Development of Nanocrystal Compressed Minitablets for Chronotherapeutic Drug Delivery
Abstract
1. Introduction
2. Results and Discussion
2.1. Compatibility Studies
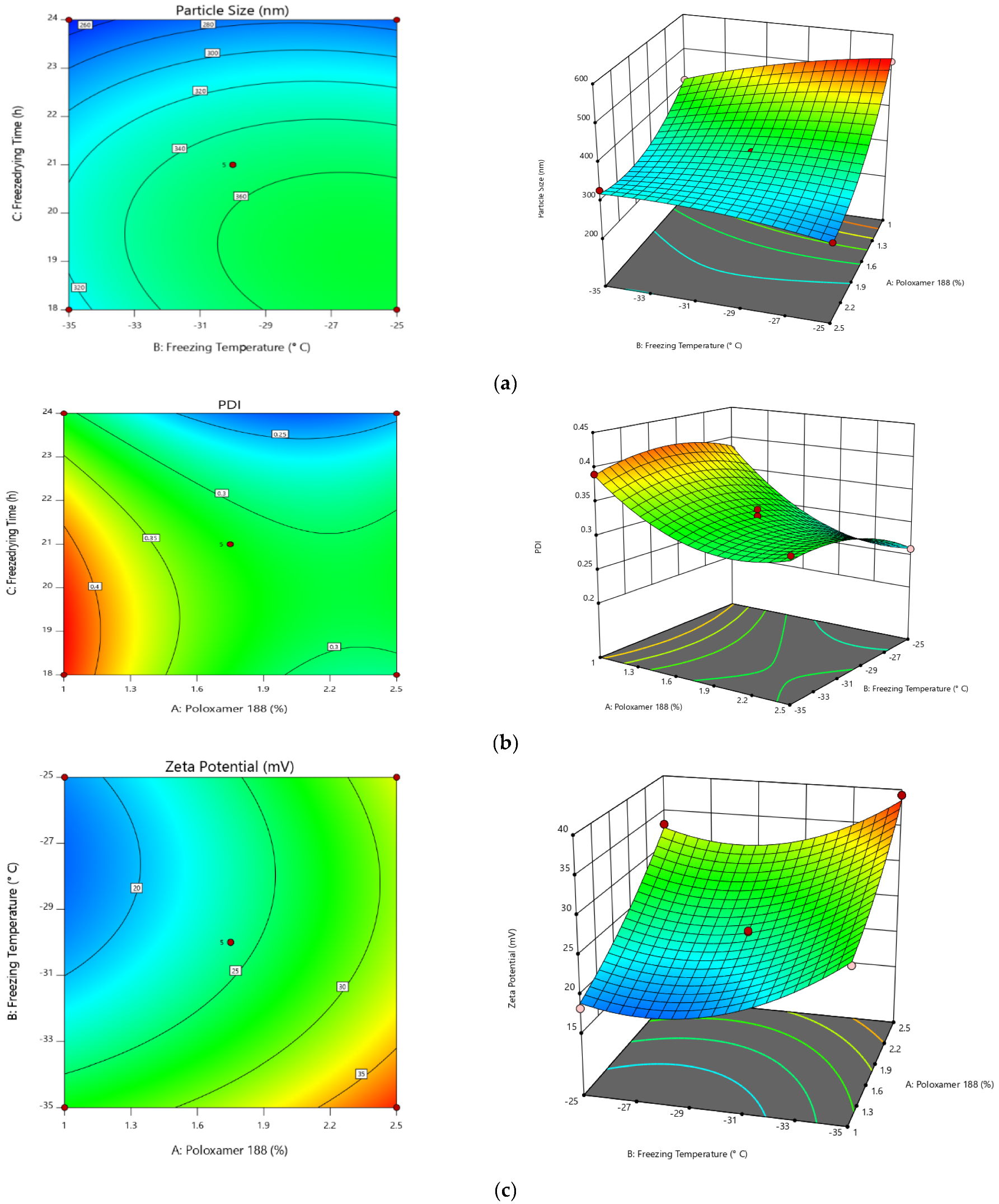
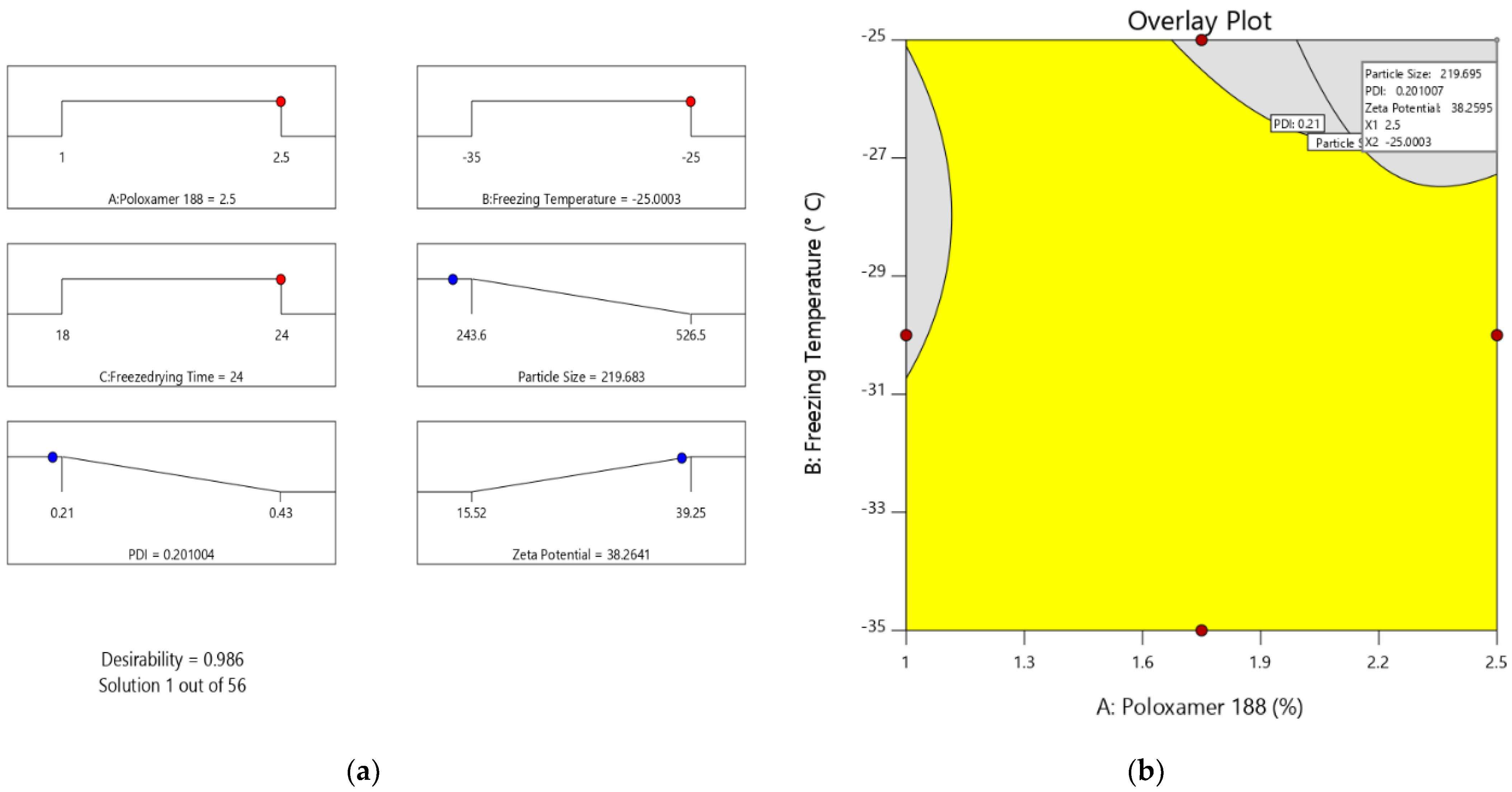
2.2. Step I: Optimization of Preparation of Valsartan Nanocrystals
2.2.1. Dissolution Study
2.2.2. Stability Studies
3. Materials and Methods
3.1. Materials
3.2. Formulation Development of “Tab in Cap” System for VS
3.3. Compatibility Studies
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.2. Differential Scanning Calorimetry (DSC) Studies
3.4. Step I: Optimization of Preparation of Valsartan Nanocrystals
Optimization
3.5. Characterization
3.6. Step II: Preparation of Valsartan Nanocrystal Minitablets
3.7. Step III: Coating of Core Mini Tablets
3.8. Step IV: Preparation of Fast Dissolving Tablets of Valsartan
3.9. Evaluation Tests for Prepared Tablets
Disintegration Test
3.10. Filling/Assembly of Mini-Tablets and F-VS into Size 1 Capsule
3.11. In Vitro Dissolution Study
3.12. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youan, B.B.C. Chronopharmaceutical drug delivery systems: Hurdles, hype or hope? Adv. Drug Deliv. Rev. 2010, 62, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Jawed, S.; Satish, C.S. Chrono-therapeutics: Recent advances and development. Int. J. Pharm. Res. 2021, 13, 475. [Google Scholar] [CrossRef]
- Gowthami, B.; Krishna, S.V.G.; Rao, D.S. Application of coating technology to chronotherapeutic drug delivery systems: Recent publications and patents. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100015. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, A.; Martchenko, S.E.; Biancolin, A.D.; Brubaker, P.L. Circadian Rhythms and the Gastrointestinal Tract: Relationship to Metabolism and Gut Hormones. Endocrinology 2020, 161, 12. [Google Scholar] [CrossRef]
- Bruguerolle, B.; Labrecque, G. Rhythmic pattern in pain and their chronotherapy. Adv. Drug Deliv. Rev. 2007, 59, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Portaluppi, F.; Lemmer, B. Chronobiology and chronotherapy of ischemic heart disease. Adv. Drug Deliv. Rev. 2007, 59, 952–965. [Google Scholar] [CrossRef]
- Latha, K.; Srikanth, V.V.; Sunil, S.A.; Srinivasa, N.R.; Uhumwangho, M.U.; Shaik, N.B.; Murthy, K.V.R. Applicability of Gum Karaya in the Preparation and In Vitro Evaluation of Losartan Potassium as Chronotherapeutic Drug Delivery System. J. Sci. Res. 2015, 7, 65–74. [Google Scholar] [CrossRef][Green Version]
- Qureshi, J.; Ali, J.; Baboota, S.; Ahuja, A.; Mallikarjun, C. Development and evaluation of chronotherapeutic drug delivery system for the management of nocturnal asthma. Trop. J. Pharm. Res. 2012, 11, 703–712. [Google Scholar] [CrossRef]
- Shukla, D.; Chakraborty, S.; Mishra, B. In vitro and in vivo evaluation of multilayered pastilles for chronotherapeutic management of nocturnal asthma. Expert Opin. Drug Deliv. 2012, 9, 9–18. [Google Scholar] [CrossRef]
- Soni, M.L.; Namdeo, K.P.; Jain, S.K.; Gupta, M.; Dangi, J.S.; Kumar, M.; Dangi, Y.S. pH-enzyme di-dependent chronotherapeutic drug delivery system of theophylline for nocturnal asthma. Chem. Pharm. Bull. 2011, 59, 191–195. [Google Scholar] [CrossRef][Green Version]
- Manjunath, P.N.; Seth, A.K.; Anand, K.R.; Vivekandan, S. Approaches of Chronotherapeutic Drug Delivery Systems and Its Impact on Circadian Rhythm. Int. J. Pharm. Res. 2021, 13, 202. [Google Scholar] [CrossRef]
- Kaur, K.; Seth, N.; Gill, N.S. Advancement In Chronotherapeutic Drug Delivery System: Marketed Technologies And Current Scenario. J. Pharm. Sci. Res. 2019, 11, 1984–1989. [Google Scholar]
- Rojas, M.; Chávez-Castillo, M.; Pírela, D.; Ortega, Á.; Salazar, J.; Cano, C.; Chacín, M.; Riaño, M.; Batista, M.J.; Díaz, E.A.; et al. Chronobiology and Chronotherapy in Depression: Current Knowledge and Chronotherapeutic Promises. Curr. Psychiatry Res. Rev. 2020, 16, 179–193. [Google Scholar] [CrossRef]
- Kashyap, S.; Bala, R.; Behl, T. Understanding the Concept of Chronotherapeutics in the Management of Diabetes Mellitus. Curr. Diabetes Rev. 2020, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sunil, S.A.; Rao, N.S.; Srikanth, M.V.; Uhumwangho, M.U.; Kumar, K.S.P.; Murthy, K.V.R. Development and evaluation of a chronotherapeutic drug delivery system of torsemide. Brazilian J. Pharm. Sci. 2011, 47, 593–600. [Google Scholar] [CrossRef]
- Saigal, N.; Baboota, S.; Ahuja, A.; Ali, J. Site Specific Chronotherapeutic Drug Delivery Systems: A Patent Review. Recent Pat. Drug Deliv. Formul. 2009, 3, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Olff, M. Traumatic stress and the circadian system: Neurobiology, timing and treatment of posttraumatic chronodisruption. Eur. J. Psychotraumatol. 2020, 11, 1833644. [Google Scholar] [CrossRef]
- Sewlall, S.; Pillay, V.; Danckwerts, M.P.; Choonara, Y.E.; Ndesendo, V.M.; du Toit, L. A Timely Review of State-of-the-Art Chronopharmaceuticals Synchronized with Biological Rhythms. Curr. Drug Deliv. 2010, 7, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Buijink, M.R.; Michel, S. A multi-level assessment of the bidirectional relationship between aging and the circadian clock. J. Neurochem. 2021, 157, 73–94. [Google Scholar] [CrossRef]
- Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Antidepressants and circadian rhythm: Exploring their bidirectional interaction for the treatment of depression. Pharmaceutics 2021, 13, 1975. [Google Scholar] [CrossRef]
- Kuila, A.; Dhandapani, N.V.; Bhowmik, H.; Soni, A.; Kumar, K.H. Chronotherapeutic drug delivery system: An emerging approach to treat circadian rhythmic related disease. Asian J. Pharm. Clin. Res. 2018, 11, 15–20. [Google Scholar] [CrossRef]
- Sunil, S.A.; Srikanth, M.V.; Rao, N.S.; Uhumwangho, M.U.; Latha, K.; Murthy, K.V.R. Chronotherapeutic Drug Delivery Systems—An Approach to Circadian Rhythms Diseases. Curr. Drug Deliv. 2011, 8, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Pena-Seijo, M.; Hermida-Ameijeiras, A.; Lopez-Paz, J.; Calvo-Gonzalez, G.; Romero-Miguez, M.; Coca-Payeras, A.; Calvo-Gomez, C. Administration time of high-dose valsartan in non dipper hypertensive patients: PP.33.306. J. Hypertens. 2010, 28, e542. [Google Scholar] [CrossRef]
- Ushijima, K.; Nakashima, H.; Shiga, T.; Harada, K.; Ishikawa, S.; Ioka, T.; Ando, H.; Fujimura, A. Different chronotherapeutic effects of valsartan and olmesartan in non-dipper hypertensive patients during valsartan treatment at morning. J. Pharmacol. Sci. 2015, 127, 62–68. [Google Scholar] [CrossRef]
- Nayak, U.Y.; Shavi, G.V.; Nayak, Y.; Averinen, R.K.; Mutalik, S.; Reddy, S.M.; Das Gupta, P.; Udupa, N. Chronotherapeutic drug delivery for early morning surge in blood pressure: A programmable delivery system. J. Control. Release 2009, 136, 125–131. [Google Scholar] [CrossRef]
- Sokar, M.; Hanafy, A.; Elkamel, A.; El-Gamal, S. Design of chronomodulated drug delivery system of valsartan: In vitro characterization. Indian J. Pharm. Sci. 2015, 77, 470–477. [Google Scholar] [CrossRef]
- Thombre, A.G.; Shah, J.C.; Sagawa, K.; Caldwell, W.B. In vitro and in vivo characterization of amorphous, nanocrystalline, and crystalline ziprasidone formulations. Int. J. Pharm. 2012, 428, 8–17. [Google Scholar] [CrossRef]
- Dening, T.J.; Rao, S.; Thomas, N.; Prestidge, C.A. Silica encapsulated lipid-based drug delivery systems for reducing the fed/fasted variations of ziprasidone in vitro. Eur. J. Pharm. Biopharm. 2016, 101, 33–42. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug Nanoparticles: Formulating Poorly Water-Soluble Compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef]
- Tashan, E.; Karakucuk, A.; Celebi, N. Optimization and in vitro evaluation of ziprasidone nanosuspensions produced by a top-down approach. J. Drug Deliv. Sci. Technol. 2019, 52, 37–45. [Google Scholar] [CrossRef]
- Karakucuk, A.; Celebi, N.; Teksin, Z.S. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. A Design of Experiment approach. Eur. J. Pharm. Sci. 2016, 95, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.N.; Karakucuk, A.; Ilbasmis-Tamer, S.; Celebi, N. Dermal flurbiprofen nanosuspensions: Optimization with design of experiment approach and in vitro evaluation. Eur. J. Pharm. Sci. 2018, 122, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.B.; Shaikh, F.; Patel, V.B.; Surti, N. Application of Experiential Design for Framing Gastroretentive Microsponges of Glipizide: Screening of Critical Variables by Plackett-Burman Design and Optimization by Box-Behnken Design. Indian J. Pharm. Educ. Res. 2021, 55, 966–978. [Google Scholar] [CrossRef]
- Rai, N.; Bal, T.; Swain, S. In vitro Evaluations of Biodegradable Polyacrylamide Grafted Moringa Bark Gum Graft Copolymer (MOG-g- PAAM) as Biomedical and Controlled Drug Delivery Device Synthesized by Microwave Accelerated free Radical Synthesis. Indian J. Pharm. Educ. Res. 2020, 54, 385–396. [Google Scholar] [CrossRef]
- Badhwar, R.; Singh, R.; Popli, H. Implementation of Quality by Design (QbD) Approach in Development of QCT-SMEDDS with Combination of AgNPs for Diabetic Foot Ulcer Management. Indian J. Pharm. Educ. Res. 2021, 55, 1207–1223. [Google Scholar]
- Ahad, H.A.; Chinthaginjala, H.; Priyanka, M.S.; Raghav, D.R.; Gowthami, M.; Jyothi, V.N. Datura stramonium Leaves Mucilage Aided Buccoadhesive Films of Aceclofenac using 32 Factorial Design with Design-Expert Software. Indian J. Pharm. Educ. Res. 2021, 55, s396–s404. [Google Scholar] [CrossRef]
- Kim, K.M. Design of Experiments for Critical Material Attributes Assessment of Linagliptin and Metformin Fixed-dose Combination Tablets. Indian J. Pharm. Educ. Res. 2021, 55, 701–708. [Google Scholar] [CrossRef]
- Naveen, N.R.; Kurakula, M.; Gowthami, B. Process optimization by response surface methodology for preparation and evaluation of methotrexate loaded chitosan nanoparticles. Mater. Today Proc. 2020, 33, 2716–2724. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R.; Patel, B.; Manne, R.; Patel, D.B. Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels 2021, 7, 96. [Google Scholar] [CrossRef]
- Abuhmaidan, Y.; Al-Majali, S. The impact of the coronavirus pandemic on mental health among al ain university students in light of some demographic variables. Psychiatr. Danub. 2021, 32, 482–490. [Google Scholar] [CrossRef]
- Xu, P.; Cao, J.; Yin, C.; Wang, L.; Wu, L. Quantum chemical study on the adsorption of megazol drug on the pristine BC3 nanosheet. Supramol. Chem. 2021, 33, 63–69. [Google Scholar] [CrossRef]
- Liu, S.; Ho, P.C. Formulation optimization of scutellarin-loaded HP-β-CD/chitosan nanoparticles using response surface methodology with Box–Behnken design. Asian J. Pharm. Sci. 2017, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Jeirani, Z.; Mohamed Jan, B.; Si Ali, B.; Mohd Noor, I.; Chun Hwa, S.; Saphanuchart, W. The optimal mixture design of experiments: Alternative method in optimizing the aqueous phase composition of a microemulsion. Chemom. Intell. Lab. Syst. 2012, 112, 1–7. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Gong, W.; Liang, Z. Quantifying the effects of fuel compositions on GDI-derived particle emissions using the optimal mixture design of experiments. Fuel 2015, 154, 252–260. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Ispaghula mucilage-gellan mucoadhesive beads of metformin HCl: Development by response surface methodology. Carbohydr. Polym. 2014, 107, 41–50. [Google Scholar] [CrossRef]
- Hooda, A.; Nanda, A.; Jain, M.; Kumar, V.; Rathee, P. Optimization and evaluation of gastroretentive ranitidine HCl microspheres by using design expert software. Int. J. Biol. Macromol. 2012, 51, 691–700. [Google Scholar] [CrossRef]
- Lai, W.F.; Huang, E.; Lui, K.H. Alginate-based complex fibers with the Janus morphology for controlled release of co-delivered drugs. Asian J. Pharm. Sci. 2021, 16, 691–700. [Google Scholar] [CrossRef]
- Rizg, W.Y.; Naveen, N.R.; Kurakula, M.; Bukhary, H.A.; Safhi, A.Y.; Alfayez, E.; Sindi, A.M.; Ali, S.; Murshid, S.S.; Hosny, K.M. QbD Supported Optimization of the Alginate-Chitosan Nanoparticles of Simvastatin in Enhancing the Anti-Proliferative Activity against Tongue Carcinoma. Gels 2022, 8, 103. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Design expert supported mathematical optimization of repaglinide gastroretentive floating tablets: In vitro and in vivo evaluation. Futur. J. Pharm. Sci. 2017, 3, 140–147. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Kurakula, M. Okra-thioglycolic acid conjugate-synthesis, characterization, and evaluation as a mucoadhesive polymer. Processes 2020, 8, 316. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. In situ gel loaded with chitosan-coated simvastatin nanoparticles: Promising delivery for effective anti-proliferative activity against tongue carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.; Šibanc, R.; Dreu, R.; Frankiewicz, M.; Sznitowska, M. Assessment of Mini-Tablets Coating Uniformity as a Function of Fluid Bed Coater Inlet Conditions. Pharmaceutics 2021, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Gowthami, B.; Krishna, S.V.G.; Rao, D.S. Formulation of Tablets in Capsule system: Statistical optimization for chronotherapeutic drug delivery of propranolol hydrochloride. J. Drug Deliv. Sci. Technol. 2021, 63, 102398. [Google Scholar] [CrossRef]
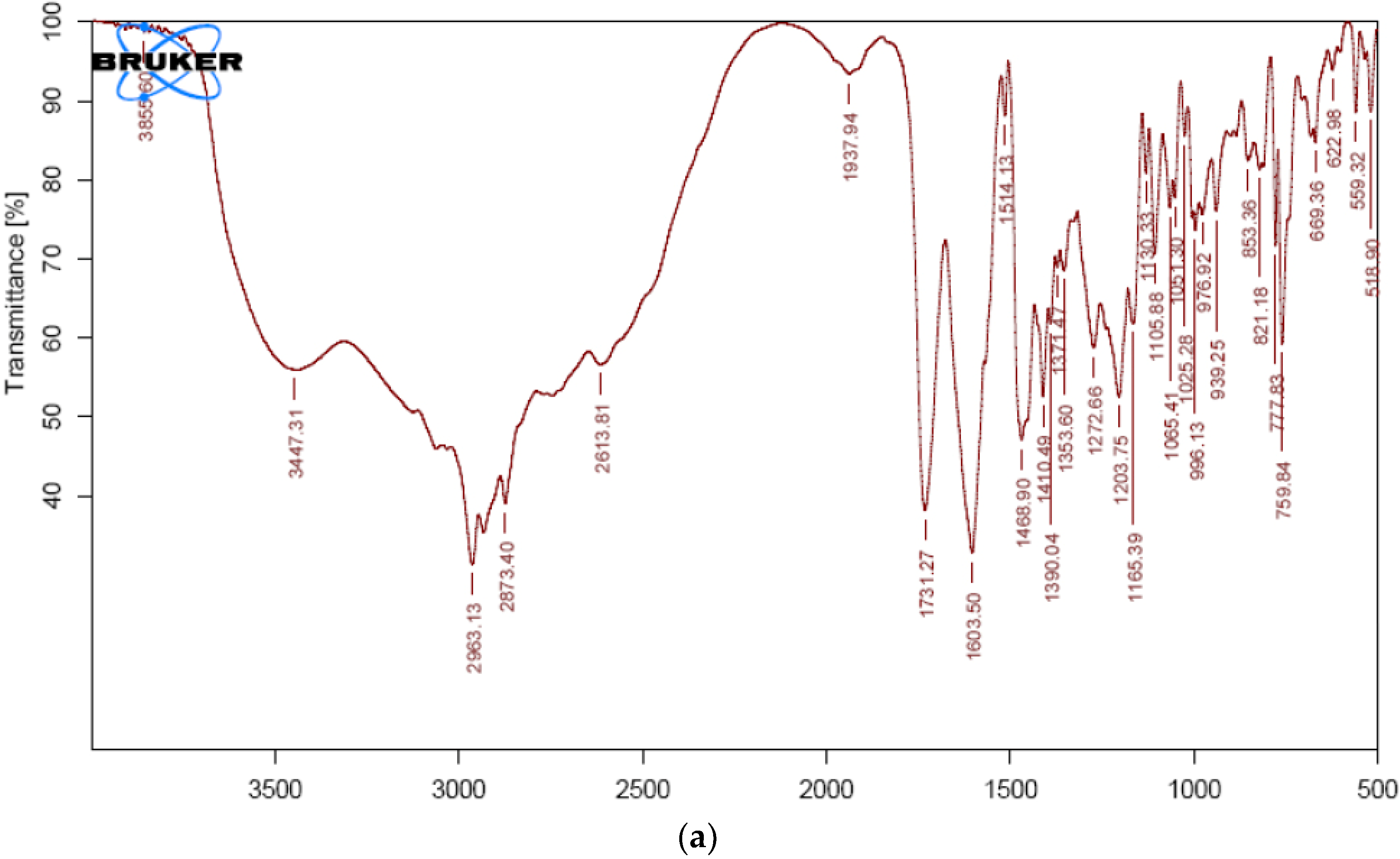





| Sample | Frequency cm−1 | Group Assigned |
|---|---|---|
| VS | 3447 | N-H functional group |
| 2963 | C-H group stretching | |
| 1731 | Carboxyl carbonyl | |
| 1603 | Amide carbonyl group | |
| 1514 | C=C aromatic group | |
| 1273 | C-O stretch | |
| VS formulation | 3425 | N-H functional group |
| 2962 | C-H group stretching | |
| 1730 | Carboxyl carbonyl | |
| 1603 | Amide carbonyl group | |
| 1513 | C=C aromatic group | |
| 1272 | C-O stretch |
| Run | A: Poloxamer 188 | B: Freezing Temperature | C: Freeze Drying Time | Particle Size | PDI | Zeta Potential |
|---|---|---|---|---|---|---|
| % | °C | h | nm | mV | ||
| 6 | 1 | −35 | 21 | 413.5 | 0.39 | 26.95 |
| 5 | 1 | −25 | 21 | 526.5 | 0.38 | 18.21 |
| 12 | 1 | −30 | 18 | 521.4 | 0.43 | 20.24 |
| 15 | 1 | −30 | 24 | 398.2 | 0.31 | 15.52 |
| 8 | 1.75 | −35 | 18 | 311.2 | 0.31 | 28.59 |
| 7 | 1.75 | −25 | 18 | 374.5 | 0.28 | 20.18 |
| 14 | 1.75 | −35 | 24 | 243.6 | 0.22 | 31.24 |
| 9 | 1.75 | −25 | 24 | 272.4 | 0.21 | 25.8 |
| 3 | 1.75 | −30 | 21 | 351.4 | 0.33 | 23.76 |
| 13 | 1.75 | −30 | 21 | 351.3 | 0.32 | 24.06 |
| 2 | 1.75 | −30 | 21 | 351.2 | 0.31 | 23.74 |
| 10 | 1.75 | −30 | 21 | 351.9 | 0.32 | 23.84 |
| 4 | 1.75 | −30 | 21 | 350.9 | 0.31 | 24.23 |
| 1 | 2.5 | −35 | 21 | 329.8 | 0.32 | 39.25 |
| 11 | 2.5 | −25 | 21 | 281.6 | 0.26 | 33.09 |
| 17 | 2.5 | −30 | 18 | 287.2 | 0.29 | 22.56 |
| 16 | 2.5 | −30 | 24 | 261.3 | 0.24 | 35.71 |
| Response | Models | R2 | Adju.R2 | Pred.R2 | Adequate Precision | Sequential p-Value | Remarks |
|---|---|---|---|---|---|---|---|
| PS | Linear | 0.7459 | 0.6873 | 0.4682 | ---- | 0.0004 | |
| 2 FI | 0.8347 | 0.7356 | 0.1509 | 51.052 | 0.2125 | ||
| Quadratic | 0.9964 | 0.9917 | 0.9423 | --- | <0.0001 | Suggested | |
| Cubic | 1.0000 | 1.0000 | --- | <0.0001 | Aliased | ||
| PDI | Linear | 0.6614 | 0.5833 | 0.3061 | --- | 0.0022 | |
| 2 FI | 0.6981 | 0.5170 | −0.4893 | --- | 0.7524 | ||
| Quadratic | 0.9914 | 0.9804 | 0.9390 | 35.781 | <0.0001 | Suggested | |
| Cubic | 0.9947 | 0.9789 | --- | 0.5413 | |||
| Linear | 0.7151 | 0.6494 | 0.4283 | 0.0008 | |||
| Zeta potential | 2 FI | 0.8491 | 0.7585 | 0.3272 | 0.0842 | ||
| Quadratic | 0.9951 | 0.9889 | 0.9261 | 46.676 | <0.0001 | Suggested | |
| Cubic | 0.9997 | 0.9988 | 0.0064 |
| Intercept | A | B | C | AB | AC | BC | A² | B² | C² | |
|---|---|---|---|---|---|---|---|---|---|---|
| Particle Size | 351.34 | −87.4625 | 19.6125 | −39.85 | −40.3 | 24.325 | −8.625 | 51.555 | −15.045 | −35.87 |
| p-values | < 0.0001 | 0.0001 | <0.0001 | <0.0001 | 0.0003 | 0.0500 | <0.0001 | 0.0039 | <0.0001 | |
| PDI | 0.318 | −0.05 | −0.01375 | −0.04125 | −0.0125 | 0.0175 | 0.005 | 0.041 | −0.0215 | −0.0415 |
| p-values | < 0.0001 | 0.0019 | <0.0001 | 0.0173 | 0.0034 | 0.2548 | <0.0001 | 0.0009 | <0.0001 | |
| Zeta Potential | 23.926 | 6.21125 | −3.59375 | 2.0875 | 0.645 | 4.4675 | 0.7425 | 1.252 | 4.197 | −1.6705 |
| p-values | <0.0001 | < 0.0001 | <0.0001 | 0.0915 | <0.0001 | 0.0591 | 0.0059 | <0.0001 | 0.0013 |
| Formulation | Average Weight (mg) | Weight Variation (%) | Hardness (kg/cm2) | Friability (%) | Disintegration (min) |
|---|---|---|---|---|---|
| VNM | 101 | ±1.25 | 7 | 0.154 | 368 |
| F-VS1 | 100 | ±0.85 | 4 | 0.268 | 14 |
| F-VS2 | 101 | ±1.55 | 5 | 0.280 | 9 |
| F-VS3 | 101 | ±1.25 | 4 | 0.325 | 8 |
| F-VS4 | 100 | ±0.65 | 4 | 0.325 | 16 |
| S. No | Formulation Code | VS-NC | MCC (PH 102) | Talc | Magnesium Stearate | HPMC K 15 M |
|---|---|---|---|---|---|---|
| 1. | VNM-1 | 10 mg | 64.2 mg | 0.4 mg | 0.4 mg | 25 mg |
| Test | Initial | 25 °C ± 2 °C + 60% ± 5% RH | 40 °C ± 2 °C + 75% ± 5% RH | ||
|---|---|---|---|---|---|
| 3 M | 6 M | 3 M | 6 M | ||
| Capsule physical appearance | Complies | Complies | Complies | Complies | Complies |
| f2 | -- | 96.08 | 94.25 | 95.28 | 93.25 |
| Factors/Independent Variables | Levels | Responses/Dependent Variables | Constraints | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Concentration of Poloxamer 188 (%)—X1 | 6 | 8 | 10 | Particle size (nm) | Minimum |
| Freezing Temperature (°C)—X2 | 70 | 80 | 90 | PDI | Minimum |
| Freeze drying Time (h)—X3 | 1.5 | 2 | 2.5 | Zeta Potential (mV) | Maximum |
| S. No | Material | Quantity |
|---|---|---|
| 1. | VS | 40 |
| 2. | Polyplasdone XL and SSG | 2 mg, 6 mg (F-VS1,F-VS2) and 3 mg, 9 mg (F-VS3, F-VS4) |
| 3. | Spray-dried lactose | 55 mg (F-VS1), 51 mg (F-VS2) 54 mg (F-VS3) and 48 mg (F-VS4) |
| 4. | Magnesium stearate | 1 mg |
| 5. | Talc | 2 mg |
| Total tablet weight | 100 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreeharsha, N.; Naveen, N.R.; Anitha, P.; Goudanavar, P.S.; Ramkanth, S.; Fattepur, S.; Telsang, M.; Habeebuddin, M.; Answer, M.K. Development of Nanocrystal Compressed Minitablets for Chronotherapeutic Drug Delivery. Pharmaceuticals 2022, 15, 311. https://doi.org/10.3390/ph15030311
Sreeharsha N, Naveen NR, Anitha P, Goudanavar PS, Ramkanth S, Fattepur S, Telsang M, Habeebuddin M, Answer MK. Development of Nanocrystal Compressed Minitablets for Chronotherapeutic Drug Delivery. Pharmaceuticals. 2022; 15(3):311. https://doi.org/10.3390/ph15030311
Chicago/Turabian StyleSreeharsha, Nagaraja, Nimbagal Raghavendra Naveen, Posina Anitha, Prakash S. Goudanavar, Sundarapandian Ramkanth, Santosh Fattepur, Mallikarjun Telsang, Mohammed Habeebuddin, and Md. Khalid Answer. 2022. "Development of Nanocrystal Compressed Minitablets for Chronotherapeutic Drug Delivery" Pharmaceuticals 15, no. 3: 311. https://doi.org/10.3390/ph15030311
APA StyleSreeharsha, N., Naveen, N. R., Anitha, P., Goudanavar, P. S., Ramkanth, S., Fattepur, S., Telsang, M., Habeebuddin, M., & Answer, M. K. (2022). Development of Nanocrystal Compressed Minitablets for Chronotherapeutic Drug Delivery. Pharmaceuticals, 15(3), 311. https://doi.org/10.3390/ph15030311








