 2, 3). Likewise, the presence of a p-fluorophenyl substituent at the chiral center of phosphine oxide derivative 12o provided a very active substrate with IC50 values of 1.46 ± 0.19 and 21.97 ± 3.42 μM in the A549 and SKOV3 cell lines, respectively (Table 3, Entry 15). Nevertheless, the parent perfluorophenyl derivative 12p showed less activity with a modest IC50 value of 20.34 ± 0.79 μM against the A549 cell line and no toxicity in the SKOV3 cell line (Table 3, Entry 16). It should be remarked that all active phosphorated 3-hydroxy 3-pyrrolin-2-ones 12 presented excellent selectivity towards malignant cells with IC50 values higher than 50 μM in nonmalignant cells in all cases.
Finally, the most active and selective γ-lactam substrates were chosen, and their cytotoxicity levels were tested against the RKO cell line (human colon epithelial carcinoma). Although most of the substrates were found to be inactive towards this cell line, compounds 8a and 12l showed significant grown inhibition activity with IC50 values of 18.67 ± 1.31 and 33.62 ± 0.41 μM, respectively (See Table S1 in the Supplementary Materials).
In order to clarify the mechanism of action of these unsaturated γ-lactam derivatives, cell morphology analysis and flow cytometry assays were performed (See Supplementary Materials). First, flow cytometry assays were conducted using compounds 4l (1 µM) and 12a (5 µM) in A549 cells, and measurements were carried out 24 h post-exposure. This assay allowed us to distinguish four separate cell populations: live cells (FL-1 and FL-3 negative), early apoptotic live cells (FL-1 positive, FL-3 negative), late apoptotic dead cells (FL-1 and FL-3 positive) and nonapoptotic dead cells (FL-1 negative, FL-3 positive). At the time of measurement, around 80% of cells exposed to compound 4l showed a positive FL-1 signal and negative FL-3 signal, indicating an early apoptotic cell population, while in cells exposed to compound 12a, the percentage of early apoptotic cells was lower, around 50% (Figure 5). At this time point, percentages of late apoptotic cells were still low in both cases with values below 10%. These results indicate that, within 24 h after exposure to compounds 4l and 12a, around 90% and 60% of cells, respectively, had activated the apoptotic mechanisms. Necrotic cells that were nonapoptotic represented less than 1% of the whole cell population for both compounds, suggesting that the majority of dead cells detected in this study were apoptotic cells. Finally, nonapoptotic live cells represented around 10% of the whole cell population. These results reveal that compound 4l has a higher capacity to induce apoptosis compared with 12a. Therefore, further cell imaging studies were carried out with the more active compound 4l.
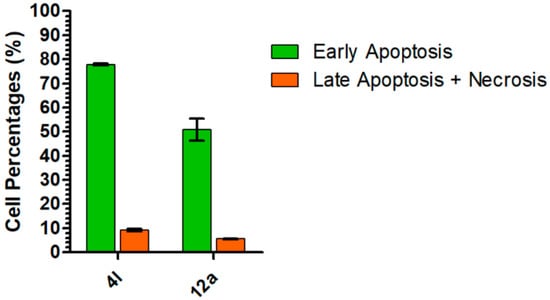
Figure 5.
Percentages of early apoptotic (green bars) and late apoptotic (orange bars) A549 cells 24 h after exposure to compounds 4l (1 µM) and 12a (5 µM). Each value represents the mean ± SD of 3 measurements.
The cell morphology of the A549 cell line was analyzed at different exposure times after the addition of three different concentrations of 3-amino 3-pyrrolin-2-one 4l to observe the cellular modifications during the treatment. The first studied concentration was 1.1 µM, which is a ten-fold higher concentration compared with the IC50 of 4l. At that high concentration, a disruption of cellular growth was noticed, especially after 6 h of exposure, and the suspension of cellular growth became gradually more pronounced over time (Figure 6A). Then, the IC50 concentration of 4l was used (0.11 µM) for this experiment, and peak cellular death was observed 24 or 48 h after the addition of the compound. Then, live cells started growing again, and higher cellular confluence was witnessed after 72 h (Figure 6B). On the contrary, the addition of 4l at 0.02 µM, a five-fold lower concentration than the measured IC50, showed cells with a healthy, uniform morphology, and cellular growth was recognizable over time, meaning that this concentration was well tolerated (Figure 6C).
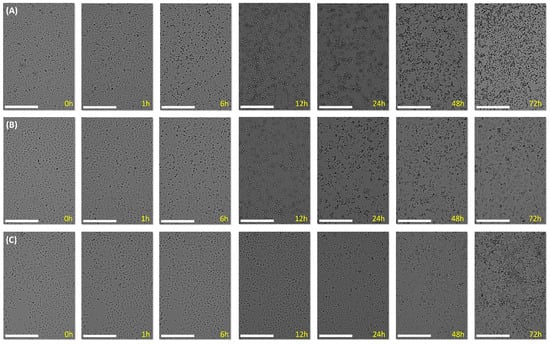
Figure 6.
Cell morphology visualization with an X4 lens at 0, 1, 6, 12, 24, 48 and 72 h after exposure to compound 4l. Scale bar: 300 µm. (A) A549 cells treated with 1.1 µM of 4l. (B) A549 cells treated with 0.11 µM of 4l. (C) A549 cells treated with 0.02 µM of 4l.
In addition, it is worth mentioning that all compounds, with the exception of 3-hydroxy γ-lactam derivative 12p, fulfilled the requirements for orally active drugs for use in humans in accordance with Lipinski’s rule of five. In addition, and according to the predictions, most of the synthesized compounds may have high levels of gastrointestinal absorption, however not all of them seem to have the ability to cross the blood–brain barrier (see Table S2 in the Supplementary Materials).
3. Material and Methods
3.1. Chemistry
3.1.1. General Experimental Information
Solvents for extraction and chromatography were of technical grade. All solvents used in reactions were freshly distilled from appropriate drying agents before use. All other reagents were recrystallized or distilled as necessary. All reactions were performed under an atmosphere of dry nitrogen. Analytical TLC was performed with silica gel 60 F254 plates. Visualization was accomplished by UV light. 1H, 13C, 31P and 19F NMR spectra were recorded on a Varian Unity Plus (Varian Inc., NMR Systems, Palo Alto, CA, USA) (at 300 MHz, 75 MHz, 120 MHz and 282 MHz, respectively) and on a Bruker Avance 400 (Bruker BioSpin GmbH, Rheinstetten, Germany) (at 400 MHz for 1H, and 100 MHz for 13C). Chemical shifts (δ) are reported in ppm relative to the residual CHCl3 (δ = 7.26 ppm for 1H and δ = 77.16 ppm for 13C NMR), and phosphoric acid (50%) was used as an external reference (δ = 0.0 ppm) for 31P NMR spectra. Coupling constants (J) are reported in Hertz. Data for the 1H NMR spectra are reported as follows: chemical shift, multiplicity, coupling constant, and integration. The multiplicity abbreviations used are as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet). 13C NMR peak assignments were supported by Distortionless Enhanced Polarization Transfer (DEPT). High resolution mass spectra (HRMS) were obtained by positive-ion electrospray ionization (ESI). Data are reported in the form m/z (intensity relative to base = 100). Infrared spectra (IR) were produced with a Nicolet iS10 Termo Scientific spectrometer (Thermo Scientific Inc., Waltham, MA, USA) as neat solids. Peaks are reported in cm−1.
3.1.2. Compound Purity Analysis
All synthesized compounds were analyzed by HPLC to determine their purity. The analyses were performed with an Agilent 1260 infinity HPLC system (Agilent, Santa Clara, CA, USA) (C-18 column, Hypersil, BDS, 5 μm, 0.4 mm × 25 mm) at room temperature. All tested compounds were dissolved in dichloromethane, and 5 μL of the sample was loaded onto the column. Ethanol:Heptane (90:10) or Ethanol:Ethyl acetated (50:50) was used as the mobile phase, and the flow rate was set at 1.0 mL/min. The maximal absorbance at the range of 190–400 nm was used as the detection wavelength. The purity of all tested lactam derivatives 4, 8, 9, 12 and 13 was >95%, which meets the purity requirement of the Journal.
3.1.3. Representative Experimental Procedures and Characterization Data for Compounds 4, 8, 9, 12 and 13
Representative Procedure for the Multicomponent Reaction of Amines 1, Aldehydes 2 and Acetylenes 3
A solution of amine 1 (4 mmol), aldehyde 2 (2 mmol), acetylene dicarboxylate derivative 3 (2 mmol) and BINOL-derived phosphoric acid (70 mg, 0.2 mmol) was stirred in the presence of anhydrous MgSO4 in Toluene (10 mL) at 110 °C for 24–72 h (see ESI). Then, the volatiles were distilled off at reduced pressure, and the crude residue was purified by column chromatography (Hexanes/AcOEt) to produce pure γ-lactams 4 and 8.
Ethyl 5-oxo-2-phenyl-1-(p-tolyl)-4-(p-tolylamino)-2,5-dihydro-1H-pyrrole-3-carboxylate (4a). The general procedure was followed using p-toluidine (1a) (429 mg, 4 mmol), benzaldehyde (2a) (204 μL, 2 mmol) and diethyl acetylenedicarboxylate (3a) (320 μL, 2 mmol). The residue was purified by column chromatography (Hexanes/AcOEt 8:2), producing 656 mg (77%) of 4a as a white solid. M.p. (Et2O) 154–155 °C. 1H NMR (400 MHz, CDCl3): δ 8.17 (bs, 1H, NH), 7.34 (d, 3JHH = 8.5 Hz, 2H, 2 × CHar), 7.26–7.21 (m, 5H, 5 × CHar), 7.12 (d, 3JHH = 8.3 Hz, 2H, 2 × CHar), 7.08 (d, J = 8.5 Hz, 2H, 2 × CHar), 7.03 (d, 3JHH = 8.3 Hz, 2H, 2 × CHar), 5.77 (s, 1H, CHN), 4.01 (q, 3JHH = 7.1 Hz, 2H, CH2 OEt), 2.33 (s, 3H, CH3 Tol), 2.23 (s, 3H, CH3 Tol), 1.01 (dd, 3JHH = 7.1 Hz, 3JHH = 7.1 Hz, 3H, CH3 OEt). 13C {1H} NMR (101 MHz, CDCl3) δ 164.7 (C=O ester), 164.1 (C=O amide), 142.7 (=Cquat), 137.2 (Cquat), 136.1 (Cquat), 135.5 (Cquat), 134.6 (Cquat), 134.2 (Cquat), 129.5 (2 × CHar), 129.1 (2 × CHar), 128.4 (2 × CHar), 128.1 (CHar), 127.83(2 × CHar), 123.2 (2 × CHar), 122.8 (2 × CHar), 108.9 (=Cquat), 63.3 (CHN), 60.2 (CH2 OEt), 21.1 (CH3), 21.0 (CH3), 14.0 (CH3 OEt). FTIR (neat) νmax: 3289 (N-H), 1701 (C=O), 1679 (C=O), 1632 (C=C). HRMS (Q-TOF) m/z calcd for C27H26N2O3 [M]+ 426.1943, found 426.1950.
5-Oxo-2-phenyl-N,1-di-p-tolyl-4-(p-tolylamino)-2,5-dihydro-1H-pyrrole-3-carboxamide (8a). The general procedure was followed using p-toluidine (1a) (429 mg, 4 mmol), benzaldehyde (2a) (204 μL, 2 mmol) and diethyl acetylenedicarboxylate (3a) (284 mg, 2 mmol). The residue was purified by column chromatography (Hexanes/AcOEt 8:2), producing 29 mg (3%) of 8a as a white solid. A higher yield (22%) was achieved using di-iso-propyl acetylenedicarboxylate (3b) (396 mg, 2 mmol). M.p. (Et2O) 226 °C (dec.). 1H NMR (300 MHz, CDCl3) δ 8.31 (bs, 1H, NH), 7.38–7.28 (m, 6H, 6 × CHar), 7.11–7.04 (m, 7H, 7 × CHar), 6.96 (d, 3JHH = 8.5 Hz, 2H, 2 × CHar), 6.84 (d, 3JHH = 8.5 Hz, 2H, 2 × CHar), 6.63 (bs, 1H, NH), 5.85 (s, 1H, CHN), 2.28 (s, 3H,CH3 Tol), 2.25 (s, 3H, CH3 Tol), 2.24 (s, 3H, CH3 Tol). 13C {1H} NMR (75 MHz, CDCl3) (75 MHz, CDCl3) δ 164.75 (C=O), 162.12 (C=O), 139.1 (=Cquat), 136.6 (Cquat), 136.1 (Cquat), 135.8 (Cquat), 134.8 (Cquat), 134.6 (Cquat), 133.9 (Cquat), 133.8 (Cquat), 129.7 (4 × CHar), 129.5 (2 × CHar), 129.4 (2 × CHar), 129.3 (CHar), 128.0 (2 × CHar), 123.3 (2 × CHar), 122.5 (2 × CHar), 119.8 (2 × CHar), 112.4 (=Cquat), 63.8 (CHN), 21.1 (CH3), 21.0 (CH3), 21.0 (CH3). FTIR (neat) νmax: 3309 (N-H), 3251 (N-H), 1685 (C=O), 1632 (C=C). HRMS (Q-TOF) m/z calcd for C27H26N2O3 [M]+ 487.22598, found 487.2255.
Representative Procedure for the Multicomponent Reaction of Amines 1, Aldehydes 2 and Phosphorated Pyruvate Derivatives 10
A solution of amine 1 (4 mmol), aldehyde 2 (2 mmol), ethyl pyruvate derivative 10 (6 mmol), BINOL-derived phosphoric acid (70 mg, 0.2 mmol) and anhydrous MgSO4 was stirred in MTBE (10 mL) at 55 °C for 48 h. The volatiles were distilled off at reduced pressure and the crude residue was purified by column chromatography (Hexanes/AcOEt) to produce pure γ-lactams 12.
Diethyl (4-hydroxy-5-oxo-2-phenyl-1-(p-tolyl)-2,5-dihydro-1H-pyrrol-3-yl)phosphonate (12a). The general procedure was followed using p-toluidine (1a) (429 mg, 4 mmol), benzaldehyde (2a) (204 μL, 2 mmol) and ethyl 3-(diethoxyphosphoryl)-2-oxopropanoate (10a) (1.513 g, 6 mmol). The residue was purified by column chromatography (Hexanes/AcOEt 3:7), producing 770 mg (96%) of 12a as a white solid. M.p. (Et2O) 145–147 °C. 1H NMR (300 MHz, CDCl3) δ 9.75 (bs, 1H, OH), 7.36 (d, 3JHH = 8.5 Hz, 2H, 2 × CHar), 7.26–7.13 (m, 5H, 5 × CHar), 7.05 (d, 3JHH = 8.3 Hz, 2H, 2 × CHar), 5.58 (d, 3JPH = 2.8 Hz, 1H, CH), 4.15 (m, 2H, OCH2), 3.70 (m, 1H, OCH2), 3.16 (m, 1H, OCH2), 2.23 (s, 3H, CH3, Tol), 1.39 (dd, 3JHH = 7.1 Hz, 3JHH = 7.1 Hz, 3H, OEt), 0.80 (dd, 3JHH = 7.1 Hz, 3JHH = 7.1 Hz, 3H, OEt). 13C NMR {1H} (75 MHz, CDCl3) δ 163.0 (d, 2JPC = 19.8 Hz = Cquat), 160.0 (d, 3JPC = 6.5 Hz, C=O), 135.7 (Cquat), 135.5 (Cquat), 134.1 (Cquat), 129.7 (2 × CHar), 129.1 (2 × CHar), 128.7 (CHar), 127.1 (2 × CHar), 121.9 (2 × CHar), 106.6 (d, 1JPC = 200.8 Hz, Cquat-P), 62.98 (d, 2JPC = 5.4 Hz, CH2O), 62.71 (CH), 62.56 (d, 2JPC = 4.5 Hz, CH2O), 21.0 (CH3), 16.5 (d, 3JPC = 6.4 Hz, CH3), 15.6 (d, 3JPC = 7.5 Hz, CH3). 31P NMR (121 MHz, CDCl3) δ 15.4. FTIR (neat) νmax: 3112 (OH), 1692 (C=O), 1663 (C=C), 1165 (P=O), 1021 (P-O-C). HRMS (ESI-TOF) m/z: [M+H]+ calcd for C21H25NO5P 402.1470; Found 402.1468.
Representative Procedure for the Hydrolysis of 3-Amino 3-Pyrrolin-2-Ones 4: Synthesis of 3-Hydroxy 3-Pyrrolin-2-Ones 13
Compound 4 (0.5 mmol) was dissolved in a 1:1 mixture of a 3 M aqueous solution of HCl and THF (10 mL), and the reaction was heated to 66 °C overnight. The reaction progress was monitored by TLC.(Scharlab, S.L, Barcelona, Spain) The resulting mixture was concentrated under reduced pressure in order to evaporate THF and then diluted with AcOEt (10 mL). The organic layer was washed with a 3M aqueous solution of NaOH (2 × 5 mL) and H2O (2 × 5 mL) and dried over anhydrous MgSO4, and the crude residue was purified by crystallization from Et2O/pentane to produce pure γ-lactams 13.
Ethyl 4-hydroxy-5-oxo-2-phenyl-1-(p-tolyl)-2,5-dihydro-1H-pyrrole-3-carboxylate (13a). The general procedure was followed, producing 0.161 g (95%) of 13a as a white solid. M.p. (Et2O) 170–172 °C. 1H-NMR (300 MHz, CDCl3) δ 9.19 (bs, 1H, OH), 7.38 (d, 3JHH = 8.2 Hz, 2H, 2 × CHar), 7.32–7.25 (m, 5H, 5 × CHar), 7.09 (d, 3JHH = 8.2 Hz, 2H, 2 × CHar), 5.74 (s, 1H, CH), 4.20 (q, 3JHH =, 7.1 Hz, 2H, CH2O), 2.26 (s, 3H, CH3 Tol), 1.20 (dd, 3JHH = 7.1Hz, 3JHH = 7.1Hz, 3H, CH3 OEt). 13C {1H} NMR (75 MHz, CDCl3) δ 165.0 (C=O ester), 162.9 (C=O amide), 156.4 (=Cquat), 135.7 (Cquat), 135.3 (Cquat), 133.7 (Cquat), 129.6 (2 × CHar), 128.6 (2 × CHar), 128.5 (CHar), 127.6 (2 × CHar), 122.4 (2 × CHar), 113.1 (=Cquat), 61.8 (CHN), 61.2 (CH2 OEt), 20.9 (CH3 Tol), 14.0 (CH3 OEt). FTIR (neat) νmax: 3425 (O–H), 1704 (C=O), 1675 (C=O), 1643 (C=C). HRMS (Q-TOF) m/z calculated for C27H26N2O3 [M]+ 337.1314, found 337.1319.
3.2. Biology
3.2.1. Materials
Reagents and solvents were used as purchased without further purification. All stock solutions of the investigated compounds were prepared by dissolving the powered materials in appropriate amounts of DMSO. The final concentration of DMSO never exceeded 5% (v/v) in reactions. The stock solution was stored at 5 °C until use.
3.2.2. Cell Culture
Human epithelial lung carcinoma cells (A549) (ATCC® CCL-185™, ATCC—Manassas, VA, USA) were grown in Kaighn’s Modification of Ham’s F-12 Medium (ATCC® 30-2004™, ATCC—Manassas, VA, USA), and lung fibroblast cells (MRC5) (ATCC® CCL-171™, ATCC—Manassas, VA, USA) were grown in Eagle’s Minimum Essential Medium (EMEM, ATCC® 30-2003™, ATCC—Manassas, VA, USA). Ephitelial ovary adenocarcinoma cells (SKOV3) (ATCC® HTB-77™, ATCC—Manassas, VA, USA) were grown in McCoy’s 5A medium (ATCC® 30-2007™, ATCC—Manassas, VA, USA), and colon carcinoma cells (RKO) (ATCC® CRL-2577™, ATCC—Manassas, VA, USA) were grown in Eagle’s Minimum Essential Medium (EMEM, ATCC® 30-2003™, ATCC—Manassas, VA, USA). All were supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich, Madrid, Spain) and 1% NORMOCIN solution (Thermo Fisher, Waltham, MA, USA). Cells were incubated at 37 °C in a 5% CO2 atmosphere and were split every 3–4 days to maintain monolayer coverage. For the cytotoxicity experiments, A549 cells were seeded in 96-well plates at a density of 2.5–3 × 103 cells per well and incubated overnight to achieve 70% confluence at the time of exposure to the cytotoxic compound.
3.2.3. Cytotoxicity Assays
Cells were incubated with different concentrations (50 µM, 30 µM, 20 µM, 10 µM, 5 µM, 2.5 µM and 1 µM) of the cytotoxic compounds for 48 h. Then, 10 µL of cell counting-kit 8 was added into each well, and cells were incubated for an additional 2 h at 37 °C in a 5% CO2 atmosphere. The absorbance of each well was determined by an Automatic Elisa Reader System (Thermo Scientific Multiskan FC, Thermo Scientific, Shangai, China) at a wavelength of 450 nm. If the obtained IC50 value was below 1 µM, the assay was repeated at lower concentrations (10 µM, 5 µM, 2.5 µM, 1 µM, 0.5 µM, 0.2 µM and 0.1 µM).
3.2.4. Evaluation of Cytotoxicity Mechanisms
Flow cytometry assays were conducted using a FACSCalibur system flow cytometer (Becton Dickinson Bioscience, San Jose, CA, USA) in order to identify apoptotic cells and differentiate them from necrotic cells. A549 cells were exposed to 1 µM and 5 µM of cytotoxic compounds 4l and 12a, and cell apoptosis and necrosis were evaluated 24 h after exposure. For that purpose, treated cells were washed with phosphate buffered saline (PBS) (Sigma-Aldrich, Madrid, Spain) and detached with trypsin/EDTA (0.25%) (Gibco, Waltham, MA, USA). Cells were centrifuged at 1100 rpm for 5 min, and then the resulting pellet was resuspended in cell growth media and transferred to specific flow cytometer tubes. Propidium iodide (Sigma-Aldrich, Madrid, Spain) at a dilution of 1:300 was used in each sample to detect necrotic cells, and eBioscience™ Annexin V Apoptosis Detection Kit FITC (Fisher Scientific, Madrid, Spain) was used to detect apoptotic cells in accordance with the manufacturer’s instructions. The fluorescent signals corresponding to necrotic cells and apoptotic cells were measured at 650 nm (FL3) and 525 nm (FL1), respectively. Nontreated cells, used as negative control samples, were displayed on a forward scatter (FSC) versus side scatter (SSC) dot plot to establish a collection gate and exclude cells debris. Cells treated with 1 µM of camptothecin (Sigma-Aldrich, Madrid, Spain) served as a positive control for apoptosis and were used to establish cytometer settings and channel compensations. The experiments were carried out in triplicate for each condition. For each sample, 10,000 events were collected.
3.2.5. Visualization of Cell Growth and Morphology
A qualitative analysis of A549 cell growth and morphology after exposure to 1.1 µM, 0.11 µM and 0.02 µM of the cytotoxic compound 4l was conducted using the CytationTM 1 Cell Imaging Multi-Mode Reader (Biotek, Winooski, VT, USA). Cell images were acquired immediately after the addition of the compound and at the following time points after exposure: 1, 6, 12, 24, 48 and 72 h.
4. Conclusions
In conclusion, two complementary multicomponent methodologies were used for the preparation of 3-amino and 3-hydroxy 3-pyrrolin-2-ones, holding a variety of substituents at the five-membered ring. This strategy allows the possibility of structural diversity in the resultant scaffold depending on the starting amine, aldehyde and pyruvate or acetylene derivative, in a single step. The obtained γ-lactam derivatives showed in vitro cytotoxicity, inhibiting the growth of human tumor cells RKO (human colon epithelial carcinoma), SKOV3 (human ovarian carcinoma) and A549 (carcinomic human alveolar basal epithelial cell), and producing low activity toward MRC5 nonmalignant lung fibroblasts. QSAR studies indicate that the cytotoxicity is enhanced, in general, by the presence of aromatic groups bearing lipophilic methyl substituents or fluorine atoms. Moreover, the presence of an ester group at C-4 of the 5-membered heterocycle provided lower IC50 values than those previously reported for unsubstituted γ-lactam derivatives. Better antiproliferative activity was obtained for small methyl esters compared with ethyl or iso-propyl esters, which suggests a crucial interaction of the ester group, which can be complicated if a bulky group is present. In addition, while increased cytotoxicity was observed for 3-amino substituted γ-lactams compared with the 3-hydroxy substituted derivatives, the antiproliferative activity of such enol derivatives was enhanced when the flat ester group was isosterically replaced by a tetrahedral phosphonate or phosphine oxide moiety. Methyl ester-substituted γ-lactam 4e, derived from p-toluidine, presented an excellent IC50 value of 1.67 µM against the A549 cell line with excellent selectivity towards the SKOV3 and RKO cell lines and nonmalignant cells. Its amide derivative 8a showed good toxicity against lung, ovarian and colon cancer cells with IC50 values of 2.97, 6.95 and 18.67 µM, respectively, as well as very high selectivity towards malignant cell lines compared with noncancerous MRC5. The combination of phenol and methoxy moieties provided excellent IC50 values of 0.11 and 1.23 µM in the A549 and SKOV3 cell lines for p-toluidine-derived 3-amino γ-lactam 4l with a 10 to 100 times higher selectivity level towards nonmalignant cells. The best level of cytotoxicity for 3-hydroxyγ-lactams was found for compound 4l with an IC50 value of 4.00 μm in the A549 cell line. Likewise, the bioisosterism approach was shown to be an excellent tool in this study, leading to increased cytotoxicity for phosphorus-substituted γ-lactams with respect to the parent carboxylate-substituted derivatives with IC50 values of 1.46 and 21.97 µM in the A549 and SKOV3 cell lines in phosphine oxide substituted γ-lactam 12o. These results may support the relevance of the isosterical substitution of carboxylic groups with tetrahedral phosphorus derivatives in view of their ability to block enzymes involved in the hydrolysis of peptides. In general terms, the γ-lactam derivatives described in this study were shown to be highly active towards the A549 cell line, while the SKOV3 and RKO cell lines were found to be more resistant. Importantly, most of the substrates showed high selectivity in cancer cells compared with nonmalignant cells. In addition, the cell morphology analysis and flow cytometry assays indicate that the main pathway by which γ-lactam derivatives induce cytotoxicity towards cancer cells is based on the activation of intracellular apoptotic mechanisms.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph15050511/s1: experimental procedures and characterization data for compounds 4, 8, 9, 12 and 13; 1H NMR, 13C NMR, 31P NMR and 19F NMR spectra of compounds 4, 8, 9, 12 and 13; HPLC chromatograms of compounds 4, 8, 9, 12 and 13; Flow cytometric assays on A-549 cells after addition of compounds 4l and 12a;e; Table S1 antiproliferative activity in RKO cell line and Table S2 calculation of Lipinski’s rule of five and prediction of ADME properties.
Author Contributions
Conceptualization, A.L.-F., X.d.C., E.M.d.M., J.M.d.l.S., C.A., F.P. and J.V.; methodology, A.L.-F., X.d.C., I.V.-B. and M.S.-R.; software, A.L.-F., X.d.C., I.V.-B. and M.S.-R.; validation, E.M.d.M. and J.V.; formal analysis, A.L.-F. and X.d.C.; investigation, A.L.-F., X.d.C., I.V.-B. and M.S.-R.; resources, E.M.d.M., J.L.P., J.M.d.l.S., C.A., F.P. and J.V.; data curation, A.L.-F. and X.d.C.; writing—original draft preparation, J.V.; writing—review and editing, A.L.-F., X.d.C., I.V.-B., M.S.-R., J.M.d.l.S., C.A., E.M.d.M., F.P. and J.V.; visualization, E.M.d.M., J.L.P., F.P. and J.V.; supervision, E.M.d.M. and J.V.; project administration, E.M.d.M. and J.V.; funding acquisition, E.M.d.M., J.M.d.l.S., C.A., J.L.P., F.P. and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support by Ministerio de Economía, Industria y Competividad (MINECO) (RTI2018-101818-B-I00) and Gobierno Vasco (GV, IT 992-16) is gratefully acknowledged. X.d.C. and A.L.-F. thank the Basque Country Government for a predoctoral grant. I.V.-B. thanks the University of the Basque Country (UPV/EHU) for a postdoctoral fellowship (ESPDOC19/47). M.S.-R. thanks the University of the Basque Country (UPV/EHU) for a pre-doctoral fellowship (PIF17/79).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Files.
Acknowledgments
The authors are thankful for the technical and human support provided by SGIker (UPV/EHU/ERDF, EU).
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Légaré, J. Population Aging: Economic and Social Consequences. In International Encyclopedia of the Social & Behavioral Sciences, 2nd ed.; Elsevier: Oxford, UK, 2015; pp. 540–544. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically active γ-lactams: Synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef]
- Schümann, J.; Hertweck, C. Molecular Basis of Cytochalasan Biosynthesis in Fungi: Gene Cluster Analysis and Evidence for the Involvement of a PKS-NRPS Hybrid Synthase by RNA Silencing. J. Am. Chem. Soc. 2007, 129, 9564–9565. [Google Scholar] [CrossRef]
- Albrecht, D.; Basler, B.; Bach, T. Preparation and Intramolecular [2+2]-Photocycloaddition of 1,5-Dihydropyrrol-2-ones and 5,6-Dihydro-1H-pyridin-2-ones with C-, N-, and O-linked Alkenyl Side Chains at the 4-Position. J. Org. Chem. 2008, 73, 2345–2356. [Google Scholar] [CrossRef]
- Chatzimpaloglou, A.; Kolosov, M.; Eckols, T.K.; Tweardy, D.J.; Sarli, V. Synthetic and Biological Studies of Phaeosphaerides. J. Org. Chem. 2014, 79, 4043–4054. [Google Scholar] [CrossRef]
- Melekhina, V.G.; Komogortsev, A.N.; Lichitsky, B.V.; Mityanov, V.S.; Fakhrutdinov, A.N.; Dudinov, A.A.; Migulin, V.A.; Nelyubina, Y.V.; Melnikova, E.K.; Krayushkin, M.M. One-pot synthesis of substituted pyrrolo [3,4-b]pyridine-4,5-diones based on the reaction of N-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-arylethyl)acetamide with amines. Beilstein J. Org. Chem. 2019, 15, 2840–2846. [Google Scholar] [CrossRef]
- Peifer, C.; Selig, R.; Kinkel, K.; Ott, D.; Totzke, F.; Schaechtele, C.; Heidenreich, R.; Roecken, M.; Schollmeyer, D.; Laufer, S. Design, Synthesis, and Biological Evaluation of Novel 3-Aryl-4-(1H-indole-3yl)-1,5-dihydro-2H-pyrrole-2-ones as Vascular Endothelial Growth Factor Receptor (VEGF-R) Inhibitors. J. Med. Chem. 2008, 51, 3814–3824. [Google Scholar] [CrossRef]
- Choi, E.; Lee, C.; Cho, M.; Seo, J.J.; Yang, J.S.; Oh, S.J.; Lee, K.; Park, S.K.; Kim, H.M.; Kwon, H.J.; et al. Property-Based Optimization of Hydroxamate-Based γ-Lactam HDAC Inhibitors to Improve Their Metabolic Stability and Pharmacokinetic Profiles. J. Med. Chem. 2012, 55, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Kirpotina, L.N.; Schepetkin, I.A.; Khlebnikov, A.I.; Ruban, O.I.; Ge, Y.; Ye, R.D.; Kominsky, D.J.; Quinn, M.T. 4-Aroyl-3-hydroxy-5-phenyl-1H-pyrrol-2(5H)-ones as N-formyl peptide receptor 1 (FPR1) antagonists. Biochem. Pharmacol. 2017, 142, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, C.; Chen, M.; Chen, K.; Wu, Y.; Wu, S. Cytotoxic Polyketides Containing Tetramic Acid Moieties Isolated from the Fungus Myceliophthora Thermophila: Elucidation of the Relationship between Cytotoxicity and Stereoconfiguration. Chem. Eur. J. 2007, 13, 6985–6991. [Google Scholar] [CrossRef] [PubMed]
- Janecka, A.; Wyre, A.; Gach, K.; Fichna, J.; Janecki, T. Natural and synthetic α-methylenelactones and α-methylenelactams with anticancer potential. Drug Discov. Today 2012, 17, 561–572. [Google Scholar] [CrossRef]
- Singh, S.B.; Goetz, M.A.; Jones, E.T.; Bills, G.F.; Giacobbe, R.A.; Herranz, L.; Stevens-Miles, S.; Williams, D.L. Oteromycin: A Novel Antagonist of Endothelin Receptor. J. Org. Chem. 1995, 60, 7040–7042. [Google Scholar] [CrossRef]
- Hazuda, D.; Blau, C.U.; Felock, P.; Hastings, J.; Pramanik, B.; Wolfe, A.; Bushman, F.; Farnet, C.; Goetz, M.; Williams, M.; et al. Isolation and Characterization of Novel Human Immunodeficiency Virus Integrase Inhibitors from Fungal Metabolites. Antivir. Chem. Chemother. 1999, 10, 63–70. [Google Scholar] [CrossRef]
- He, H.; Yang, H.Y.; Bigelis, R.; Solum, E.H.; Greenstein, M.; Carter, G.T. Pyrrocidines A and B, new antibiotics produced by a filamentous fungus. Tetrahedron Lett. 2002, 43, 1633–1636. [Google Scholar] [CrossRef]
- Zhuang, C.; Miao, Z.; Zhu, L.; Dong, G.; Guo, Z.; Wang, S.; Zhang, Y.; Wu, Y.; Yao, J.; Sheng, C.; et al. Discovery, Synthesis, and Biological Evaluation of Orally Active Pyrrolidone Derivatives as Novel Inhibitors of p53–MDM2 Protein–Protein Interaction. J. Med. Chem. 2012, 55, 9630–9642. [Google Scholar] [CrossRef]
- Huang, W.; Dong, Z.; Wang, F.; Peng, H.; Liu, J.-Y.; Zhang, J.-T. A Small Molecule Compound Targeting STAT3 DNA-Binding Domain Inhibits Cancer Cell Proliferation, Migration, and Invasion. ACS Chem. Biol. 2014, 9, 1188–1196. [Google Scholar] [CrossRef]
- Koz’minykh, V.O.; Igidov, N.M.; Zykova, S.S.; Kolla, V.E.; Shuklina, N.S.; Odegova, T. Synthesis and pharmacological activity of 3-hydroxy-1,5-diaryl-4-pivaloyl-2,5-dihydro-2-pyrrolone. Pharm. Chem. J. 2002, 36, 188–191. [Google Scholar] [CrossRef]
- Tarnavsky, S.S.; Dubinina, G.G.; Golovach, S.M.; Yarmoluk, S.M. Antitumor activity among derivatives of the 3-chloro-4-(3-hydroxyanilino)-2,5-dihydropyrrole-2,5-dione. Biopolym. Cell. 2003, 19, 548–552. [Google Scholar] [CrossRef][Green Version]
- Sánchez, C.; Zhu, L.; Braña, A.F.; Salas, A.P.; Rohr, J.; Méndez, C.; Salas, J.A. Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc. Nat. Acad. Sci. USA 2005, 102, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, Z.; Han, X.; Liu, J.; Wang, W. In vitro cytotoxicity screening to identify novel anti-osteosarcoma therapeutics targeting pyruvate dehydrogenase kinase 2. Bioorg. Med. Chem. Lett. 2019, 29, 126665. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, N.; Petronijević, J.; Janković, N.; Baskić, D.; Popović, S.; Todorović, D.; Matić, S.; Bogdanović, G.A.; Vraneš, M.; Tot, A.; et al. Synthesis, characterization, anticancer evaluation and mechanisms of cytotoxic activity of novel 3-hydroxy-3-pyrrolin-2-ones bearing thenoyl fragment: DNA, BSA interactions and molecular docking study. Bioorg. Chem. 2019, 88, 102954. [Google Scholar] [CrossRef]
- Arencibia, J.M.; Fröhner, W.; Krupa, M.; Pastor-Flores, D.; Merker, P.; Oellerich, T.; Neimanis, S.; Schmithals, C.; Köberle, V.; Süß, E.; et al. An Allosteric Inhibitor Scaffold Targeting the PIF-Pocket of Atypical Protein Kinase C Isoforms. ACS Chem. Biol. 2017, 12, 564–573. [Google Scholar] [CrossRef]
- Yang, D.; Huang, C.; Liao, H.; Zhang, H.; Wu, S.; Zhu, Q.; Zhou, Z.-Z. Discovery of Dihydropyrrol-2-ones as Novel G0/G1-Phase Arresting Agents Inducing Apoptosis. ACS Omega 2019, 4, 17556–17560. [Google Scholar] [CrossRef]
- del Corte, X.; López-Francés, A.; Maestro, A.; Villate-Beitia, I.; Sainz-Ramos, M.; Martínez de Marigorta, E.; Pedraz, J.; Palacios, F.; Vicario, J. A Multicomponent Protocol for the Synthesis of Highly Functionalized γ-Lactam Derivatives and Their Applications as Antiproliferative Agents. Pharmaceuticals 2021, 14, 782. [Google Scholar] [CrossRef]
- Palacios, F.; Vicario, J.; Aparicio, D. An efficient synthesis of achiral and chiral cyclic dehydro-α-amino acid derivatives through nucleophilic addition of Amines to β,γ-unsaturated α-keto esters. Eur. J. Org. Chem. 2006, 2006, 2843–2850. [Google Scholar] [CrossRef]
- del Corte, X.; Maestro, A.; Vicario, J.; Martínez de Marigorta, E.; Palacios, F. Brønsted-Acid-Catalyzed Asymmetric Three-Component Reaction of Amines, Aldehydes, and Pyruvate Derivatives. Enantioselective Synthesis of Highly Functionalized γ-Lactam Derivatives. Org. Lett. 2018, 20, 317–320. [Google Scholar] [CrossRef]
- del Corte, X.; López-Francés, A.; Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Brønsted Acid Catalyzed Multicomponent Synthesis of Phosphorated and Fluorinated γ-Lactam Derivatives. J. Org. Chem. 2020, 85, 14369–14383. [Google Scholar] [CrossRef]
- del Corte, X.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. A Brønsted Acid-Catalyzed Multicomponent Reaction for the Synthesis of Highly Functionalized γ-Lactam Derivatives. Molecules 2019, 24, 2951. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.M.; Kurth, M.J.; Shaw, J.T.; Younai, A. Diversity-Oriented Synthesis; Trabocchi, A., Ed.; Wiley: New York, NY, USA, 2013; pp. 29–57. [Google Scholar]
- Schreiber, S.L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Rybak, T.; Verdelet, T. Multicomponent Hetero-[4 + 2] Cycloaddition/Allylboration Reaction: From Natural Product Synthesis to Drug Discovery. Acc. Chem. Res. 2016, 49, 2489–2500. [Google Scholar] [CrossRef]
- de Moliner, F.; Kielland, N.; Lavilla, R.; Vendrell, M. Modern Synthetic Avenues for the Preparation of Functional Fluorophores. Angew. Chem. Int. Ed. 2017, 56, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Gein, V.L.; Popov, A.V.; Kolla, V.E.; Popova, N.A. Synthesis and biological activity of 1,5-diaryl-3-alkylamino-4-carboxymethyl-2,5-dihydropyrrol-2-ones and 1,5-diaryl-4-carboxymethyl-tetrahydropyrrol-2,3-diones. Pharmazie 1993, 48, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Gein, V.L.; Popov, A.V.; Kolla, V.E.; Popova, N.A.; Potemkin, K.D. Synthesis and biological activity of 1,5-diaryl-3-arylamino-4-carboxymethyl-2,5-dihydro-2-pyrrolones and 1,5-diaryl-4-carboxymethyltetrahydropyrrole-2, 3-diones. Pharm. Chem. J. 1993, 27, 343–346. [Google Scholar] [CrossRef]
- Khalaf, A.I.; Waigh, R.D.; Drummond, A.J.; Pringle, B.; McGroarty, I.; Skellern, G.G.; Suckling, C.J. Distamycin Analogues with Enhanced Lipophilicity: Synthesis and Antimicrobial Activity. J. Med. Chem. 2004, 47, 2133–2156. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, F.; Li, Y. Synthesis and anti-biofilm activities of dihydro-pyrrol-2-one derivatives on Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2015, 25, 597–601. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, L.; Chen, Z.; Zheng, S.; Shu, H.; Li, J.; Jiang, H.; Liu, S. A novel class of small-molecule caspase-3 inhibitors prepared by multicomponent reactions. Eur. J. Med. Chem. 2012, 54, 232–238. [Google Scholar] [CrossRef]
- Li, B.; Wever, W.J.; Walsh, C.T.; Bowers, A.A. Dithiolopyrrolones: Biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat. Prod. Rep. 2014, 31, 905–923. [Google Scholar] [CrossRef]
- Ma, K.; Wang, P.; Fu, W.; Zhou, L.; Chu, Y.; Ye, D.; Wan, X. Rational design of 2-pyrrolinones as inhibitors of HIV-1 integrase. Bioorg. Med. Chem. Lett. 2011, 21, 6724–6727. [Google Scholar] [CrossRef] [PubMed]
- Pace, P.; Spieser, S.A.H.; Summa, V. 4-Hydroxy-5-pyrrolinone-3-carboxamide HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3865–3869. [Google Scholar] [CrossRef] [PubMed]
- Gein, V.L.; Kasimova, N.N.; Voronina, E.V.; Gein, L.F. Synthesis and antimicrobial activity of 5-aryl-4-acyl-1-(N,N-dimethylaminoethyl)-3-hydroxy-3-pyrrolin-2-ones. Pharm. Chem. J. 2001, 35, 151–154. [Google Scholar] [CrossRef]
- Gein, V.L.; Kasimova, N.N.; Panina, M.A.; Voronina, E.V. Synthesis and antibacterial activity of 1-(5-aryl-4-benzoyl-3-hydroxy-2-oxo-3-pyrrolin-1-yl)-2-(3-benzoylmethylene-2-oxopiperazin-1-yl)ethanes. Pharm. Chem. J. 2006, 40, 410–412. [Google Scholar] [CrossRef]
- Gein, V.L.; Pitirimova, S.G.; Voronina, E.V.; Porseva, N.Y.; Pantsurkin, V.I. Synthesis and antibacterial activity of 1-substituted 5-aryl-4-aroyl-3-hydroxy-3-pyrrolin-2-ones. Pharm. Chem. J. 1997, 31, 603–605. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X.; Zheng, G.; Huang, L. Synthesis and activity in enhancing long-term potentiation (LTP) of Clausenamide stereoisomers. Bioorg. Med. Chem. Lett. 2009, 19, 2112–2115. [Google Scholar] [CrossRef]
- Gein, V.L.; Shumilovskikh, E.V.; Andreichikov, Y.S.; Saraeva, R.F.; Korobchenko, L.V.; Vladyko, G.V.; Boreko, E.I. Synthesis of 4-substituted 1-methyl-5-aryl- and 1,5-diaryltetrahydropyrrole-2,3-diones and their antiviral action. Pharm. Chem. J. 1991, 25, 884–887. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosphonate biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable Potential of the Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Karl, D.M. Phosphorus, the Staff of Life. Nature 2000, 406, 31–33. [Google Scholar] [CrossRef]
- Engel, R. Handbook of Organophosphorus Chemistry; M. Dekker Inc.: New York, NY, USA, 1992. [Google Scholar]
- Kafarski, P.; Lejezak, B. Biological activity of aminophosphonic acids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Hoagland, R.E. Biologically Active Natural Products; Culter, H.G., Ed.; ACS Symposium Series 380; American Chemical Society: Washington, DC, USA, 1988; p. 182. [Google Scholar]
- Recio, R.; Vengut-Climent, E.; Mouillac, B.; Orcel, H.; López-Lázaro, M.; Calderón-Montaño, J.M.; Álvarez, E.; Khiar, N.; Fernández, I. Design, synthesis and biological studies of a library of NK1-Receptor Ligands Based on a 5-arylthiosubstituted 2-amino-4,6-diaryl-3-cyano-4H-pyran core: Switch from antagonist to agonist effect by chemical modification. Eur. J. Med. Chem. 2017, 138, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Bégué, J.P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Liew, S.K.; Malagobadan, S.; Arshad, N.M.; Nagoor, N.H. A Review of the Structure-Activity Relationship of Natural and Synthetic Antimetastatic Compounds. Biomolecules 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Elford, H.L.; Van’t Riet, B. Phenols and Polyphenols as Antioxidants and Anticancer Agents. In Eicosanoids and Other Bioactive Lipids in Cancer and Radiation Injury. Developments in Oncology; Honn, K.V., Marnett, L.J., Nigam, S., Walden, T.L., Eds.; Springer: Boston, MA, USA, 1991; Volume 67. [Google Scholar]
- Trabbic, C.J.; George, S.M.; Alexander, E.M.; Du, S.; Offenbacher, J.M.; Crissman, E.J.; Overmeyer, J.H.; Maltese, W.A.; Erhardt, P.W. Synthesis and biological evaluation of isomeric methoxy substitutions on anti-cancer indolyl-pyridinyl-propenones: Effects on potency and mode of activity. Eur. J. Med. Chem. 2016, 122, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Metwally, K.; Khalil, A.; Sallam, A.; Pratsinis, H.; Kletas, D.; El Sayed, K. Structure–activity relationship investigation of methoxy substitution on anticancer pyrimido[4,5-c]quinolin-1(2H)-ones. Med. Chem. Res. 2013, 22, 4481–4491. [Google Scholar] [CrossRef]
- Patani, G.A.; Lavoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Ridaforolimus. Drugs R D 2010, 10, 165–178. [CrossRef][Green Version]
- Rivera, V.M.; Squillace, R.M.; Miller, D.; Berk, L.; Wardwell, S.D.; Ning, Y.; Pollock, R.; Narasimhan, N.I.; Iuliucci, J.D.; Wang, F.; et al. Ridaforolimus (AP23573; MK-8669), a Potent mTOR Inhibitor, Has Broad Antitumor Activity and Can Be Optimally Administered Using Intermittent Dosing Regimens. Mol. Cancer Ther. 2011, 10, 1059–1071. [Google Scholar] [CrossRef]
- Markham, A. Brigatinib: First Global Approval. Drugs 2017, 77, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-S.; Liu, S.; Zou, D.; Thomas, M.; Wang, Y.; Zhou, T.; Romero, J.; Kohlmann, A.; Li, F.; Qi, J.; et al. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J. Med. Chem. 2016, 59, 4948–4964. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).