Using a Caenorhabditis elegans Parkinson’s Disease Model to Assess Disease Progression and Therapy Efficiency
Abstract
1. Introduction
2. Results
2.1. Synuclein Plaques Accumulate as Worms Age, Affecting Mobility but Not Overall Lifespan
2.2. An Affordable and Simple Platform to Assess Disease Progression and Therapy Efficiency
2.3. Levodopa Exposure Recovers the Mobility Defect in a C. elegans PD Model, While the α-Synuclein Aggregates Are Not Affected
2.4. Screening of Small-Molecule Modulators for a Positive Effect on the Hallmarks of PD in C. elegans
3. Discussion
4. Materials and Methods
4.1. Strains and Nematode Preparation
4.2. Small-Molecule Modulators
4.3. Treatment
4.4. Thrashing Assay and Analysis
4.5. Fluorescent Microscopy
4.6. Quantification of Plaques
4.7. Lifespan Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkison pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic—A call to action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Szczepski, K.; Lachowicz, J.I.; Jaremko, L.; Emwas, A.-H.; Jaremko, M. Aggregation of biologically important peptides and proteins: Inhibition or acceleration depending on protein and metal ion concentrations. R. Soc. Chem. Adv. 2020, 10, 215–227. [Google Scholar] [CrossRef]
- Moons, R.; Konijnenberg, A.; Mensch, C.; van Elzen, R.; Johannessen, C.; Maudsley, S.; Lambeir, A.-M.; Sobott, F. Metal ions shape α-synuclein. Sci. Rep. 2020, 10, 16293. [Google Scholar] [CrossRef]
- Buell, A.K.; Galvagnion, C.; Gasper, R.; Sparr, E.; Vendruscolo, M.; Knowles, T.P.J.; Linse, S.; Dobson, C.M. Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2014, 111, 7671–7676. [Google Scholar] [CrossRef]
- Duarte, C.M.; Jaremko, L.; Jaremko, M. Hypothesis: Potentially Systemic Impacts of Elevated CO2 on the Human Proteome and Health. Front. Public Health 2020, 8, 543322. [Google Scholar] [CrossRef]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Parkinson’s Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef]
- Hornykiewicz, O. A brief history of levodopa. J. Neurol. 2010, 257, 249–252. [Google Scholar] [CrossRef]
- Cotzias, G.C.; van Woert, M.H.; Schiffer, L.M. Aromatic amino acids and modification of Parkinsonism. N. Engl. J. Med. 1967, 276, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Cotzias, G.C.; Papsvasiliou, P.S.; Gellene, R. Modification of Parkinsonism—Chronic treatment with L-dopa. N. Engl. J. Med. 1969, 280, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W. Levodopa is the best symptomatic therapy for PD: Nothing more, nothing less. Mov. Disord. 2019, 34, 812–815. [Google Scholar] [CrossRef]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s Disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.S.; Lang, A.E. Pharmacological treatmenr of Parkinson Disease: A review. JAMA 2014, 311, 1670–1783. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Ives, N.; Rick, C.; Patel, S.; Gray, A.; Jenkinson, C.; McIntosh, E.; Wheatley, K.; Williams, A.; Clarke, C.E. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease. Lancet 2014, 384, 1196–1205. [Google Scholar] [PubMed]
- Stoker, T.B.; Torsney, K.M.; Barker, R.A. Emerging treatment approaches for Parkinson’s Disease. Front. Neurosci. 2018, 12, 693. [Google Scholar] [CrossRef]
- Teil, M.; Arotcarena, M.-L.; Faggiani, E.; Laferriere, F.; Bezard, E.; Dehay, B. Targeting α-Synuclein for PD Therapeutics: A Pursuit on All Fronts. Biomolecules 2020, 10, 391. [Google Scholar] [CrossRef]
- Shaye, D.D.; Greenwald, I. Ortholist: A compendium of C. elegans genes with human orthologs. PLoS ONE 2011, 6, e20085. [Google Scholar] [CrossRef]
- Hillier, L.W.; Coulson, A.; Murray, J.I.; Bao, Z.; Sulston, J.E.; Waterston, R.H. Genomics in C. elegans: So many genes, such a little worm. Genome Res. 2005, 15, 1651–1660. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.J.; Jarrell, T.A.; Brittin, C.A.; Wang, Y.; Bloniarz, A.E.; Yakovlev, M.A.; Nguyen, K.C.Q.; Tang, T.-H.; Bayer, E.A.; Duerr, J.S.; et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 2019, 571, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Culetto, E.; Sattelle, D.B. A role for Caenorhabditis elegans in understadnign the function and interactions of human disease. Hum. Mol. Genet. 2000, 9, 769–877. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Le, W. Modeling neurodegenerative diseases in Caenorhabditis elegans. Exp. Neurol. 2013, 250, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.A.; Willicott, C.W.; Caldwell, G.A. Modeling neurodegeneration in Caenorhabditis elegans. Dis. Models Mech. 2020, 13, dmm046110. [Google Scholar] [CrossRef] [PubMed]
- Soto, C. Unfolding the role of protein misfolsing in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- van Ham, T.J.; Thijssen, K.L.; Breitling, R.; Hofstra, R.M.W.; Plasterk, R.H.A.; Nollen, E.A.A. C. elegans model identifies genetics modifiers of a-synuclein inclusion formation during aging. PLoS Genet. 2008, 3, e1999927. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Gabel, C.; Feree, A.; Guillily, M.; Ebata, A. Watching worms whither: Modeling neurdegeneratino in C. elegans. Prog. Mol. Biol. Transl. Sci. 2011, 100, 499–514. [Google Scholar]
- Schmidt, E.; Seifert, M.; Baumeister, R. Caenorhabditis elegans as a model system for Parkinson’s Disease. Neurodegener. Disord. 2007, 4, 199–217. [Google Scholar] [CrossRef]
- Harrington, A.J.; Hamamichi, S.; Caldwell, G.A.; Caldwell, K.A. C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s Disease. Dev. Dyn. 2010, 239, 1282–1295. [Google Scholar]
- Maulik, M.; Mitra, S.; Bult-Ito, A.; Taylor, B.E.; Vayndorf, E.M. Behavioral Phenotyping and Pathological Indicators of Parkinson’s Disease in C. elegans Models. Front. Genet. 2017, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.F.; van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Parkinson’s Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Bodhicharla, R.; Nagarajan, A.; Winter, J.; Adenle, A.; Nazir, A.; Brady, D.; Vere, K.; Richens, J.; O’Shea, P.; Bell, D.R.; et al. Effects of α-synuclein overexpression in transgenic Caenorhabditis elegans strains. CNS Neurol. Disord. Drug Targets 2012, 11, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Koyama, A.; Gengyo-Ando, K.; Masuda, M.; Kowa, H.; Tsunoda, M.; Mitani, S.; Iwatsubo, T. Familial Parkinson mutant alpha-synuclein causes dopamine neuron dysfunction in transgenic Caenorhabditis elegans. J. Biol. Chem. 2006, 281, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Giunti, S.; Andersen, N.; Rayes, D.; De Rosa, M.J. Drug disovery: Insights from the invertebrate Caenorhabitis elegans. Pharmacol. Res. Perspect. 2021, 9, e00721. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, D.E. Parkinson’s Disease. Lancet 2015, 386, P896–P912. [Google Scholar] [CrossRef]
- Pujols, J.; Pena-Diaz, S.; Lazaro, D.F.; Peccati, F.; Pinheiro, F.; Gonzalez, D.; Carija, A.; Navarro, S.; Conde-Gimenez, M.; Garcia, J.; et al. Small molecule inhibits α-synuclein aggregation, disrupts amyloid fibrils, and prevents degeneration of dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 10481–10486. [Google Scholar] [CrossRef]
- Pena-Diaz, S.; Pujols, J.; Conde-Gimenez, M.; Carija, A.; Dalfo, E.; Garcia, J.; Navarro, S.; Pinheiro, F.; Santos, J.; Salvatella, X.; et al. ZPD-2, a small compound that inhibitis a-synuclin amyloid aggregation and its seeded polymerisation. Front. Mol. Neurosci. 2019, 12, 306. [Google Scholar] [CrossRef]
- Sofela, S.; Sahloul, S.; Song, Y.-A. Biophysical analysis of drug efficacy on C. elegans models for neurodegenerative and neuromuscular diseases. PLoS ONE 2021, 16, e0246496. [Google Scholar]
- Braungart, E.; Gerlach, M.; Riederer, P.; Baumeister, R.; Hoener, M.C. Caenorhabditis elegans MPP+ model of Parkinson’s Disease for high-throughput drug screenings. Neurodegener. Dis. 2004, 1, 175–183. [Google Scholar] [CrossRef]
- Sohrabi, S.; Mor, D.E.; Kaletsky, R.; Keyes, W.; Murphy, C.T. High-throughput behavioural screen in C. elegans reveals Parkinson’s disease drug candidates. Commun. Biol. 2020, 4, 203. [Google Scholar] [CrossRef] [PubMed]
- Manalo, R.V.M.; Medina, P.M.B. Caffeine Protects Dopaminergic Neurons from Dopamine-Induced Neurodegeneration via Synergistic Adenosine-Dopamine D2-Like Receptor Interactions in Transgenic Caenorhabditis elegans. Front. Neurosci. 2018, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Mitra, S.; Hunter, S.; Hunstiger, M.; Oliver, S.R.; Bult0Ito, A.; Taylor, B.E. Sir-2.1 mediated attenuation of α-synuclein expression by Alaskan bog blueberry polyphenols in a transgenic model of Caenorhabditis elegans. Sci. Rep. 2018, 8, 10216. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-W.; Tasai, R.-T.; Liu, S.-P.; Chen, C.-S.; Tsai, M.-C.; Chien, S.-H.; Hung, H.-S.; Lin, S.-Z.; Shyu, W.C.; Fu, R.-H. Neuroprotective Effects of Betulin in Pharmacological and Transgenic Caenorhabditis elegans Models of Parkinson’s Disease. Cell Transplant. 2017, 26, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Haque, R.; Jadiya, P.; Shamsuzzama; Kumar, L.; Nazir, A. Ida-1, the Caenorhabditis elegans ortholog of mammalian diabetes autoantigen IA-3, potentially acts as a common modulator between Parkinson’s Disease and diabetes: Role of daf-2 /daf-16 insulin like signalling pathway. PLoS ONE 2014, 9, e113986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laine, R.F.; Sinnige, T.; Ma, K.Y.; Haack, A.J.; Poudel, C.; Gaida, P.; Curry, N.; Perni, M.; Nollen, E.A.A.; Dobson, C.M.; et al. Fast flourescence lifetime imging reveals the aggregation processes of alpha-synuclein and polyglutamine in aging Caenorhabditis elegans. ACS Chem. Biol. 2019, 14, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Neuropathology, biochemitsry, and biophysics of alpha-synuclein aggregation. J. Neurochem. 2007, 103, 17–37. [Google Scholar]
- Chen, K.S.; Menezes, K.; Rodgers, J.B.; O’Hara, D.M.; Tran, N.; Fujisawa, K.; Ishikura, S.; Khodaei, S.; Chau, H.; Cranston, A.; et al. Small molecule inhibitors of α-synuclein oligomers identified by targeting early dopamine-mediated motor impairment in C. elegans. Mol. Neurodegener. 2021, 16, 77. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Elkouzi, A.; Vendam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease—Repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef]
- Paleari, D.; Rossi, G.A.; Nicolini, G.; Olivieri, D. Ambroxol: A multifaceted molecule with additional therapeutic potentials in respiratory disorders of childhood. Expert Opin. Drug Discov. 2011, 6, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Ragnoli, B. Ambroxol in the 21st century: Pharmacological and clinical update. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, M.; Mi, N.; Yang, W.; Li, X.; Wang, P.; Yin, N.; Li, Y.; Yue, F.; Chan, P.; et al. Increased oligomerization and phosphorylation of α-synuclein are associated with decreased activity of glucocerebrosidase and protein phosphatase 2A in aging monkey brains. Neurobiol. Aging 2015, 39, 2649–2659. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Zunke, F.; Tsunemi, T.; Toker, N.T.; Jeon, S.; Burbulla, L.F.; Patnaik, S.; Sidransky, E.; Marugan, J.J.; Sue, C.M.; et al. Activation of b-glucocerebrosidase reduces pathological α-synclein and restores lysosomal function in Parkinson’s patient midbrian neurons. J. Neurosci. 2016, 36, 7693–7706. [Google Scholar] [CrossRef] [PubMed]
- Migdalska-Richards, A.; Daly, L.; Bezard, E.; Schapira, A.H.V. Ambroxol effects in glucocerebrosidaase and α-synuclein transgenic mice. Ann. Neurol. 2016, 80, 766–775. [Google Scholar] [CrossRef]
- Migdalska-Richards, A.; Ko, W.K.D.; Li, Q.; Bezard, E.; Schapira, A.H.V. Oral ambroxol increases brain glucocerebrosidase activity in a non-human primate. Synapse 2017, 71, e21967. [Google Scholar] [CrossRef] [PubMed]
- Mullin, S.; Smith, L.; Lee, K.; D’Souzza, G.; Woodgate, P.; Elflein, J.; Hallqvist, J.; Toffoli, M.; Streeter, A.; Hosking, J.; et al. Ambroxol for the Treatment of Patients with Parkinson Disease with and without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial. JAMA Neurol. 2020, 77, 427–434. [Google Scholar] [CrossRef]
- Silveira, C.R.A.; MacKinley, J.; Coleman, K.; Li, Z.; Finger, E.; Bartha, R.; Morrow, S.A.; Wlels, J.; Borrie, M.; Tirona, R.G.; et al. Ambroxol as a novel disease-modifying treatment for Parkinson’s disease dementia: Protocol for a single-centre, randomized, double-blind, placebo-controlled trial. BMC Neurol. 2019, 19, 20. [Google Scholar] [CrossRef]
- Kim, M.; Knoefler, D.; Quarles, E.; Jakob, U.; Bazopoulu, D. Automated phenotyping and lifespan assessment of a C. elegans model of Parkinson’s Disease. Transl. Med. Aging 2020, 4, 38–44. [Google Scholar] [CrossRef]
- Wood, S.J.; Wypych, J.; Steavenson, S.; Louis, J.-C.; Citron, M.; Biere, A.L. α-Synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J. Biol. Chem. 1999, 274, 19509–19512. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meany, D.F.; Trojanowski, J.Q.; Lee, V.M.-Y. Exogenous α-Synuclein Fibrils Induce Lewy Body Pathology Leading to Synaptic Dysfunction and Neuron Death. Neuron 2012, 72, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, F.; Kim, T.-E.; Bill, A.; Dettmer, U. Dynamic behaviours of alpha-synuclein and tau in the cellular context: New mechanistic insights and therapeutic opportunities in neurodegeneration. Neurobiol. Dis. 2019, 132, 104543. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, S.; Sahay, S.; Murray, K.A.; Morgan, S.; Guenther, E.L.; Jiang, L.; Williams, C.K.; Vinters, H.V.; Goedert, M.; Eisenberg, D.S. Inhibition of synucleinopathic seedng by rationally designed inhibitors. eLife 2020, 2020, e46775. [Google Scholar] [CrossRef] [PubMed]
- Glenn, C.F.; Chow, D.K.; David, L.; Cooke, C.A.; Gami, M.S.; Iser, W.B.; Henselman, K.B.; Goldberg, I.G.; Wolkow, C.A. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, 1251–1260. [Google Scholar] [CrossRef]
- Herndon, L.A.; Schmeissner, P.J.; Dudaronek, J.M.; Brown, P.A.; Listner, K.M.; Sakano, Y.; Paupard, M.C.; Hall, D.H.; Driscoll, M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002, 419, 808–814. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Pisciotta, M.S.; Laudisio, A.; Monaco, M.R.L.; Onder, G.; Brandi, V.; Fusco, D.; Di Capua, B.; Ricciardi, D.; Bernabei, R.; et al. Sarcopenia in Parkinson Disease: Comparison of Different Criteria and Association with Disease Severity. J. Am. Med. Dir. Assoc. 2018, 19, 523–527. [Google Scholar] [CrossRef]
- Cai, Y.; Feng, F.; Wei, Q.; Jiang, Z.; Ou, R.; Shang, H. Sarcopenia in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 598035. [Google Scholar] [CrossRef]
- Perni, M.; Galavgnion, C.; Maltsev, A.; Meisi, G.; Muller, M.B.D.; Challa, P.K.; Kirkegaard, J.B.; Flagmeier, P.; Cohen, S.I.; Cascella, R.; et al. A natural product inhibits the initiation of α-synuclein aggregation and suppresses its toxicity. Proc. Natl. Acad. Sci. USA 2017, 114, e1009–e1017. [Google Scholar] [CrossRef]
- Kim, H.; Calatayud, C.; Guha, S.; Ferandez-Caeasa, I.; Berkowitz, L.; Carballo-Carbajal, I.; Ezquerra, M.; Fernandez-Santiago, R.; Kapahi, P.; Raya, A.; et al. The small GTPase RAC1/CED-10 is essential in maintaining dopaminergic neuron function and survival aginst alpha-synuclein-induced toxicity. Nolecular Neurobiol. 2018, 55, 7533–7552. [Google Scholar]
- Olanow, C.W.; Kieburtz, K.; Rascol, O.; Poewe, W.; Schapira, A.H.; Emre, M.; Nissinen, H.; Leinonen, M.; Stocchi, F.; Investigators, S.-P. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov. Disord. 2013, 28, 1064–1071. [Google Scholar] [CrossRef]
- Poewe, W.; Antonini, A.; Zijlmans, J.C.M.; Burkhard, P.R.; Vingerhoets, F. Levodopa in the treatment of Parkinson’s disease: An old drug still going strong. Clin. Interv. Aging 2010, 5, 229–238. [Google Scholar] [PubMed]
- Gupta, D.K.; Hang, X.; Liu, R.; Hasan, A.; Feng, Z. Levodopa-induced motor and dopamine receptor chnages in Caenorhabditis elegans overexpressing human alpha-synuclein. Neurodegener. Dis. 2016, 16, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Salat, D.; Tolosa, E. Levodopa in the treatment of Parkinson’s Disease: Current status and new developments. J. Parkinson’s Dis. 2013, 3, 255–269. [Google Scholar] [CrossRef]
- Knutsson, E.; Martensson, A. Quantitative effects of L-dopa on different types of movements and muscle tone in Parkinsonian patients. Scand. J. Rehabil. Med. 1971, 3, 121–130. [Google Scholar] [PubMed]
- Carlsson, A.; Lindqvist, M.; Magnusson, T. 3, 4-Dihydroxyphenylalanine and 5-hydroxytryptamine as reserpine antagonists. Nature 1957, 180, 1200. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Magalhaes, J.; Shen, C.; Chau, K.-Y.; Hughes, D.; Mehta, A.; Foltynie, T.; Cooper, J.M.; Abramov, A.Y.; Gegg, M.; et al. Ambroxol improves lyosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain 2014, 137, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Kim, H.W.; Lee, H.K.; Jeon, B.J.; Sung, S.H. Ameliorative effect of betulin from Betyla platyphylla bark on scopolamine-induced amnesic mice. Biosci. Biotechnol. Biochem. 2015, 80, 166–171. [Google Scholar] [CrossRef]
- Monti, B.; Gatta, V.; Piretti, F.; Raffaelli, S.S.; Virgilli, M.; Contestabile, A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s Disease: Involvment of alpha-synuclein. Neurotox. Res. 2009, 17, 130–141. [Google Scholar] [CrossRef]
- Ximenes, J.C.M.; Neves, K.R.T.; Leal, L.K.A.M.; do Carmo, M.R.S.; Brito, G.A.d.C.; Naffah-Mazzacoratti, M.d.G.; Cavalheiro, É.A.; Viana, G.S.d.B. Valproic Acid Neuroprotection in the 6-OHDA Model of Parkinson’s Disease Is Possibly Related to Its Anti-Inflammatory and HDAC Inhibitory Properties. J. Neurodegener. Dis. 2015, 2015, 313702. [Google Scholar] [CrossRef]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.D.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef]
- McFarland, K.; Spalding, T.A.; Hubbard, D.; Ma, J.-N.; Olsson, R.; Burstein, E.S. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem. Neurosci. 2013, 3, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Kowal, N.; Indurthi, D.; Ahring, P.; Chebib, M.; Olafsdottir, E.; Balle, T. Novel Approach for the Search for Chemical Scaffolds with Activity at Both Acetylcholinesterase and the α7 Nicotinic Acetylcholine Receptor: A Perspective on Scaffolds with Dual Activity for the Treatment of Neurodegenerative Disorders. Molecules 2019, 24, 446. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Hutchinson, M.; Larsen, J.P. Cognitive, psychiatric and motor response to galantamine in Parkinson’s disease with dementia. Int. J. Geriatr. Psychiatry 2003, 18, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Litvinenko, I.V.; Odinak, M.M.; Mogil’naya, V.I.; Emelin, A.Y. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial). Neurosci. Behav. Physiol. 2008, 38, 937. [Google Scholar] [CrossRef]
- Paleacu, D. Tetrabenazine in the treatment of Huntington’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 545–551. [Google Scholar]
- de Tommaso, M.; Serpino, C.; Sciruicchio, V. Management of HD: Role of tetrabenazine. Ther. Clin. Risk Manag. 2011, 7, 123–129. [Google Scholar] [CrossRef]
- Brusa, L.; Orlacchio, A.; Stefani, A.; Galati, S.; Pierantozzi, M.; Iani, C.; Mercuri, N.B. Tetrabenazine improves levodopa-induced peak-dose dyskinesias in patients with Parkinson’s disease. Funct. Neurol. 2013, 28, 101–105. [Google Scholar]
- Wong, S.Q.; Jones, A.; Dodd, S.; Grimes, D.; Barclay, J.W.; Marson, A.G.; Cunliffe, V.T.; Burgoyne, R.D.; Sills, G.J.; Morgan, A. A Caenorhabditis elegans assay of seizure-like activity optimised for identifying antiepileptic drugs and their mechanisms of action. J. Neurosci. Methods 2018, 309, 132–142. [Google Scholar] [CrossRef]
- Calaharro, F.; Holden-Dye, L.; O’Conner, V. Impact of drug solvents on C. elegans pharyngeal pumping. Toxicol. Rep. 2021, 8, 1240–1247. [Google Scholar] [CrossRef]
- Giacomotto, J.; Segalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef]
- Apfeld, J.; Fonatna, W. Age-Dependence and Aging-Dependence: Neuronal Loss and Lifespan in a C. elegans Model of Parkinson’s Disease. Biology 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wagner-Valladolid, S.; Stephens, A.D.; Jung, R.; Poudel, C.; Sinnige, T.; Lechler, M.C.; Schlorit, N.; Lu, M.; Laine, R.F.; et al. Intrinsically aggregation-prone proteins form amyloid-like aggregates and contribute to tissue aging in Caenorhabditis elegans. eLife 2019, 8, e43059. [Google Scholar] [CrossRef] [PubMed]
- David, D.C.; Ollikainen, N.; Trinidad, J.C.; Cary, M.P.; Burlingame, A.L.; Kenyon, C. Widespread Protein Aggregation as an Inherent Part of Aging in C. elegans. PLoS Biol. 2010, 8, e1000450. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Balasubramaniam, M.; Suri, P.; Mackintosh, S.G.; Tackett, A.J.; Sullivam, D.H.; Shmookler Reis, R.J.; Dennis, R.A. Proteins that accumulate with age in human skeletal-muscle aggregates contribute to declines in muscle mass and function in Caenorhabditis elegans. Aging 2016, 8, 3486–3496. [Google Scholar] [CrossRef]
- Cermak, N.; Yu, S.K.; Clark, R.; Huang, Y.-C.; Baskoylu, S.N.; Flavell, S.W. Whole-organism behavioral profiling reveals a role for dopamine in state-dependent motor program coupling in C. elegans. eLife 2020, 2020, e57093. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Liu, Y.; Hassinan, C.; Ailion, M.; Zhao, Z.; Wang, T.; Chen, Z.; Bai, J. Dopamine receptor DOP-1 engages a sleep pathway to modulate swimming in C. elegans. iScience 2021, 24, 102247. [Google Scholar] [CrossRef]
- Chase, D.L.; Pepper, J.S.; Koelle, M.R. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat. Neurosci. 2004, 7, 1096–1103. [Google Scholar] [CrossRef]
- Berthet, A.; Porras, G.; Doudnikoff, E.; Stark, H.; Cador, M.; Bezard, E.; Bloch, B. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J. Neurosci. 2009, 29, 4829–4835. [Google Scholar] [CrossRef]
- Guigoni, C.; Doudnikoff, E.; Li, Q.; Bloch, B.; Bezard, E. Altered D1 dopamine receptor trafficking in parkinsonian and dyskinetic nonhuman primates. Neurol. Dis. 2007, 26, 452–463. [Google Scholar]
- Schwarz, P.B.; Peever, J.H. Dopamine triggers skeletal muscle tone by activing D1-like receotors on somatic motoneurons. J. Neurophysiol. 2011, 106, 1299–1309. [Google Scholar] [CrossRef][Green Version]
- Verschuur, C.V.M.; Suwijn, S.R.; Boel, J.A.; Post, B.; Bloem, B.R.; van Hilten, J.J.; van Laar, T.; Tissingh, G.; Munts, A.G.; Deuschl, G.; et al. Randomized Delayed-Start Trial of Levodopa in Parkinson’s Disease. N. Engl. J. Med. 2019, 380, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, G.; Brotchie, J.M.; Grandas, F.; Nomoto, M.; Goetz, C.G. Levodopa-induced dyskinesias. Mov. Disord. 2007, 22, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Mucibabic, M.; Steneberg, P.; Lidh, E.; Straseviciene, J.; Ziolkowska, A.; Dahl, U.; Lindahl, E.; Edlunch, H. α-Synuclein promotes IAPP fibril formation in vitro and β-cell amyloid formation in vivo in mice. Sci. Rep. 2020, 10, 20438. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Shah, M.; Saraogi, I. Molecular Aspects of Insulin Aggregation and Various Therapeutic Interventions. ACS Bio. Med. Chem. 2022. [Google Scholar] [CrossRef]
- Xie, X.; Chamoli, M.; Bhaumik, D.; Sivapatham, R.; Angeli, S.; Andersen, J.K.; Lithgow, G.L.; Schilling, B. Quantification of Insoluble Protein Aggregation in Caenorhabditis elegans during Aging with a Novel Data-Independent Acquisition Workflow. J. Vis. Exp. 2020, 162, e61366. [Google Scholar] [CrossRef]
- Zurlo, E.; Kumar, P.; DMeisl, G.; Dear, A.J.; Mondal, D.; Claessens, M.M.A.E.; Knowles, T.P.J.; Huber, M. In situ kinetic measurements of α-synuclein aggregation reveal large population of short-lived oligomers. PLoS ONE 2021, 16, e0245548. [Google Scholar] [CrossRef]
- Srivastava, T.; Raj, R.; Dubey, A.; Kumar, D.; Chaturvedi, R.K.; Sharma, S.K.; Pritya, S. Fast kinetics of environmentally induced α-synuclein aggregation mediated by structural alteration in NAC region and result in structure dependent cytotoxicity. Sci. Rep. 2020, 10, 18412. [Google Scholar] [CrossRef]
- Iljina, M.; Garcia, G.A.; Horrocks, M.H.; Tosatto, L.; Choi, M.L.; Ganzinger, K.A.; Ambramov, A.Y.; Gandhi, S.; Wood, N.W.; Cremades, N.; et al. Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading. Proc. Natl. Acad. Sci. USA 2016, 113, e1206–e1215. [Google Scholar] [CrossRef]
- Kautu, B.B.; Carrasquilla, A.; Hicks, M.L.; Caldwell, K.A.; Caldwell, G.A. Valproic acid ameliorates C. elegans dopaminergic neurodegeneration with implications for ERK- MAPK signaling. Neurosci. Lett. 2013, 541, 116–119. [Google Scholar] [CrossRef]
- Monti, B.; Mercatelli, D.; Contestabile, A. Valproic acid neuroprotection in 6-OHDA lesioned rat, a model for parkinson’s disease. HOAJ Biol. 2012, 1, 4. [Google Scholar] [CrossRef]
- Sramek, J.J.; Frackiewicz, E.J.; Cutler, N.R. Review of the acetylcholinesterase inhibitor galanthamine. Expert Opin. Investig. Drugs 2005, 9, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- van Laar, T.; De Deyn, P.P.; Aarsland, D.; Barone, P.; Galvin, J.E. Effects of cholinesterase inhibitors in Parkinson’s disease dementia: A review of clinical data. CNS Neurosci. Ther. 2011, 17, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Owesson-White, C.A.; Roitman, M.F.; Sombers, L.A.; Belle, A.M.; Keithley, R.B.; Peele, J.L.; Carelli, R.M.; Wightman, R.M. Sources Contributing to the Average Extracellular Concentration of Dopamine in the Nucleus Accumbens. J. Neurochem. 2012, 121, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Duerr, J.S.; Frisby, D.L.; Gaskin, J.; Duke, A.; Asermely, K.; Huddleston, D.; Eiden, L.E.; Rand, J.B. The cat-1 Gene of Caenorhabditis elegans Encodes a Vesicular Monoamine Transporter Required for Specific Monoamine-Dependent Behaviors. J. Neurosci. 1999, 19, 72–84. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. Elegans. Wormbook 2006, 11, 1–11. [Google Scholar] [CrossRef]
- Hughes, S.; Kolsters, N.; van de Klashorst, D.; Kreuter, E.; Berger Buter, K. An extract of Rosaceae, Solanaceae and Zingiberaceae increases health span and mobility in Caenorhabditis elegans. BMC Nutr. 2022, 8, 5. [Google Scholar] [CrossRef]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.-S.; Lee, S.-J.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 56147–56152. [Google Scholar] [CrossRef]
- Klang, I.M.; Schilling, B.; Sorensen, D.J.; Sahu, A.K.; Kapahi, P.; Andersen, J.K.; Swoboda, P.; Killilea, D.W.; Gibson, B.W.; Lithgow, G.J. Iron promotes protein insolubiliity and aging in C. elegans. Aging 2014, 6, 975–988. [Google Scholar] [CrossRef]
- Schmidt, M.Y.; Chamoli, M.; Lithgow, G.J.; Andersen, J.K. Swimming exercise reduces native α-synuclein protein species in a transgenic C. elegans model of Parkinson’s disease. Micropublication Biol. 2021, 2021, 17912. [Google Scholar]
- Laranjeiro, R.; Harinath, G.; Hewitt, J.E.; Hartman, J.H.; Royal, M.A.; Meyer, J.N.; Vanapalli, S.A.; Drsicoll, M. Swim exercise in Caenorhabditis elegans extends neuromuscular and gut healthspan, enhances learning ability, and protects against neurodegeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 23829–23839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Snoek, B.L.; Sterken, M.G.; Riksen, J.A.G.; Stastna, J.J.; Kammenga, J.E.; Harvey, S.C. Genetic Background Modifies Phenotypic and Transcriptional Responses in a C. Elegans Model of Α-Synuclein Toxicity. BMC Genom. 2019, 20, 232. [Google Scholar] [CrossRef]
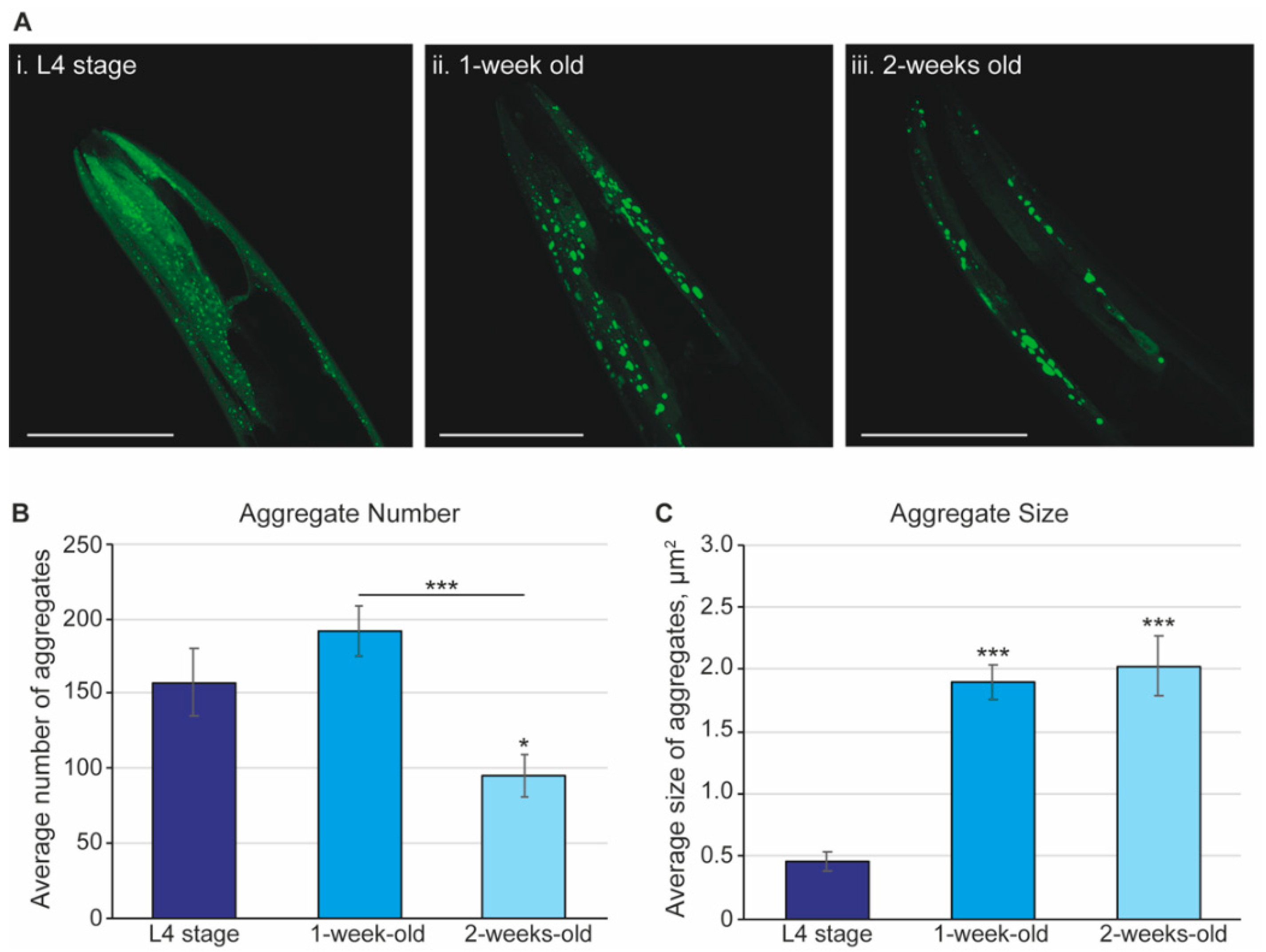
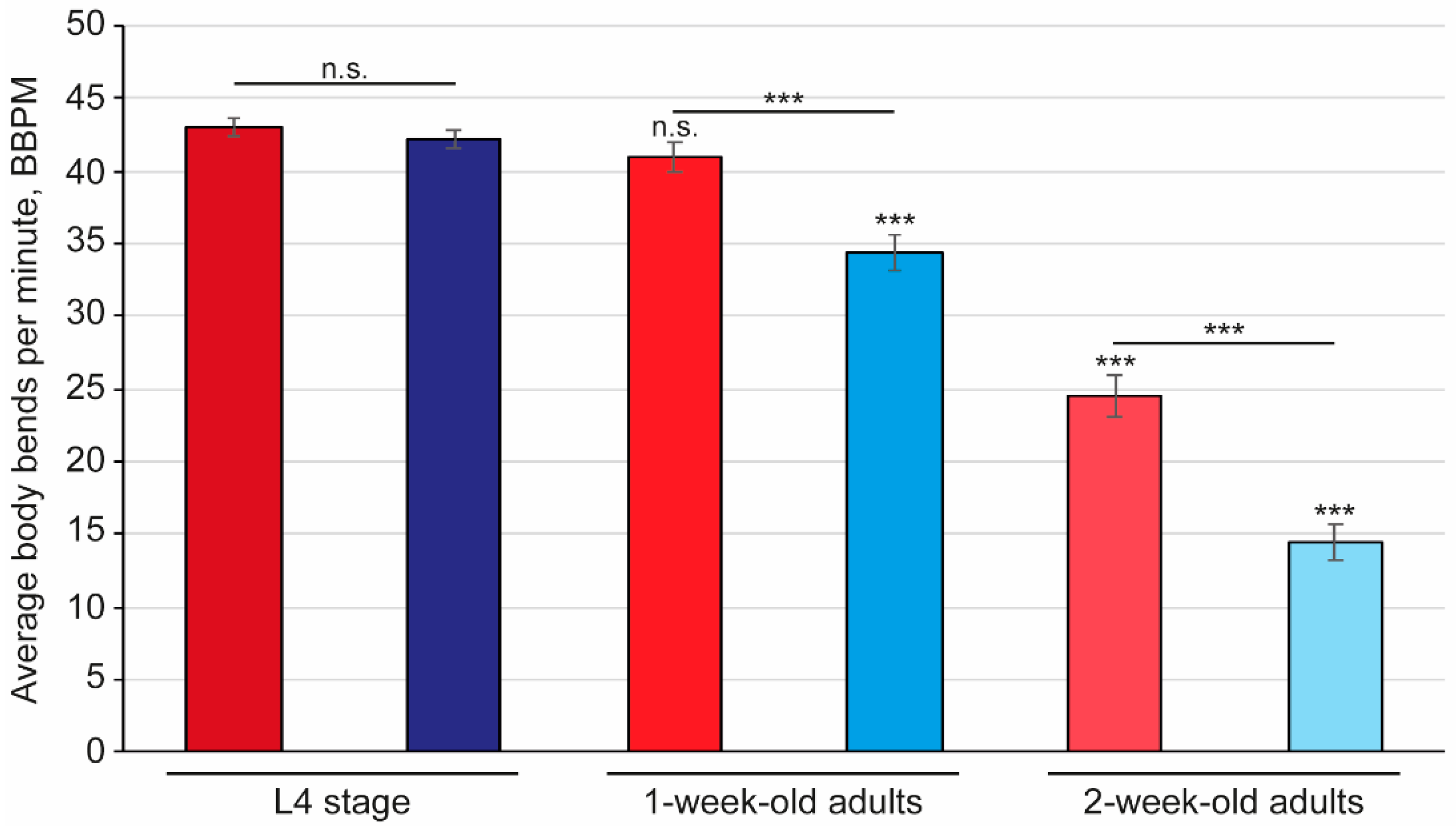
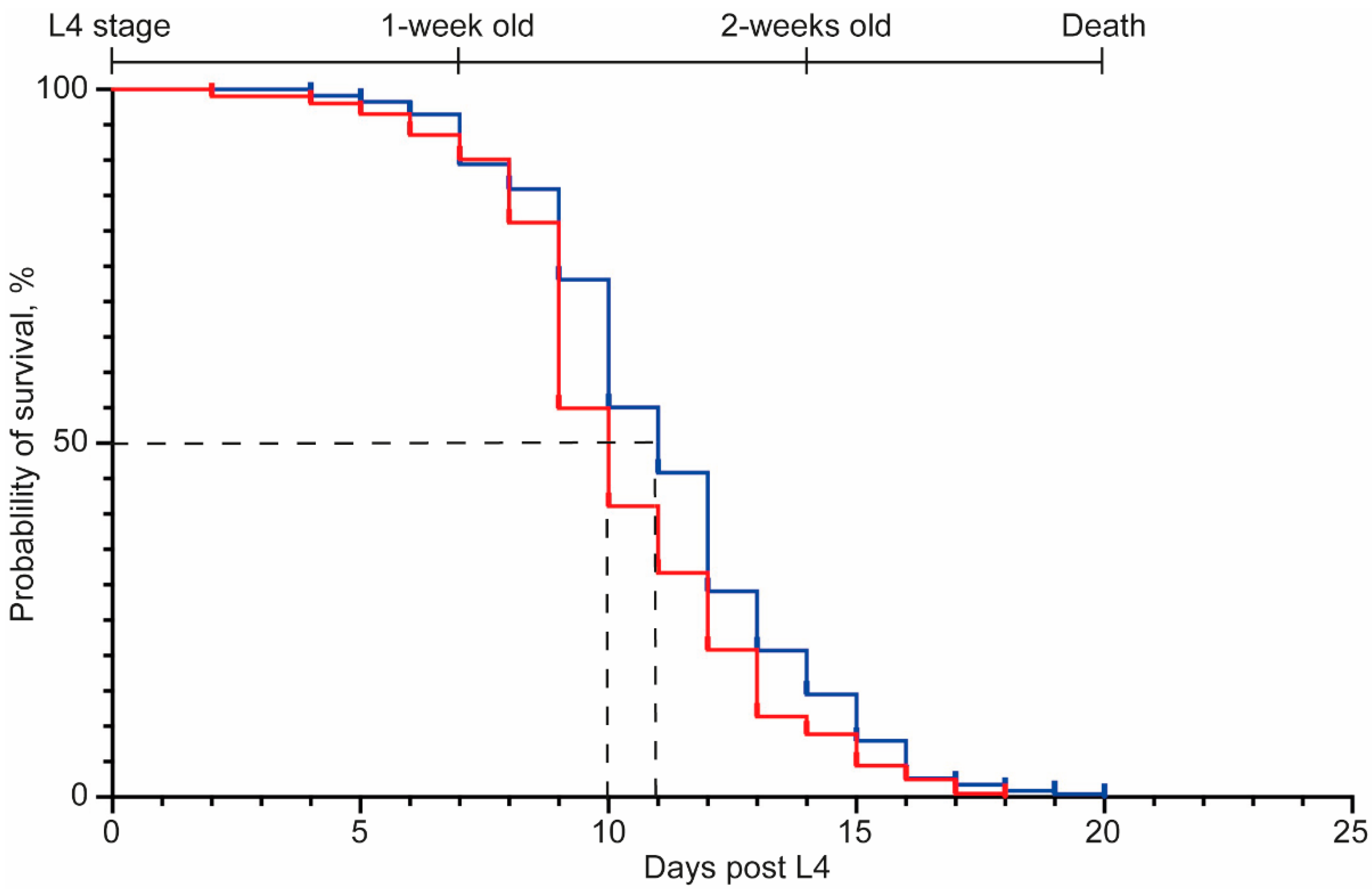


| N2 (Wild-Type) | NL5901 (α-Synuclein) | |||||
|---|---|---|---|---|---|---|
| 0.1 mM | 1 mM | 3 mM | 0.1 mM | 1 mM | 3 mM | |
| Control | 32 | 27 | ||||
| 0.1% DMSO | 32 | 37 | ||||
| 1% DMSO | 38 | 37 | ||||
| 3% DMSO | 31 | 22 | ||||
| Ambroxol | 33 | 34 | n/a | 23 | 21 | n/a |
| Betulin | 40 | 38 | n/a | 40 | 42 | n/a |
| Bexarotene | 33 | 41 | 32 | 33 | 39 | 38 |
| Galantamine | 22 | 29 | 32 | 21 | 17 | 20 |
| Tetrabenazine | 36 | 39 | n/a | 24 | 22 | n/a |
| Valproic acid | 40 | 39 | 46 | 34 | 41 | 40 |
| Drug | Solvent | Order Number | Molecular Weight (g/mol) | Company | Disease |
|---|---|---|---|---|---|
| Ambroxol | DMSO | A9797 | 378.10 | Sigma Aldrich | Parkinson’s |
| Betulin | DMSO | B9757 | 442.72 | Sigma Aldrich | Parkinson’s |
| Bexarotene | DMSO | SML0282 | 348.48 | Sigma Aldrich | Alzheimer’s |
| Galantamine | Water | Y0001279 | 368.27 | Sigma Aldrich | Alzheimer’s |
| Levodopa | Water | PHR1271 | 197.19 | Merck | Parkinson’s |
| Tetrabenazine | DMSO | T2952 | 317.42 | Sigma Aldrich | Huntington’s |
| Valproic Acid | Water | PHR1061 | 144.21 | Sigma Aldrich | Alzheimer’s Parkinson’s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, S.; van Dop, M.; Kolsters, N.; van de Klashorst, D.; Pogosova, A.; Rijs, A.M. Using a Caenorhabditis elegans Parkinson’s Disease Model to Assess Disease Progression and Therapy Efficiency. Pharmaceuticals 2022, 15, 512. https://doi.org/10.3390/ph15050512
Hughes S, van Dop M, Kolsters N, van de Klashorst D, Pogosova A, Rijs AM. Using a Caenorhabditis elegans Parkinson’s Disease Model to Assess Disease Progression and Therapy Efficiency. Pharmaceuticals. 2022; 15(5):512. https://doi.org/10.3390/ph15050512
Chicago/Turabian StyleHughes, Samantha, Maritza van Dop, Nikki Kolsters, David van de Klashorst, Anastasia Pogosova, and Anouk M. Rijs. 2022. "Using a Caenorhabditis elegans Parkinson’s Disease Model to Assess Disease Progression and Therapy Efficiency" Pharmaceuticals 15, no. 5: 512. https://doi.org/10.3390/ph15050512
APA StyleHughes, S., van Dop, M., Kolsters, N., van de Klashorst, D., Pogosova, A., & Rijs, A. M. (2022). Using a Caenorhabditis elegans Parkinson’s Disease Model to Assess Disease Progression and Therapy Efficiency. Pharmaceuticals, 15(5), 512. https://doi.org/10.3390/ph15050512






